Supplemental Digital Content is available in the text.
Keywords: animals; atherosclerosis; cholesterol; diet, Western; mice
Objective:
NR4A orphan receptors have been well studied in vascular and myeloid cells where they play important roles in the regulation of inflammation in atherosclerosis. NR4A1 (nerve growth factor IB) is among the most highly induced transcription factors in B cells following BCR (B-cell receptor) stimulation. Given that B cells substantially contribute to the development of atherosclerosis, we examined whether NR4A1 regulates B-cell function during atherogenesis.
Approach and Results:
We found that feeding Ldlr−/− mice a Western diet substantially increased Nr4a1 expression in marginal zone B (MZB) cells compared with follicular B cells. We then generated Ldlr−/− mice with complete B- or specific MZB-cell deletion of Nr4a1. Complete B-cell deletion of Nr4a1 led to increased atherosclerosis, which was accompanied by increased T follicular helper cell–germinal center axis response, as well as increased serum total cholesterol and triglycerides levels. Interestingly, specific MZB-cell deletion of Nr4a1 increased atherosclerosis in association with an increased T follicular helper–germinal center response but without any impact on serum cholesterol or triglyceride levels. Nr4a1−/− MZB cells showed decreased PDL1 (programmed death ligand-1) expression, which may have contributed to the enhanced T follicular helper response.
Conclusions:
Our findings reveal a previously unsuspected role for NR4A1 in the atheroprotective role of MZB cells.
Graphic Abstract:
A graphic abstract is available for this article.
Highlights.
NR4A1 is a new target that modulates B-cell functions in atherosclerosis.
Nr4a1 deletion in B2 cells increases atherosclerosis and is associated with increased Tfh-GC response and circulating lipid levels.
NR4A1 expression in MZB cells regulates PDL1 expression, limiting the Tfh-GC response and protecting from atherosclerosis.
Atherosclerosis is a chronic inflammatory disease involving interactions between vascular, circulating, and immune cells. B cells play an important role in the chronic immunoinflammatory response, producing antibodies and regulating T-cell and natural killer–cell activation. The role of B cells in atherosclerosis is complex, with atherogenic and protective roles assigned for distinct B-cell subsets.1 B2 cells comprise the phenotypically distinct follicular B (FOB) cells of the spleen and the lymph nodes and the marginal zone B (MZB) cell population of the spleen. While depletion of all B2 cells reduces atherosclerosis,2–4 we have recently discovered that selective depletion of MZB cells promotes atherosclerosis.5 In response to a high-fat/high-cholesterol (HF/HC) diet, MZB cells activate an atheroprotective programme, limiting T follicular helper (Tfh) cell motility in a PDL1 (programmed death ligand-1)-dependent manner.5
NR4A1 (nerve growth factor IB; also called Nur77) is a member of the NR4A orphan nuclear receptor subfamily. NR4A1 has been involved in negative selection of thymocytes, differentiation of regulatory T cells, and development of Ly6C− monocytes.6 However, little is known about its role in B cells besides the fact that it is rapidly induced upon BCR (B-cell receptor) signaling activation7 and that it may regulate the survival of self-reactive B cells.8 NR4A1 also plays important but complex roles in atherosclerosis, at least in part, due to its impact on vascular cell,9 monocyte,6,10,11 and T-cell biology.6,12 We have now addressed the role of NR4A1 specifically in B cells in the development of atherosclerosis.
