Supplemental Digital Content is available in the text.
Keywords: follow-up studies, inflammation, middle aged, mortality, thromboembolism
Objective:
Coronavirus disease 2019 (COVID-19) can infect patients in any age group including those with no comorbid conditions. Understanding the demographic, clinical, and laboratory characteristics of these patients is important toward developing successful treatment strategies.
Approach and Results:
In a retrospective study design, consecutive patients without baseline comorbidities hospitalized with confirmed COVID-19 were included. Patients were subdivided into ≤55 and >55 years of age. Predictors of in-hospital mortality or mechanical ventilation were analyzed in this patient population, as well as subgroups. Stable parameters in overall and subgroup models were used to construct a cluster model for phenotyping of patients. Of 1207 COVID-19–positive patients, 157 met the study criteria (80≤55 and 77>55 years of age). Most reliable predictors of outcomes overall and in subgroups were age, initial and follow-up d-dimer, and LDH (lactate dehydrogenase) levels. Their predictive cutoff values were used to construct a cluster model that produced 3 main clusters. Cluster 1 was a low-risk cluster and was characterized by younger patients who had low thrombotic and inflammatory features. Cluster 2 was intermediate risk that also consisted of younger population that had moderate level of thrombosis, higher inflammatory cells, and inflammatory markers. Cluster 3 was a high-risk cluster that had the most aggressive thrombotic and inflammatory feature.
Conclusions:
In healthy patient population, COVID-19 remains significantly associated with morbidity and mortality. While age remains the most important predictor of in-hospital outcomes, thromboinflammatory interactions are also associated with worse clinical outcomes regardless of age in healthy patients.
Graphic Abstract:
A graphic abstract is available for this article.
Highlights.
Coronavirus disease 2019 (COVID-19) adversely affects the older population and those with risk factors; however, it can be fatal and is associated with significant in-hospital mortality or need for mechanical ventilation in healthier patients and in the young population.
d-dimer and LDH (lactate dehydrogenase) elevation and their re-elevation seem to be linked to worse in-hospital outcomes regardless of age in the healthier patient population.
Aging process, inflammation-induced thrombosis, endothelial infection, or multiple repetitive successive pathological insults of different mechanisms can contribute to the elevation and re-elevation of d-dimer and LDH.
Unsupervised cluster model using age, d-dimer, and LDH initial and follow-up values produced 3 clusters in terms of in-hospital outcomes that could be identified as low, intermediate, and high risk. The 3 clusters were distinct in their thrombosis, inflammation, and end-organ damage behavior.
Understanding of such pathological processes and their impact on patients with and without comorbidities among all age categories is crucial toward developing successful treatment strategies.
Coronavirus disease 2019 (COVID-19) is caused by the recently identified coronavirus also known as severe acute respiratory syndrome coronavirus 2 (or SARS-CoV-2).1 Since December 2019, COVID-19 has caused a global outbreak and is still spreading quickly in >100 countries.2 Our understanding of this disease has been increasing with time. A disease entity that was initially identified as a primarily respiratory illness has slowly emerged as a systemic syndrome that causes endothelial dysfunction leading to microthrombosis and severe inflammatory response leading to a cytokine storm.3 However, the pathophysiological and clinical characterization of the disease is still evolving. The disease still remains significantly heterogeneous both in its demographic characteristics and clinical features. One example of such heterogeneity is that this disease was thought to vastly affect the older population with significant comorbidities such as hypertension and diabetes mellitus.4 However, in our clinical experience, we have started to see significant morbidity and mortality in younger and otherwise healthy patient population. With the lack of clinical and pathophysiological studies in this subgroup of healthier patients with COVID-19, we sought to initiate a retrospective study in patients hospitalized with COVID-19 infection. The goal of this study was to characterize the clinical and laboratory findings and understand predictors of outcome in this patient population.
Methods
In a retrospective study design, consecutive adult patients admitted to our hospital between March 15 and April 23, 2020, with confirmed COVID-19 were reviewed. Patients were included if they had no baseline comorbidities. Patients were excluded if their age was <18 years, body mass index ≥30 kg/m2, or if they had ≥1 baseline comorbidities, defined as history of hypertension, diabetes mellitus, bronchial asthma, chronic obstructive pulmonary disease, HIV infection, chronic liver disease, hepatitis B or C viral infection, congestive heart failure, coronary artery disease, chronic kidney disease, end-stage renal disease, smoking, malignancy, or any other chronic condition. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Population, Patient Triage, and Comparisons
Patients with symptoms suggestive of COVID-19 infection were identified in the emergency department. After initial assessment, basic clinical data including time from symptom onset and admission laboratory investigations were obtained. In addition, all patients had pulmonary imaging (chest radiograph or computed tomography of the chest) done. Patients subsequently had a nasal swab for COVID-19 RNA to confirm the infection with SARS-Cov-2. Electronic medical charts were reviewed for the presence of baseline comorbidities, initial and subsequent laboratory values, treatment and therapeutic modalities, need for mechanical ventilation, and in-hospital mortality.
All laboratory data were obtained on the same day of admission, while follow-up laboratory values were obtained for LDH (lactate dehydrogenase) on the second day of admission and for D-dimer if a change in the clinical condition (change in the requirement of oxygen or vital signs or clinical suspension of thromboembolic episode).
Patients were then classified based on their age as younger (≤55 years of age) or older (>55 [older] years of age). We selected a 55-year-old cutoff value to differentiate between younger and older patients based in the US Census Bureau report, which defines older adults as ≥55 years of age and elderly as ≥65 years of age.5
Subgroups were compared for their demographic, clinical, and laboratory variables and outcomes. The primary end point was in-hospital mortality and need for mechanical ventilation.
