Abstract
Background
Bladder cancer (BCa) is the most common urinary malignancy. The standard surgical treatment for patients with muscle‐invasive BCa is cystectomy plus urinary diversion. Ileal conduit (IC) or orthotopic neobladder (ON), which have different indications, are the most commonly performed urinary diversions.
Methods and materials
We sampled 5480 BCa patients from the Surveillance, Epidemiology, and End Results (SEER) database from 2004 to 2015. Kaplan‐Meier method with the log‐rank test was used to assess cancer‐specific survival (CSS) and overall survival (OS). Univariate and multivariate Cox's proportional hazard model was conducted to evaluate the hazard ratio of cancer‐specific mortality and all‐cause mortality before and after propensity score matching (PSM).
Results
We identified 5480 patients who received radical cystectomy (RC) plus IC (n = 5071) or ON (n = 409) with a median follow‐up period of 33 months (interquartile range, 13‐78 months). Patients in the ON group tended to be male and younger, with a higher percentage of married individuals, early pathological T stage, lymphadenectomy, and non‐radiotherapy (all P < .05). After 1:1 PSM, 409 matched pairs were selected. Univariate and multivariate analysis showed that the ON group had better CSS and OS probabilities than the IC group in the overall cohort [hazard ratio (HR): 0.692, 95% confidence intervals (CI): 0.576‐0.831, P < .001; HR: 0.677, 95% CI: 0.579‐0.793, P < .001 respectively]. However, subgroup analysis revealed that only patients with pathological T2 stage benefited from ON diversion after PSM in the context of CSS (P = .016) and OS (P <.001).
Conclusions
Young, married, and male patients with early pathological T stage, especially T2 stage, were more suitable to receive RC plus ON surgery, which could improve their probability of survival.
Keywords: bladder cancer, ileal conduit, orthotopic neobladder, radical cystectomy, SEER
Young, married, and male patients with early pathological T stage, especially T2 stage, were more suitable to receive RC plus ON surgery, which might improve the survival probabilities.
![]()
1. INTRODUCTION
Bladder cancer (BCa) is the most common malignancy in the urinary system and the 4th most commonly diagnosed cancer in males in the United States. 1 According to the latest European Association of Urology guideline on muscle‐invasive bladder cancer (MIBC), radical cystectomy (RC) with urinary diversion is the standard treatment recommended for patients with MIBC T2‐T4a, N0‐Nx, M0, and selected patients with high‐risk nonmuscle‐invasive bladder cancer (NMIBC). 2 Urinary diversion is the second important step after RC. There are currently three alternative types of urinary diversion, including abdominal diversion, urethral diversion, and rectosigmoid diversion. Age, comorbidity, cardio‐pulmonary function, cognitive function, patient's social support, and preference are all important factors that should be considered when choosing the type of urinary diversion. Nowadays, the most commonly performed urinary diversions are ileal conduit (IC) or orthotopic neobladder (ON). 2 , 3 Previous studies have aimed to assess the impact of IC and ON diversions on the health‐related quality‐of‐life (HRQoL) of BCa patients. Although no significant differences in QoL were observed between continent and incontinent urinary diversion, ON was shown to provide a better body image and normal urethral voiding.
In a multicenter study that included 2501 patients undergoing RC for BCa, regardless of the urinary diversion that was used, the actuarial 5‐year cancer‐specific survival (CSS) and overall survival (OS) were 67% and 47% respectively. 4 Oncological outcomes after RC depend on specific variables, including tumor stage, lymph node yield and positivity, lymphovascular invasion (LVI), surgical margin status, and neoadjuvant chemotherapy. Nevertheless, Yossepowitch and his colleagues reported that ON diversion did not compromise the oncological outcomes of cystectomy, because no CSS or recurrence‐free survival (RFS) differences were identified between the ON (n = 214) and IC (n = 269) cohorts when stratified by pathological stage. 5 To the best of our knowledge, there have been no randomized controlled trials or retrospective studies on a large population aimed at comparing the oncological outcomes between IC and ON diversion. Consequently, the aim of this study is to use data from the Surveillance, Epidemiology, and End Results (SEER) database, which represents 28% of the United States’ population, to compare the CSS and OS of patients with IC or ON diversion. Ultimately, the aim is to provide a relatively good level of evidence for urologists and patients to make the decision about which urinary diversion to use.
2. MATERIALS AND METHODS
2.1. Data source and study population
The study cases in this study were retrieved from 18 SEER cancer registries using SEER*STAT 8.3.6 software. Patients were included if they met the following criteria (n = 7531): (a) year of diagnosis between 2004 and 2015; (b) using the International Classification of Diseases for Oncology 3/WHO site recode: C67.0‐67.9 (urinary bladder); (c) using the surgery codes C61 and C64 (radical cystectomy plus IC or ON). The following exclusion criteria were also applied: (a) patients with a prior history of other malignancy (n = 2021); (b) unknown death status (n = 30) (Figure 1). A total of 5480 patients were included and their clinicopathological data were retrospectively analyzed.
FIGURE 1.
