Abstract
Chronic graft-versus-host disease (cGVHD) is major cause of morbidity and mortality following allogeneic hematopoietic cell transplantation (HCT). Ixazomib is an oral, second-generation, proteasome inhibitor (PI) that has been shown in preclinical models to prevent GVHD. We conducted a phase I/II trial in 57 subjects to evaluate the safety and efficacy of ixazomib administration for cGVHD prophylaxis in patients undergoing allogeneic HCT. Oral ixazomib was administered on a weekly basis for a total of four doses, beginning days +60 through +90, to recipients of matched sibling donor (MRD, n=25) or unrelated donor (MUD, n=26) allogeneic HCT in phase II portion of the study, once the recommended phase II dose of 4 mg was identified in phase I (n=6). All patients received peripheral blood graft and standard GVHD prophylaxis of tacrolimus and methotrexate. Ixazomib administration was safe and well-tolerated, with thrombocytopenia, leukopenia, gastrointestinal complaints and fatigue as the most common adverse events (>10%). In phase II (n=51), the cumulative incidence of cGVHD at 1-year was 36% (95%CI, 19-54) in MRD cohort, and 39% (95%CI, 21-56) in the MUD cohort. One-year cumulative incidence of non-relapse mortality (NRM) and relapse were 0% and 20% (95%CI, 8-36) in the MRD cohort, respectively. In the MUD cohort, the respective NRM and relapse rates were 4% (0-16) and 34% (17-52). The outcomes on the study were compared post-hoc with contemporaneous matched CIBMTR controls. This post-hoc analysis showed no significant improvement in cGVHD rates in both the MRD (HR 0.85, p=0.64) or MUD cohorts (HR 0.68, p=0.26) on the study compared to CIBMTR controls. B-cell activating factor (BAFF) plasma levels were significantly higher after ixazomib dosing in those who remained cGVHD-free compared to those developed cGVHD. This study shows that the novel strategy of short-course oral ixazomib following allogeneic HCT is safe but did not demonstrate significant improvement in cGVHD incidence in recipients of MRD and MUD transplantation when compared to matched CIBMTR controls. This study is registered at www.clinicaltrials.gov as NCT02250300.
Keywords: Ixazomib, allogeneic hematopoietic cell transplantation, graft-versus-host disease
Introduction
Chronic graft-versus-host disease (cGVHD) is a significant cause of late morbidity and mortality after allogeneic hematopoietic cell transplantation (HCT)1,2. Chronic GVHD affects 30-60% of allogeneic HCT recipients3, depending on the degree of histocompatibility between donor and recipient, type of graft, recipient age and GVHD prophylaxis4–6. The incidence of cGVHD is increasing, likely due to frequent use of peripheral blood (PB) grafts, increasing number of reduced intensity conditioning (RIC) transplants in older patients and use of unrelated donors3. Symptoms generally present in the first year after HCT and are associated with significant impairment of patient-reported quality-of-life and late mortality. Despite advances in the understanding of the complex pathobiology of cGVHD, there have been few advances in the management of cGVHD and no agent is currently approved for preventing cGVHD7. Therefore, effective prophylaxis against cGVHD is an area of unmet need.
Extensive preclinical and clinical data suggest a role for proteasome inhibitors (PIs) in preventing cGVHD after allogeneic HCT1,8–13. Proteasome Inhibitors have immunomodulatory effects through inhibition of dendritic cells (DC), as well as key T- and B-cell subsets that play a role in the pathogenesis of cGVHD1,14. The prototypical proteasome inhibitor, bortezomib exerts a variety of biological effects through NF-κB inhibition, a key regulator of cytokine signaling and T-cell activation, proliferation, and apoptosis15–19. Bortezomib, a PI, as a result, selectively depletes proliferating alloreactive T lymphocytes, reduces T-helper (Th-) 1 cytokines and Interleukin (IL)-6 levels, and blocks antigen-presenting cell (APC) activation, while sparing regulatory T-cells (Tregs)1,14,20–22. PIs also have inhibitory effects on B-cells and plasma cells, which is relevant as B-cell dysregulation with increased allo-antibody production plays a pivotal role in the pathogenesis of cGVHD2,23–27.
Ixazomib (Ninlaro®, Takeda Pharmaceutical Company Limited) is an oral PI currently approved for the treatment of relapsed/refractory myeloma28,29. Preclinical studies have shown that ixazomib has a similar selectivity and potency in terms of proteasome inhibition to bortezomib30. Ixazomib has also been shown to inhibit DC maturation, decrease proinflammatory cytokine production through the down-regulation of NF-κB dependent transcription and thereby, modulate GVHD in preclinical model in a schedule-dependent fashion1. Considering these immunomodulatory effects and the inhibitory effects of PIs on B-cells and plasma cells24,25, we conducted a phase I/II study designed to investigate the safety and efficacy of ixazomib as a pharmacologic prophylaxis for cGVHD in patients receiving standard acute GVHD (aGVHD) prophylaxis after allogeneic HCT.
Patients and Methods
This prospective clinical trial (ClinicalTrials.gov number, NCT02250300) was approved by the Medical College of Wisconsin Institutional Review Board. Written, informed consent was obtained from patients before enrollment.
Inclusion and Exclusion Criteria
Adult patients with hematological malignancies having undergone allogeneic HCT using an HLA-identical (matched for HLA-A, -B, -C, and -DRB1) sibling (MRD) or unrelated donor (MUD) with a PB graft were eligible. Patients with active and uncontrolled infections, abnormal renal (creatinine clearance <40ml/min), hepatic (serum bilirubin >2 mg/dl, serum AST and ALT >3 times upper limit of normal), pulmonary (DLCO or FEV1 <40% of predicted), or cardiac (left ventricular ejection fraction <40%) function, poor Karnofsky Performance Score (KPS) (<60), with active ≥ grade 3 peripheral neuropathy or grade 2 with pain or history of ixazomib intolerance or allergy were excluded. Patients undergoing ex vivo or in vivo T-cell depleted allogeneic HCT were not eligible. In addition, patients with post-allogeneic HCT disease relapse or progression, active grade III-IV aGVHD, active steroid-refractory aGVHD, cGVHD and those receiving anti-B-cell monoclonal antibodies (such as rituximab) before the first dose of ixazomib prophylaxis were not eligible. For eligibility details, refer to the Supplemental Appendix A. Patients were enrolled after they had undergone allogeneic HCT.
Treatment and GVHD Prophylaxis
This was a phase I/II study: during the run-in phase I portion patients undergoing both sibling and unrelated donor allogeneic HCT were enrolled in the same arm to determine the dose-limiting toxicity (DLT) and maximum tolerated dose (MTD). During the phase II portion, patients were enrolled in two separate and independent cohorts: A. matched sibling donor transplants and B. matched unrelated donor transplants. The two cohorts were analyzed separately. The transplant conditioning intensity and regimen were at the discretion of the treating physician. Acute GVHD prophylaxis regimen was a combination of tacrolimus and methotrexate. During both phase I and phase II, four oral (capsules) doses of ixazomib were administered to subjects on a weekly basis for a total of four doses (i.e., on days 1, 8, 15 and 22), starting day+60 to +90 following allogeneic HCT.
The phase I portion followed a 3+3 design with escalating dose levels (3 dose levels [DL]; DLminus 1=2.3 mg, DL1=3 mg and DL2=4 mg) of ixazomib to determine the MTD and recommended phase II dose (RP2D). Dose escalation to DL2 (4 mg) was allowed if no DLT occurred in the first 3 evaluable patients at DL1, and if only 1 subject out of 6 had a DLT (refer to the study protocol Supplementary Appendix). DL2 was considered MTD if 0 of 3 subjects experienced DLT. No intra-patient dose escalation was permitted. Toxicity was evaluated according to National Cancer Institute Common Toxicity Criteria for Adverse Events, version 4.0 (NCI CTCAE v4.0). DLT was defined as grade 2 peripheral neuropathy with pain or grade 3 or greater peripheral neuropathy, any grade 3-4 non-hematologic toxicity, drop in donor myeloid-cell chimerism by more than 50% or unexplained graft rejection at day +90 (in the absence of disease relapse), grade 4 neutropenia, grade 3 neutropenia with fever and/or infection, grade 4 thrombocytopenia lasting >7 days, all definitely or probably related to ixazomib (Supplemental Table 2). DLT observation period was from first dose of ixazomib to 28 days after the last dose. Dose escalation to level 2 required completion of DLT observation period for the last patient at dose level 1. The phase II portion utilized the MTD for ixazomib, determined from the phase I portion of the study. Oral ixazomib was continued until one of the following criteria was met: patient completed four doses of ixazomib, development of grade III-IV aGVHD, severe cGVHD, any DLT possibly, probably or definitely related to ixazomib.