Statistical Analysis
Continuous variables were expressed as mean±SD, and nominal and categorical variables were expressed as numbers (%). The independent samples Student t test and 1-way ANOVA were used to compare the mean values of different groups, and χ2 test was used for comparison of nominal and categorical variables. Predictors of mortality and mechanical ventilation were checked using univariate and multivariate Cox regression models. The most stable predictors in all models were used to construct an SPSS unsupervised 2-step cluster model to test the presence of the natural subgroups and were subsequently compared for characterization of each cluster of patients. Kaplan-Meier survival curves were used to test the difference in cumulative in-hospital outcomes in different clusters. For all statistical tests, P<0.05 was considered statistically significant. All analyses were performed with commercially available software (SPSS, version 23.0; SPSS, Inc).
Results
During the study period, 1207 patients with confirmed COVID-19 were identified. The baseline demographic, clinical, laboratory, and imaging criteria for all patients are summarized in Table I in the Data Supplement. Of these patients, 7 patients were excluded as their age was <18 years, and 1050 patients were excluded due to the presence of ≥1 comorbidities (734 [70%] with hypertension, 549 [52%] with diabetes mellitus, 306 [29%] patients were smokers, 79 [8%] with HIV infection, 154 [15%] with asthma, 119 [11%] with chronic obstructive pulmonary disease, 7 [1%] with chronic liver disease, 4 [0.4%] with hepatitis B infection, 50 [5%] with hepatitis C infection, 117 [11%] with congestive heart failure, 125 [12%] with coronary artery disease, 98 [9%] with chronic kidney disease, 82 [8%] with end-stage renal disease, and 12 [1%] patients with other causes).
One hundred fifty-seven patients met the study criteria and were included for final analysis. The mean age of the study population was 52.6±17 years. Eighty (50.9%) patients were ≤55 years of age, and 46 (29%) were women. Of the study population, 105 (67%) patients received hydroxychloroquine/azithromycin combination, and 13 (8%) patients received tocilizumab. Thirty (19%) patients were mechanically ventilated, and 25 (16%) patients died during to the hospital course. Of note, while all initial laboratory data were obtained at the day of admission, the second LDH values were obtained on the second day of admission and the second D-dimer was obtained within a week from the first obtained D-dimer in 137 (87%) patients (44 [32%] on the second day, 43 [31%] on the third day, 21 [15%] on the fourth day, 15 [11%] on the fifth day, 7 [5%] on the sixth day, and 7 [5%] on the seventh day), while the remaining patients had their D-dimer checked in the second week from the admission (overall mean difference between the first and second D-dimer was 3.9±7.8 days).
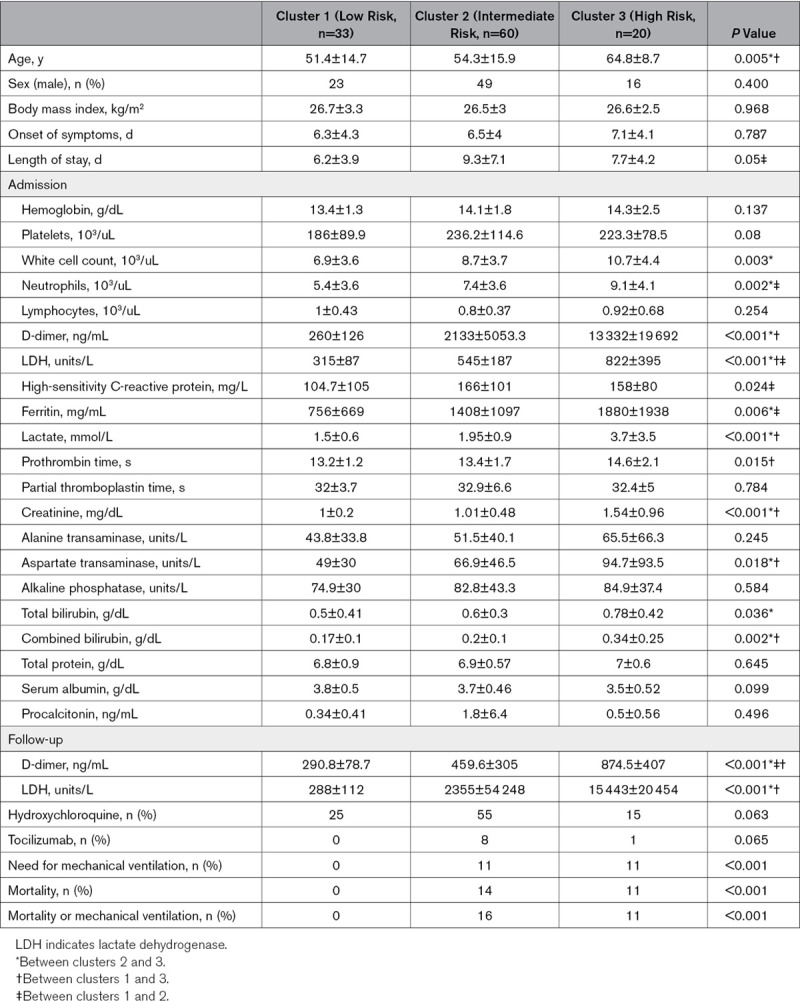
When patients were classified based on their age, it was found that patients >55 years of age had lower lymphocyte count, and lower albumin levels. Older patients were also noted to have higher D-dimer, LDH (lactate dehydrogenase), lactate, ferritin, and creatinine levels (Table 1). There were no differences between the two groups with regard to sex, body mass index, and laboratory values including high-sensitivity C-reactive protein, hemoglobin levels, white cell count, platelet count, prothrombin time, partial thromboplastin time, bilirubin, serum protein, and procalcitonin levels (Table 1). The two groups were similar in terms of medication use. Older patient subgroup had higher mortality and higher need for mechanical ventilation (Table 1).
Table 1.
