Abstract
Major surgery due to traumatic injury can activate early systemic postoperative pro-inflammatory responses and postoperative immunosuppression. However, the interaction between them is complex and not entirely clear. This study was performed in postoperative patients admitted to the intensive care unit (ICU) to elucidate the correlation between the systemic cellular immunity function and circulating cytokines levels in the early postoperative period.
Twenty-four cases of postoperative patients admitted to the ICU were enrolled in this study. Twelve hours after admission, blood routine examination and measurement of circulating cytokines (interleukin-2 [IL-2], IL-4, IL-6, IL-10, IL-17A, interferon-γ, tumor necrosis factor-alpha [TNF-α], TNF-β, granulocyte-colony stimulating factor [G-CSF], and granulocyte-macrophage colony-stimulating factor [GM-CSF]) were performed. The correlation analysis between cytokines levels and absolute peripheral blood lymphocyte count or lymphocytes/neutrophils ratio was analyzed.
The cytokines (IL-2, IL-4, IL-6, IL-10, IL-17A, TNF-α, G-CSF, and GM-CSF) levels were increased above the normal upper limit at 12 hours after surgery. The number of leukocytes and neutrophils were markedly increased. In contrast, the absolute count and relative ratio of lymphocytes decreased below the lower normal limit. Spearman correlation analysis showed a moderate negative correlation between absolute peripheral blood lymphocyte count and IL-2 or IL-4 level. A low-negative correlation between absolute peripheral blood lymphocyte count and GM-CSF levels was detected. We also found that lymphocytes/neutrophils ratio was also negatively correlated with plasma IL-2, IL-4, or GM-CSF level.
In ICU patients with compromised immune function in the early postoperative period, the elevated levels of IL-2, IL-4, and GM-CSF may be the compensatory responses to systemic immunosuppression.
Keywords: cytokines, intensive care, lymphocytes, postoperative immune suppression
1. Introduction
Surgical trauma due to major surgery can activate early systemic pro-inflammatory responses, characterized by excessive production of proinflammatory cytokines, neutrophil activation and microvascular adherence, microcirculatory disturbance, increased vascular permeability, and cell-mediated immune dysfunction.[1–3] The proinflammatory response could induce a systemic anti-inflammatory response that in turn results in surgical trauma-induced immunosuppression, which makes individuals susceptible to postoperative infections.[3–6] The severity of the surgical trauma is shown to be positively associated with the degree of postoperative immunosuppression and the duration of immunosuppression.[7,8] Evidences have demonstrated that the absolute count of peripheral blood lymphocytes can serve as a biomarker for immunosuppression.[9,10] Postoperative lymphopenia could reach a nadir from 2 hours to 2 days after surgery and is associated with compromised immune function and increased risk of postoperative pneumonia.[9] However, the interaction between postoperative proinflammatory responses and postoperative immunosuppression is complex, and the correlation between the absolute peripheral blood lymphocyte count and cytokines levels in patients with early postoperative immune dysfunction remains unclear.
In this study, we collected blood samples from patients who had undergone elective surgery at 12 hours after surgery to measure the circulating cytokines levels, and the absolute count and percentage of blood lymphocytes. We further analyzed the correlation between cytokines levels and absolute peripheral blood lymphocyte count or lymphocytes/neutrophils ratio. This study could help us understand the relationship between postoperative proinflammatory responses and postoperative immunosuppression, and could provide a theoretical basis for immunomodulatory therapy for postoperative immunosuppression.
2. Methods
2.1. Patients
Postoperative patients admitted to the comprehensive intensive care unit (ICU) were enrolled between June 2018 and January 2019 in Union Hospital, Tongji Medical College, Huazhong University of Science and Technology. Eligibility criteria for inclusion were described as follows: patients aged between 18 and 80 years; patients transferred directly to ICU after surgery; the absolute counts of peripheral blood lymphocytes <1.1 × 109 cells/L at 12 hours after surgery. The lower limit of normal for the absolute peripheral blood lymphocyte count is 1.1 × 109 cells/L, measured in the laboratory of the Wuhan Union Hospital. Patients with chronic renal insufficiency treated with intermittent dialysis, patients with acute renal failure or pregnant, and lactating women were excluded. A total of 24 patients met these criteria and were included (Fig. 1). The protocol and study procedures were approved by the Medical Ethics Committee, Tongji Medical College, Huazhong University of Science and Technology (#2018/S255). Before the study, written informed consent was obtained from each subject.
Figure 1.