Endpoints
Primary endpoints were safety (phase I) and cumulative incidence of cGVHD of any severity at 1 year following allogeneic HCT (phase II) using MRD or MUD. Secondary endpoints included cumulative incidence of relapse, non-relapse mortality (NRM), relapse-free survival (RFS), and overall survival (OS), immune reconstitution following HCT, and B-cell activating factor (BAFF levels; before and after ixazomib dosing) as a potential biomarker to evaluate an association with GVHD. Consensus Conference Criteria31 and the National Institutes of Health (NIH) Consensus Development Project Criteria32 were used for grading aGVHD and cGVHD, respectively. While not mandated by the study protocol, but in addition to having treating physician-assessed rates, an independent GVHD review panel adjudicated the diagnosis and grading/severity of cGVHD in all enrolled subjects. The Protocol Principal Investigator was blinded to the adjudication panel and was not permitted to modify independent GVHD Review Panel’s assessment.
Immune Reconstitution, B-cell Activating Factor, and Donor-Cell Chimerism
For immune reconstitution assays peripheral blood mononuclear cells (PBMC) were obtained from EDTA-anticoagulated whole blood samples obtained on days +100, +180 and +365 post transplantation (described in detail in the Supplemental Appendix). In this analysis, CD4+ T-cells were defined as CD3+CD4+; CD8+ T-cells as CD3+CD8+; CD4+ naïve T-cells as CD3+CD45RA+CD45RO−CD4+; Tregs as CD3+CD4+CD25+CD127−; Natural Killer (NK)-cells as CD3−CD56+CD16+ and B-cells as CD19+.
BAFF is a TNF superfamily member (TNFSF13B) best known for its role in the survival and maturation of B cells. We determined the effects of ixazomib on plasma BAFF and BAFF:CD19+B-cell ratio by analyzing baseline (pre-ixazomib) and post-ixazomib administration plasma samples. To detect human BAFF, the Quantikine Human BAFF Immunoassay solid-phase ELISA was employed. Briefly, human serum samples prediluted 2-5 folds were pipetted into wells precoated with monoclonal antibody specific for BAFF. After washing away any unbound substances, an enzyme-linked polyclonal antibody specific for BAFF was added to the wells. Following a wash to remove any unbound antibody-enzyme reagent, a substrate solution was added to the wells and color developed in proportion to the amount of BAFF bound onto the wells. Human BAFF concentrations were then calculated based on the standard curve. Lineage-specific donor-cell chimerism analysis was performed on days +30, +100, +180 and +360 post-HCT. Complete donor chimerism was defined as presence of ≥95% donor cells.
Statistical Considerations
Due to the differences in the incidence of cGVHD after MRD and MUD allogeneic HCT, the phase II portion of the study was designed to accrue the two cohorts separately but in parallel. The study used a Minimax Simon two-stage design, to test the null hypothesis H0: p≥0.55 versus the alternate H1: p≤0.30 for sibling donor allogeneic HCT and for matched unrelated donor HCT, the null hypothesis H0: p≥0.65 versus the alternate H1: p≤0.40, where p is the probability of cGVHD at 1-year. OS and PFS were estimated using the Kaplan-Meier method. OS was defined as the time from HCT to death from any cause, and surviving patients were censored at last follow-up. PFS from HCT was calculated using death and disease relapse as events. The cumulative incidences of NRM and relapse risk were estimated by considering these two events as competing risks33. The cumulative incidence of cGVHD was estimated with death without GVHD as competing risks33. Comparisons were made of serum BAFF levels and BAFF: CD19+B-cell ratios between pre- and approximately 100 days post-ixazomib administration samples, by using a Wilcoxon signed-rank test. All p-values were two-sided. For detailed statistical plan, refer to the Supplemental Appendix B.
Matched CIBMTR Controls
The protocol by design enrolled a patient population that survived at least two months post-HCT (without relapse or severe aGVHD) that possibly had a lower risk of cGVHD (relative to an unselected population at day zero of transplantation). To address this bias, we planned post-hoc to compare the outcomes of study patients to contemporaneous, matched controls from the Center for International Blood and Marrow Transplant Research (CIBMTR ®) database surviving at least two months post-HCT without relapse or severe aGVHD. For patients enrolled in the phase II portion of this trial, a total of 195 matched controls (who survived two months post-HCT without relapse or severe aGVHD) were selected from the CIBMTR database using selection criteria described in Supplemental Table 1.
To compare the study population against CIBMTR controls, multivariable analysis was performed using marginal Cox regression model for OS, RFS, NRM, relapse and cGVHD, stratified by donor (MRD vs. MUD). The assumption of proportional hazards for each factor in the Cox model was tested using time-dependent covariates. A backward stepwise model selection approach was used to identify all significant risk factors. Covariates that were significant at a 5% level were kept in the final model. The main effect (case vs. control group) was kept in all model building steps. Adjusted probabilities of RFS and OS, and adjusted cumulative incidence functions of cGVHD, NRM and relapse were calculated using the multivariate models, stratified on main effect and weighted by the pooled sample proportion value for each prognostic factor. These adjusted probabilities estimated likelihood of outcomes in populations with similar prognostic factors. Statistical analyses were performed by SAS statistical software, version 9.4 (Cary, NC).
Results
Study Cohort
Between January 2015 and March 2018, 57 patients were enrolled, after undergoing allogeneic HCT. In the phase I cohort, six patients were enrolled and two DLs were tested (DL1 at 3 mg and DL2 at 4 mg). Baseline characteristics of all study patients are presented in Table 1. Median patient age was 57 years (range, 27-73). Median number of ixazomib doses administered was 4 (range, 1-4). Eighteen patients (31.6%) had a KPS of ≥90, 29 (50.9%) had HCT-CI of ≥3. Thirty-seven patients (64.9%) had myeloid malignancy, whereas 20 (35%) had lymphoid malignancy (including 6 patients with multiple myeloma). Nineteen patients had a prior autologous transplant. Eighteen patients (31.6%) received myeloablative conditioning (MAC). All patients received a PB graft. Median time from transplant to start oral ixazomib was 68 days (range, 60-90). Before starting ixazomib, 14 patients had a history of grade I-II aGVHD, including 4 patients with active grade I-II aGVHD at the time of first dose of ixazomib. No patient had steroid-refractory aGVHD or cGVHD before the first dose of ixazomib. Median follow-up of survivors is 21.6 months (range, 12-37.3 months).
Table 1.
Baseline Characteristics of the Clinical Trial (Phase I/II) Patients
| Phase I N=6 (%) | Phase II MRD N=25 | Phase II MUD N=26 | |
|---|---|---|---|
| Male (%) | 5 (83%) | 15 (79%) | 13 (68%) |
| Median age, years (range) | 66 (54-73) | 55 (27-71) | 56 (27-69) |
| Median doses of ixazomib (range) | 4 (3-4) | 4 (1-4) | 4 (1-4) |
| Causes of missed ixazomib doses | nausea/diarrhea=1 | nausea/vomiting=3; aGVHD=1; acute kidney injury=1 | thrombocytopenia=4; nausea/vomiting/diarrhea=3; relapse=2; others=2 |
| Ixazomib start day, median (range) | 71 (66-73) | 68 (60-90) | 67 (60-90) |
| Median KPS at enrollment (range) | 75 (70-90) | 80 (70-90) | 80 (60-100) |
| Median HCT-CI (range) | 4 (2-5) | 3 (0-6) | 2 (0-4) |
| Diagnosis | |||
| Leukemia/MDS/MPN (%) | 4 (67) | 13 (52) | 20 (77) |
| Lymphoma (%) | 2 (33) | 9 (36) | 3 (11) |
| Multiple Myeloma (%) | 3 (12) | 3 (11) | |
| Prior autograft (%) | 3 (50) | 10 (40) | 6 (23) |
| HLA match (%) | 6 (100) | ||
| 8/8 | 25 (100) | 24 (92) | |
| 10/10 | 2 (8) | ||
| DRI | |||
| High | 1 (17) | 2 (8) | 1 (4) |
| Intermediate | 1 (17) | 15 (60) | 18 (69) |
| Low | 4 (67) | 8 (32) | 7 (27) |
| Conditioning | |||
| Reduced Intensity (%) | 6 (100) | 18 (72) | 15 (57) |
| Fludarabine/Busulfan2 | 6 (100) | 12 (48) | 10 (38) |
| Fludarabine/Melphalan | 6 (24) | 5 (19) | |
| Myeloablative (%) | 7 (28) | 11 (42) | |
| Fludarabine/Busulfan4 (%) | 5 (20) | 5 (19) | |
| Busulfan/Cyclophosphamide2(%) | 0 (0) | 2 (8) | |
| Cyclophosphamide/TBI (%) | 2 (8) | 4 (15) | |
| Planned acute GVHD prophylaxis | |||
| Tacrolimus/Methotrexate (%) | 6 (100) | 25 (100) | 26 (100) |
| PB graft (%) | 6 (100) | 25 (100) | 26 (100) |
| ABO mismatch (%) | 1 (17) | 10 (40) | 17 (65) |
| Acute GVHD before ixazomib | |||
| Patients with grade I-II aGVHD before starting ixazomib (%) | 0 | 5 (20) | 9 (35) |
| Patients with active aGVHD at the start of ixazomib (% of GVHD cases before ixazomib) | 0 | 2 (40) | 2 (22) |
| Median follow-up of survivors, months (range) | 36 (24-37) | 17 (12-36) | 21 (12-37) |
Abbreviations: aGVHD, acute Graft-versus-Host Disease; KPS, Karnofsky Performance Score; HCT-CI, hematopoietic cell transplantation-comorbidity index; MDS, myelodysplastic syndrome; MPN, myeloproliferative neoplasm; PB, peripheral blood; wt, weight.