Demographic, Clinical, and Laboratory Data for All Patients and Subgroups
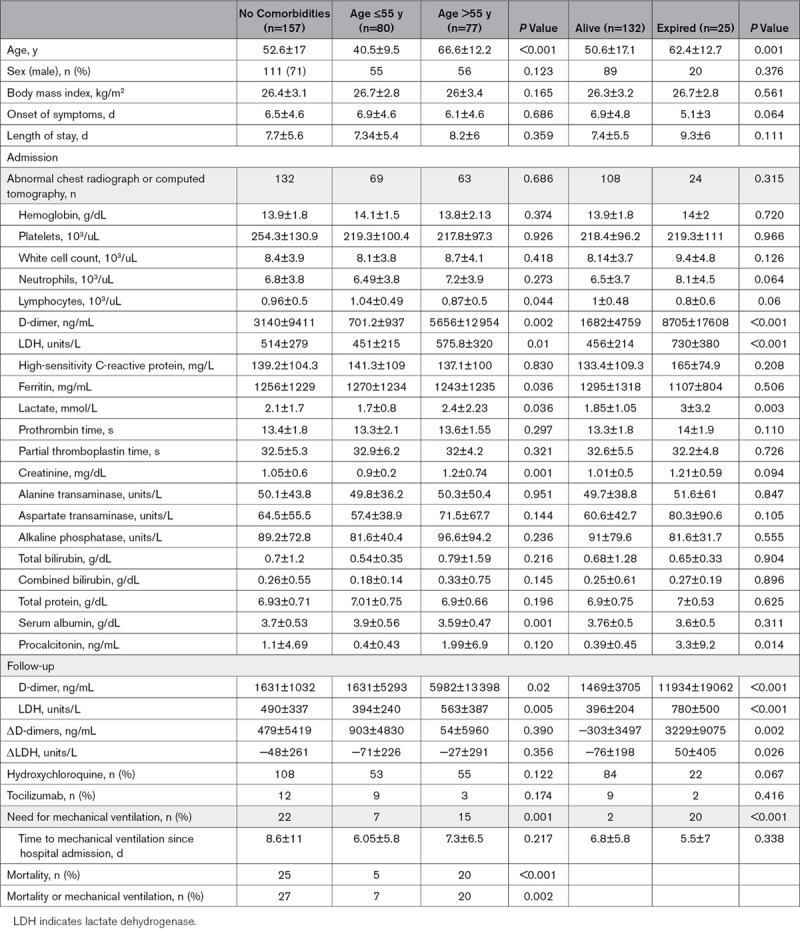
In a subgroup analysis of patients who died, we noted that these patients were older and had higher D-dimer, LDH, lactate, and procalcitonin levels (Table 1).
Predictors of In-Hospital Mortality
The overall predictors of composite of in-hospital mortality or need for mechanical ventilation in patients without comorbidities are summarized in Table 2. Univariate Cox regression model showed that the independent predictors of in-hospital mortality were age, D-dimer, and LDH levels. Multivariate Cox regression models, however, showed that D-dimer and LDH levels were the only predictors of in-hospital mortality, while age lost statistical significance. In patients ≤55 years of age with no comorbidities (younger healthier patients), the predictors of in-hospital mortality in the univariate Cox regression model were lower hemoglobin, higher neutrophil count, D-dimer, LDH, and total bilirubin level. In the multivariate regression analysis, neutrophil count, D-dimer, and LDH levels were the only independent predictors of in-hospital mortality (Table 2).
Table 2.
Cox Regression Models for Predictors of the Composite of Mechanical Ventilation or Death in Younger and Older Patients
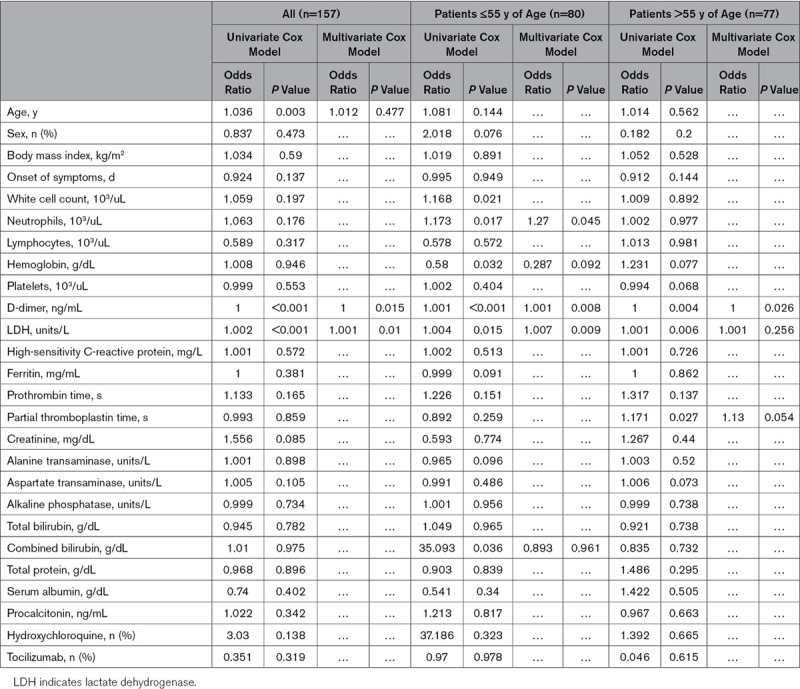
In older patients’ subgroup, higher D-dimers, LDH, and partial thromboplastin time levels were the predictors of in-hospital mortality by univariate Cox regression model. Multivariate regression analysis, however, showed that only higher D-dimer predicted in-hospital mortality (Table 2).
Follow-Up D-Dimer and LDH
Most consistent predictors of the composite outcomes regardless of age in all models were found to be D-dimer and LDH. Follow-up values of D-dimer and LDH were found to be significantly higher in patients >55 years of age and in patients who died, compared with patients ≤55 years of age and those who survived (P=0.02 and 0.005, respectively; Table 1). We also analyzed ΔD-dimer and ΔLDH and noted that they were not significantly different between younger and older patient subgroups (P=0.390 and 0.356, respectively). However, these values were significantly higher in patients who died compared with patients who survived (P=0.002 and 0.026, respectively; Table 1).