Materials and Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Animals
All experiments were approved by the Home Office, United Kingdom, and were performed under Personal Project Licence 80/2426. All mice were on C57Bl/6J background. Cd79aCre/+, µMT, Nr4a1−/−, and Ldlr–/– mice were originally from The Jackson Laboratory. Rbpjkflox/flox mice were kindly provided by Tasuku Honjo.13 To generate an atherosclerotic mouse model that lacks MZB cells, Cd79aCre/+; Rbpjkflox/flox were crossed with Ldlr–/– to generate Ldlr−/−; Cd79aCre/+; RBPflox/flox mice. For atherosclerosis experiments, we generated 2 different mice models. For deletion of Nr4a1 in all B cells, 6- to 8-week-old Ldlr–/– mice were lethally irradiated (two 5.5 Gy fractioned doses) and injected with a mixed bone marrow (BM) chimera containing 80% uMT plus either 20% WT (wild type) C57Bl/6J (to reconstitute with WT B cells) or 20% Nr4a1−/− (to reconstitute with Nr4a1−/− B cells). We only used male mice to limit the number of animals used in our experiments. In subsequent experiments, we used both males and females. For deletion of Nr4a1 selectively in MZB cells, we developed a partial irradiation model in atherosclerotic mice lacking MZB cells. Recipient Ldlr−/−; Cd79aCre/+; RBPflox/flox male and female mice were partially irradiated (1 single dose of 4 Gy) and injected intravenously with BM from Nr4a1−/− or WT C57Bl/6J male and female mice, respectively, which we expected to result in reconstitution of MZB cell compartment with cells coming from the donors (Nr4a1−/− or WT), while the rest of immune cells (including FOB, T, myeloid, etc) would be mostly from the recipient. To validate this partial irradiation model, we used the CD45 (cluster of differentiation 45) congenic lineage tracing system. We exposed recipient CD45.2+; CD45.1− male and female mice lacking MZB cells (LDLr−/−; Cd79a Cre/+; RBPflox/flox) to different doses of irradiation (from 4 to 7 Gy), then injected CD45.2−; CD45.1+ male and female BM donor cells, respectively, to allow us to track the reconstitution of B-cell subsets using flow cytometry. A single dose of 4 Gy resulted in reconstitution of the majority of MZB cells (>99%) with donor CD45.2−; CD45.1+ cells, while the majority of the FOB cells and the rest of the BM-derived cells (including other lymphocytes [FSClow] and myeloid [FSChi] cells; 90%) were from the CD45.2+; CD45.1− recipient (Figure I in the Data Supplement).
For atherosclerotic experiments, all recipient mice were randomly assigned to different experimental groups based on their weight at the beginning of the experiment. After 6 weeks of recovery, mice were started on HF/HC (21% fat, 0.15% cholesterol) for 8 weeks.
Extent and Composition of Atherosclerotic Lesions
The lesions in the root of the aorta beneath all 3-valve leaflets were analyzed. To measure atherosclerotic plaque size, we stained paraffin-embeded sections with Masson trichrome. To characterize atherosclerotic lesion composition, we stained sections for macrophages (Mac-3 [lysosome-associated membrane protein 2], 1:200, clone M3/84; Santa Cruz), T cells (polyclonal CD3, 1:100; Dako), and neutrophils (Ly6G [lymphocyte antigen 6 complex locus G6D], 1:100, clone 1AB; BD). We performed each staining on a single slide (3 sections for each staining) from approximately the same level of the aortic root for all experimental and control mice. Computer-assisted analysis (Image-J) was used to determine lesion size and areas or cell counts for specific cell types. All slides for each experiment were analyzed by the same blinded observer to minimize variations. All measurements were performed by manual selection of stained pixel thresholds and presented as percent positive (stained) area relative to the entire intimal area. CD4+ T and Ly6G+ cells were counted, and the data are presented as the number of T cells per total number of cells or as the total number of cells per section, respectively, in atherosclerotic plaques and adventitia. Representative images of immunofluorescence staining are included in Figure II in the Data Supplement. Whole aortas were prepared en face for Oil Red O staining to measure lipid deposition and quantification of lesion size using J-Microvision.
Flow Cytometry
Single-cell suspensions of homogenized spleen and inguinal lymph nodes were stained with fluorophore-conjugated antibodies and analyzed using an LSRII Fortessa (Becton, Dickinson and Company) or FACSCantoII (BD) flow cytometer as described previously.5 Representative plots of gating strategies can be found in Figure II in the Data Supplement.
Determination of Circulating Antibodies
Specific antibody titers to given antigens in plasma were determined by chemiluminescent ELISA as described previously.14,15
Determination of Serum Lipid Levels
Total cholesterol, triglycerides, and HDL-C were measured by an enzymatic method in a Siemens Dimensions RxL analyzer.
Purification of MZB and FOB
Single-cell suspensions were prepared from spleens by dissociation through 70-µm filters using a syringe plunger. For MZB and FOB cell purification, B cell–enriched populations were separated by Automacs using a B-cell purification kit (Miltenyi Biotech), and B cells were stained for 15 minutes at 4°C with anti-CD23-phycoerythrine and anti-CD21-fluorescein isothiocyanate. After washing and staining with 7-AAD for cell viability, MZB (CD21hiCD23low) and FOB (CD23hiCD21low) were sorted using an AriaIII Cell, Influx, or FACSJazz sorter (BD). Purity of both populations was higher than 95%.
BCR Stimulation
Purified sorted MZB and FOB from WT or Nr4a1−/− mice were stimulated for 30 minutes, 3 hours, or 6 hours with F(ab)2 fragment of the anti-IgM (eBioscience; 10 ug/mL).
Quantitative Real-Time Polymerase Chain Reaction
For gene expression analysis, RNA from sorted MZB and FOB cells was isolated using RNAeasy mini kit (Qiagen). qRT-PCR was performed using Quantitect whole transcriptome kit. qRT-PCR was performed with SYBR-green primers. Primer sequences are listed in Table I in the Data Supplement.