High-resolution typing at HLA-A, -B, -C and –DRB1
Disease-risk classification based on the standard criteria (available at: https://www.cibmtr.org/ReferenceCenter/Statistical/Tools/Pages/DRI.aspx [Last accessed Nov 22, 2019])
Compliance and toxicity
Ixazomib was well tolerated. No DLT was observed in phase I; treatment-emergent adverse events (AEs) were all grade 1-2; elevated alanine aminotransferase (n=1) and aspartate aminotransferase (n=1), and thrombocytopenia (n=1) (Table 2). Based on the phase I run-in, ixazomib dose of 4 mg was selected for the phase II expansion. Thirty-six patients (70.6%) in phase II received 4 doses of ixazomib. Six, seven and two patients missed 1, 2 and 3 ixazomib doses, respectively. The reasons for missing doses of ixazomib in 15 patients were following: nausea/vomiting/diarrhea, n=6; thrombocytopenia, n=4; acute kidney injury, n=2, microangiopathic hemolysis, n=1; infection, n=1; aGVHD, n=1; disease relapse, n=2. One patient withdrew consent and discontinued ixazomib after two doses. AEs potentially (possibly, probably, or definitely) related to ixazomib are shown in Table 5. Grade 3 AEs in phase II were nausea (n=3), diarrhea (n=3), abdominal pain (n=3), hyponatremia (n=3), catheter-related infection (n=3), thrombocytopenia (n=12), leukopenia (n=3), neutropenia (n=3), lymphopenia (n=9). No grade 4-5 toxicity related to ixazomib was observed in the study.
Table 2.
Adverse events (possibly, probably or definitely) related to ixazomib in the study patients.
| Adverse event | Any Grade n (%) | Grade 1-2 n (%) | Grade 3 n (%) |
|---|---|---|---|
| Phase I (N=6) | |||
| Thrombocytopenia | 1 (17) | 1 (17) | 0 |
| Aspartate Aminotransferase Increased | 1 (17) | 1 (17) | 0 |
| Alanine aminotransferase Increased | 1 (17) | 1 (17) | 0 |
| Alkaline phosphatase Increased | 1 (17) | 1 (17) | 0 |
| Phase II (N=51) | |||
| Thrombocytopenia | 14 (27) | 10 (20) | 4 (8) |
| Leukopenia | 5 (10) | 4 (8) | 1 (2) |
| Neutropenia | 3 (6) | 2 (4) | 1 (2) |
| Lymphopenia | 4 (8) | 1 (2) | 3 (6) |
| Diarrhea | 17 (33) | 16 (31) | 1 (2) |
| Nausea | 17 (33) | 16 (31) | 1 (2) |
| Vomiting | 10 (20) | 10 (20) | 0 |
| Fatigue | 7 (14) | 7 (14) | 0 |
| Fever | 3 (6) | 3 (6) | 0 |
| Bloating | 2 (4) | 2 (4) | 0 |
| Edema limbs | 3 (6) | 3 (6) | 0 |
| Acute kidney injury | 2 (4) | 2 (4) | 0 |
| Rise in creatinine | 1 (2) | 1 (2) | 0 |
| Dehydration | 1 (2) | 1 (2) | 0 |
| Abdominal pain | 2 (4) | 1 (2) | 1 (2) |
| Testicular pain | 1 (2) | 1 (2) | 0 |
| Dysgeusia | 2 (4) | 2 (4) | 0 |
| Hyponatremia | 1 (2) | 0 | 1 (2) |
| Catheter-related infection | 1 (2) | 0 | 1 (2) |
| Weight gain | 1 (2) | 1 (2) | 0 |
| Skin rash | 2 (4) | 2 (4) | 0 |
| Muscle pain | 1 (2) | 1 (2) | 0 |
| Cough | 1 (2) | 1 (2) | 0 |
| Gout | 1 (2) | 1 (2) | 0 |
| Dry Mouth | 1 (2) | 1 (2) | 0 |
| Gastritis | 1 (2) | 1 (2) | 0 |
Table 5.
Kinetics of donor cell chimerism and immune reconstitution after allogeneic transplant
| Variable (Reference range) | Day +30 | Day +100 | Day +180 | Day +365 |
|---|---|---|---|---|
| Post-transplantation donor cell chimerism in the MRD cohort | ||||
| T-cell (CD3+) chimerism | 90 (0-100) | 94 (21-100) | 97.5 (49-100) | 100 (82-100) |
| Myeloid (CD33+) chimerism | 100 (91-100) | 100 (83-100) | 100 (79-100) | 100 (98-100) |
| Post-transplantation donor cell chimerism in the MUD cohort | ||||
| T-cell (CD3+) chimerism | 93 (76-100) | 96 (81.3-100) | 100 (84.3-100) | 100 (0-100) |
| Myeloid (CD33+) chimerism | 100 (89-100) | 100 (1-100) | 100 (1-100) | 100 (0-100) |
| Immune reconstitution (MRD and MUD cohort data combined) | ||||
| IgM (40-230 mg/dL) | 40.5 (5-585) | 32.5 (0-139) | 36 (0-320) | 54 (5-462) |
| IgA (70-400 mg/dL) | 119 (0-2849) | 79.5 (0-1973) | 92.5 (0-1404) | 71 (0-696) |
| IgG (700-1600 mg/dL) | 577.5 (50-2597) | 526 (287-4266) | 573 (191-1921) | 577 (66-2820) |
| CD3+/CD4+, median (range) (410-1540/μL) | 248 (0-1632) | 326 (0-1100) | 379 (73-1372) | |
| CD3+/CD8+, median (range) (230-1090/μL) | 235 (0-2034) | 293 (0-2251) | 341 (28-2540) | |
| CD4+ naive T-cells‡, median (range) (145-768/μL) | 56.5 (0-512) | 58 (0-330) | 55 (0-288) | |
| Regulatory T-cells#, median (range) (19-41/μL) | 33 (10-70) | 40 (1-99) | 54 (8-292) | |
| CD19+/CD20+ B-cells, median (range) (0-559/μL) | 24.5 (0-480) | 32 (0-946) | 104.5 (0-1250) | |
| CD16+/CD56+ NK-cells, median (range) (42-447/μL) | 167 (36-469) | 173 (0-810) | 175.5 (4-882) | |
Data presented are median (range).
CD4+ naive T cell subsets defined as CD3+/CD45RA+/CD45RO−/CD4+.
Regulatory T cells defined as CD3+CD4+CD25med-highCD127low.
MRD: matched related donor, MUD: matched unrelated donor
Chronic GVHD
All patients in the phase II cohort were evaluable for cGVHD. In the MRD cohort (n=25), cGVHD developed in 14 patients. Median time to the onset of cGVHD was 328 days (range, 135-932). Eight patients had moderate (n=6) or severe (n=2) cGVHD up to last follow up. The adjusted cumulative incidence of cGVHD at 12 months was 36% (95% Confidence Interval [CI], 19-54%) (Figure 1A) and the incidence of moderate/severe cGVHD was 28% (95%CI, 19-54%) (Table 3). Six patients with cGVHD had prior aGVHD. In the two patients with severe cGVHD, organ involvement included mouth (n=1), and liver (n=2), whereas the following organs were involved in the 6 patients with moderate cGVHD: skin (n=4), mouth (n=4), eyes (n=2), liver (n=2), and lungs (n=2) (Table 4).
Figure 1.