Receiver operating characteristic was done for initial and follow-up (second) D-dimer and LDH, as well as their delta values (Figure 1), and we found that the best predictors for composite outcomes for initial, follow-up, and ΔD-dimer were when their values were >461, 491, and 798 ng/mL, respectively. Similarly, the best predictors for initial, follow-up, and ΔLDH were when their values were >467, 505, and 128 units/L, respectively.
Figure 1.
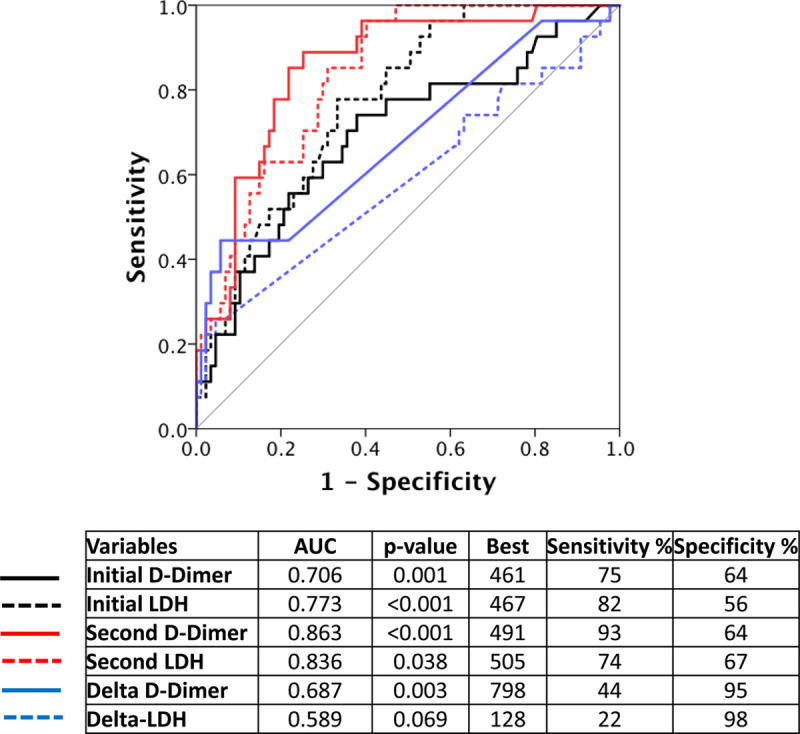
Receiver operating characteristics curve for best predictors of in-hospital outcomes. AUC indicates area under the curve; and LDH, lactate dehydrogenase.
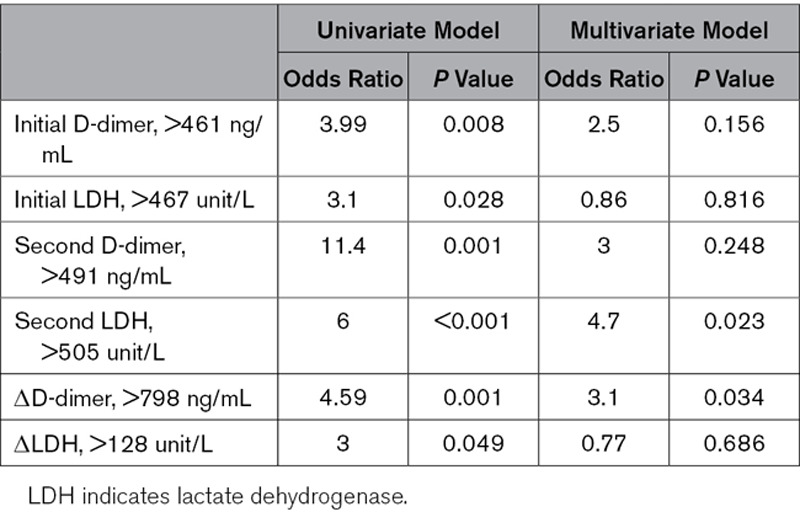
Based on these cutoff values, Cox regression models were repeated with adjustment for both age and sex for all patients (Table 3), and it was noted that all adjusted variables can significantly predict outcomes based on univariate models, whereas in multivariate models, only adjusted ΔD-dimer >798 ng/mL and repeat LDH level of >505 units/L predicted mortality.
Table 3.
Cox Regression for Prediction of the Composite of Need of Mechanical Ventilation or Death Adjusted for Age and Sex in All Patients
Cluster Model Prediction of Composite Outcome Based on Age, D-Dimer, and LDH
Next, a 2-step cluster model was initiated for all patients based on age, initial d-dimer >461 ng/mL, follow-up d-dimer >491 ng/mL, initial LDH >467 unit/L, and follow-up LDH >505 unit/L (Figure 2). The output revealed that patients were classified into 3 different clusters. Based on Kaplan-Meier curve for survival free of the composite outcomes, it was noted that cluster 1 was a low-risk cluster, cluster 2 was an intermediate-risk cluster, and cluster 3 was a high-risk cluster (mortality and mechanical ventilation: 0 [0%], 16 [27%], and 11 [55%], respectively; P<0.001; Table 4). Post hoc examination of the clusters revealed the following characteristics for each cluster:
Figure 2.