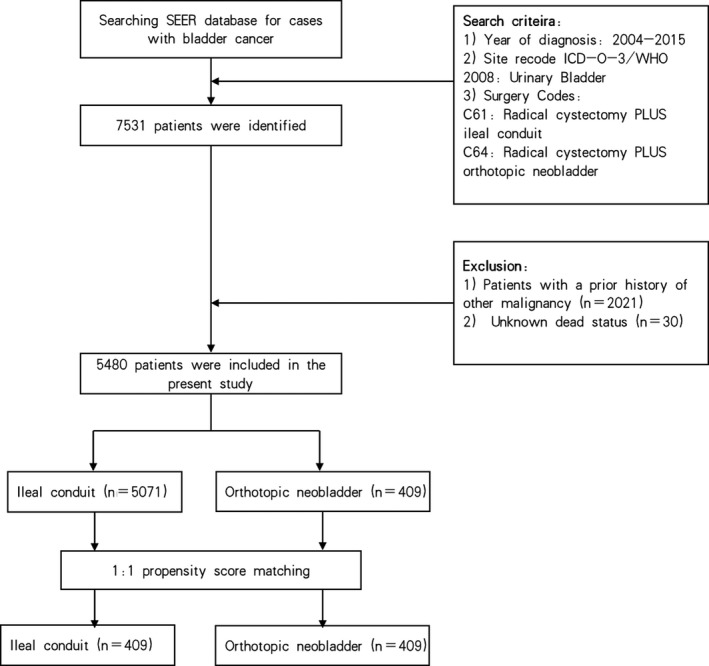
Flow‐chart demonstrating the approach used to identify patients with bladder cancer registered in the SEER database from 2004 to 2015
2.2. Variables definition
Demographic characteristics included age at diagnosis, gender, marital status, and race. Tumor characteristics included tumor location, histology, TNM status, stage, grade, LVI, and lymphadenectomy. Treatment information included surgery approach, radiotherapy, and chemotherapy. Marital status was defined as married, unmarried, or unknown. Unmarried included never married, separated/divorced, and widowed patients. Race was categorized as white, black, Asian/Pacific Islander, and American Indian/Alaskan Native. Tumor location was defined as primary tumor located in the trigone, dome, lateral wall, anterior wall, posterior wall, bladder neck, ureteric orifice, urachus, overlapping lesions, and other locations not otherwise specified of the bladder. BCa histology was classified as transitional cell carcinoma, squamous cell carcinoma, and other rare types. Pathological TNM status was assessed according to the American Joint Committee on Cancer, sixth edition. T stages were divided into subgroups as T0‐Tis‐Ta‐T1, T2, T3, T4, and Tx/NA for further analysis. Tumor grade was assigned according to the SEER database as grades I, II, III, IV, and unknown. According to the “RX Summ‐Scope Reg LN Sur (2003+)” column in the SEER database, lymphadenectomy included 1 to 3 removed regional lymph nodes, 4 or more removed regional lymph nodes, and unknown number of removed regional lymph nodes. Surgical approach was categorized as RC plus ID or ON according to the “RX Summ‐Surg Prim Site (1998+).”
2.3. Statistical analyses
SPSS version 23.0 (IBM Corp, Armonk, NY) was used for all statistical analyses and propensity score matching (PSM). Chi‐square test and Mann‐Whitney U tests were used to determine the significant differences between categorical and continuous variables and surgical approach. The CSS and OS curves were created by the Kaplan‐Meier method with the log‐rank test. To estimate the impact of IC or ON on prognosis, univariate and multivariate cox regression analysis were carried out, and the results were presented as hazards ratios (HR) and 95% confidence intervals (95% CI). A fewer number of patients were treated by ON than by IC diversion (409 vs 5071). Moreover, there were also imbalances in baseline characteristics, such as age, gender, marital status, T stage, lymphadenectomy and radiotherapy; therefore, PSM was used for more objective comparison. For PSM, patients receiving IC or ON were matched 1:1 with a caliper set at 0. The matching algorithm was nearest neighbor matching, and the estimation algorithm was logistic regression. The PSM was described and adjusted for different variables, including age, gender, sex, marital status, TNM stages, lymphadenectomy, radiotherapy, and chemotherapy. All reported P‐values were two‐tailed and a P < .050 was considered significant.
3. RESULTS
3.1. Comparison of baseline clinicopathological characteristics before and after PSM
A total of 5480 BCa patients who underwent RC plus IC (n = 5071) or ON (n = 409) were included (Table S1). There were no significant differences in terms of race, primary tumor site, histology, N stage, M stage, grade, and chemotherapy between the two different urinary diversions (all P > .050). However, patients in the ON group tended to be younger (median year: 63 vs 69, P < .001) and male (97.1% vs 86.9%, P < .001), with a higher percentage of married individuals (73.3% vs 64.1%, P < .001), early T stage (T0‐T2: 59.6% vs 49.3%, P < .001), lymphadenectomy (97.1% vs 91.3%, P < .001), and nonradiotherapy (98.5% vs 96.1%, P = .012) before PSM. After 1:1 PSM adjusting for age, gender, sex, marital status, TNM stages, lymphadenectomy, radiotherapy, and chemotherapy, there were no significant differences between the IC and ON groups (all P > .050, Table 1).