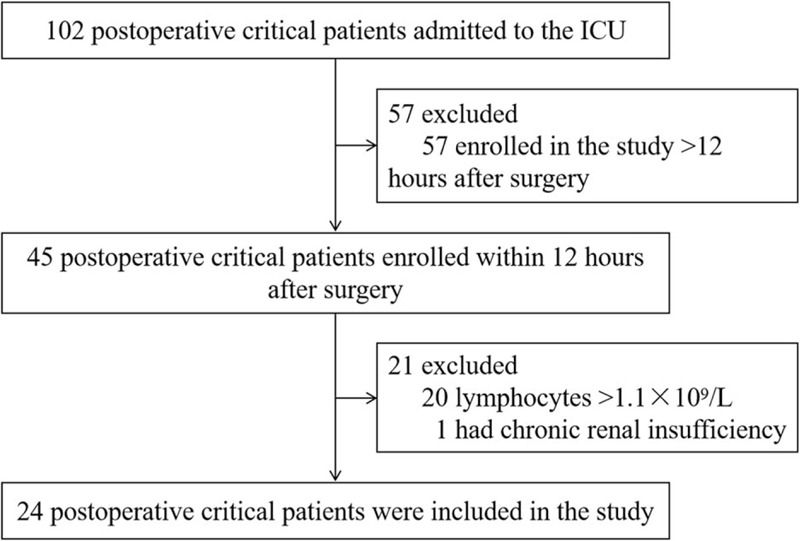
Study flow diagram. ICU = intensive care unit.
2.2. Sample collection and processing
Venous blood samples were collected from the patients at 12 hours after surgery for blood routine examination and measurement of plasma cytokines. All samples were processed within the first 4 hours after collection.
2.3. Blood routine examination
Venous blood samples were sent to Clinical Laboratory Department of Union Hospital for leukocyte counts, neutrophil count and percentage, lymphocyte count and percentage, monocyte count and percentage, and platelet counts detection using standard biochemical techniques.
2.4. Cytokine Assays
Plasma samples were analyzed using AimPlex assay kits (QuantoBio, Beijing, China) according to manufacturer instruction to measure the plasma levels of cytokines, including interleukin-2 (IL-2), IL-4, IL-6, IL-10, IL-17A, interferon-γ (IFN-γ), tumor necrosis factor-alpha (TNF-α), TNF-β, granulocyte-colony stimulating factor (G-CSF), and granulocyte-macrophage colony stimulating factor (GM-CSF).
2.5. Statistical analysis
The data collected were analyzed using SPSS version 20.0 software (SPSS, Tokyo, Japan). Data were expressed according to their scaling as arithmetic mean ± standard deviation (SD), median (25%, 75% quartiles), or frequencies (%). Spearman rank correlation test was used to assess the relationship between the absolute peripheral blood lymphocyte count and cytokines levels. P < .05 was considered statistically significant.
3. Results
3.1. Study population
During June 2018 and January 2019, 102 postoperative critical patients were admitted to ICU, of whom 78 patients were considered ineligible, including 57 patients who were enrolled in this study >12 hours after surgery, 20 patients whose absolute peripheral blood lymphocyte count >1.1 × 109 cells/L, and 1 patient who had chronic renal insufficiency treated with intermittent dialysis. Twenty-four patients met these criteria and were included (Fig. 1).
Basic patient characteristics, the types of surgery, and the reasons for ICU admission are summarized in Table 1.
Table 1.
Patient characteristics.

3.2. Leukocyte count and subtypes and plasma cytokine levels
The plasma levels of IL-2, IL-4, IL-6, IL-10, IL-17A, TNF-α, G-CSF, and GM-CSF were all increased above the normal upper limit at 12 hours after surgery (Table 2). The levels of IL-2 and IL-6 were markedly increased >10 times, and the levels of IL-17A and G-CSF were increased >4 times upper normal limit (Table 2).
Table 2.
Plasma levels of cytokines at 12 hours after surgery.

The absolute count of leukocytes and neutrophils were markedly increased above the normal upper limit at 12 hours after surgery (Table 3). In contrast, the count and percentage of lymphocytes were decreased below the lower normal limit (Table 3). However, the absolute count of monocytes and platelets showed no changes (Table 3).
Table 3.
Blood routine parameters at 12 hours after surgery.