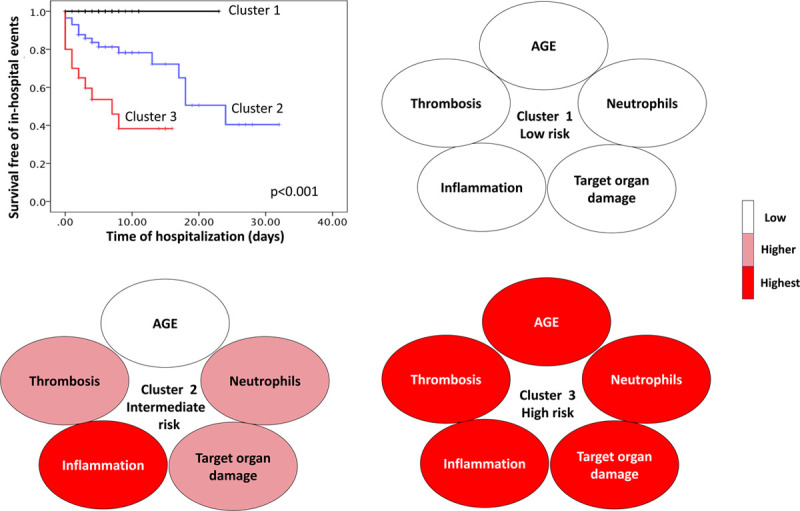
Unsupervised computational cluster model. The model was initiated after feature extraction using best predictors of in-hospital outcomes from Cox regression model and receiver operator characteristic curve, namely age, initial and follow-up d-dimer above 461 and 491 ng/mL, respectively, and initial and follow-up lactate dehydrogenase 467 505 units/L, respectively. The model output showed 3 clusters. Based on Kaplan-Meier curve (upper left), cluster 1 was a low-risk cluster with zero outcomes (black line), cluster 2 was intermediate risk (blue line), and cluster 3 was high risk (red line). Post hoc analysis for cluster description based on domains of age, thrombosis, inflammatory cells, inflammatory markers, and target organ damage showed a progressively worsening profile from clusters 1 to 3.
Table 4.
Comparison Between Different Clusters for Demographic, Clinical, and Laboratory Variables, As Well As Treatment Options and Outcomes
Age
Clusters 1 and 2 were significantly younger than cluster 3 (51.4±14.7, 54.3±15.9, and 64.8±8.7 years of age, respectively; P=0.005; Table 4).
Thrombosis Burden
Thrombosis burden based on d-dimer level was found to progressively increase from cluster 1 to clusters 2 and 3 (260±126, 2133±5053.3, and 13332±19692 ng/mL, respectively; P<0.001; Table 4).
Blood Cells
While hemoglobin and platelet levels were not different between clusters, white cell count was the lowest in cluster 1 compared with the other two clusters (6.9±3.6, 8.7±3.7, and 10.7±4.4 10/3mL, respectively; P=0.002; Table 4). Deferential white count showed that this difference was mainly due to neutrophil count, which progressively increased from cluster 1 to cluster 3 (5.4±3.6, 7.4±3.6, and 9.1±4.1 103/mL, respectively; P=0.002; Table 4), while lymphocyte count was not different between clusters.
Inflammatory Markers
It was noted that for acute-phase reactants such as high-sensitivity C-reactive protein and ferritin significantly and progressively increased from cluster 1 to cluster 3 (high-sensitivity C-reactive protein: 104.7±105, 166±101, and 158±80 mg/d, respectively, P=0.024; ferritin: 756±669, 1408±1097, and 1880±1938 mg/mL, respectively, P=0.006; Table 4).
Target Organ Damage and Tissue Damage
Parameters suggestive of target organ damage such as renal function tests and liver function tests and parameters of tissue hypoperfusion such as lactate levels were checked for all clusters. Target organ damage and tissue hypoperfusion were the hallmark of cluster 3 as suggested by difference in creatinine (cluster 1, 1±0.2 mg/dL; cluster 2, 1.01±0.48 mg/dL; cluster 3, 1.54±0.96 mg/dL; P<0.001), aspartate aminotransferase (cluster 1, 49±30 units/L; cluster 2, 66.9±46.5 units/L; cluster 3, 94.7±93.5 units/L; P=0.018), total bilirubin (cluster 1, 0.5±0.41 mg/dL; cluster 2, 0.6±0.3 mg/dL; cluster 3, 0.78±0.42 mg/dL; P=0.036), direct bilirubin (cluster 1, 0.17±0.1 mg/dL; cluster 2, 0.2±0.1 mg/dL; cluster 3, 0.34±0.25 mg/dL; P=0.002), and lactate levels (cluster 1, 1.5±0.6 ng/mL; cluster 2, 1.95±3.7 ng/mL; cluster 3,3.7±3.5 ng/mL; P<0.001; Table 4). In addition, tissue damage as suggested by LDH levels progressively increased from cluster 1 to clusters 2 and 3 (cluster 1, 315±87 unit/L; cluster 2, 545±187 unit/L; cluster 3, 822±395 unit/L; P<0.001; Table 4).
Discussion
To the best of our knowledge, this is the first report describing the demographic, clinical, and laboratory parameters of healthier patient population hospitalized with COVID-19. The main findings of the current study can be summarized as follows: the main predictors of in-hospital outcomes (mortality or need for mechanical ventilation) are older age and high D-dimer and LDH levels. D-dimer and LDH seemed to be the most consistent predictors of adverse outcomes regardless of age. Moreover, the predictive ability of both variables increased when these were followed up during hospitalization with the best sensitivity acquired by second high D-dimer measurement and the best specificity observed for significant positive change of D-dimer (ΔD-dimer) on subsequent testing. Finally, when patients were classified with an unsupervised cluster using their age, initial, and follow-up D-dimer, and LDH, the model output created 3 different phenotypes with distinct in-hospital outcomes. Importantly, two of these clusters were relatively younger patients who were differentiated based on their D-dimer, inflammatory markers, and parameters of end-organ damage, and the third cluster consisted of older patients with the significantly worst clinical and laboratory profile.