TABLE 1.
Clinical and pathological characteristics of BCa patients between different urinary diversion after PSM
| Characteristic | Urinary diversion, % of patients | Chi‐square or Z | P value | |
|---|---|---|---|---|
| Ileal conduit (n = 409) | Orthotopic neobladder (n = 409) | |||
| Age, median (IQR) | 63 (56‐69) | 63 (55‐69) | −0.789 | .430 |
| Gender | 0.440 | .507 | ||
| Male | 97.8 | 97.1 | ||
| Female | 2.2 | 2.9 | ||
| Race | 7.585 | .108 | ||
| White | 90.2 | 89.7 | ||
| Black | 5.6 | 2.9 | ||
| AI | 0.5 | 0.5 | ||
| API | 3.4 | 6.6 | ||
| Unknown | 0.2 | 0.2 | ||
| Marital status | 3.743 | .154 | ||
| Married | 73.1 | 73.3 | ||
| No | 26.7 | 25.2 | ||
| Unknown | 0.2 | 1.5 | ||
| Primary tumor site | 11.331 | .254 | ||
| Trigone of bladder | 6.8 | 3.9 | ||
| Dome of bladder | 5.4 | 3.7 | ||
| Lateral wall of bladder | 17.1 | 17.1 | ||
| Anterior wall of bladder | 1.2 | 2.4 | ||
| Posterior wall of bladder | 7.3 | 7.8 | ||
| Bladder neck | 1.7 | 3.7 | ||
| Ureteric orifice | 1.2 | 1.5 | ||
| Urachus | 0.0 | 0.5 | ||
| Overlapping lesion of bladder | 20.0 | 20.0 | ||
| Bladder, NOS | 39.1 | 39.4 | ||
| Histology | 1.580 | .454 | ||
| Transitional cell carcinoma | 94.4 | 92.2 | ||
| Squamous cell carcinoma | 2.0 | 2.7 | ||
| Other types | 3.7 | 5.1 | ||
| Pathological T stage | 0.168 | .997 | ||
| T0‐Tis‐Ta‐T1 | 15.2 | 14.4 | ||
| T2 | 44.7 | 45.2 | ||
| T3 | 27.6 | 28.4 | ||
| T4 | 11.5 | 11.0 | ||
| Tx/NA | 1.0 | 1.0 | ||
| Pathological N stage | 3.525 | .317 | ||
| N0 | 81.2 | 79.0 | ||
| N1 | 11.0 | 9.5 | ||
| N2 | 6.6 | 10.0 | ||
| N3 | 0.0 | 0.0 | ||
| Nx/NA | 1.2 | 1.5 | ||
| Pathological M stage | 4.328 | .115 | ||
| M0 | 96.6 | 94.6 | ||
| M1 | 2.0 | 4.4 | ||
| Mx/NA | 1.5 | 1.0 | ||
| Grade | 4.916 | .296 | ||
| I | 0.7 | 0.7 | ||
| II | 4.6 | 4.2 | ||
| III | 26.9 | 34.0 | ||
| IV | 63.1 | 57.2 | ||
| Unknown | 4.6 | 3.9 | ||
| Lymphadenectomy | 2.687 | .261 | ||
| No | 2.7 | 2.7 | ||
| Yes | 96.1 | 97.1 | ||
| Others | 1.2 | 0.2 | ||
| Adjuvant radiation | 2.020 | .155 | ||
| No | 99.5 | 98.5 | ||
| Yes | 0.5 | 1.5 | ||
| Adjuvant chemotherapy | 0.127 | .721 | ||
| No | 60.6 | 59.4 | ||
| Yes | 39.4 | 40.6 | ||
Abbreviations: AI, American Indian/Alaskan Native; API, Asian/Pacific Islander; BCa, bladder cancer; IQR, interquartile range; NA, not available; NOS, not otherwise specified; PSM, propensity score matching.
3.2. Univariate and multivariate analyses of the predictors of cancer‐specific mortality and all other‐cause mortality before PSM
The median follow‐up period was 33 months (interquartile range: 33‐78 months), with a total of 3034 (59.8%) and 170 (41.6%) deaths in the IC and ON groups respectively. Among all of the deaths, 2178 (43%) and 127 (31.1%) patients died specifically from BCa in the IC and ON groups respectively. The 5‐year CSS and OS probabilities for the IC group and the ON group were 55%, 72.9%, and 45.1%, 66.0% respectively (Figure 2A and F, both P < .001). When stratified by T stages, patients with each T stage in the ON group also showed longer CSS and OS than those in the IC group (Figure 2B‐2E and G‐J).
FIGURE 2.