Statistical Analysis
Values are expressed as means±SEM. Based on pilot experiments, we found that MZB deficiency increases lesion size by 2-fold. Use of the equation for Student t test for the difference of 2 means gives us an n=6 to detect a 50% increase in lesion size with α=0.05 and 90% power. Where data sets passed normality tests, differences between values were examined using parametric 2-tailed unpaired Student t test, other data sets by using nonparametric Mann-Whitney U test; and were considered significant at P<0.05 for the B-cell model where only males were used. In the partial irradiation model, we used both males and females and performed 2-way ANOVA to determine comparisons between genotypes. When sex did not have a significant effect on the results, we pooled the male and female data. When sex had a significant effect, data were not pooled, and Bonferroni post hoc analysis was performed to find differences between groups.
Results
NR4A1 Deletion in B Cells Exacerbates Atherosclerosis
We reconstituted lethally irradiated Ldlr−/− male mice with a mixed BM containing 80% of BM cells from µMT male mice (which cannot produce B cells) supplemented with either 20% BM from WT C57Bl/6J mice (referred to as the WT B-cell group) or 20% of BM cells from Nr4a1−/− mice (referred to as the Nr4a1−/− B-cell group). MZB and FOB cells were reconstituted equally in both groups (Figure III in the Data Supplement). After recovery, mice were put on a high-fat/high-cholesterol diet for 8 weeks. Specific Nr4a1 deletion in B cells markedly accelerated the development of atherosclerosis in the aortic sinus (Figure 1A) and the aortic arch (Figure III in the Data Supplement). Serum levels of total cholesterol and triglycerides were also significantly increased in the Nr4a1−/− B-cell group (Figure 1B through 1D), which could account, at least in part, for the increased atherosclerosis seen in these mice. Analysis of plaque composition showed a trend toward increased %necrotic core area but a similar percentage of macrophages and CD3+ T cells in lesions of Nr4a1−/− compared with WT B-cell mice (Figure III in the Data Supplement). In the adventitia, we found similar percentages of CD3+ T cells and a trend toward increased percentages of neutrophils (Ly6G+) in the Nr4a1−/− compared with WT B-cell mice (Figure III in the Data Supplement). We did not find any difference in blood immune cell subsets (including B cells, T cells, neutrophils, and monocytes) between both groups (Figure III in the Data Supplement).
Figure 1.
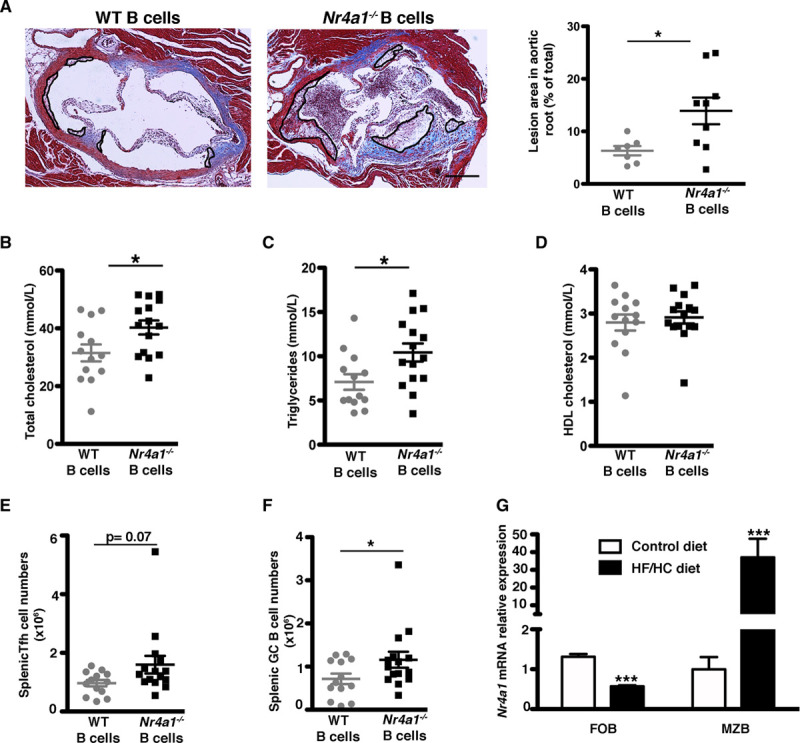
Nr4a1 (nerve growth factor IB) deletion in B cells increases atherosclerosis development. Ldlr−/− mice were transplanted with a mixed chimera containing 80% µMT +20% WT (wild type; for reconstitution with WT B cells) or 20% Nr4a1−/− (for reconstitution with Nr4a1−/− B cells) BM and fed a high-fat/high-cholesterol (HF/HC) diet for 8 wk (A–F). A, Representative Masson trichrome staining and quantification of atherosclerotic plaques in aortic roots (each symbol represents one mouse, and the horizontal bars are group mean±SEM with n=7 and n=9, respectively, in each group). Original magnification, ×10. Scale bars=500 μm. B–D, Total plasma cholesterol, triglycerides, and HDL (high-density lipoprotein) cholesterol levels. E and F, Total numbers of T follicular helper (Tfh) cells (CD3+ CD4+ CD44+ CD62L− ICOS+ CXCR5+ PD1+) and germinal center (GC) B cells (B220+ CD19+ CD95+ GL7+; n=13–15 in each group). G, Nr4a1 relative expression in sorted marginal zone B (MZB) and follicular B (FOB) cells from Ldlr−/− mice fed a standard laboratory or HF/HC diet for 8 wk (n=3–4 in each group). For A–G, 2-tailed unpaired Student t test or 2-way ANOVA followed by Bonferroni post hoc analysis, *P<0.05 and ***P<0.001. CD indicates cluster of differentiation; CXCR5, C-X-C chemokyne receptor-5; ICOS, inducible T-cell coestimulator; and PD1, programmed cell death protein 1.
