Abstract
Colorectal cancers generally consist of multiple subclones. These subclones have their own unique characteristics, resulting in intratumor heterogeneity (ITH). As the discussion of ITH has advanced, a model describing the relationship of ITH to the tumor has gradually emerged. ITH can be divided into two types of intraprimary tumor heterogeneity and intraindividual tumor heterogeneity, the former for further understanding of tumor composition, and the latter for providing more information about evolutionary patterns. With the rapid development of new methods, such as next‐generation, polyguanine region sequencing, and Image detection, researchers may unravel the secrets underlying ITH. The higher the ITH of the tumor, the richer the interaction between the subclones maybe, or the greater the chance of the tumor getting more powerful subclones may be, thus increasing the malignant potential of the tumor. Existing evidence suggests that ITH may increase the ability of tumors to resist treatment and can be used as an independent influence on the prognosis of colorectal cancer. We reviewed 80 recent studies to give researchers a new perspective on colorectal cancer. There is still a limited amount of research in this area. Further study of the relationship between ITH and clinical endpoints may lead to the development of new treatment strategies.
Keywords: clonal evolution, colorectal cancer, intratumor heterogeneity, prognosis, review, sequence analysis
Intratumor heterogeneity may reveal the course of tumor development, progression, and metastasis, and may affect prognosis. The concept of intraprimary tumor heterogeneity and intraindividual tumor heterogeneity proposed by us may help to further enhance researchers' understanding of this field.
1. INTRODUCTION
Colorectal cancer (CRC) is the fourth most commonly occurring cancer among men. Globally, it is the third and fourth leading cause of cancer‐related deaths in men and women, respectively, and it represents one of the major diseases that threatens human health. 1 , 2 The uncontrollable proliferation and metastasis of cancer cells within major organs, such as the liver and lung, are the leading causes of death in CRC patients. 3 , 4 Surgical resection is generally used for CRC patients that do not present with metastasis, however, resection of the primary lesions is often insufficient. 5 , 6 Cancer recurrence in major organs following resection remains a significant cause for treatment failure. To improve clinical outcome in patients with recurrent CRC, oncologists rely on gene testing to select eligible drugs. During this process, they are often surprised to find that there are differences in mutated sites and biomarkers between tumors from the primary site and the matched metastasis. 7 Over time, researchers have discovered that colorectal cancers are not homogeneous, but rather heterogeneous, consisting of many different cells or subclones of which different gene expression profiles among them. 8 These differences within the tumor are referred to as intratumor heterogeneity (ITH). Heterogeneity endows tumors with multiple capabilities and biological characteristics, making them more prone to metastasis, recurrence, and drug resistance. 9
In the classic CRC development pathway, an adenoma undergoes a shift to become an adenocarcinoma. Successive mutations occur in the APC, TGF‐beta, RAS, and TP53 genes during this process. 10 As tumor cells divide, these mutations occur randomly, accumulate and are transmitted to their offspring, resulting in multiple subclones with different genotypes. 11 Upon inspection, the primary tumor now consists of a collection of different subclones. At this stage, the heterogeneity in the primary tumor lesion may be referred to as intraprimary tumor heterogeneity (IPTH) (Figure 1). IPTH can provide us with a clearer understanding of the internal composition of tumors. Furthermore, cancer is so pernicious that it will never be confined to its original position. There are several existing modes of cancer metastasis including parallel progression, linear progression, dormancy mode, and tumor self‐seeding. 12 However, ITH also occurs at the metastatic lesion regardless of the specific mode. 13 The differences between the primary and matched metastatic lesions are known as intraindividual tumor heterogeneity (IITH) (Figure 1). IITH may act as a tracker to explore patterns and mechanisms of cancer metastasis. For the first time, the borderline between IPTH and IITH can be distinguished and this will provide a theoretical foundation for future research.
FIGURE 1.
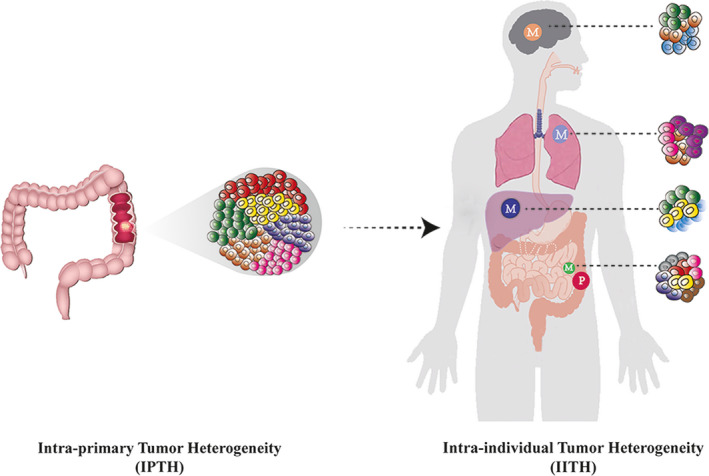
Intratumor heterogeneity (IPTH) and intraindividual heterogeneity (IITH) in colorectal cancer. (IPTH) This schematic image simulates a primary tumor in the descending colon composed of six distinct subclones (red, yellow, green, light brown, blue, and pink). Within the tumor, these subclones are clustered in different parts of the tumor. Each subclone grows in its own space and can be mixed at the boundary. The heterogeneity of primary tumors results from these subclones. (IITH) As a tumor progresses, different subclones may metastasize to perienteric lymph nodes and distant organs such as liver, lung, and brain. Perienteric lymph nodes were metastasized by subclones of four primary tumors (yellow, red, blue, and pink), with the birth of two private subclones (brown and gray, respectively). The liver was metastasized from subclones of two primary tumors (yellow and green, respectively) and a private subclone (sky blue) was born. Similarly, the lung has a subclone of two primary tumors (pink and light brown, respectively) and a private subclone (purple). There are two primary subclones of the brain (green and light brown) and a private subclone (sky blue). The schematic diagram indicates that all metastases are related to the primary tumor. The metastasis of lung and liver was similar to that of the perienteric lymph nodes, with the possibility of linear metastasis. Brain metastasis was different from perienteric lymph nodes, suggesting parallel metastasis. There are even replants from liver metastases. (P: primary site; M: metastatic site)
In recent years, the development of next‐generation sequencing (NGS) technology has facilitated the study of ITH. Many studies related to ITH have been published. In this review, we summarize the research progress made with respect to ITH in CRC. ITH may be the key to understanding the molecular basis for the evolution of CRC and other cancers.