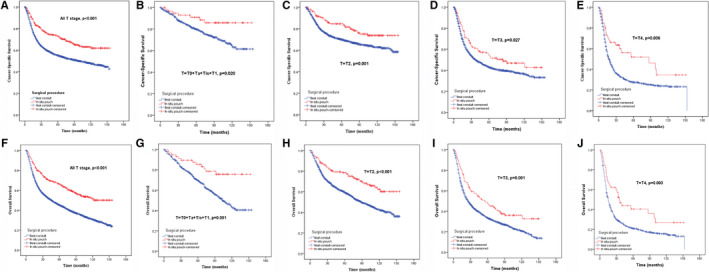
Influence of the urinary diversion on Cancer‐Specific Survival (A‐E) and Overall Survival (F‐J) in Bladder Cancer patients stratified by pathological T stage before propensity score matching
Before PSM, univariate analyses revealed that age, marital status, primary tumor site, histology, TNM stages, grade, lymphadenectomy, urinary diversion, and radiotherapy were all associated with cancer‐specific mortality (CSM) and all‐cause mortality (ACM) (Tables S2 and S3). After controlling for other factors, urinary diversion was an independent risk factor for CSM and ACM in the multivariate analyses [ON vs IC, HR: 0.692, 95% CI: 0.576‐0.831, P < .001; HR: 0.677, 95% CI: 0.579‐0.793, P < .001 respectively] (Tables S2 and S3).
3.3. Impact of IC vs ON on CSS and OS after PSM
After 1:1 PSM, cox regression analyses showed that ON was a protective factor for CSS and OS for all T stages by multivariate analysis (ON vs ID, HR: 0.741, 95% CI: 0.583‐0.941, P = .014; HR: 0.671, 95% CI: 0.548‐0.823, P < .001, respectively) (Table 2, Figure 3A and Table 3, Figure 3F respectively). However, when stratified by T stages, subgroup analysis revealed that only patients with pathological T2 stage obtained survival benefit from ON after PSM in terms of CSS (P = .016) and OS (P < .001) (Figure 3B‐3E and Figure 3G‐3J).
TABLE 2.
Univariate and multivariate regression analyses for CSM after PSM
| Characteristic | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Age | 1.015 | 1.002‐1.028 | .019* | 1.012 | 0.999‐0.025 | .073* |
| Gender | ||||||
| Female | Ref. | .040 | Ref. | .077 | ||
| Male | 0.497 | 0.255‐0.968 | 0.542 | 0.274‐1.069 | ||
| Race | ||||||
| White | Ref. | .499 | ||||
| Black | 1.077 | 0.604‐1.922 | .801 | |||
| AI | 0.750 | 0.105‐5.344 | .774 | |||
| API | 0.546 | 0.281‐1.062 | .075 | |||
| Unknown | 0.001 | 0.001‐1.003 | .946 | |||
| Marital status | ||||||
| Married | Ref. | .179 | ||||
| No | 1.229 | 0.944‐1.601 | .125 | |||
| Unknown | 0.373 | 0.052‐2.662 | .325 | |||
| Primary tumor site | ||||||
| Trigone of bladder | Ref. | .938 | ||||
| Dome of bladder | 0.788 | 0.372‐1.668 | .533 | |||
| Lateral wall of bladder | 0.833 | 0.482‐1.439 | .512 | |||
| Anterior wall of bladder | 0.331 | 0.077‐1.427 | .138 | |||
| Posterior wall of bladder | 0.980 | 0.532‐1.806 | .949 | |||
| Bladder neck | 0.598 | 0.222‐1.610 | .309 | |||
| Ureteric orifice | 0.917 | 0.310‐2.710 | .875 | |||
| Urachus | 0.001 | 0.001‐1.002 | .948 | |||
| Overlapping lesion of bladder | 0.863 | 0.508‐1.468 | .588 | |||
| Bladder, NOS | 0.896 | 0.544‐1.475 | .666 | |||
| Histology | ||||||
| Transitional cell carcinoma | Ref. | .005* | Ref. | .077 | ||
| Squamous cell carcinoma | 0.415 | 0.133‐1.294 | .130 | 0.421 | 0.134‐1.326 | .139 |
| Other types | 1.956 | 1.227‐3.119 | .005* | 1.583 | 0.919‐2.727 | .098 |
| Pathological T stage | ||||||
| T0‐Tis‐Ta‐T1 | Ref. | <.001* | Ref. | <.001* | ||
| T2 | 1.737 | 1.060‐2.847 | .028* | 1.618 | 0.986‐2.655 | .057 |
| T3 | 4.020 | 2.469‐6.545 | <.001* | 3.066 | 1.857‐5.062 | <.001* |
| T4 | 5.442 | 3.199‐9.257 | <.001* | 4.315 | 2.510‐7.419 | <.001* |
| Tx/NA | 4.203 | 1.430‐12.358 | .009* | 2.915 | 0.370‐22.986 | .310 |
| Pathological N stage | ||||||
| N0 | Ref. | <.001* | Ref. | <.001* | ||
| N1 | 2.854 | 2.083‐3.910 | <.001* | 1.990 | 1.432‐2.765 | <.001* |
| N2 | 3.299 | 2.351‐4.628 | <.001* | 2.615 | 1.843‐3.711 | <.001* |
| N3 (n = 0) | ||||||