We then characterized the innate and the adaptive immune responses in the spleen. Splenic dendritic cell, monocyte, and neutrophil levels were similar between the 2 groups of mice (Figure III in the Data Supplement). Nr4a1−/− B-cell mice showed a trend toward increased Tfh cell numbers (P=0.07) and had significantly increased germinal center (GC) B cells (P<0.05) compared with mice with WT B cells (Figure 1E and 1F). All other T-cell subsets including T effector memory, T regulatory cells, and T follicular regulatory cells were not significantly altered between the two groups (Figure IV in the Data Supplement).
We have previously demonstrated that B2 cells play different roles in atherosclerosis, with MZB cells being atheroprotective through limitation of Tfh cell formation (MZB cell deletion leads to increased Tfh)5—a phenotype that is similar to the present phenotype observed for B cell–specific depletion of Nr4a1−/−. Furthermore, expression of Nr4a1 was significantly upregulated in purified MZB cells but downregulated in FOB cells from LDLr−/− mice consuming a high-fat/high-cholesterol diet, in comparison with mice fed a standard laboratory diet (Figure 1G). Therefore, we addressed the role of NR4A1 specifically in MZB cells during the development of atherosclerosis.
NR4A1 Deletion Selectively in MZB Cells Exacerbates Atherosclerosis
To study the role of Nr4a1 in MZB cells and its impact on atherosclerosis, we generated an atherosclerotic mouse model in which Nr4a1−/− was specifically deleted in MZB cells. For this aim, we generated Ldlr−/− mice on Cd79aCre/+; RBPflox/flox background (mice that do not have MZB cells, as described previously5). After partial irradiation (4 Gy), Ldlr−/−; Cd79aCre/+; RBPflox/flox male and female mice were injected with WT or Nr4a1−/− BM from males and females, respectively, resulting in mice reconstituted with WT MZB cells (the WT MZB cell group) or with MZB cells in which Nr4a1 has been deleted (the Nr4a1−/− MZB cell group; see Materials and Methods section for a more detailed explanation of the model and Figure I in the Data Supplement). Both groups had the same numbers of MZB and FOB cells after reconstitution (Figure I in the Data Supplement).
After 8 weeks on a high-fat/high-cholesterol diet, deletion of Nr4a1 specifically in MZB cells led to increased development of atherosclerosis in the aortic sinus (Figure 2A) and increased lipid deposition in the aortic arches (Figure V in the Data Supplement), without altering serum levels of total cholesterol, HDL-C, or triglycerides, compared with mice with WT MZB cells (Figure 2B through 2D). Females had increased atherosclerotic plaques and decreased HDL-C levels than males—a phenotype that has been reported previously in LDLr−/− mice.16 Analysis of plaque composition showed a trend toward increased %necrotic core area in the Nr4a1−/− MZB cell group while there were no differences in percentages of Mac-3+ macrophages and CD3+ T cells between both groups (Figure V in the Data Supplement). No significant differences were found in the percentages of CD3+ T cells and neutrophils in the adventitia between the 2 groups (Figure V in the Data Supplement).
Figure 2.