Despite the great experience gained in the clinical characterization and diagnosis, COVID-19 remains a dynamic disease process for which our understanding continues to evolve from what was initially identified as a primarily respiratory illness toward a systemic syndrome that involves multiorgan endothelial dysfunction strongly linked to microthrombosis and severe inflammatory response. The heterogeneity of the disease is demonstrated by the fact that it continues to significantly affect all age groups and not just the older population or those with comorbidities, particularly diabetes mellitus and hypertension.6 Recent studies showed that children and young adults who died had also had baseline comorbidities and that those who did not have baseline comorbidities could be managed on general wards and were discharged successfully.7,8
Demographic, Laboratory, and Clinical Characteristics of the Disease
In our study, of 1207 patients admitted with COVID-19 during the earlier period of the pandemic, 157 of them (13%) had no baseline comorbidities, which was slightly more than that reported by the Centers for Disease Control (7.9%).9 Twenty-seven (17%) of all patients reported here had in-hospital outcomes. Importantly, 80 of these patients were <55 years of age, of whom 7 (9%) had in-hospital outcomes, suggesting that rates of serious adverse outcomes are not as low as initially expected at the beginning of the pandemic. While age remained an important predictor of mortality, sex failed to predict mortality overall and in subgroups. When patients were classified based on their age, it was found that older patients (>55 years of age) had lower lymphocyte count, serum albumin, and platelet count and higher LDH, creatinine, and lactic acid, and the most remarkable difference between both groups was the significantly elevated D-dimer levels in the older group. This was confirmed in the Cox regression models when only D-dimer and LDH were the stable predictors of mortality regardless of the age. This suggests that the primary pathophysiology in this group of patients is thrombotic in nature while all other changes can be secondary. The fact that the follow-up D-dimer and ΔD-dimer levels in hospital were the parameters that could predict mortality with high sensitivity and specificity, respectively, suggests that this thrombotic burden is dynamic and continues to rebound during the course of the disease. When unsupervised cluster model was applied to all patients, an important link between the inflammatory status and thrombosis was uncovered pointing toward age-related progressive worsening of inflammatory state coupled with the inflammatory cells and the inflammatory markers that seems linked to higher mortality. It is important to note that such natural classification of patients revealed that younger patients may not be similar in their presentation in the disease and only those who have higher thromboinflammatory burden are at an increased risk for in-hospital outcomes, suggesting that there may be underlying physiological or genetic factors that enhance such process in some individuals versus the others. On the other hand, the cluster model suggested that in older age, this thromboinflammatory relationship seems to be the most aggressive, leading to higher in-hospital outcomes.
Proposed Pathophysiological Mechanisms
SARS-Cov-2 entry into the cells is mediated by ACE-2 (angiotensin-converting enzyme 2) receptor on cellular membranes,10 abolishing ACE-2 enzyme activity.11 The expression of ACE-2 receptors may decrease with advanced age.12 This may lead to a decreased metabolism of angiotensin II and a systemic imbalance between angiotensin II (proinflammatory/procoagulant) and angiotensin 1-7 (anti-inflammatory/anticoagulant), causing a worse baseline proinflammatory/procoagulant state in older patients that is aggravated by COVID-19 infection, which induces further downregulation of ACE-2 in older patients (Figure 3).
Figure 3.
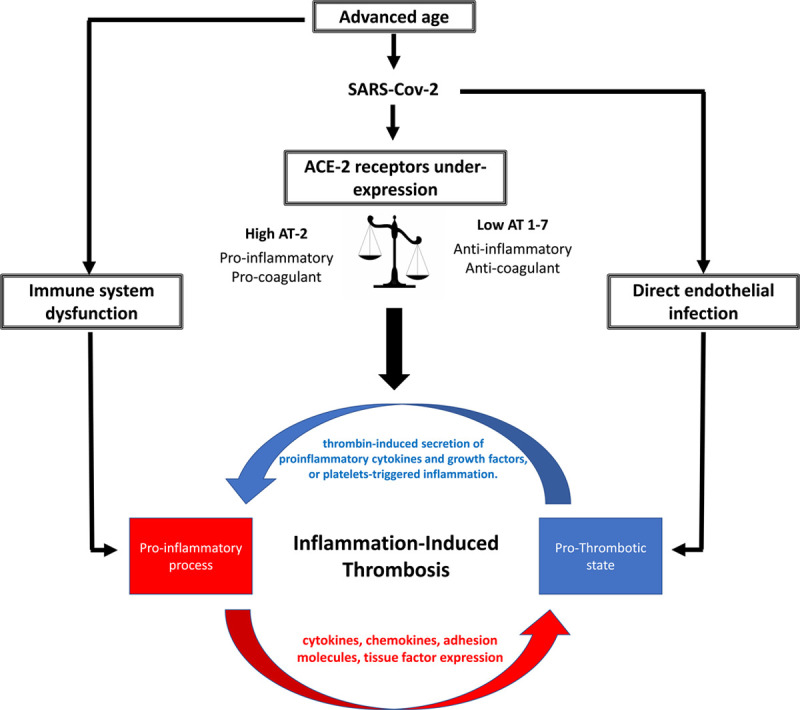
Proposed mechanisms of thromboinflammatory response in patients with no risk factor. ACE-2 indicates angiotensin-converting enzyme 2; AT-2, angiotensin-2; and SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2.
The aging process can also impact the immune system leading to a process of a less-competent immune system referred to as immune senescence,13 which incorporates dysfunction in cellular signaling that directly affects the ability to contain infections and in case of COVID-19 can lead to dysfunctional (decreased or overexerted) response to viral load and inability to control the viral spread.
Another possible explanation is inflammation-induced thrombosis (IIT)—a phenomenon reported in several inflammatory and autoimmune diseases.14 The pathogenesis of IIT is complicated and involves bidirectional interactions between the inflammatory state and the coagulation system (Figure 3). Reports have shown that IIT can be initiated either on the proinflammatory end or the procoagulant end. An overlap of both ends of IIT may occur, causing a vicious cycle that leads to rebound inflammation and thrombosis with a baseline increased proinflammatory state as explained previously. This may explain why in our study the predictive ability of D-dimer elevation may shed light on that a re-elevation of D-dimer may signify multiple episodes of thrombosis that is linked to high mortality in those patients. Importantly, such IIT is also reported to develop in the absence of vascular endothelial damage, which may explain the pathology in younger patients observed in our study.