| Nx/NA | 2.067 | 0.850‐5.030 | .109 | 1.454 | 0.237‐8.905 | .686 |
| Pathological M stage | ||||||
| M0 | Ref. | .012* | Ref. | .673 | ||
| M1 | 2.257 | 1.317‐3.868 | .003* | 1.067 | 0.599‐1.903 | .826 |
| Mx/NA | 1.244 | 0.463‐3.340 | .665 | 0.270 | 0.014‐5.228 | .387 |
| Grade | ||||||
| I | Ref. | .477 | ||||
| II | 0.650 | 0.138‐3.059 | .585 | |||
| III | 1.064 | 0.262‐4.329 | .931 | |||
| IV | 1.190 | 0.295‐4.797 | .807 | |||
| Unknown | 1.311 | 0.296‐5.811 | .721 | |||
| Lymphadenectomy | ||||||
| No | Ref. | .906 | ||||
| Yes | 1.030 | 0.486‐2.182 | .939 | |||
| Others | 0.664 | 0.082‐5.396 | .702 | |||
| Urinary diversion | ||||||
| Ileal conduilt | Ref. | .042* | Ref | .014* | ||
| Orthotopic neobladder | 0.781 | 0.616‐0.991 | 0.741 | 0.583‐0.941 | ||
| Adjuvant radiation | ||||||
| No | Ref. | .003* | Ref. | .070 | ||
| Yes | 3.864 | 1.589‐9.393 | 2.389 | 0.932‐6.126 | ||
| Adjuvant chemotherapy | ||||||
| No | Ref. | .011* | Ref. | .395 | ||
| Yes | 1.362 | 1.073‐1.728 | 0.890 | 0.681‐1.164 | ||
Abbreviations: AI, American Indian/Alaskan Native; API, Asian/Pacific Islander; CI, confidence interval; CSM, cancer‐specific mortality; HR, hazard ratio; NA, not available; NOS, not otherwise specified; PSM, propensity score matching; Ref., reference.
Statistically significant.
FIGURE 3.
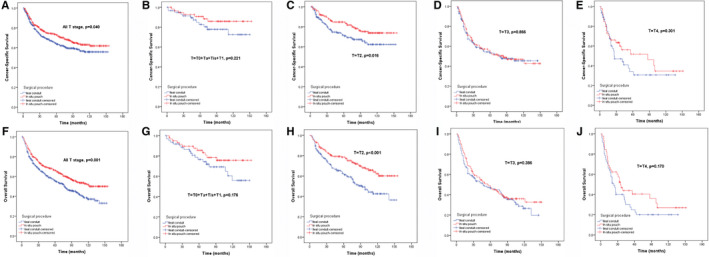
Influence of the urinary diversion on Cancer‐Specific Survival (A‐E) and Overall Survival (F‐J) in Bladder Cancer patients stratified by pathological T stage after propensity score matching
TABLE 3.
Univariate and multivariate regression analyses for ACM after PSM
| Characteristic | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Age | 1.025 | 1.014‐1.036 | <.001* | 1.021 | 1.010‐1.033 | <.001* |
| Gender | ||||||
| Female | Ref. | .041* | Ref. | .054 | ||
| Male | 0.535 | 0.293‐0.976 | 0.551 | 0.300‐1.012 | ||
| Race | ||||||
| White | Ref. | .172 | ||||
| Black | 1.007 | 0.610‐1.662 | .977 | |||
| AI | 1.127 | 0.281‐4.528 | .866 | |||
| API | 0.463 | 0.254‐0.844 | .012* | |||
| Unknown (n = 2) | ||||||
| Marital status | ||||||
| Married | Ref. | .032* | Ref. | .100 | ||
| No | 1.315 | 1.055‐1.639 | .015* | 1.276 | 1.015‐1.605 | .037* |
| Unknown | 0.536 | 0.133‐2.154 | .379 | 0.733 | 0.181‐2.958 | .662 |
| Primary tumor site | ||||||
| Trigone of bladder | Ref. | .813 | ||||
| Dome of bladder | 0.804 | .470 | ||||
| Lateral wall of bladder | 0.780 | .269 | ||||
| Anterior wall of bladder | 0.416 | .101 | ||||
| Posterior wall of bladder | 0.741 | .257 | ||||
| Bladder neck | 0.540 | .145 | ||||
| Ureteric orifice | 0.597 | .334 | ||||
| Urachus (n = 2) | ||||||
| Overlapping lesion of bladder | 0.726 | 0.470‐1.120 | .148 | |||
| Bladder, NOS | 0.809 | 0.541‐1.211 | .303 | |||
| Histology | ||||||
| Transitional cell carcinoma | Ref. | .040* | Ref. | .084 | ||
| Squamous cell carcinoma | 0.382 | 0.143‐1.023 | .056 | 0.381 | 0.141‐1.029 | .057 |
| Other types | 1.451 | 0.925‐2.277 | .105 | 1.331 | 0.796‐2.227 | .276 |
| Pathological T stage | ||||||
| T0‐Tis‐Ta‐T1 | Ref. | <.001* | Ref. | <.001* | ||
| T2 | 1.656 | 1.123‐2.442 | .011* | 1.552 | 1.051‐2.292 | .027 |
| T3 | 3.307 | 2.243‐4.875 | <.001* | 2.584 | 1.732‐3.854 | <.001* |
| T4 | 4.520 | 2.939‐6.951 | <.001* | 3.718 | 2.394‐5.774 | <.001* |
| Tx/NA | 2.642 | 0.932‐7.487 | .068 | 0.647 | 0.135‐3.098 | .586 |
| Pathological N stage | ||||||
| N0 | Ref. | <.001* | Ref. | <.001* | ||
| N1 | 2.307 | 1.739‐3.062 | <.001* | 1.577 | 1.171‐2.123 | .003* |