2. ITH REFLECTS THE EVOLUTIONARY PATHWAY OF CRC
With respect to ITH, it is important to understand the evolutionary path of cancer. ITH results from branched evolution, which is the natural product of mutation. 14 Mutations accompany tumor development during the growth of CRC and occur over the evolutionary course of a tumor. Because most mutations are neutral, they continue to spread as tumor cells proliferate. Over time, mutations accumulate and genotypes become abundant and diverse. 15 Accumulation of new mutations eventually leads to qualitative transformation and promotes tumor evolution. 16 Due to genetic drift, the variant allele frequency (VAF) may gradually increase in tumor cells. 17 The emergence of selection caused by various factors further changes the proportion of mutant genotypes in CRC. Eventually new subclones will carry these mutations because of the proliferation of tumor cells. 18 As a result, ITH emerges from CRC.
Although ITH appears to amplify the effect of mutations under selection, the timing of the specific mutation that leads to the creation of the new subclone is critical. It is possible that the biological characteristics of tumors are established early in the course of development, and tumor progression plays a role in subsequent amplification and selection. 19 In the early stage of tumorigenesis, there are public mutations shared by all subclones and private mutations that occur during the process of replication. Private mutations produced in the early stage of tumorigenesis are trunk mutations, which gradually evolve into multiple subclones that are distributed to different regions of the tumor. 20 Although new private mutations may occur during tumor cell proliferation, Korolev et al 21 reported that the continual selective sweep that leads to the alteration of subclone structure is rare. Therefore, it is difficult for subsequent private mutations to produce new subclones. Some studies further support this idea. Patients with precancerous lesions accumulated more driver mutations and exhibited a higher VAF compared with advanced CRC patients. 22 Multiple trunk APC mutations and heterogeneity of KRAS mutations were also found in early colorectal adenomas, suggesting that ITH is already present in such lesions. 23 The early evolutionary progress of CRC is branched, while most mutations in advanced CRC are neutral. Time is a natural process that enables the expansion of the early selection effect.
In addition to coding DNA, there are other factors that can produce ITH. In the field of noncoding DNA, the impact of microsatellite instability (MSI) status to ITH is somewhat controversial. Studies have found that ITH in MSS is higher than MSI‐H, which may be due to the ITH of MSI‐H tumors reduced by tumor infiltrating lymphocytes and M1 macrophages. 24 , 25 While some researchers have discovered that CRC patients with MSI‐H have higher ITH, this may pertain to the increased mutation rate caused by mismatch repair gene defects. 26 Chromosome instability (CIN) can also cause ITH through the gain or loss of large segments of the tumor genome, or by a change in the ploidy of chromosomes. 27 ITH resulting from these phenomena may also provide more information with respect to the evolutionary pathway of CRC.
3. GENE SEQUENCING, IMAGING, AND CELL CULTURE ARE THREE "WINDOWS" TO EXPLORE ITH
The ITH of CRC is represented by subclones distributed in different spaces. 28 In order to identify these subclones, the most common method is to obtain tumor VAF. This relies on NGS to detect DNA information or RNA sequencing to indirectly reflect genetic information to obtain single nucleotide variant (SNV), indel, or copy number variation (CNV) to calculate VAF. 29 Cancer cell fraction (CCF) also needs to be taken into account to assess the reliability of VAF. 30 After the subclones of each sample have been identified, it is common to construct phylogenetic trees from multiple samples using Bayesian methods in order to obtain more information about the evolutionary patterns of tumors. 31 Once a specific value of bootstrapper (usually greater than 70) is set, each sample composed of multiple subclones can be recombined into a different trunk, and the number of branches of the evolutionary tree and the genetic distance between the samples can be determined. 32 In addition, other algorithms for constructing the phylogenetic trees, such as Binary tree, 33 are also emerging gradually. In recent years, single‐cell sequencing has provided a hopeful method for building evolutionary trees by providing cell‐level genetic information, which has been partially confirmed in some studies. 34 , 35 Some researchers also try to explore ITH from other perspectives than subclones. For example, Oesper et al 36 developed an algorithm known as “tumor heterogeneity analysis” that can assess the level of CNV heterogeneity within a tumor.