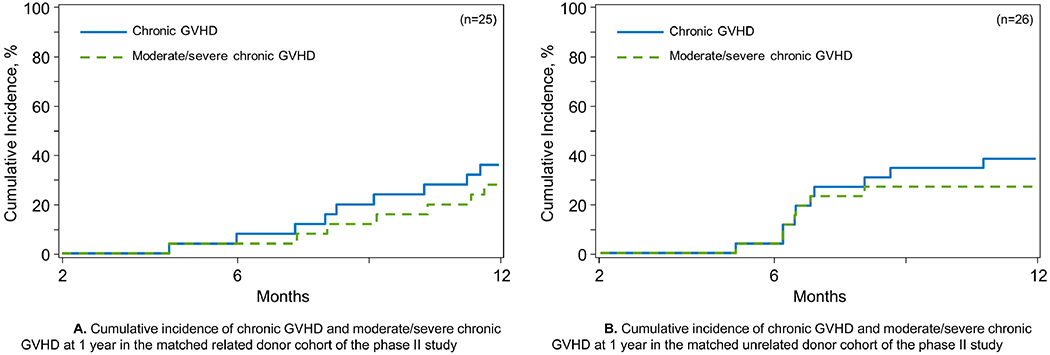
A. Cumulative incidence of chronic GVHD and moderate/severe chronic GVHD at 1 year in the matched related donor cohort of the phase II study B. Cumulative incidence of chronic GVHD and moderate/severe chronic GVHD at 1 year in the matched unrelated donor cohort of the phase II study
Table 3.
Adjusted (for age) outcomes after allogeneic transplant receiving ixazomib plus standard graft-versus-host disease (GVHD) prophylaxis on the study.
| Outcomes | Phase II MRD (n=25) Probability (95% CI) | Phase II MUD (n=26) Probability (95% CI) |
|---|---|---|
| Chronic GVHD, 1 year | 36% (19-54) | 39% (21-56) |
| Moderate/Severe Chronic GVHD, 1 year | 28% (13-46) | 27% (12-44) |
| Acute GVHD, grade II-IV, 100 days | 20% (8-37) | 15% (5-31) |
| Acute GVHD, grade III-IV, 100 days | 8% (1-22) | 12% (3-26) |
| Acute GVHD, grade II-IV, 180 days | 28% (13-46) | 23% (10-40) |
| Acute GVHD, grade III-IV, 180 days | 8% (1-22) | 15% (5-31) |
| Non-relapse mortality, 1 year | 0% | 4% (0-16) |
| Relapse, 1 year | 20% (8-37) | 35% (18-52) |
| Relapse-free survival, 1 year | 80% (58-91) | 62% (40-77) |
| Overall survival, 1 year | 92% (72-98) | 89% (68-96) |
Table 4.
Frequency and severity of chronic graft-versus-host disease and its organ distribution in the matched related and unrelated donor cohorts of the phase II study
| A. Matched related donor | |
|---|---|
| Severity of Chronic Grade (n) | Organs involved (n) |
| Mild chronic GVHD (6) | Eyes (1) |
| Mouth (4) | |
| Liver (3) | |
| Moderate chronic GVHD (6) | Eyes (2) |
| Mouth (4) | |
| Skin (4) | |
| Liver (2) | |
| Lungs (2) | |
| Severe chronic GVHD (2) | Mouth (1) |
| Liver (2) | |
| B. Matched unrelated donor | |
| Severity of Chronic Grade (n) | Organs involved (n) |
| Mild chronic GVHD (2) | Eyes (1) |
| Liver (1) | |
| Moderate chronic GVHD (5) | Eyes (2) |
| Mouth (2) | |
| Skin (3) | |
| Liver (2) | |
| GIT (1) | |
| Joints/fascia (1) | |
| Severe chronic GVHD (4) | Eyes (2) |
| Mouth (2) | |
| Skin (2) | |
| Liver (2) | |
| Lungs (1) | |
Abbreviations: GIT, gastrointestinal tract; GVHD, graft-versus-host disease.
In the MUD cohort (n=26), eleven patients developed cGVHD up to last follow up; of these 11, nine had moderate (n=5) or severe (n=4) cGVHD. Six patients with cGVHD had prior aGVHD. Median time to onset of cGVHD was 210 days (range, 155-539). The adjusted cumulative incidence of cGVHD at 12 months was 39% (95%CI, 21-56%) (Figure 1B) and was 27% (95%CI, 12-44) for moderate/severe cGVHD (Table 3). In the nine patients with moderate or severe cGVHD, the following organs were affected: skin (n=5), mouth (n=3), eyes (n=4), fascia/joints (n=1), gut (n=1), liver (n=4), lungs (n=1) (Table 4).
Acute GVHD
In the MRD cohort, a total of 11 patients developed aGVHD in the MRD cohort, of whom 3 had grade III aGVHD (skin, n=1, gut, n=1, skin + gut, n=1) and none had grade IV aGVHD. The median time to onset of aGVHD was 72 days (range, 23-244). Four MRD patients had aGVHD before initiating ixazomib (grade I [skin], n=3, grade II [gut], n=1), of whom two had active GVHD (grade I, n=2) at the time of first dose of ixazomib, managed with topical corticosteroids alone. The cumulative incidence rates of grade II-IV aGVHD in the MRD cohort at days +100 and +180 were 20% (95%CI, 8-37%) and 28% (95%CI, 13-46%), respectively (Table 3). The cumulative incidence of grade III-IV aGVHD was 8% (95%CI, 1-22%) for both time points.
In the MUD cohort, a total of 15 patients developed aGVHD at a median of 49 days after allogeneic HCT (range, 21-425), including 6 developing grade III-IV aGVHD. Nine patients had a history of aGVHD before starting oral ixazomib (grade I, n=6; grade II, n=3; ; all involving skin) and two had active GVHD at the time of first dose of ixazomib (grade I, n=1, grade II, n=1, both skin). The cumulative incidence rates of grade II-IV aGVHD in the MUD cohort at days +100 and +180 were 15% (95%CI, 5-31%) and 23% (95%CI, 10-40%), respectively (Table 3). The respective figures for grade III-IV aGVHD were 12% (95%CI, 3-26) and 15% (95%CI, 5-31%).
Relapse and Survival
The adjusted cumulative incidence of NRM at 1 year was 0% and 4% (95%CI, 0-16%) in the MRD and MUD cohorts, respectively (Table 3). At 1 year, the adjusted cumulative incidence of relapse was 20% (95%CI, 8-37%) and 35% (95%CI, 18-52%) in the MRD and MUD cohorts, respectively (Table 3). The adjusted 1-year OS and RFS for MRD cohort were 92% (95%CI, 72-98%) and 80% (95%CI, 59-91%), respectively (Table 3). For the MUD cohort, the 1-year OS and RFS were 88% (68-96%) and 62% (42-77%), respectively (Table 3). At data cut-off, fourteen patients in the study had died (phase I, n=1; phase II, MRD; n=6, MUD, n=7). Causes of death are summarized in Supplemental Table 2.
Engraftment Kinetics, Immune reconstitution and BAFF Profiles
There were no primary graft failures or rejections after starting ixazomib in the non-relapsing patients. In the MRD and MUD cohorts, the median donor myeloid cell (CD33+) chimerism was 100% at all test time points (Table 5). The median donor T cell (CD3+) chimerism in the MRD and MUD cohorts on days +100, +180, and +365 were 90%, 94%, and 97.5% and 93%, 96%, and 100%, respectively (Table 5). Recipient immune reconstitution data are summarized in Table 5. For the entire cohort the median immunoglobulin G levels were >500 mg/dL at all tested time points. Reconstitution of the T-cell compartment was prompt. The median CD4+ cell count was above 200/μL from day +100 onward, and median CD8+ T cell count was within normal limits from day +100 onward. The median Treg (CD4+CD25+CD127−) cell count was 30/μL day +100 onward. Median CD19+ B cell and CD16+/CD56+ natural killer (NK) cell count at the 1-year mark were 104.5/μL and 175.5/μL, respectively (Table 5).
For the phase II cohort, plasma BAFF levels following ixazomib dosing were found to be significantly elevated (median, 4431.6 pg/mL; range, 1176.4-20,665.8) than at baseline (median, 2989.2 pg/mL; range, 840.4-8880.0) (Supplemental Figure 1). There were no statistically significant differences in the baseline (pre-ixazomib) BAFF levels in those who eventually developed cGVHD later (n=25) vs. those who did not (n=26) (median, 4046.3 g/mL and 2458.8 pg/mL, respectively, p=0.24) (Figure 2A). Among patients who did not develop cGVHD (n=26), significant elevation of BAFF levels after ixazomib exposure was observed (median, 6968 pg/mL; range, 1750.9-20,665.8), compared to baseline values (median, 2458.8 pg/mL; range, 840.4-8880.0) (p=0.0013) (Figure 2B). No significant difference was observed between pre- and post-ixazomib BAFF levels in patients who developed cGVHD (4046.3 vs. 4254.9 pg/mL) (Figure 2C). Post-ixazomib BAFF levels were significantly higher in patients who did not develop cGVHD (median, 6968.8 pg/mL) versus those who did (4254.9 pg/mL) (p=0.0102) (Figure 2D). In the group of patients that did not develop cGVHD (regardless of donor cohort), CD19+ B-cell counts did not increase after ixazomib use (median 18/μL [range, 0-400/μL] and 19.5/μL [0-262/μL], pre- and post-ixazomib, respectively; p=0.76). In contrast, in patients who developed cGVHD, CD19+ B-cell counts increased from a median of 12/μL (range, 0-480/μL) to 42/μL (range, 0-214/μL) with the use of ixazomib (p=0.001) (Supplemental Figures 2A–B). There were, however, no significant differences in BAFF:CD19+B-cell ratios between pre- and post-ixazomib in patients who developed cGVHD (median, 338.7 vs. 105.5; Figure 2E) or remained cGVHD-free (median, 89.6 vs. 330.9; Figure 2F). In addition, no significant differences were found between pre- and post-ixazomib Tregs in those with no cGVHD (median, 34 vs. 33, p=0.96; Figure 2G) and those who developed cGVHD (median, 36 vs. 39.5, p=0.36; Figure 2H).