| N2 | 2.885 | 2.144‐3.882 | <.001* | 2.401 | 1.768‐3.261 | <.001* |
| N3 (n = 0) | ||||||
| Nx/NA | 2.007 | 0.947‐4.252 | .069 | 3.284 | 1.050‐10.270 | .041* |
| Pathological M stage | ||||||
| M0 | Ref. | .024* | Ref. | .982 | ||
| M1 | 1.970 | 1.211‐3.206 | .006* | 0.975 | 0.571‐1.664 | .926 |
| Mx/NA | 1.127 | 0.466‐2.724 | .791 | 0.849 | 0.128‐5.644 | .866 |
| Grade | ||||||
| I | Ref. | .511 | ||||
| II | 0.615 | 0.204‐1.853 | .388 | |||
| III | 0.746 | 0.275‐2.023 | .564 | |||
| IV | 0.872 | 0.325‐2.343 | .786 | |||
| Unknown | 0.822 | 0.275‐2.459 | .726 | |||
| Lymphadenectomy | ||||||
| No | Ref. | .961 | ||||
| Yes | 0.956 | 0.525‐1.743 | .884 | |||
| Others | 0.806 | 0.179‐3.639 | .780 | |||
| Urinary diversion | ||||||
| Ileal conduit | Ref. | .001* | Ref. | <.001* | ||
| Orthotopic neobladder | 0.705 | 0.576‐0.862 | 0.671 | 0.548‐0.823 | ||
| Adjuvant radiation | ||||||
| No | Ref. | .002* | Ref. | .033* | ||
| Yes | 3.523 | 1.567‐7.919 | 2.526 | 1.080‐5.908 | ||
| Adjuvant chemotherapy | ||||||
| No | Ref. | .098 | ||||
| Yes | 1.187 | 0.969‐1.455 | ||||
Abbreviations: ACM, all‐cause mortality; AI, American Indian/Alaskan Native; API, Asian/Pacific Islander; CI, confidence interval; HR, hazard ratio; NA, not available; NOS, not otherwise specified; PSM, propensity score matching; Ref., reference.
Statistically significant.
4. DISCUSSION
RC and urinary diversion are two important steps of the operation for BCa patients. There are different choices of urinary diversions after RC, including uretero‐cutaneostomy, IC, continent cutaneous urinary diversion, ureterocolonic diversion, and ON according to the patients’ preference, performance status, life expectancy, and oncological control. Among these urinary diversions, IC and ON are the most commonly utilized ones in the clinical practice. 2 , 3 As a continent diversion, ON offers many advantages and represents an excellent option for urinary diversion for appropriate cases. However, large studies based on the United States and European populations have demonstrated that the utilization of orthotopic diversion has decreased over time. 6 , 7 The reason for this phenomenon may be due to increased adoption of robotic radical cystectomy and increased utilization of intracorporeal urinary diversion. 3 Intracorporeal ON is a difficult technique, and thus, is associated with a higher rate of high‐grade complications. 8 , 9 Complications due to urinary diversion can prolong the hospital stay, increase hospitalization expenses, and impact all‐other cause survival. Different kinds of complications related to urinary diversion after RC have been reported, but most are due to the use of the bowel for urinary diversion. Complications related to IC diversion include urinary tract infections, pyelonephritis, ureteroileal leakage and stenosis, and stomal problems. 10 , 11 In two studies with large populations, complications associated with ON included diurnal and nocturnal incontinence, ureterointestinal stenosis, metabolic disorders, and vitamin B12 deficiency. 12 , 13 One previous retrospective study revealed that there was no significant difference in the decline of estimated glomerular filtration rate between IC and ON in patients with preoperative chronic kidney disease. 14 In this study, we focus on the impact of IC and ON diversion on the oncological outcomes of BCa patients. Our aim is to provide some decision‐making guidance to urologists and patients regarding urinary diversion.