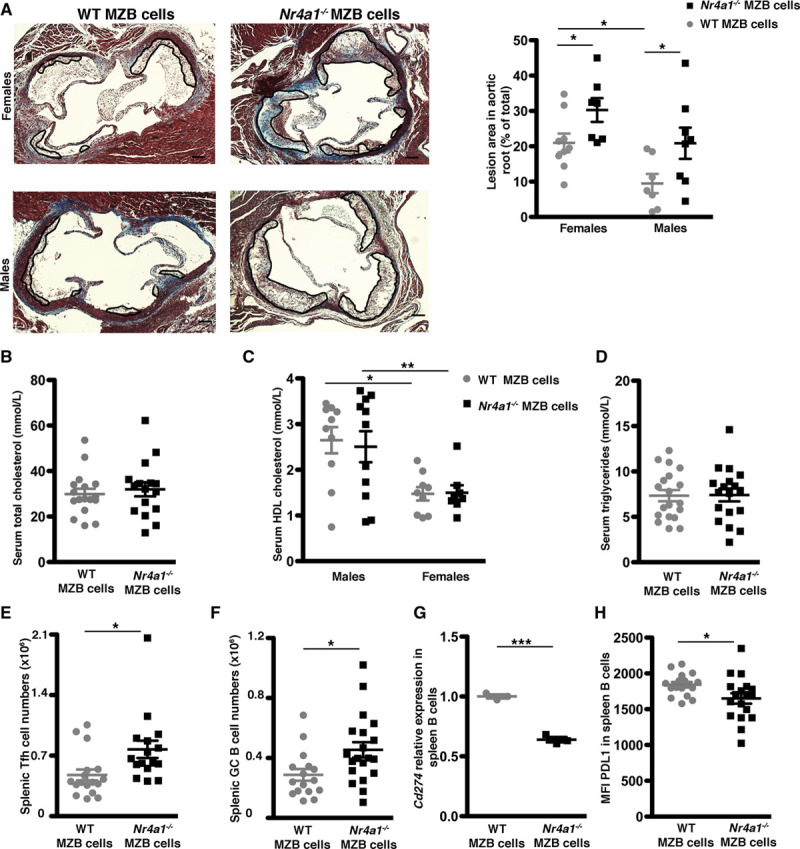
Nr4a1 (nerve growth factor IB) deletion in marginal zone B (MZB) cells increases atherosclerosis development. Ldlr−/−; Cd79aCre/+; Rbpjkflox/flox were partially irradiated (Materials and Methods in the Data Supplement) and transplanted with WT (wild type; for reconstitution with WT MZB cells) or Nr4a1−/− (for reconstitution with Nr4a1−/− MZB cells) BM and fed a high-fat/high-cholesterol diet for 8 wk (A–H). A, Representative Masson trichrome staining and quantification of atherosclerotic plaques in aortic roots (each symbol represents one mouse, and horizontal bars are group mean±SEM with n=16–18 in each group). Original magnification, ×10. Scale bars=500 μm. B–D, Total plasma cholesterol, HDL (high-density lipoprotein) cholesterol, and triglycerides levels. E and F, Total numbers of T follicular helper (Tfh) and germinal center (GC) B cells. Cd274 (Pdl1 [programmed death ligand-1] gene) expression, quantified by qRT-PCR (n=5 in each group) in sorted MZB cells (G) and PDL1 protein expression, quantified by flow cytometry in splenic MZB cells (H; n=16–18 in each group). For A–G, 2-tailed unpaired Student t test or 2-way ANOVA followed by Bonferroni post hoc analysis, *P<0.05 and **P<0.01. MFI indicates mean fluorescence intensity; and PDL1, programmed cell death ligand-1.
Interestingly, Nr4a1−/− MZB-cell mice had significantly increased splenic Tfh and GC B-cell numbers (Figure 2E and 2F) compared with WT MZB-cell mice, without detectable changes in serum antibody titers against modified LDL (low-density lipoprotein; Figure V in the Data Supplement). Despite our limitations to comprehensively assess the antibody response, we hypothesize that other Tfh cell functions may account for their atherogenic role, as discussed previously.5 Nr4a1−/− MZB cell group also had significantly increased T regulatory cells and T follicular regulatory CD25pos cells, while T effector memory, T follicular regulatory CD25neg cells, dendritic cells, neutrophils, and monocytes were not significantly altered (Figure VI in the Data Supplement).