In recent years, noncoding DNA has been used by researchers to track ITH. Naxerova et al 32 proposed the construction of phylogenetic trees using polyguanine, a hypervariable region in DNA. This may be a more convenient way to determine the source of ITH compared with the use of coding DNA. Base insertions and deletions in the polyguanine region can be detected. A similar number of insertions and deletions in the same polyguanine tract may be used as markers specific to a subclone. The authors included 22 patients and found that the insertion and deletion within the polyguanine region could be detected in 91% of the tumors. After establishing phylogenetic trees, it was evident that the primary sites of multiple tumor metastases are composed of multiple subclones, and the metastatic tumor is related to a specific subclone within the primary tumor. 8 In colorectal cancer, the polyguanine technique is robust for determining the relationship between lymphatic and distant metastasis. In a study of 17 patients, lymphatic and distant metastases arose from two independent subclones from the primary lesion in 11 patients. Of the remaining six patients, lymphatic and distant metastasis were homologous. This provides more evidence for a linear (Tumor cells move from the primary lesion to the lymph nodes and subsequently to the distant organs orderly) or parallel (Tumor cells independently metastasize to lymph nodes and distant organs) metastasis model in colorectal cancer. In fact, Naxerova et al 32 suggested that different patients with colorectal cancer may have different metastasis patterns.
When studying ITH, multiregion sampling is usually adopted. With respect to tumor sampling, multiple angles should be considered to cover multiple tissues at different depths. 37 Some studies have indicated that sampling at three tumor‐rich sites rich may reduce the negative results of DNA testing, increase the accuracy of gene detection and improve overall ITH assessment. 38 Sequencing tumor tissue DNA by multiregion sampling, however, may also be challenging. The contrast sampling method of tumor center, tumor periphery, and normal tissue obtained by diagnostic puncture method cannot achieve the function of multipoint sampling to obtain the information of intratumor heterogeneity. 39 In addition, it is very difficult to obtain a multipoint sample that meets the test requirements in vivo. Therefore, many researchers have proposed the use of imaging to detect ITH to solve the problem. A method called “cancer rainbow” can fluorescently barcode somatic mutations, enabling the visualization of the expansion and diffusion of oncogenes. This allows direct observation of ITH within tumor tissue. 40 Different levels of VEGFR‐2 expression can also be determined in tumor tissue by molecular MRI. 41 18F‐FDG (18F‐flurodeoxyglucose) PET can track ITH based on blood flow and the metabolic rate of tumors. 42 In addition, in vitro culturing of cells from multiple tumor sites can also be used to assess ITH indirectly. 43 Such methods enrich the means of detecting ITH and may also establish a foundation for the clinical application of ITH in the future.
4. UNDERSTANDING CRC FROM THE PERSPECTIVE OF IPTH AND IITH
According to results from Jones et al 44 and Mao et al, 45 at least 50% of CRC patients have more than one heterogeneous region within their tumor. It is unclear whether this is the result of genetic or epigenetic differences, although all of them exhibit IPTH. Researchers have found that subclones containing KRAS and NRAS mutations can exist at different sites in primary CRC tumors. 46 It is also possible that private APC, TP53, and ERBB4 mutations exist in subclones from different tumor regions. 47 In tumors with mutations of KRAS, NRAS, PIK3CA, or BRAF, the proportion of VAF with different mutations in tumor cell samples of the same patient varies significantly, which may also reflect the ITH of tumor cells. 48 With respect to cell lines cultured in vitro, homogeneity is not observed either. It has been reported that there are three subclones in HT29 cells and four in the SW480 cell line. 49 The studies above suggest that a tumor is a population consisting of different genotypes. Epigenetically, the expression levels of micro‐RNAs, such as miR‐92a and miR‐375, are significantly different in different areas of the tumor. 50 Outside of the nucleus, private mutations and a high level of ITH can be detected in mitochondrial DNA (mtDNA) in different regions of the tumor tissue. 51 In addition, the immune component of the tumor microenvironment may also have an impact on ITH. For example, infiltration of CD8+ and PD1+ T cells was significantly different in regions within a tumor. 52
One of the most important features of advanced CRC is metastasis. IITH can provide us with more biological information about primary and matched metastatic lesions. Some studies have shown that KRAS, NRAS, BRAF, and PIK3CA mutations are homogenous in primary tumors and matched primary and metastatic lesions. 53 , 54 Metastatic lesions inherit multiple subclones from the primary site and the level of ITH in these lesions is relatively small. VAF in these lesions was significantly higher compared with the primary site. 13 , 55 The discussion above indicates that metastatic lesions are the result of subclones from the primary site that traveled from their original location to implant at distant sites. Furthermore, subclones in lymphatic metastases consist of early, middle, and late stage tumor cells during tumor progression, indicating that subclones from the primary site metastasize to lymph nodes at different points in the timeline. 56 However, different metastatic lesions may contain different subclones from the primary site. In a study of 17 primary and matched metastasis samples, 6 of 17 tumors maintained the same origin of lymphatic and distant metastasis, while 11 of 17 tumors had different ancestors. 32 CRC patients presenting with multiple primary sites may have a unique type of IITH. Using somatic copy number variation (SCNV) analysis, we found that a different primary site originated from different ancestors and their clone composition was also unique. 57