Consistent with a previous study, 5 this study showed that the ON cohort comprised younger and male patients and with a significantly higher percentage of early T stages compared to the IC cohort. Age alone is not a criterion for choosing continent ON diversion, but age >80 years is often considered to be the threshold. 2 However, the optimal cut‐off value for age is still disputed. In our opinion, younger patients with BCa would have longer life expectancy, better tolerance for a complicated orthotopic diversion technique, and easier recovery from postoperational complications. A higher male‐to‐female ratio has also been observed among patients undergoing ON substitution in some large series from experience centers. 15 , 16 Male BCa patients are more suitable to receive ON substitution after RC due to the following reasons: (a) the incidence rate of BCa is higher in men than in women (male to female ratio 3:1) 1 ; (b) the urethra of men is longer than that of women, which would, to some extent, ensure urinary continence after ON substitution. Frozen sections of pelvic lymph nodes and surgical margins have to be performed during the operation if ON diversion is planned. Positive surgical margins, N2 or N3 disease would exclude orthotopic neobladder reconstruction, 2 , 3 which may be the potential reason why early T stages are found in the ON group.
There have been many studies on the impact of ON and IC diversions on the HRQoL of BCa patients, but the influence of ON diversion on cancer control has not been adequately studied. One systemic review, which included 29 studies, found no significant differences in QoL after radical cystectomy between continent and incontinent urinary diversion. 17 However, by maintaining body image and normal urethral voiding in suitable patients, ON could offer a better QoL. In a relatively large study with 214 ON and 269 IC patients, Yossepowitch and his colleagues demonstrated that ON diversion had a more favorable CSS and OS compared to IC diversion (P = .04 and P = .001, respectively). However, in the subgroup analysis this statistical significance disappeared when further stratified into organ confined and nonorgan confined diseases. 5 This was the only study on the impact of ON and IC diversions on oncological outcomes that we could find. In contrast with the above study, this study found better 5‐year CSS and 5‐year OS in the ON group for either all T stages together or T stage subgroups. Furthermore, given the discrepancies between the two groups, we performed 1:1 PSM to adjust for age, gender, sex, marital status, TNM stages, lymphadenectomy, radiotherapy, and chemotherapy. After PSM, multiple cox regression analyses showed that ON was a protective factor for CSS and OS for all T stages. However, when stratified for pathological T stages, subgroup analysis revealed that only patients with pathological T2 stage obtained CSS (P = .016) and OS (P < .001) benefit from ON diversion.
This study has some limitations as well as some strengths. Among limitations, first, the study was of a retrospective nature, which means that the significant survival outcomes could be the effect of strict selection criteria for ON diversion. As a consequence, a randomized controlled trial to compare the long‐term oncological outcomes between ON diversion and IC diversion is important and urgent. Second, the documentation on lymphadenectomy was not very precise about the scope of lymph node dissection, which may have had an impact on survival outcomes. 18 Third, there was no documentation on BCa recurrence in the SEER database; thus, we could not analyze recurrence‐free survival. Moreover, we did not control for performance status or any prognostic risk score at baseline. Consequently, as patients who have better performance status may be eligible for ON vs IC, this may result in selection bias. Nonetheless, to the best of our knowledge, the strength of this study is that it is the largest one to compare survival outcomes between the two groups based on the SEER cancer registries, which cover approximately 28% of the population of the United States. Most clinical trials have excluded patients who had a history of other prior malignancy, since this may have an impact on survival outcomes. 19 , 20 Based on this, and to ensure the reliability of our results, we also excluded patients with previous tumor history. Finally, the PSM method also increased the accuracy of this study.
5. CONCLUSIONS
As a continent diversion, ON diversion showed some advantages in terms of survival outcomes compared to IC diversion. Young, married, and male patients with early pathological T stage, especially T2 stage, were more suitable to receive RC plus ON surgery, which could improve their probability of survival.
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
AUTHOR CONTRIBUTIONS
Xiaohong Su: Data curation, formal analysis, investigation, methodology, project administration, writing‐original draft. Kaihui Wu, Shuo wang and Wei Su: Data curation, formal analysis, investigation. Bingkun Li and Chuanyin Li: Data curation, formal analysis, investigation. Xiangming Mao: Conceptualization, project administration, supervision, and writing‐review and editing.
Supporting information
TableS1‐S3
Su X, Wu K, Wang S, et al. The impact of orthotopic neobladder vs ileal conduit urinary diversion after cystectomy on the survival outcomes in patients with bladder cancer: A propensity score matched analysis. Cancer Med. 2020;9:7590–7600. 10.1002/cam4.3404
Funding information
This work was supported by grants from the National Natural Science Foundation of China (81773277), and Science and Technology Planning Project of Guangzhou (201803010014).
Contributor Information
Bingkun Li, Email: 25840416@qq.com.
Xiangming Mao, Email: mxm631221@126.com.
DATA AVAILABILITY STATEMENT
The raw data underlying this paper is available upon request to from the corresponding author.