NR4A1 Regulates PDL1 Expression
We found that MZB cells from the Nr4a1−/− MZB group expressed lower levels of the PDL1 encoding gene (Cd274) and PDL1 protein (Figure 2G and 2H) compared with those from the WT MZB group, which could, at least in part, explain why the Nr4a1−/− MZB cell group also showed increased Tfh cells.5
To further examine the link between NR4A1 and PDL1, we performed in vitro experiments using sorted MZB cells from total WT and Nr4a1−/− mice. MZB cells from Nr4a1−/− mice expressed lower levels of PDL1 than WT MZB cells at baseline (Figure VII in the Data Supplement). As described previously, Nr4a117 and Cd2745 significantly increased upon BCR activation (Figure VII in the Data Supplement). BCR-induced pBTK (Bruton tyrosine kinase) was not altered in the absence of NR4A1 (Figure VII in the Data Supplement). BCR-induced Cd274 gene expression was significantly downregulated in the absence of NR4A1 (Figure VII in the Data Supplement). However, BCR-mediated regulation of MZB cell surface PDL1 protein expression was not altered in the absence of NR4A1 (Figure VII in the Data Supplement). These data suggest that the impact of NR4A1 deletion on PDL1 expression in vivo was independent of BCR activation.
Discussion
To the best of our knowledge, this is the first experimental study addressing the role of NR4A1 in B cells during atherosclerosis. Our data show that NR4A1 expression in MZB cells regulates PDL1 expression, limiting the Tfh-GC response and protecting from atherosclerosis. A recent work performed in a cancer line has shown that NR4A1 binds to the GC motif of the PDL1 promoter and regulates its gene expression,18 further supporting the possibility of a direct regulation of PDL1 expression by NR4A1 in MZB cells. Previous studies suggested that PDL1 expression in antigen-presenting cells might limit proatherogenic immune responses.19 Whether NR4A1 also regulates PDL1 expression in immune cells, other than MZB cells, is worth of investigation.
Interestingly, NR4A1 may regulate PDL1 in a BCR-independent manner, opening up an opportunity to modulate NR4A1-dependent pathways in B cells without affecting the BCR signaling pathway. Furthermore, human MZB-like cells express high levels of NR4A120 and PDL1,5 and, therefore, we speculate that NR4A1 may have a similar role in human B cells, although further studies are required to determine whether this is indeed the case. The potential use of Bis-Indole-NR4A1 antagonists as anticancer treatments is starting to emerge,18,21 and, therefore, we would advise to take into consideration their effect on B cells to avoid or monitor potential undesirable cardiovascular side effects associated to anticancer treatments.
NR4A1 has previously been reported to play a very important role in B-cell survival.8,22 Nevertheless, in both of our B-cell models of Nr4a1 deletion, we did not find any difference in B-cell numbers, making it unlikely that NR4A1 regulates B-cell survival during atherosclerosis. This is in agreement with a recent finding that NR4A1 regulates B-cell survival in a T cell–independent response but not in a T cell–dependent response.23 In our models, we could not study the potential contribution of NR4A1 in B1 cells because these cells do not reconstitute well after irradiation, and, therefore, further experiments are needed to elucidate the role of NR4A1 in B1 cells during atherosclerosis.
Surprisingly, NR4A1 deletion in all B2 cells not only regulates the Tfh-GC response but also the circulating lipid levels. Thus, B2 cells other than MZB cells could be responsible for this effect on lipid parameters. B cells express several receptors, such as LDLR (LDL receptor), CD1d, and scavenger receptors, that could recognize and remove lipids.24 Moreover, previous studies modulating B cells have shown changes in cholesterol levels,25,26 but none of the previous atherosclerotic models targeting NRA1 in monocytes have shown a significant effect on lipid levels.10,11 Thus, the specific role of B cells in lipid metabolism is yet to be clarified, and further experiments are required.
Overall, our data demonstrate that NR4A1 in B cells is an attractive target to modulate the development of atherosclerotic disease.
Acknowledgments
We acknowledge Prof Anna Petrunkina, Natalia Savinykh, Nika Romashova, and Simon McCallum, in the Phenotyping Hub of the Department of Medicine (University of Cambridge), for their help in flow cytometry and sorting and Maria Ozsvar Kozma (Department of Laboratory Medicine, Medical University of Vienna, Austria) for help in antibody measurements. We also acknowledge Keith Burling and all the Core Biomedical Assay Laboratory in Addenbrooke Hospital for lipid measurements.
Sources of Funding
This work was supported by the British Heart Foundation (BHF) Project grant PG/17/73/33251 to M. Nus and Z. Mallat and by the European Union grant RG71687 to Z. Mallat. We were also supported by the Cambridge BHF Centre of Research Excellence and NIHR Cambridge Biomedical Research Centre.
Disclosures
None.