Recent pathological reports have shown that viral elements exist within endothelial cells of patients with COVID-19 associated with accumulation of inflammatory cells, causing endotheliitis.15 Endothelial dysfunction occurring in COVID-19 can shift the vascular equilibrium toward more vasoconstriction, subsequent organ hypoperfusion, and inflammation with associated procoagulant state. This can explain the elevated lactate, LDH, and inflammatory markers that seem to be related to the severity of the disease in our study. In patients with COVID-19, endotheliitis can lead to activation of the coagulation cascade to form fibrin meshes in an attempt to contain the viral spread by forming microthrombi in situ,16 which can potentially dislodge systemically leading to arterial and venous thrombosis. As such, the integrity of the endothelial function and immune system in the younger people is crucial for a regulated immune response good enough to control the virus spread by forming microthrombi for viral entrapment, and this in turn may partially lead to a relative quiescent systemic inflammatory state in the younger healthier patients. On the other hand, direct endothelial infection can also aggravate the baseline endothelial dysfunction that can exist because of comorbidities or due to aging,17 which may partially explain the high levels of D-dimers and inflammatory response in the older patients in our study.
An alternate explanation for the change in D-dimer with time can be the multiple injuries, or multiple hit to the endothelium by different successive mechanisms while the disease progresses. The successive hits can be composed of endothelial infection with the associated thrombo-entrapment of the virus, inflammatory response to the virus mediated by IIT, and hypoxia- or hypotension-induced thrombosis.18
Phenotypes of the Disease in Patients Without Comorbidities
In our study, we have attempted to uncover the natural distribution of parameters between patients to understand phenotyping, and for that, we have used a machine learning–based unsupervised cluster model. Computational clustering is an exploratory statistical method designed to uncover natural groups within data that would otherwise be invisible using traditional classification methods. Unsupervised clustering, particularly, separates patients into groups without a priori classification. In our study, unsupervised 2-step cluster analysis was used. In the first step, small preclusters were created, which are then merged according to the greatest change in the distance measure in the second step into the most meaningful clusters. The parameters used for clustering in our study were those that had the best prediction of mortality in terms of Cox regression models and receiver operating characteristic curves, namely age, initial and follow-up D-dimer, and LDH levels. The model spit out 3 distinct clusters in terms of in-hospital outcomes that could be identified as low, intermediate, and high risk. The most interesting finding was that the low- and intermediate-risk clusters were identical in terms of age (both mostly represent younger patients) and other demographic variables; however, the low-risk cluster had no in-hospital outcomes and was characterized by the better profile across all predefined disease domains, namely thrombosis, inflammation, inflammatory cells, and end-organ damage. On the other hand, the intermediate-risk cluster showed an intermediate level of thrombosis, elevation of inflammatory cells, and target organ damage and a higher level of inflammatory markers. The high-risk cluster had the worst profile across all domains and had older age. Such clusters seem reasonably explained based on the previously proposed mechanisms. It seemed that the presumed more preserved endothelial function in the intermediate-risk group leads to a less thrombosis burden compared with the high-risk group in which the mechanisms of thromboinflammation were the most aggressive probably because of a worse state of endothelial dysfunction created by more pronounced aging process as explained previously.
Study Limitations
We acknowledge the following limitations: first, the sample size is small, which can affect the results of all models. However, the incidence and prevalence of COVID-19 in patients with no comorbidities remain small, making it difficult to gather information from an appropriate number of patients. As such, the findings of our study should be confirmed by further larger multicenter studies. Second, the data and variables collected for our patients depended on the laboratory analyses that were done in the initial stages of the disease. Other relevant laboratory findings that can confirm our hypothesis such as troponin levels, and longer trends of D-dimers and LDH, as well as echocardiography, computed tomography chest with contrast, or other imaging modalities, were not available for the vast majority of the patients. Moreover, the time between the first and second D-dimer evaluation was based on clinical suspicion only, as our study was not powered to detect the effect of time to change of D-dimer clinical outcomes; further studies need to address this limitation. Further studies should focus on recruiting patients after measuring these variables to get more insight into the pathophysiological mechanisms. Third, most of the presumed mechanisms in the current study are just assumptions, and despite that pathological reports exist, they are still rarely done, and to confirm the presumed mechanisms, more detailed pathological reports are needed. Fourth, the 2-step cluster model that was used in our study functions best in the presence of large scale data, and as such, results from this model should be treated with care. Traditionally, sample size selection is based on the number of variables fed into the cluster model. The best approach to assess the appropriateness of the sample size is to determine whether the dimensionality of the data is not too high for the number of cases to be grouped. The minimal sample size to include should be no <2k cases (k=number of variables), preferably 5×2k.19,20 In our analysis, the number of variables fed into the model was 5, and accordingly, the appropriate sample size for a relatively stable model would be anywhere between 32 and 160. Thus, our sample size of 157 patients would be sufficient. However, validation of all the models described in this report needs to be done on larger scale studies before any of these findings can be adopted into clinical practice. Fifth, the differences between the laboratory values in the conventional analysis (Table 1), despite statistically significant, were only subtle, and the mean values of parameters such as those of neutrophils, lymphocytes, creatinine, and liver function tests appear to be normal. However, considerable number of patients had abnormal values of these parameters when categorically classified (Table II in the Data Supplement). It is to be noted that when patients were classified based on the unsupervised cluster model using D-dimer, LDH, and age, the differences between these variables changed, demonstrating how a cluster analysis without a priori assumptions can change our look into laboratory results based on the natural distribution of the classifying attributes, compared with conventional methods of comparison, which depends on artificial separation of data based on arbitrary cutoff values such as age of 55 years used in the current study.
Finally, while D-dimer elevates as the result of thrombolysis, which represents an indirect evidence of thrombosis and thus used clinically to exclude thromboembolic episodes, D-dimer can also be elevated in a variety of other situations including, pregnancy, inflammation, malignancy, trauma, postsurgical treatment, liver disease (decreased clearance), and heart disease.21–23 It is also frequently high in hospitalized patients. Given that the population studied is healthy at baseline excludes most of the nonthrombotic causes of D-dimer elevation and, moreover, the high levels for patients on presentation and follow-up during their hospital stay, is somewhat not familiar to those noted for hospitalized patients.