Figure 2.
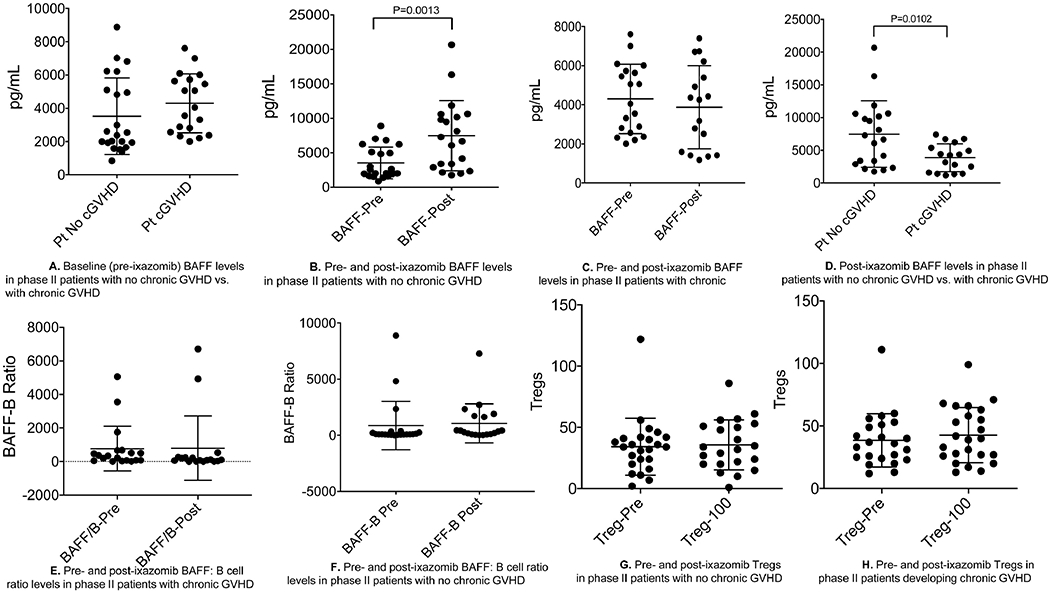
A. Baseline (pre-ixazomib) BAFF levels in phase II patients with no chronic GVHD vs. with chronic GVHD B. Pre- and post-ixazomib BAFF levels in phase II patients with no chronic GVHD C. Pre- and post-ixazomib BAFF levels in phase II patients with chronic D. Post-ixazomib BAFF levels in phase II patients with no chronic GVHD vs. with chronic GVHD E. Pre- and post-ixazomib BAFF: B cell ratio levels in phase II patients with chronic GVHD F. Pre- and post-ixazomib BAFF: B cell ratio levels in phase II patients with no chronic GVHD G. Pre- and post-ixazomib Tregs in phase II patients with no chronic GVHD H. Pre- and post-ixazomib Tregs in phase II patients developing chronic GVHD
Control population
Contemporaneous matched CIBMTR controls underwent allogeneic HCT during the same time period as the trial population, and included patients surviving without grade III-IV aGVHD or disease relapse for at least day+60 post-HCT (Supplemental Table 1). Baseline clinical characteristics of the control cohorts can be found in Supplemental Tables 3 and 4. The 51 cases from the phase II cohorts were matched with 195 controls using a 1:4 match comparison based on following criteria: donor type (matched sibling vs. matched unrelated donor), disease (acute leukemia; chronic leukemia; lymphoma; multiple myeloma; myelodysplastic syndrome/myeloproliferative neoplasm), disease status (chemo-sensitive vs. -resistant), conditioning intensity (MAC vs. RIC), conditioning regimens (Cy/12Gy TBI, Bu/Cy, Flu/Bu, Flu/Mel), HCT-CI (0 vs. 1-2. vs. ≥3), patient age (±10 years). Twenty-five cases from the MRD cohort were matched with 92 CIBMTR controls and 26 cases from the MUD cohort were matched with 103 controls (Supplemental Tables 3 and 4).
Matched-pair analysis adjusted for age showed that risk of cGVHD in the MRD cohort was similar to the matched controls (hazard ratio [HR] for cGVHD or death, 0.85; 95%CI, 0.42-1.71, p=0.64) (Table 6, Supplemental Figure 3A). The risk of cGVHD in the MUD cohort was also not significantly different from that in the matched controls (HR, 0.68; 95%CI, 0.34-1.33, p=0.26) (Table 6, Supplemental Figure 4A). Compared to the matched controls, the risk of NRM was significantly lower in the MRD cohort (HR 0.0, p<0.01), but not significantly different in the MUD (HR 2.19; 95%CI, 0.19-24.59, p=0.53) cohort (Table 6, Supplemental Figures 3B, 4B). Matched-pair analysis did not show any significant difference in the risk of relapse between the two cohorts and their controls (HR 0.59, 95%CI, 0.23-1.52, p=0.27 for MRD; HR 1.64, 95%CI, 0.74-3.59, p=0.22 for MUD) (Table 6, Supplemental Figures 3C, 4C). No statistically significant differences were observed between the two cohorts and their respective matched controls with regards to RFS and OS (Table 6 and Supplemental Table 5, Supplemental Figures 3D–E, 4D–E).
Table 6.
Results of matched-pair analysis comparing key outcomes between phase II study patients and the contemporaneous matched CIBMTR controls.
| A. Matched Sibling donor cohort | Case HR (95% CI) | Matched Control HR (Reference) | P-value |
|---|---|---|---|
| Chronic GVHD | 0.85 (0.42-1.71) | 1.0 | 0.64 |
| Moderate/Severe Chronic GVHD | 1.04 (0.48-2.23) | 1.0 | 0.93 |
| Acute GVHD, grade II-IV | 0.86 (0.41-1.79) | 1.0 | 0.68 |
| Acute GVHD, grade III-IV | 1.19 (0.32-4.39) | 1.0 | 0.79 |
| Non-Relapse Mortality | 0.00 | 1.0 | <0.01 |
| Relapse | 0.59 (0.23-1.52) | 1.0 | 0.27 |
| Relapse-Free Survival | 0.47 (0.19-1.20) | 1.0 | 0.11 |
| Overall Survival | 0.34 (0.08-1.39) | 1.0 | 0.13 |
| B. Matched Unrelated donor cohort | Case HR (95% CI) | Matched Control HR (Reference) | P-Value |
| Chronic GVHD | 0.68 (0.34-1.33) | 1.0 | 0.26 |
| Moderate/Severe Chronic GVHD | 0.83 (0.41-1.71) | 1.0 | 0.62 |
| Acute GVHD, grade II-IV | 0.78 (0.36-1.68) | 1.0 | 0.53 |
| Acute GVHD, grade III-IV | 4.73 (1.12-19.97) | 1.0 | 0.03 |
| Non-Relapse Mortality | 2.19 (0.19-24.59) | 1.0 | 0.53 |
| Relapse | 1.64 (0.74-3.59) | 1.0 | 0.22 |
| Relapse-Free Survival | 1.68 (0.79-3.57) | 1.0 | 0.18 |
| Overall Survival | 0.71 (0.20-2.53) | 1.0 | 0.60 |
Discussion
Chronic GVHD is a multi-organ disease characterized by immune dysregulation and is a leading cause of late morbidity and mortality from allogeneic HCT3,34–37. It leads to increased symptom burden, impaired quality-of-life, and prolonged immunosuppressive therapy with resultant toxicities35–39. Extending the duration of prophylactic immunosuppression has not been effective in preventing cGVHD40,41. T-cell depletion (in vivo42–44 or ex vivo45) has shown efficacy against cGVHD, but has not unequivocally improved OS and may increase the risk of relapse (especially in RIC allogeneic HCT)46–48. Clinical trials using B-cell depletion using rituximab post-HCT for preventing cGVHD have shown promise49,50. The post-transplant cyclophosphamide (PTCY) platform is an effective strategy that has been associated with lower incidence of cGVHD in two recent prospective clinical trials12,51,52.