5. ITH MAY PROVIDE TUMORS WITH SPECIFIC ABILITIES THAT RESULT IN WORSE OUTCOMES
There appears to be a significant correlation between ITH and cancer prognosis. In colorectal cancer patients, ITH index values correlated significantly with clinical prognosis. 58 A decrease in overall survival (OS) associated with increased ITH has been found in a variety of cancers. 59 For example, shorter OS and progression‐free survival (PFS) were observed in ovarian cancer patients with high ITH. 60 In breast cancer patients, elevated estrogen receptor (ER) and ITH was found to be twice as fatal compared with patients exhibiting low ER and ITH. 61 Similar results have been observed in CRC. Patients with higher ITH have shorter PFS. 58 In metastatic CRC, the 3‐year OS and PFS of patients with low ITH in metastatic lesions were 66% and 23%, respectively, while patients exhibiting high ITH had shorter OS (18%) and PFS (5%). 62 When using a tool called mutant‐allele tumor heterogeneity (MATH) to assess ITH in CRC patients, we also found that MATH was an independent risk factor for males. 63 High ITH was also associated with a higher incidence of liver metastasis in CRC. 64
In general, the biological characteristics of a tumor are determined by the dominant subclone population within, while a smaller proportion of subclones have little effect. The relationship between tumor subclones, however, is complex. Some subclones can promote proliferation of all the tumor cells by altering the tumor microenvironment. 65 By implanting breast cancer cells with different genotypes from luminal and basal sites into wild‐type mice, researchers found the efficiency of tumorigenesis of a single type was very low. However, the mixed population at a 1:1 ratio could effectively induce tumor formation with clones comprised of genotypes from both sites. 66 There seems to be a cooperative relationship between subclones in tumors. Each subclone acts like a gear in a machine that does its job. The population of a single subclone does not determine its overall importance. Rather, a small proportion of subclones within the tumor may play a vital role. Removal of these cells may lead to decreased tumor growth. 67 The reason for this phenomenon may be that a specific subclone can change the immunogenicity of a tumor. 68 In fact, it has been shown that there is a negative correlation between ITH and immune cell infiltration and tumors with higher ITH exhibit a lower level of immune T cell infiltration. 69 , 70
However, there are some controversies concerning how subclones affect cancer prognosis. Some researchers have found that compared to tumors with only one or two subclones, those with more than two subclones are associated with a poorer overall survival rate. However, no additional risk was observed in tumors with more than four subclones. 69 Some studies have found that ITH has little effect on the growth rate of tumors when there are three or fewer subclones. But when the number of subclone increases to 6 or 12, the rate of tumor proliferation increases significantly. 70 At present, the exact reason for the impact of ITH on prognosis is unclear. One possible explanation is that high ITH results in the interaction between subclones to induce a more complex tumor with a worse prognosis. It is also possible that high ITH results in an increased chance of obtaining more malignant subclones. Further study is needed in order to prove our hypotheses and observations.
6. ITH MAY CONFERS RESISTANCE TO TREATMENT BY PROVIDING SELECTABLE SUBCLONES OR COOPERATION BETWEEN SUBCLONES
ITH is closely linked to tumor resistance to treatment and tumors with high ITH exhibit greater drug resistance. 71 When CRC patients receive neoadjuvant chemoradiotherapy, increased ITH results in the failure of follow‐up treatment. 72 Various primary treatments may exert a strong selective effect and subclones may respond differently to treatment according to their genotype. 73 Tumor cells that are susceptible to treatment will be eliminated as they cannot produce more offspring. 53 Groups of resistant cells will then gain more survival advantages after intervention. 74 Successive interventions may result in multiple selection of subclones with the highest level of resistance. As selections continue, the resistance to treatment will become stronger, and finally, the tumor will be uncontrollable (Figure 2). In addition, the development of CRC is likely to be a process that takes more than 20 years. In contrast, tumor metastasis may occur in the early stage of the tumor and remain dormant until activated. 11 The effect of treatment intervention can change the proportion of the subclone population and create more space for these cells to grow. 75 After obtaining space, the incubated cells begin to proliferate. They can also evolve into new subclones which transform the original sensitive group into a resistant colony after treatment. 76 This also provides an explanation for the emergence of novel subclones carrying KRAS mutations in liver metastases from wild‐type RAS CRC patients treated with chemotherapy. 7
FIGURE 2.