REFERENCES
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA: Cancer J Clin. 2020;70:7‐30. [DOI] [PubMed] [Google Scholar]
- 2. Witjes JA, Bruins HM, Cathomas R, et al. European association of urology guidelines on muscle‐invasive and metastatic bladder cancer: summary of the 2020 guidelines. Eur Urol. 2020. 10.1016/j.eururo.2020.03.055 [DOI] [PubMed] [Google Scholar]
- 3. Almassi N, Bochner BH. Ileal conduit or orthotopic neobladder: selection and contemporary patterns of use. Curr Opin Urol. 2020;30:415‐420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nuhn P, May M, Sun M, et al. External validation of postoperative nomograms for prediction of all‐cause mortality, cancer‐specific mortality, and recurrence in patients with urothelial carcinoma of the bladder. Eur Urol. 2012;61:58‐64. [DOI] [PubMed] [Google Scholar]
- 5. Yossepowitch O, Dalbagni G, Golijanin D, et al. Orthotopic urinary diversion after cystectomy for bladder cancer: implications for cancer control and patterns of disease recurrence. J Urol. 2003;169:177‐181. [DOI] [PubMed] [Google Scholar]
- 6. Bachour K, Faiena I, Salmasi A, et al. Trends in urinary diversion after radical cystectomy for urothelial carcinoma. World J Urol. 2018;36:409‐416. [DOI] [PubMed] [Google Scholar]
- 7. Groeben C, Koch R, Baunacke M, et al. Urinary diversion after radical cystectomy for bladder cancer: comparing trends in the US and Germany from 2006 to 2014. Ann Surg Oncol. 2018;25:3502‐3509. [DOI] [PubMed] [Google Scholar]
- 8. Hussein AA, Elsayed AS, Aldhaam NA, et al. A comparative propensity score‐matched analysis of perioperative outcomes of intracorporeal vs extracorporeal urinary diversion after robot‐assisted radical cystectomy: results from the International Robotic Cystectomy Consortium. BJU Int. 2020;126:265‐272. [DOI] [PubMed] [Google Scholar]
- 9. Hosseini A, Mortezavi A, Sjoberg S, et al. Robot‐assisted intracorporeal orthotopic bladder substitution after radical cystectomy: perioperative morbidity and oncological outcomes ‐ a single institution experience. BJU Int. 2020. 10.1111/BJU.15112 [DOI] [PubMed] [Google Scholar]
- 10. Nieuwenhuijzen JA, de Vries RR, Bex A, et al. Urinary diversions after cystectomy: the association of clinical factors, complications and functional results of four different diversions. Eur Urol. 2008;53:834‐844. [DOI] [PubMed] [Google Scholar]
- 11. Madersbacher S, Schmidt J, Eberle JM, et al. Long‐term outcome of ileal conduit diversion. J Urol. 2003;169:985‐990. [DOI] [PubMed] [Google Scholar]
- 12. Hautmann RE, Volkmer BG, Schumacher MC, et al. Long‐term results of standard procedures in urology: the ileal neobladder. World J Urol. 2006;24:305‐314. [DOI] [PubMed] [Google Scholar]
- 13. Stein JP, Skinner DG. Results with radical cystectomy for treating bladder cancer: a 'reference standard' for high‐grade, invasive bladder cancer. BJU Int. 2003;92:12‐17. [PubMed] [Google Scholar]
- 14. Gershman B, Eisenberg MS, Thompson RH, et al. Comparative impact of continent and incontinent urinary diversion on long‐term renal function after radical cystectomy in patients with preoperative chronic kidney disease 2 and chronic kidney disease 3a. Int J Urol. 2015;22:651‐656. [DOI] [PubMed] [Google Scholar]
- 15. Hautmann RE, de Petriconi RC, Pfeiffer C, et al. Radical cystectomy for urothelial carcinoma of the bladder without neoadjuvant or adjuvant therapy: long‐term results in 1100 patients. Eur Urol. 2012;61:1039‐1047. [DOI] [PubMed] [Google Scholar]
- 16. Neuzillet Y, Yonneau L, Lebret T, et al. The Z‐shaped ileal neobladder after radical cystectomy: an 18 years experience with 329 patients. BJU Int. 2011;108:596‐602. [DOI] [PubMed] [Google Scholar]
- 17. Yang LS, Shan BL, Shan LL, et al. A systematic review and meta‐analysis of quality of life outcomes after radical cystectomy for bladder cancer. Surg Oncol. 2016;25:281‐297. [DOI] [PubMed] [Google Scholar]
- 18. Patel DN, Luu M, Zumsteg ZS, et al. Development and validation of an improved pathological nodal staging system for urothelial carcinoma of the bladder. Eur Urol Oncol. 2019;2:656‐663. [DOI] [PubMed] [Google Scholar]
- 19. Al‐Husseini MJ, Saad AM, Mohamed HH, et al. Impact of prior malignancies on outcome of colorectal cancer; revisiting clinical trial eligibility criteria. BMC Cancer. 2019;19:863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Laccetti AL, Pruitt SL, Xuan L, et al. Effect of prior cancer on outcomes in advanced lung cancer: implications for clinical trial eligibility and accrual. J Natl Cancer Inst. 2015;107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TableS1‐S3
Data Availability Statement
The raw data underlying this paper is available upon request to from the corresponding author.