Conclusions
In patients without baseline comorbidities, COVID-19 can be fatal and is associated with significant in-hospital outcomes defined as in-hospital mortality or need for mechanical ventilation. While age remains the most important predictor, D-dimer and LDH elevation at baseline and follow-up during the hospital stay seems to be linked to worse in-hospital outcomes regardless of age in these patients. The underlying mechanisms seem to be related to an aging process, IIT, endothelial infection, or multiple hit from different successive pathological insults. Understanding of such pathological processes and their impact on patients with and without comorbidities among all age categories is crucial toward developing successful treatment strategies.
Disclosures
None.
Supplementary Material
Nonstandard Abbreviations and Acronyms
- ACE-2
- angiotensin-converting enzyme 2
- COVID-19
- coronavirus disease 2019
- IIT
- inflammation-induced thrombosis
- LDH
- lactate dehydrogenase
These authors contributed equally to this article.
The Data Supplement is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/ATVBAHA.120.314845.
For Disclosures, see page 2774.
References
- 1.Lai CC, Shih TP, Ko WC, Tang HJ, Hsueh PR. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges. Int J Antimicrob Agents. 2020; 55:105924 doi: 10.1016/j.ijantimicag.2020.105924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Srivastava N, Baxi P, Ratho RK, Saxena S. Global trends in epidemiology of coronavirus disease 2019 (COVID-19). Coronavirus Disease 2019 (COVID-19). 20209–21. doi: 10.1007/978-981-15-4814-7_2 [Google Scholar]
- 3.England JT, Abdulla A, Biggs CM, Lee AYY, Hay KA, Hoiland RL, Wellington CL, Sekhon M, Jamal S, Shojania K, et al. Weathering the COVID-19 storm: lessons from hematologic cytokine syndromes. Blood Rev. 2020100707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li B, Yang J, Zhao F, Zhi L, Wang X, Liu L, Bi Z, Zhao Y. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol. 2020; 109:531–538. doi: 10.1007/s00392-020-01626-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shapiro K.Classifying age groups. 2004. https://www.medscape.com/viewarticle/495441.
- 6.Zhang L, Yan X, Fan Q, Liu H, Liu X, Liu Z, Zhang Z. D-dimer levels on admission to predict in-hospital mortality in patients with COVID-19. J Thromb Haemost. 2020; 18:1324–1329. doi: 10.1111/jth.14859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Team CC-R. Coronavirus disease 2019 in children - United States, February 12-April 2, 2020. MMWR Morb Mortal Wkly Rep. 2020; 69:422–426. doi: 10.15585/mmwr.mm6914e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu X, Zhang L, Du H, Zhang J, Li YY, Qu J, Zhang W, Wang Y, Bao S, Li Y, et al. ; Chinese Pediatric Novel Coronavirus Study Team. SARS-CoV-2 infection in children. N Engl J Med. 2020; 382:1663–1665. doi: 10.1056/NEJMc2005073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.COVIDView: a weekly surveillance summary of U.S. COVID-19 activity. 2020 www.cdc.gov/coronavirus/2019-ncov/covid-data/covidview/index.html.
- 10.Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020; 181:271–280.e8. doi: 10.1016/j.cell.2020.02.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang H, Penninger JM, Li Y, Zhong N, Slutsky AS. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020; 46:586–590. doi: 10.1007/s00134-020-05985-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.AlGhatrif M, Cingolani O, Lakatta EG. The dilemma of coronavirus disease 2019, aging, and cardiovascular disease: insights from cardiovascular aging science. JAMA Cardiol. 2020; 5:747–748. doi: 10.1001/jamacardio.2020.1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garrido A, Cruces J, Ceprián N, Vara E, de la Fuente M. Oxidative-inflammatory stress in immune cells from adult mice with premature aging. Int J Mol Sci. 2019; 20:769 doi: 10.3390/ijms20030769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aksu K, Donmez A, Keser G. Inflammation-induced thrombosis: mechanisms, disease associations and management. Curr Pharm Des. 2012; 18:1478–1493. doi: 10.2174/138161212799504731 [DOI] [PubMed] [Google Scholar]
- 15.Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, Mehra MR, Schuepbach RA, Ruschitzka F, Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020; 395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Badimon L, Vilahur G. Neutrophil extracellular traps: a new source of tissue factor in atherothrombosis. Eur Heart J. 2015; 36:1364–1366. doi: 10.1093/eurheartj/ehv105 [DOI] [PubMed] [Google Scholar]
- 17.Herrera MD, Mingorance C, Rodríguez-Rodríguez R, Alvarez de Sotomayor M. Endothelial dysfunction and aging: an update. Ageing Res Rev. 2010; 9:142–152. doi: 10.1016/j.arr.2009.07.002 [DOI] [PubMed] [Google Scholar]
- 18.Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, Vanstapel A, Werlein C, Stark H, Tzankov A, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in COVID-19. N Engl J Med. 2020; 383:120–128. doi: 10.1056/NEJMoa2015432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Formann A. Die latent-class-analyse: Einführung in theorie und anwendung. 1984, Weinheim W. Germany: Beltz; 2002 [Google Scholar]
- 20.Dolnicar Sara: A review of unquestioned standards in using cluster analysis for data-driven market segmentation 2002. [Google Scholar]
- 21.Foëx BA, Blythe A. Towards evidence based emergency medicine: best BETs from the manchester royal infirmary. BET 2: D-dimer levels during normal menstrual cycle. Emerg Med J. 2014; 31:863–864. doi: 10.1136/emermed-2014-204199.2 [DOI] [PubMed] [Google Scholar]
- 22.Cervellin G, Bonfanti L, Picanza A, Lippi G. Relation of D-dimer and troponin I in patients with new-onset atrial fibrillation. Am J Cardiol. 2014; 114:1129–1130. doi: 10.1016/j.amjcard.2014.07.029 [DOI] [PubMed] [Google Scholar]
- 23.Spring JL, Winkler A, Levy JH. The influence of various patient characteristics on D-dimer concentration in critically ill patients and its role as a prognostic indicator in the intensive care unit setting. Clin Lab Med. 2014; 34:675–686. doi: 10.1016/j.cll.2014.06.015 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