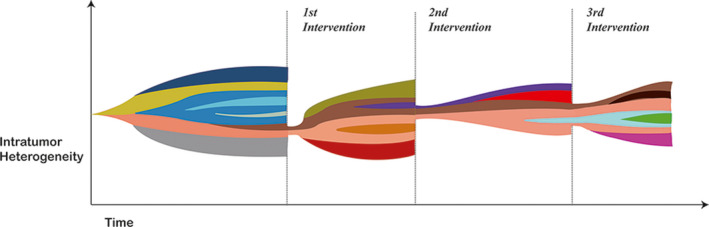
The level of intratumor heterogeneity during a series of interventions. A series of interventions serves as the selection pressure. There are no interventions in the beginning, thus subclones inside the tumor continue to increase due to neutral evolution. These subclones differ from each other genetically or epigenetically. The level of intratumor heterogeneity (the diversity of subclone population) gradually increases as time passes. After the first intervention, most subclones were wiped out while two subclones (colored in pink and brown), which have the ability to produce more offspring than others, continue to prosper and evolve into more genetically or epigenetically different subclones. After the second intervention, most subclones are eliminated while two (colored in pink and brown) remain to proliferate. The remaining subclones continue to evolve. The same process occurs during the third intervention. As the tumor is treated, the probability for the emergence of intervention‐resistant tumor cells gradually increases. (The color of the subclones gradually deepened.) It is also possible that intervention‐resistant tumor may obtain some biological characteristics that worsens the prognosis of the patients
Apart from providing more resistant subclones by heterogeneous tumors in the face of selection, the interaction between subclones will also lead to stronger drug resistance. RASwt CRC is usually sensitive to cetuximab, while KRASmut often results in drug resistance. The drug‐resistant phenotype of the KRASmut subclone may not be limited to this specific subclone. Researchers found that TGF‐alpha and amphiregulin produced by tumor cells harboring a KRAS mutation can induce KRASwt cells to grow continuously during drug exposure. 68 PFS is similar in patients irrespective of the frequency of KRAS mutation. 45 In a CRC patient cohort receiving neoadjuvant therapy, mutations in APC, TP53, ABCA13, MUC16, or THSD4 are always present in the drug‐resistant population. 77 This phenomenon has been verified in renal cell carcinoma patients using primary and matched metastatic lesions grown in nude mice. 78 This evidence suggests that subclones from heterogeneous tumor subclones can cooperate with each other, creating a more powerful tumor.
7. LIMITATIONS AND PROSPECTS
There are some limitations that exist in the field of tumor biology from the perspective of ITH. Intratumor heterogeneity has not always been observed in tumors. For example, in a study of 88 breast cancer patients in which cells were collected from different regions of the tumors, only a few samples exhibited detectable ITH and the level of ITH was not associated with overall survival. 79 The exploration of ITH is often derived from the inference of samples. Therefore, the sampling quality and the ability to take estimates on samples as a whole represents a limitation. Genotypic changes caused by a single point mutation may not be the cause of ITH. ITH may originate from mesoscale gene (about 10bp scale) or CNVs. 28 , 80 Therefore, it is possible that regular detection methods may fail to gather key information regarding ITH. The inference of ITH largely depends on specific algorithms. Existing algorithms that assess ITH must be evaluated to improve accuracy. Finally, the reason for the ITH effect on tumors is largely unknown and further studies are needed to explore these hidden mechanisms. Filling the information gap between ITH and clinical practice will benefit more patients. Despite many obstacles, continued understanding of ITH will enable us to trace the evolutionary pathway during tumor progression. In the area of CRC, this will facilitate our interpretation of disease development and prognosis. It may be said that one may not be afraid of long roads ahead with ITH, but afraid of short ambition.
CONFLICT OF INTEREST
The authors have no financial or other conflict of interest to disclose.
AUTHOR CONTRIBUTIONS
Zicheng Zheng was involved in conceptualization and writing—original draft. Tao Yu was involved in conceptualization, visualization, and writing—review and editing. Xinyu Zhao, Xin Gao, and Yao Zhao were involved in data curation. Gang Liu was involved in conceptualization, project administration, supervision, and writing—review and editing.
ACKNOWLEDGMENT
We thank Edanz Group (https://en‐author‐services.edanzgroup.com/) for editing a draft of this manuscript.
Zheng Z, Yu T, Zhao X, Gao X, Zhao Y, Liu G. Intratumor heterogeneity: A new perspective on colorectal cancer research. Cancer Med. 2020;9:7637–7645. 10.1002/cam4.3323
Zicheng Zheng and Tao Yu contributed to this work equally and should be regarded as co‐first authors.
DATA AVAILABILITY STATEMENT
No additional data are contained in this review.