Supplementary Material
Nonstandard Abbreviations and Acronyms
- BCR
- B-cell receptor
- BM
- bone marrow
- FOB
- follicular B
- GC
- germinal center
- LDL
- low-density lipoprotein
- LDLR
- low-density lipoprotein receptor
- MZB
- marginal zone B
- Tfh
- T follicular helper
- WT
- wild type
This manuscript was sent to William C. Sessa, Senior Consulting Editor, for review by expert referees, editorial decision, and final disposition.
The Data Supplement is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/ATVBAHA.120.314607.
For Sources of Funding and Disclosures, see page 2604.
References
- 1.Nus M, Tsiantoulas D, Mallat Z. Plan B (-cell) in atherosclerosis. Eur J Pharmacol. 2017; 816:76–81. doi: 10.1016/j.ejphar.2017.09.002 [DOI] [PubMed] [Google Scholar]
- 2.Ait-Oufella H, Herbin O, Bouaziz JD, Binder CJ, Uyttenhove C, Laurans L, Taleb S, Van Vré E, Esposito B, Vilar J, et al. B cell depletion reduces the development of atherosclerosis in mice. J Exp Med. 2010; 207:1579–1587. doi: 10.1084/jem.20100155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kyaw T, Cui P, Tay C, Kanellakis P, Hosseini H, Liu E, Rolink AG, Tipping P, Bobik A, Toh BH. BAFF receptor mAb treatment ameliorates development and progression of atherosclerosis in hyperlipidemic ApoE(-/-) mice. PLoS One. 2013; 8:e60430 doi: 10.1371/journal.pone.0060430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sage AP, Tsiantoulas D, Baker L, Harrison J, Masters L, Murphy D, Loinard C, Binder CJ, Mallat Z. BAFF receptor deficiency reduces the development of atherosclerosis in mice–brief report. Arterioscler Thromb Vasc Biol. 2012; 32:1573–1576. doi: 10.1161/ATVBAHA.111.244731 [DOI] [PubMed] [Google Scholar]
- 5.Nus M, Sage AP, Lu Y, Masters L, Lam BYH, Newland S, Weller S, Tsiantoulas D, Raffort J, Marcus D, et al. Marginal zone B cells control the response of follicular helper T cells to a high-cholesterol diet. Nat Med. 2017; 23:601–610. doi: 10.1038/nm.4315 [DOI] [PubMed] [Google Scholar]
- 6.Hamers AA, Hanna RN, Nowyhed H, Hedrick CC, de Vries CJ. NR4A nuclear receptors in immunity and atherosclerosis. Curr Opin Lipidol. 2013; 24:381–385. doi: 10.1097/MOL.0b013e3283643eac [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zikherman J, Parameswaran R, Weiss A. Endogenous antigen tunes the responsiveness of naive B cells but not T cells. Nature. 2012; 489:160–164. doi: 10.1038/nature11311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tan C, Mueller JL, Noviski M, Huizar J, Lau D, Dubinin A, Molofsky A, Wilson PC, Zikherman J. Nur77 links chronic antigen stimulation to B cell tolerance by restricting the survival of self-reactive B cells in the periphery. J Immunol. 2019; 202:2907–2923. doi: 10.4049/jimmunol.1801565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.You B, Jiang YY, Chen S, Yan G, Sun J. The orphan nuclear receptor Nur77 suppresses endothelial cell activation through induction of IkappaBalpha expression. Circ Res. 2009; 104:742–749. doi: 10.1161/CIRCRESAHA.108.192286 [DOI] [PubMed] [Google Scholar]
- 10.Hamers AA, Vos M, Rassam F, Marinković G, Marincovic G, Kurakula K, van Gorp PJ, de Winther MP, Gijbels MJ, de Waard V, et al. Bone marrow-specific deficiency of nuclear receptor Nur77 enhances atherosclerosis. Circ Res. 2012; 110:428–438. doi: 10.1161/CIRCRESAHA.111.260760 [DOI] [PubMed] [Google Scholar]
- 11.Chao LC, Soto E, Hong C, Ito A, Pei L, Chawla A, Conneely OM, Tangirala RK, Evans RM, Tontonoz P. Bone marrow NR4A expression is not a dominant factor in the development of atherosclerosis or macrophage polarization in mice. J Lipid Res. 2013; 54:806–815. doi: 10.1194/jlr.M034157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hanna RN, Shaked I, Hubbeling HG, Punt JA, Wu R, Herrley E, Zaugg C, Pei H, Geissmann F, Ley K, et al. NR4A1 (Nur77) deletion polarizes macrophages toward an inflammatory phenotype and increases atherosclerosis. Circ Res. 2012; 110:416–427. doi: 10.1161/CIRCRESAHA.111.253377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tanigaki K, Han H, Yamamoto N, Tashiro K, Ikegawa M, Kuroda K, Suzuki A, Nakano T, Honjo T. Notch-RBP-J signaling is involved in cell fate determination of marginal zone B cells. Nat Immunol. 2002; 3:443–450. doi: 10.1038/ni793 [DOI] [PubMed] [Google Scholar]