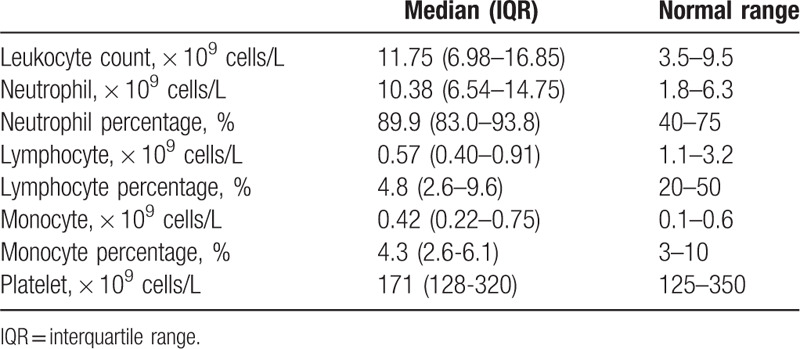
3.3. The correlation between plasma cytokines and lymphocytes
The correlation between plasma cytokines and the absolute peripheral blood lymphocyte count in patients at 12 hours after surgery was shown in Table 4. The analysis of spearman rank correlation showed that IL-2, IL-4, and GM-CSF were negatively correlated with the absolute peripheral blood lymphocyte count. We found a moderate negative correlation between absolute peripheral blood lymphocyte count and the levels of IL-2 (r = −0.718, P < .001) or IL-4 (r = −0.617, P = .001). A low negative correlation between absolute peripheral blood lymphocyte count and GM-CSF levels was detected (r = −0.414, P = .019). However, we found a nonsignificant correlation between absolute peripheral blood lymphocyte count and the levels of other cytokines, including IL-6 (r = 0.263, P = .214), IL-10 (r = −0.397, P = .055), IL-17A (r = 0.192, P = .368), TNF-α (r = −0.313, P = .137), or G-CSF (r = −0.303, P = .150).
Table 4.
Correlation between plasma cytokines and absolute peripheral blood lymphocyte count at 12 hours after surgery.
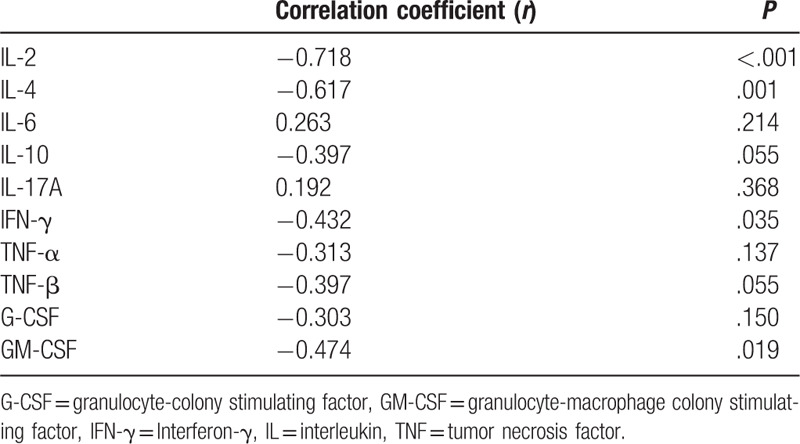
3.4. The correlation between plasma cytokines and lymphocytes/neutrophils ratio
Table 5 showed the correlation between plasma cytokines and lymphocytes/neutrophils ratio in patients at 12 hours after surgery. We found that lymphocytes/neutrophils ratio was negatively correlated with plasma IL-2 (r = −0.499, P = .013), IL-4 (r = −0.554, P = .005), IL-10 (r = −0.538, P = .007), or GM-CSF (r = −0.439, P = .032) level.
Table 5.
Correlation between plasma cytokines and leukocytes/neutrophils ratio at 12 hours after surgery.
4. Discussion
Our present study confirmed that postoperative patients admitted to the ICU in the early postoperative period suffered from both systemic proinflammatory responses and immunosuppression. IL-2, IL-4, and GM-CSF were negatively correlated with the absolute peripheral blood lymphocyte count. The plasma IL-2, IL-4, or GM-CSF level was also negatively correlated with lymphocytes/neutrophils ratio.
Studies have reported that neutrophils, a first line of defense in innate immunity, increase in number after surgery.[2,3] Consistently, our data showed that the absolute neutrophil count was markedly increased above the normal upper limit in the early postoperative period. The absolute peripheral blood lymphocyte count can serve as a biomarker for immunosuppression.[9,10] In our present study, the absolute peripheral blood lymphocyte count was decreased below the lower normal limit, indicating the existence of postoperative immunosuppression in the very early postoperative period.
Postoperative immunosuppression, particularly a loss of cell-mediated immunity, is common and is associated with a variety of complications, such as delayed wound healing, infection, sepsis, cancer recurrence and multiple organ dysfunction.[3–6,11,12] Interestingly, among the cytokines with elevated plasma levels, IL-2, IL-4, and GM-CSF were negatively correlated with the absolute peripheral blood lymphocyte count or lymphocytes/neutrophils ratio.