REFERENCES
- 1. Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7‐30. [DOI] [PubMed] [Google Scholar]
- 2. Fitzmaurice C, Allen C, Barber RM et al; Global Burden of Disease Cancer Collaboration . Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability‐adjusted life‐years for 32 cancer groups, 1990 to 2015: a systematic analysis for the global burden of disease study. JAMA Oncol. 2017;3(4):524‐548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Creasy JM, Sadot E, Koerkamp BG et al Actual 10‐year survival after hepatic resection of colorectal liver metastases: what factors preclude cure? Surgery. 2018;163:1238‐1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tomlinson JS, Jarnagin WR, DeMatteo RP et al Actual 10‐year survival after resection of colorectal liver metastases defines cure. J Clin Oncol. 2007;25:4575‐4580. [DOI] [PubMed] [Google Scholar]
- 5. Benson AB, Venook AP, Al‐Hawary MM et al NCCN guidelines insights: colon cancer, version 2.2018. J Natl Compr Canc Netw. 2018;16:359‐369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Benson AB, Venook AP, Al‐Hawary MM et al Rectal cancer, version 2.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2018;16:874‐901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Baretti M, Personeni N, Destro A, Santoro A, Rimassa L. Emergence of KRAS‐mutation in liver metastases after an anti‐EGFR treatment in patient with colorectal cancer: are we aware of the therapeutic impact of intratumor heterogeneity? Cancer Biol Ther. 2018;19:659‐663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Naxerova K, Brachtel E, Salk JJ et al Hypermutable DNA chronicles the evolution of human colon cancer. Proc Natl Acad Sci USA. 2014;111:E1889‐E1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Greaves M. Evolutionary determinants of cancer. Cancer Discov. 2015;5:806‐820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yang L, Wang S, Lee JJ et al An enhanced genetic model of colorectal cancer progression history. Genome Biol. 2019;20:168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hu Z, Ding J, Ma Z et al Quantitative evidence for early metastatic seeding in colorectal cancer. Nat Genet. 2019;51:1113‐1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Naxerova K, Jain RK. Using tumour phylogenetics to identify the roots of metastasis in humans. Nat Rev Clin Oncol. 2015;12:258‐272. [DOI] [PubMed] [Google Scholar]
- 13. Kim TM, Jung SH, An CH et al Subclonal genomic architectures of primary and metastatic colorectal cancer based on intratumoral genetic heterogeneity. Clin Cancer Res. 2015;21:4461‐4472. [DOI] [PubMed] [Google Scholar]
- 14. Griffiths RC. The frequency spectrum of a mutation, and its age, in a general diffusion model. Theor Popul Biol. 2003;64:241‐251. [DOI] [PubMed] [Google Scholar]
- 15. Williams MJ, Werner B, Barnes CP, Graham TA, Sottoriva A. Identification of neutral tumor evolution across cancer types. Nat Genet. 2016;48:238‐244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sottoriva A, Barnes CP, Graham TA. Catch my drift? Making sense of genomic intra‐tumour heterogeneity. Biochim Biophys Acta Rev Cancer. 2017;1867:95‐100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kimura M. The neutral theory of molecular evolution. Cambridge, England: Cambridge University Press; 1987. [Google Scholar]
- 18. Turajlic S, Sottoriva A, Graham T, Swanton C. Resolving genetic heterogeneity in cancer. Nat Rev Genet. 2019;20:404‐416. [DOI] [PubMed] [Google Scholar]
- 19. Sottoriva A, Kang H, Ma Z et al A Big Bang model of human colorectal tumor growth. Nat Genet. 2015;47:209–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Torrecilla S, Sia D, Harrington AN et al Trunk mutational events present minimal intra‐ and inter‐tumoral heterogeneity in hepatocellular carcinoma. J Hepatol. 2017;67:1222‐1231. [DOI] [PubMed] [Google Scholar]
- 21. Korolev KS, Muller MJ, Karahan N, Murray AW, Hallatschek O, Nelson DR. Selective sweeps in growing microbial colonies. Phys Biol. 2012;9:026008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Saito T, Niida A, Uchi R et al A temporal shift of the evolutionary principle shaping intratumor heterogeneity in colorectal cancer. Nat Commun. 2018;9:2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gausachs M, Borras E, Chang K et al Mutational heterogeneity in APC and KRAS arises at the crypt level and leads to polyclonality in early colorectal tumorigenesis. Clin Cancer Res. 2017;23:5936‐5947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Narayanan S, Kawaguchi T, Peng X et al Tumor infiltrating lymphocytes and macrophages improve survival in microsatellite unstable colorectal cancer. Sci Rep. 2019;9:13455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Suzuki Y, Ng SB, Chua C et al Multiregion ultra‐deep sequencing reveals early intermixing and variable levels of intratumoral heterogeneity in colorectal cancer. Mol Oncol. 2017;11:124‐139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Raynaud F, Mina M, Tavernari D, Ciriello G. Pan‐cancer inference of intra‐tumor heterogeneity reveals associations with different forms of genomic instability. PLoS Genet. 2018;14:e1007669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jamal‐Hanjani M, Wilson GA, McGranahan N et al Tracking the evolution of non‐small‐cell lung cancer. N Engl J Med. 2017;376:2109‐2121. [DOI] [PubMed] [Google Scholar]
- 28. Mamlouk S, Childs LH, Aust D et al DNA copy number changes define spatial patterns of heterogeneity in colorectal cancer. Nat Commun. 2017;8:14093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Korber V, Yang J, Barah P et al Evolutionary trajectories of IDH(WT) glioblastomas reveal a common path of early tumorigenesis instigated years ahead of initial diagnosis. Cancer Cell. 2019;35(4):692‐704.e12. [DOI] [PubMed] [Google Scholar]