In this phase I/II study, we evaluated the safety and efficacy of a novel strategy of oral ixazomib for prevention of cGVHD in allogeneic peripheral blood HCT patients receiving tacrolimus/methotrexate for aGVHD prophylaxis. In phase II, ixazomib was dosed at 4 mg orally, on a weekly basis for at most four doses, beginning day+60 through +90 (median, day+68). This strategy was feasible and was not associated with serious toxicities or increased relapse rates. We report a 1-year cumulative incidence of cGVHD of 36% (95%CI, 19-54%) in the MRD cohort and 39% (95%CI, 21-56%) in the MUD cohort. While the study did meet its primary endpoint of reducing the 1-year cumulative incidence of cGVHD by 25% (in the MUD cohort), after the trial completed accrual we recognized that the original protocol had an inherent selection bias arising from including only patients surviving at least two months after HCT without relapse or severe aGVHD, and that could have selected for a trial population with an anticipated lower cGVHD risk (compared to unselected patients at day zero of transplantation). In order to address this selection bias, we compared post-hoc the outcomes in the phase II cohorts with contemporaneous CIBMTR matched controls selected to match the eligibility criteria of the study participants, and did not observe any significant improvement in cGVHD risk with the use of a short-course of ixazomib in both the MRD and MUD cohorts compared to respective controls (HR 0.85, p=0.64; HR 0.68, p=0.26, respectively): the controls were 1.18 times (95%CI, 0.58-2.38) and 1.47 times (95%CI, 0.75-2.94) more likely to develop cGVHD than MRD and MUD cases, but this did not reach statistical significance (p=0.64 and 0.26, respectively) as the analysis was not powered to detect relatively small effect sizes.
Koreth et al. used short-course bortezomib (on days +1, +4, and +7) for aGVHD prophylaxis after peripheral blood HCT in a phase I/II trial and showed that 180-day cumulative incidence of grades II-IV aGVHD was 22% (95%CI, 11-35) and 1-year cumulative incidence of cGVHD was 29% (95%CI, 16-43)13. Two-year cumulative incidence of NRM and relapse were 11% (95%CI, 4-22) and 38% (95%CI, 24-52), respectively. Bortezomib-treated patients had similar rates of NRM and survival as those of contemporaneous HLA-matched RIC HCT8,13,53. However, the randomized, phase II BMT CTN 1203, comparing three GVHD preventive regimens (PTCY, bortezomib and maraviroc) with a contemporaneous CIBMTR controls, did not demonstrate an improved acute or cGVHD rates with bortezomib-based prophylaxis12. The cumulative incidence of cGVHD at 1-year was 39% (90%CI, 30-48) for tacrolimus, methotrexate, and bortezomib and 38% (90%CI, 33-43) for the control group of tacrolimus and methotrexate12. Based on the preclinical and clinical rationale for use of PIs in prevention of GVHD, studies have examined the utility of PIs in cGVHD management. A single-arm, single-center, phase II study was conducted in 22 patients using bortezomib to complement the immunomodulatory activity of prednisone in the first-line treatment of cGVHD10. Bortezomib was administered intravenously on days 1, 8, 15, 22 of 35-days cycle, for a study duration of 15 weeks. The regimen was well-tolerated and achieved a high response at week 15 (80%, including 2 (10%) complete and 14 (70%) partial responses) and allowed a decrease in median prednisone dose from 50 mg/day to 20 mg/day at week 15. An irreversible PI, carfilzomib, administered intravenously once-weekly for six 28-day cycles was evaluated in a single-arm multicenter phase II trial (n=20) with the primary endpoint of 6-month treatment failure (composite of death, relapse, additional immunosuppression)54. The study results revealed that carfilzomib therapy was poorly tolerated with low adherence to the planned therapy and high rate of discontinuation, driven by unresolved toxicity and treatment failure with additional immunosuppression therapy. Failure-free survival at 12 months was 32%.
Ixazomib is rapidly absorbed, has high oral bioavailability and has a long terminal half-life of 9.5 days55. The kinetics of early B-cell recovery (as early as day +60 to +90 post-HCT) and potential role of B cells in the pathophysiology of cGVHD were the main rationale behind ixazomib schedule adopted in the current protocol56,57. The timing of ixazomib was extrapolated from data demonstrating depletion of allogeneic donor B-cells after prophylactic anti-B-cell therapy delivered 2-3 months post-HCT in a study evaluating activity of rituximab in prevention of cGVHD49. In addition, initiating ixazomib earlier than day +60 could have increased the probability of cytopenias, gastrointestinal AEs and potentially severe aGVHD based on data from murine models58. The timing and schedule of ixazomib in our study was largely empiric. We cannot rule out the possibility that an extended prophylaxis duration could have been more effective in preventing cGVHD, especially when considering the recently reported favorable outcomes of cGVHD treatment with this agent by Pidala et al59. A limitation of the study was that the CIBMTR controls were matched to the study patients for most relevant baseline clinical characteristics (disease, disease status, conditioning regimen, HCT-CI and patient’s age) besides donor type, but there were other elements that could not be matched such as KPS and individual organ functions required for study participation and referred to in the eligibility criteria.
Improvement in cGVHD patients with rituximab supports the role of B-cells in cGVHD pathogenesis60,61. Serving as APCs, donor B-cells stimulate donor CD4+ T-cell expansion, autoreactivity, IL-7Rα expression, and survival27. These changes significantly boost donor CD4+ T cell capacity in mediating autoimmune features of cGVHD27. In the context of B-cell dysfunction, BAFF is thought to be critical for B-cell survival and activation based on preclinical models62,63. B-cell reconstitution in patients with cGVHD is delayed, and these patients have elevated plasma BAFF: B-cell ratios63. BAFF levels are elevated immediately following allogeneic HCT but decrease with B cell recovery49,62. The significant increase in BAFF levels observed post-ixazomib in patients without cGVHD (Figure 2B) could be related to the effect of ixazomib on the likely swift B-cell reconstitution that otherwise would have happened in these patients50. Similar to our observations of BAFF level elevations post-ixazomib in patients not developing cGVHD, Cutler et al. showed in a phase II study of rituximab for cGVHD prevention that BAFF levels were significantly higher in cGVHD-free patients at 9 and 12 months50. It was also shown in that study that BAFF/B-cell ratios were significantly higher in cGVHD patients than in cGVHD-free patients at 24 months. We were limited in this trial as we did not follow the BAFF levels and BAFF: CD19+ B-cell ratios over an extended period after HCT.
There is an inherent bias in any single-arm clinical trial of a post-HCT intervention that enrolls participants after HCT due to exclusion of patients who suffered significant adverse event prior to the onset of the intervention. Likewise, our trial excluded patients with active grade III-IV and/or steroid-refractory aGVHD at the time of initiation of the study drug (as such subjects were not eligible for the study). It is, therefore, worth mentioning that minimizing the selection bias by conducting a landmark analysis with contemporary matched controls is a strength of this study and allowed us to put results of a single arm study in perspective. This study results also illustrated an important finding that became obvious as a result of a comparison with the matched controls: despite achieving decreased cGVHD rates in both donor groups and meeting the primary endpoint in the MUD cohort, the comparative analysis showed that ixazomib used at the given schedule and duration lacked efficacy. This can be partially explained by the fact that this was, in effect, a landmark analysis in which outcomes in patients who survived up until at least day +60 without active grade III-IV or steroid-refractory aGVHD (at the time of initiating ixazomib) in the study cohort were compared with matched CIBMTR controls surviving until day+60 without relapse and without developing grade III-IV aGVHD.
In conclusion, this prospective phase II study using a novel strategy of oral ixazomib for cGVHD prophylaxis administered in four weekly doses starting between days +60 through +90 post-transplant did not demonstrate a reduction in cGVHD risk relative to matched controls. Further exploration of a short course of oral ixazomib using the schedule employed in this study is not warranted. Potential benefit of ixazomib as cGVHD prophylaxis with an alternate schedule and duration cannot be ruled out.
Supplementary Material
Highlights.
Administration of an oral proteasome inhibitor, ixazomib in combination with tacrolimus and methotrexate for prevention of chronic GVHD is safe and convenient but failed to show efficacy when compared to contemporaneous matched CIBMTR controls.
B-cell activating factor (BAFF), critical for B-cell survival and maturation, was elevated after administration of ixazomib in chronic-GVHD free patients indicating B-cell depleting effect of ixazomib.