- 14.Binder CJ, Hörkkö S, Dewan A, Chang MK, Kieu EP, Goodyear CS, Shaw PX, Palinski W, Witztum JL, Silverman GJ. Pneumococcal vaccination decreases atherosclerotic lesion formation: molecular mimicry between Streptococcus pneumoniae and oxidized LDL. Nat Med. 2003; 9:736–743. doi: 10.1038/nm876 [DOI] [PubMed] [Google Scholar]
- 15.Chou MY, Fogelstrand L, Hartvigsen K, Hansen LF, Woelkers D, Shaw PX, Choi J, Perkmann T, Bäckhed F, Miller YI, et al. Oxidation-specific epitopes are dominant targets of innate natural antibodies in mice and humans. J Clin Invest. 2009; 119:1335–1349. doi: 10.1172/JCI36800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rinninger F, Heine M, Singaraja R, Hayden M, Brundert M, Ramakrishnan R, Heeren J. High density lipoprotein metabolism in low density lipoprotein receptor-deficient mice. J Lipid Res. 2014; 55:1914–1924. doi: 10.1194/jlr.M048819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsiantoulas D, Kiss M, Bartolini-Gritti B, Bergthaler A, Mallat Z, Jumaa H, Binder CJ. Secreted IgM deficiency leads to increased BCR signaling that results in abnormal splenic B cell development. Sci Rep. 2017; 7:3540 doi: 10.1038/s41598-017-03688-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karki K, Wright GA, Mohankumar K, Jin UH, Zhang XH, Safe S. A bis-indole-derived NR4A1 antagonist induces PD-L1 degradation and enhances anti-tumor immunity. Cancer Res. 2020; 80:1011–1023. doi: 10.1158/0008-5472.CAN-19-2314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gotsman I, Grabie N, Dacosta R, Sukhova G, Sharpe A, Lichtman AH. Proatherogenic immune responses are regulated by the PD-1/PD-L pathway in mice. J Clin Invest. 2007; 117:2974–2982. doi: 10.1172/JCI31344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doyon-Laliberté K, Chagnon-Choquet J, Byrns M, Aranguren M, Memmi M, Chrobak P, Stagg J, Poudrier J, Roger M. NR4A expression by human marginal zone B-cells. Antibodies (Basel). 2019; 8:50 doi: 10.3390/antib8040050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hedrick E, Li X, Cheng Y, Lacey A, Mohankumar K, Zarei M, Safe S. Potent inhibition of breast cancer by bis-indole-derived nuclear receptor 4A1 (NR4A1) antagonists. Breast Cancer Res Treat. 2019; 177:29–40. doi: 10.1007/s10549-019-05279-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park K, Nowyhed H, Hedrick C. Nr4a1 (Nur77) regulates B cell survival and activation. (P1453). J Immunol. 2013; 1901 suppl174.9 LP–174.9. http://www.jimmunol.org/content/190/1_Supplement/174.9.abstract.23197258 [Google Scholar]
- 23.Tan C, Hiwa R, Mueller JL, Vykunta V, Hibiya K, Noviski M, Huizar J, Brooks J, Garcia J, Heyn C, et al. “A negative feedback loop mediated by the NR4A family of nuclear hormone receptors restrains expansion of B cells that receive signal one in the absence of signal two.” bioRxiv. 2020. doi: 10.1101/2020.03.31.017434 [Google Scholar]
- 24.Echeverri Tirado LC, Yassin LM. B cells interactions in lipid immune responses: implications in atherosclerotic disease. Lipids Health Dis. 2017; 16:30 doi: 10.1186/s12944-016-0390-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Douna H, Amersfoort J, Schaftenaar FH, Kroon S, van Puijvelde GHM, Kuiper J, Foks AC. Bidirectional effects of IL-10+ regulatory B cells in Ldlr-/- mice. Atherosclerosis. 2019; 280:118–125. doi: 10.1016/j.atherosclerosis.2018.11.019 [DOI] [PubMed] [Google Scholar]
- 26.Jackson SW, Scharping NE, Jacobs HM, Wang S, Chait A, Rawlings DJ. Cutting edge: BAFF overexpression reduces atherosclerosis via TACI-dependent B cell activation. J Immunol. 2016; 197:4529–4534. doi: 10.4049/jimmunol.1601198 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