- 30. Luo Z, Fan X, Su Y, Huang YS. Accurity: accurate tumor purity and ploidy inference from tumor‐normal WGS data by jointly modelling somatic copy number alterations and heterozygous germline single‐nucleotide‐variants. Bioinformatics. 2018;34:2004‐2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hall BG. Comparison of the accuracies of several phylogenetic methods using protein and DNA sequences. Mol Biol Evol. 2005;22:792‐802. [DOI] [PubMed] [Google Scholar]
- 32. Naxerova K, Reiter JG, Brachtel E et al Origins of lymphatic and distant metastases in human colorectal cancer. Science. 2017;357:55‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hajirasouliha I, Mahmoody A, Raphael BJ. A combinatorial approach for analyzing intra‐tumor heterogeneity from high‐throughput sequencing data. Bioinformatics. 2014;30:i78‐i86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Roerink SF, Sasaki N, Lee‐Six H et al Intra‐tumour diversification in colorectal cancer at the single‐cell level. Nature. 2018;556:457‐462. [DOI] [PubMed] [Google Scholar]
- 35. Saadatpour A, Lai S, Guo G, Yuan GC. Single‐cell analysis in cancer genomics. Trends Genet. 2015;31:576‐586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Oesper L, Mahmoody A, Raphael BJ. THetA: inferring intra‐tumor heterogeneity from high‐throughput DNA sequencing data. Genome Biol. 2013;14:R80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Salk JJSS, Risques RA, Crispin DA, Li L, Bronner MP, Brentnall TA, Rabinovitch PS, Horwitz MS, Loeb LA. Clonal expansions in ulcerative colitis identify patients with neoplasia. Proc Natl Acad Sci. 2009;8(106):20871‐20876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nelson AC, Boone J, Cartwright D et al Optimal detection of clinically relevant mutations in colorectal carcinoma: sample pooling overcomes intra‐tumoral heterogeneity. Mod Pathol. 2018;31:343‐349. [DOI] [PubMed] [Google Scholar]
- 39. Kundig P, Giesen C, Jackson H et al Limited utility of tissue micro‐arrays in detecting intra‐tumoral heterogeneity in stem cell characteristics and tumor progression markers in breast cancer. J Transl Med. 2018;16:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Boone PG, Rochelle LK, Ginzel JD et al A cancer rainbow mouse for visualizing the functional genomics of oncogenic clonal expansion. Nat Commun. 2019;10:5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ho DW, Tsui YM, Sze KM et al Single‐cell transcriptomics reveals the landscape of intra‐tumoral heterogeneity and stemness‐related subpopulations in liver cancer. Cancer Lett. 2019;459:176‐185. [DOI] [PubMed] [Google Scholar]
- 42. Tixier F, Groves AM, Goh V et al Correlation of intra‐tumor 18F‐FDG uptake heterogeneity indices with perfusion CT derived parameters in colorectal cancer. PLoS One. 2014;9:e99567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Arnadottir SS, Jeppesen M, Lamy P et al Characterization of genetic intratumor heterogeneity in colorectal cancer and matching patient‐derived spheroid cultures. Mol Oncol. 2018;12:132‐147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jones HG, Jenkins G, Williams N et al Genetic and epigenetic intra‐tumour heterogeneity in colorectal cancer. World J Surg. 2017;41:1375‐1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mao C, Wu XY, Yang ZY et al Concordant analysis of KRAS, BRAF, PIK3CA mutations, and PTEN expression between primary colorectal cancer and matched metastases. Sci Rep. 2015;5:8065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jeantet M, Tougeron D, Tachon G et al High intra‐ and inter‐tumoral heterogeneity of RAS mutations in colorectal cancer. Int J Mol Sci. 2016;17(12):2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kogita A, Yoshioka Y, Sakai K et al Inter‐ and intra‐tumor profiling of multi‐regional colon cancer and metastasis. Biochem Biophys Res Commun. 2015;458:52–56. [DOI] [PubMed] [Google Scholar]
- 48. Normanno N, Rachiglio AM, Lambiase M et al Heterogeneity of KRAS, NRAS, BRAF and PIK3CA mutations in metastatic colorectal cancer and potential effects on therapy in the CAPRI GOIM trial. Ann Oncol. 2015;26:1710‐1714. [DOI] [PubMed] [Google Scholar]
- 49. Wangsa D, Braun R, Schiefer M et al The evolution of single cell‐derived colorectal cancer cell lines is dominated by the continued selection of tumor‐specific genomic imbalances, despite random chromosomal instability. Carcinogenesis. 2018;39:993‐1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Jepsen RK, Novotny GW, Klarskov LL, Christensen IJ, Riis LB, Hogdall E. Intra‐tumor heterogeneity of microRNA‐92a, microRNA‐375 and microRNA‐424 in colorectal cancer. Exp Mol Pathol. 2016;100:125‐131. [DOI] [PubMed] [Google Scholar]
- 51. Kazdal D, Harms A, Endris V et al Prevalence of somatic mitochondrial mutations and spatial distribution of mitochondria in non‐small cell lung cancer. Br J Cancer. 2017;117:220–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Legras A, Roussel H, Mangiameli G et al mutational diversity of lung cancer and associated lymph nodes. an exploratory prospective study of 4 resected cIIIA‐N2. Pathol Oncol Res. 2019;25:319‐325. [DOI] [PubMed] [Google Scholar]
- 53. Brannon AR, Vakiani E, Sylvester BE et al Comparative sequencing analysis reveals high genomic concordance between matched primary and metastatic colorectal cancer lesions. Genome Biol. 2014;15:454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Petaccia de Macedo M, Melo FM, Ribeiro HSC, et al KRAS mutation status is highly homogeneous between areas of the primary tumor and the corresponding metastasis of colorectal adenocarcinomas: one less problem in patient care. Am J Cancer Res. 2017;7:1978‐1989. [PMC free article] [PubMed] [Google Scholar]