Acknowledgments
This work is supported in part by research funding by Takeda Pharmaceutical Company Limited (to M.H.). Correlative studies were performed at Medical Collee of Wisconsin. We thank MCW Clinical Trials Office for regulatory support, and our patients, donors, Blood and Marrow Transplant nurses and advanced practice providers.
Conflicts of interest:
SC: Honorarium: Takeda Pharmaceuticals; AD: institutional research funding from Merck, Prothena, Sanofi, EDO Mundipharma, TeneoBio, Consulting fees: Prothena, Pfizer, Imbrium, Akcea; BD: Personal fees and honorarium from Amgen, Takeda, GSK, Janssen, Celgene; TSF: Research Funding: Millennium, Kyowa, TG Therapeutics, Protola, Curis, Novartis. Consulting: Genentech, Adaptive Biotechnologies, AbbVie, Verstaem. Speaking: Genentech, Sanofi, Seattle Genetics, AstraZeneca, Celgene, Adaptive Biotechnologies; PNH: Grants and personal fees from Takeda, Celgene, BMS, Amgen, Sanofi, Karyopharm, Incyte; MH reports: Research Support/Funding: Takeda Pharmaceutical Company; Spectrum Pharmaceuticals. Consultancy: Incyte Corporation; Research Support: Novartis, Kite Pharma, Celgene/BMS, BMS. Compensation: Amgen (Consulting), Medigene (Consulting), Celgene/BMS (Consulting); NNS: Personal fees from Kite, Celgene, Incyte, Cellectar, Miltenyi; ADC Therapeutics; Celgene; Pharmacyclics, Magenta Therapeutics, Omeros, AbGenomics, Verastem, TeneoBio. Speaker’s Bureau: Sanofi Genzyme, AstraZeneca. The remaining authors declare no relevant competing interests.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented at the 59th Annual Meeting of the American Society of Hematology, Atlanta, GA, December 8-11, 2017; and the Transplant and Cell Therapy Meeting, Houston, TX, February 13-17, 2019.
REFERENCES
- 1.Koreth J, Alyea EP, Murphy WJ, et al. : Proteasome Inhibition and Allogeneic Hematopoietic Stem Cell Transplantation: A Review. Biology of Blood and Marrow Transplantation 15:1502–1512, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pai C-CS, Chen M, Mirsoian A, et al. : Treatment of chronic graft-versus-host disease with bortezomib. Blood 124:1677–1688, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arai S, Arora M, Wang T, et al. : Increasing incidence of chronic graft-versus-host disease in allogeneic transplantation: a report from the Center for International Blood and Marrow Transplant Research. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation 21:266–274, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Antin JH, Kim HT, Cutler C, et al. : Sirolimus, tacrolimus, and low-dose methotrexate for graft-versus-host disease prophylaxis in mismatched related donor or unrelated donor transplantation. Blood 102:1601–1605, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Nash RA, Antin JH, Karanes C, et al. : Phase 3 study comparing methotrexate and tacrolimus with methotrexate and cyclosporine for prophylaxis of acute graft-versus-host disease after marrow transplantation from unrelated donors. Blood 96:2062–8, 2000 [PubMed] [Google Scholar]
- 6.Ratanatharathorn V, Nash RA, Przepiorka D, et al. : Phase III study comparing methotrexate and tacrolimus (prograf, FK506) with methotrexate and cyclosporine for graft-versus-host disease prophylaxis after HLA-identical sibling bone marrow transplantation. Blood 92:2303–14, 1998 [PubMed] [Google Scholar]
- 7.Cutler CS, Koreth J, Ritz J: Mechanistic approaches for the prevention and treatment of chronic GVHD. Blood 129:22–29, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koreth J, Stevenson KE, Kim HT, et al. : Bortezomib, tacrolimus, and methotrexate for prophylaxis of graft-versus-host disease after reduced-intensity conditioning allogeneic stem cell transplantation from HLA-mismatched unrelated donors. Blood 114:3956–3959, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hideshima T, Richardson P, Chauhan D, et al. : The proteasome inhibitor PS-341 inhibits growth, induces apoptosis, and overcomes drug resistance in human multiple myeloma cells. Cancer Res 61:3071–6, 2001 [PubMed] [Google Scholar]
- 10.Herrera AF, Kim HT, Bindra B, et al. : A Phase II Study of Bortezomib Plus Prednisone for Initial Therapy of Chronic Graft-versus-Host Disease. Biology of Blood and Marrow Transplantation 20:1737–1743, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koreth J, Kim HT, Lange PB, et al. : Bortezomib-based immunosuppression after reduced-intensity conditioning hematopoietic stem cell transplantation: randomized phase II results. Haematologica 103:522–530, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bolaños-Meade J, Reshef R, Fraser R, et al. : Three prophylaxis regimens (tacrolimus, mycophenolate mofetil, and cyclophosphamide; tacrolimus, methotrexate, and bortezomib; or tacrolimus, methotrexate, and maraviroc) versus tacrolimus and methotrexate for prevention of graft-versus-host disease with haemopoietic cell transplantation with reduced-intensity conditioning: a randomised phase 2 trial with a non-randomised contemporaneous control group (BMT CTN 1203). The Lancet Haematology 6:e132–e143, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koreth J, Stevenson KE, Kim HT, et al. : Bortezomib-Based Graft-Versus-Host Disease Prophylaxis in HLA-Mismatched Unrelated Donor Transplantation. Journal of Clinical Oncology 30:3202–3208, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun K, Welniak LA, Panoskaltsis-Mortari A, et al. : Inhibition of acute graft - versus-host disease with retention of graft-versus-tumor effects by the proteasome inhibitor bortezomib. Proceedings of the National Academy of Sciences of the United States of America 101:8120–8125, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dai Y, Rahmani M, Grant S: Proteasome inhibitors potentiate leukemic cell apoptosis induced by the cyclin-dependent kinase inhibitor flavopiridol through a SAPK/JNK- and NF-kappaB-dependent process. Oncogene 22:7108–22, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Finn PW, Stone JR, Boothby MR, et al. : Inhibition of NF-κB-Dependent T Cell Activation Abrogates Acute Allograft Rejection. The Journal of Immunology 167:5994–6001, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Li Q, Verma IM: NF-kappaB regulation in the immune system. Nat Rev Immunol 2:725–34, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Sunwoo JB, Chen Z, Dong G, et al. : Novel proteasome inhibitor PS-341 inhibits activation of nuclear factor-kappa B, cell survival, tumor growth, and angiogenesis in squamous cell carcinoma. Clin Cancer Res 7:1419–28, 2001 [PubMed] [Google Scholar]
- 19.Wang X, Luo H, Chen H, et al. : Role of proteasomes in T cell activation and proliferation. J Immunol 160:788–801, 1998 [PubMed] [Google Scholar]
- 20.Blanco Bn, Pérez-Simón JA, Sénchez-Abarca LI, et al. : Bortezomib induces selective depletion of alloreactive T lymphocytes and decreases the production of Th1 cytokines. Blood 107:3575–3583, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Nencioni A, Schwarzenberg K, Brauer KM, et al. : Proteasome inhibitor bortezomib modulates TLR4-induced dendritic cell activation. Blood 108:551–558, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Pai C-CS, Hsiao H-H, Sun K, et al. : Therapeutic Benefit of Bortezomib on Acute Graft-versus-Host Disease Is Tissue Specific and Is Associated with Interleukin-6 Levels. Biology of Blood and Marrow Transplantation 20:1899–1904, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Allen JL, Fore MS, Wooten J, et al. : B cells from patients with chronic GVHD are activated and primed for survival via BAFF-mediated pathways. Blood 120:2529–2536, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mulder A, Heidt S, Vergunst M, et al. : Proteasome inhibition profoundly affects activated human B cells. Transplantation 95:1331–7, 2013 [DOI] [PubMed] [Google Scholar]
- 25.Neubert K, Meister S, Moser K, et al. : The proteasome inhibitor bortezomib depletes plasma cells and protects mice with lupus-like disease from nephritis. Nature Medicine 14:748–755, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Srinivasan M, Flynn R, Price A, et al. : Donor B-cell alloantibody deposition and germinal center formation are required for the development of murine chronic GVHD and bronchiolitis obliterans. Blood 119:1570–1580, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Young JS, Wu T, Chen Y, et al. : Donor B Cells in Transplants Augment Clonal Expansion and Survival of Pathogenic CD4<sup>+</sup> T Cells That Mediate Autoimmune-like Chronic Graft-versus-Host Disease. The Journal of Immunology 189:222–233, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moreau P, Masszi T, Grzasko N, et al. : Oral Ixazomib, Lenalidomide, and Dexamethasone for Multiple Myeloma. New England Journal of Medicine 374:1621–1634, 2016 [DOI] [PubMed] [Google Scholar]
- 29.Chhabra S: Novel Proteasome Inhibitors and Histone Deacetylase Inhibitors: Progress in Myeloma Therapeutics. Pharmaceuticals (Basel) 10, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kupperman E, Lee EC, Cao Y, et al. : Evaluation of the Proteasome Inhibitor MLN9708 in Preclinical Models of Human Cancer. Cancer Research 70:1970–1980, 2010 [DOI] [PubMed] [Google Scholar]
- 31.Przepiorka D, Weisdorf D, Martin P, et al. : 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant 15:825–8, 1995 [PubMed] [Google Scholar]
- 32.Jagasia MH, Greinix HT, Arora M, et al. : National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group Report. Biology of Blood and Marrow Transplantation 21:389–401. e1, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gooley TA, Leisenring W, Crowley J, et al. : Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Statistics in Medicine 18:695–706, 1999 [DOI] [PubMed] [Google Scholar]
- 34.Eapen M, Logan BR, Confer DL, et al. : Peripheral blood grafts from unrelated donors are associated with increased acute and chronic graft-versus-host disease without improved survival. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation 13:1461–1468, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fraser CJ, Bhatia S, Ness K, et al. : Impact of chronic graft-versus-host disease on the health status of hematopoietic cell transplantation survivors: a report from the Bone Marrow Transplant Survivor Study. Blood 108:2867–2873, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee SJ, Vogelsang G, Flowers MED: Chronic graft-versus-host disease. Biology of Blood and Marrow Transplantation 9:215–233, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Pidala J, Kurland B, Chai X, et al. : Patient-reported quality of life is associated with severity of chronic graft-versus-host disease as measured by NIH criteria: report on baseline data from the Chronic GVHD Consortium. Blood 117:4651–4657, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arai S, Jagasia M, Storer B, et al. : Global and organ-specific chronic graft-versus-host disease severity according to the 2005 NIH Consensus Criteria. Blood 118:4242–4249, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee SJ, Klein JP, Barrett AJ, et al. : Severity of chronic graft-versus-host disease: association with treatment-related mortality and relapse. Blood 100:406–14, 2002 [DOI] [PubMed] [Google Scholar]
- 40.Kansu E, Gooley T, Flowers MED, et al. : Administration of cyclosporine for 24 months compared with 6 months for prevention of chronic graft-versus-host disease: a prospective randomized clinical trial. Blood 98:3868–3870, 2001 [DOI] [PubMed] [Google Scholar]
- 41.Mengarelli A, Iori AP, Romano A, et al. : One-year cyclosporine prophylaxis reduces the risk of developing extensive chronic graft-versus-host disease after allogeneic peripheral blood stem cell transplantation. Haematologica 88:315–23, 2003 [PubMed] [Google Scholar]
- 42.Bacigalupo A, Lamparelli T, Barisione G, et al. : Thymoglobulin Prevents Chronic Graft-versus-Host Disease, Chronic Lung Dysfunction, and Late Transplant-Related Mortality: Long-Term Follow-Up of a Randomized Trial in Patients Undergoing Unrelated Donor Transplantation. Biology of Blood and Marrow Transplantation 12:560–565, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Barge RMY, Starrenburg CWJ, Falkenburg JHF, et al. : Long-term follow-up of myeloablative allogeneic stem cell transplantation using Campath ‘in the bag’ as T-cell depletion: the Leiden experience. Bone Marrow Transplantation 37:1129–1134, 2006 [DOI] [PubMed] [Google Scholar]
- 44.Finke J, Bethge WA, Schmoor C, et al. : Standard graft-versus-host disease prophylaxis with or without anti-T-cell globulin in haematopoietic cell transplantation from matched unrelated donors: a randomised, open-label, multicentre phase 3 trial. The Lancet Oncology 10:855–864, 2009 [DOI] [PubMed] [Google Scholar]
- 45.Soiffer RJ, Weller E, Alyea EP, et al. : CD6+ Donor Marrow T-Cell Depletion as the Sole Form of Graft-Versus-Host Disease Prophylaxis in Patients Undergoing Allogeneic Bone Marrow Transplant From Unrelated Donors. Journal of Clinical Oncology 19:1152–1159, 2001 [DOI] [PubMed] [Google Scholar]
- 46.Kanate AS, Mussetti A, Kharfan-Dabaja MA, et al. : Reduced-intensity transplantation for lymphomas using haploidentical related donors vs HLA-matched unrelated donors. Blood 127:938–47, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Soiffer RJ, LeRademacher J, Ho V, et al. : Impact of immune modulation with anti–T-cell antibodies on the outcome of reduced-intensity allogeneic hematopoietic stem cell transplantation for hematologic malignancies. Blood 117:6963–6970, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chhabra S, Liu Y, Hemmer MT, et al. : Comparative Analysis of Calcineurin Inhibitor–Based Methotrexate and Mycophenolate Mofetil–Containing Regimens for Prevention of Graft-versus-Host Disease after Reduced-Intensity Conditioning Allogeneic Transplantation. Biology of Blood and Marrow Transplantation 25:73–85, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Arai S, Sahaf B, Narasimhan B, et al. : Prophylactic rituximab after allogeneic transplantation decreases B-cell alloimmunity with low chronic GVHD incidence. Blood 119:6145–6154, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cutler C, Kim HT, Bindra B, et al. : Rituximab prophylaxis prevents corticosteroid-requiring chronic GVHD after allogeneic peripheral blood stem cell transplantation: results of a phase 2 trial. Blood 122:1510–1517, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Luznik L, O’Donnell PV, Symons HJ, et al. : HLA-Haploidentical Bone Marrow Transplantation for Hematologic Malignancies Using Nonmyeloablative Conditioning and High-Dose, Posttransplantation Cyclophosphamide. Biology of Blood and Marrow Transplantation 14:641–650, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.De Jong CN, Meijer E, Bakunina K, et al. : Post-Transplantation Cyclophosphamide after Allogeneic Hematopoietic Stem Cell Transplantation: Results of the Prospective Randomized HOVON-96 Trial in Recipients of Matched Related and Unrelated Donors. Blood 134:1–1, 2019. 31273001 [Google Scholar]
- 53.Koreth J, Kim HT, Lange PB, et al. : A Bortezomib-Based Regimen Offers Promising Survival and Graft-versus-Host Disease Prophylaxis in Myeloablative HLA-Mismatched and Unrelated Donor Transplantation: A Phase II Trial. Biology of Blood and Marrow Transplantation 21:1907–1913, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pidala J, Jaglowski S, Im A, et al. : Carfilzomib for Treatment of Refractory Chronic Graft-versus-Host Disease: A Chronic GVHD Consortium Pilot Phase II Trial. Biology of Blood and Marrow Transplantation 26:278–284, 2020 [DOI] [PubMed] [Google Scholar]
- 55.Gupta N, Diderichsen PM, Hanley MJ, et al. : Population Pharmacokinetic Analysis of Ixazomib, an Oral Proteasome Inhibitor, Including Data from the Phase III TOURMALINE-MM1 Study to Inform Labelling. Clinical Pharmacokinetics 56:1355–1368, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Blazar BR, Murphy WJ, Abedi M: Advances in graft-versus-host disease biology and therapy. Nature Reviews Immunology 12:443–458, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shimabukuro-Vornhagen A, Hallek MJ, Storb RF, et al. : The role of B cells in the pathogenesis of graft-versus-host disease. Blood 114:4919–4927, 2009 [DOI] [PubMed] [Google Scholar]
- 58.Vodanovic-Jankovic S, Hari P, Jacobs P, et al. : NF-κB as a target for the prevention of graft-versus-host disease: comparative efficacy of bortezomib and PS-1145. Blood 107:827–834, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pidala J, Bhatt VR, Hamilton B, et al. : Ixazomib for treatment of refractory chronic graft vs. host disease: A Chronic GVHD Consortium phase II trial: Ixazomib for chronic GVHD treatment. Biol Blood Marrow Transplant, 2020 [DOI] [PubMed] [Google Scholar]
- 60.Cutler C, Miklos D, Kim HT, et al. : Rituximab for steroid-refractory chronic graft-versus-host disease. Blood 108:756–62, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ratanatharathorn V, Ayash L, Reynolds C, et al. : Treatment of chronic graft-versus-host disease with anti-CD20 chimeric monoclonal antibody. Biol Blood Marrow Transplant 9:505–11, 2003 [DOI] [PubMed] [Google Scholar]
- 62.Hakim FT, Rehman N, Dickinson J, et al. : Elevated BAFF Is Correlated with Inflammatory Processes in Chronic Graft Versus Host Disease and Supports Increases in Transitional B Cells. Blood 112:465–465, 2008 [Google Scholar]
- 63.Sarantopoulos S, Stevenson KE, Kim HT, et al. : Altered B-cell homeostasis and excess BAFF in human chronic graft-versus-host disease. Blood 113:3865–3874, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