- 55. Wei Q, Ye Z, Zhong X et al Multiregion whole‐exome sequencing of matched primary and metastatic tumors revealed genomic heterogeneity and suggested polyclonal seeding in colorectal cancer metastasis. Ann Oncol. 2017;28:2135‐2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ulintz PJ, Greenson JK, Wu R, Fearon ER, Hardiman KM. Lymph node metastases in colon cancer are polyclonal. Clin Cancer Res. 2018;24:2214‐2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lee WK, Lee SG, Yim SH et al Whole exome sequencing identifies a novel hedgehog‐interacting protein G516R mutation in locally advanced papillary thyroid cancer. Int J Mol Sci. 2018;19(10):2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Oh BY, Shin HT, Yun JW et al Intratumor heterogeneity inferred from targeted deep sequencing as a prognostic indicator. Sci Rep. 2019;9:4542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Morris LG, Riaz N, Desrichard A et al Pan‐cancer analysis of intratumor heterogeneity as a prognostic determinant of survival. Oncotarget. 2016;7:10051‐10063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Schwarz RF, Ng CK, Cooke SL et al Spatial and temporal heterogeneity in high‐grade serous ovarian cancer: a phylogenetic analysis. PLoS Med. 2015;12:e1001789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lindstrom LS, Yau C, Czene K et al Intratumor heterogeneity of the estrogen receptor and the long‐term risk of fatal breast cancer. J Natl Cancer Inst. 2018;110:726‐733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Sveen A, Loes IM, Alagaratnam S et al Intra‐patient inter‐metastatic genetic heterogeneity in colorectal cancer as a key determinant of survival after curative liver resection. PLoS Genet. 2016;12:e1006225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Zhang J, Yan S, Liu X et al Gender‐related prognostic value and genomic pattern of intra‐tumor heterogeneity in colorectal cancer. Carcinogenesis. 2017;38:837‐846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Joung JG, Oh BY, Hong HK et al Tumor heterogeneity predicts metastatic potential in colorectal cancer. Clin Cancer Res. 2017;23:7209‐7216. [DOI] [PubMed] [Google Scholar]
- 65. Marusyk A, Tabassum DP, Altrock PM, Almendro V, Michor F, Polyak K. Non‐cell‐autonomous driving of tumour growth supports sub‐clonal heterogeneity. Nature. 2014;514:54‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Cleary AS, Leonard TL, Gestl SA, Gunther EJ. Tumour cell heterogeneity maintained by cooperating subclones in Wnt‐driven mammary cancers. Nature. 2014;508:113‐117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Inda MM, Bonavia R, Mukasa A et al Tumor heterogeneity is an active process maintained by a mutant EGFR‐induced cytokine circuit in glioblastoma. Genes Dev. 2010;24:1731‐1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Hobor S, Van Emburgh BO, Crowley E, Misale S, Di Nicolantonio F, Bardelli A. TGFalpha and amphiregulin paracrine network promotes resistance to EGFR blockade in colorectal cancer cells. Clin Cancer Res. 2014;20:6429‐6438. [DOI] [PubMed] [Google Scholar]
- 69. Andor N, Graham TA, Jansen M et al Pan‐cancer analysis of the extent and consequences of intratumor heterogeneity. Nat Med. 2016;22:105‐113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Wolf Y, Bartok O, Patkar S et al UVB‐induced tumor heterogeneity diminishes immune response in melanoma. Cell. 2019;179(1):219‐235.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Sharma A, Cao EY, Kumar V et al Longitudinal single‐cell RNA sequencing of patient‐derived primary cells reveals drug‐induced infidelity in stem cell hierarchy. Nat Commun. 2018;9:4931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Bettoni F, Masotti C, Correa BR et al The effects of neoadjuvant chemoradiation in locally advanced rectal cancer‐the impact in intratumoral heterogeneity. Front Oncol. 2019;9:974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Reinartz R, Wang S, Kebir S et al Functional subclone profiling for prediction of treatment‐induced intratumor population shifts and discovery of rational drug combinations in human glioblastoma. Clin Cancer Res. 2017;23:562‐574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Venkatesan S, Swanton C, Taylor BS, Costello JF. Treatment‐induced mutagenesis and selective pressures sculpt cancer evolution. Cold Spring Harb Perspect Med. 2017;7(8):a026617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Kreso A, O'Brien CA, van Galen P et al Variable clonal repopulation dynamics influence chemotherapy response in colorectal cancer. Science. 2013;339:543‐548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Hong SP, Chan TE, Lombardo Y et al Single‐cell transcriptomics reveals multi‐step adaptations to endocrine therapy. Nat Commun. 2019;10:3840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Frydrych LM, Ulintz P, Bankhead A et al Rectal cancer sub‐clones respond differentially to neoadjuvant therapy. Neoplasia. 2019;21:1051‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Hong B, Yang Y, Guo S et al Intra‐tumour molecular heterogeneity of clear cell renal cell carcinoma reveals the diversity of the response to targeted therapies using patient‐derived xenograft models. Oncotarget. 2017;8:49839‐49850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Yang F, Cao L, Sun Z et al Evaluation of breast cancer stem cells and intratumor stemness heterogeneity in triple‐negative breast cancer as prognostic factors. Int J Biol Sci. 2016;12:1568‐1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Buisson R, Langenbucher A, Bowen D et al Passenger hotspot mutations in cancer driven by APOBEC3A and mesoscale genomic features. Science. 2019;364(6447):eaaw2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No additional data are contained in this review.