IL-2, also known as “T cell growth factor,” is produced primarily by activated CD4 + T cells following exposure to antigen. IL-2 could orchestrate the differentiation of T helper (Th) 1 cells and the production of type 1 cytokines like interferon-gamma (IFN-γ).[13–17] IL-2 is also shown to be able to activate macrophages, induce the differentiation of various killer cells (cytotoxic T cells and natural killer [NK] cells), and enhance the ability of the body to clear infection.[18] Recombinant human IL-2 protein has been approved for the treatment of kidney cancer and melanoma as early as 1992 and 1998, respectively.[19] IL-4 can promote the polarization of antigen-stimulated naive Th cells into Th2 cells and propagates Th2 responses as opposed to Th1 responses, which plays an important role in the secretion of Th2 cytokines such as IL-4 itself and IL-10 and thus suppresses the proinflammatory milieu.[20] Hyperinflammation is negatively associated with systemic immune function.[21,22] Therefore, the increase in IL-4 level after surgery might exert an important role in the recovery of immunosuppression through downregulating hyperinflammation. Similar to the immunomodulatory effects of IL-2, IL-4 can enhance the release of GM-CSF or G-CSF from T-lymphocytes, and contribute to the proliferation and differentiation of hematopoietic progenitors directly and indirectly.[23] In addition, IL-4/IL-13 signaling could direct early thymic progenitors to mature toward dendritic cells (DCs).[24] GM-CSF, an important hematopoietic growth factor and immune modulator, can stimulate the proliferation and differentiation of myeloid stem cells into granulocytes and macrophages, and GM-CSF is also critical for the development and maturation of DCs.[25,26] Recombinant GM-CSF protein has been widely used in the treatment of clinical diseases, and plays an important role in the immune regulation of various diseases such as sepsis, autoimmune diseases and tumors.[27–29] IL-4 combined with GM-CSF can inhibit the function of myeloid derived suppressor cells (MDSCs) by shifting them into mature DCs.[30] DCs, the most potent antigen presenting cells in the peripheral immune system, can trigger the activation of T lymphocytes and play an essential role in T cell-dependent adaptive immune response against infectious agents, such as virus.[31–34] GM-CSF and IL-4 can induce the differentiation of DCs.[35] Human IL-4/GM-CSF could stimulate induction of CD209+ DCs and effective CD4+ T cell priming in humanized mice.[36] Monocyte-derived dendritic cells (Mo-DCs) can promote Th1 and CD8+ T cell responses.[37] Monocyte-derived DCs induced by IL-4/GM-CSF from patients with rheumatoid arthritis could increase the differentiation of Th17 cells through releasing high levels of IL-6 and IL-23.[38] In our present study, IL-2, IL-4, and GM-CSF were negatively correlated with the absolute peripheral blood lymphocyte count or lymphocytes/neutrophils ratio. The increase in the plasma IL-2, IL-4, and GM-CSF may be the compensatory response to the increased cytokines-induced immunosuppression. Previous study found that during severe postoperative immunosuppression, postoperative alterations of innate immune cells could recover on day 2 to 5 after surgery; however, the alterations of acquired immune cells (lymphocytes, T cells, Th cells, and cytotoxic T cells) recover later than innate immunity.[39] We speculate that the inadequate compensatory response of increase in IL-2, IL-4, and GM-CSF in the early postoperative period may contribute to the sustained immunosuppression. Elevation of IL-2, IL-4, and GM-CSF by their respective recombinant proteins in the early postoperative period may be a good therapeutic strategy for the treatment of sustained immunosuppression.
There were some notable limitations in our study. First, it was a single-center study with a small sample size. Future prospective studies with a large sample size are needed to validate the results. Second, the correlation between blood cytokines and lymphocytes in patients before major surgery was not studied.
In the future, we will furtherly investigate the correlation between absolute peripheral blood lymphocyte count and plasma cytokines (IL-2, IL-4, and GM-CSF) in severe postoperative immunosuppression or severe sepsis-induced immunosuppression to confirm the important roles of adequate compensatory elevation of IL-2, IL-4, and GM-CSF in the recovery of immunosuppression.
5. Conclusion
In the early postoperative period, the absolute count and relative ratio of lymphocytes decreased below the lower normal limit. Plasma cytokines (IL-2, IL-4, and GM-CSF) were negatively correlated with the absolute peripheral blood lymphocyte count or lymphocytes/neutrophils ratio, indicating a possible compensatory response to the increased cytokines-induced immunosuppression.
Author contributions
Conceptualization: Shiying Yuan, Jiancheng Zhang.
Data curation: Jiancheng Zhang, Xiaoyan Chen.
Formal analysis: Jiancheng Zhang.
Investigation: Jiancheng Zhang, Xiaoyan Chen.
Methodology: Jiancheng Zhang.
Project administration: Jiancheng Zhang, Xiaoyan Chen.
Resources: Jiancheng Zhang.
Software: Yujing Zhang.
Supervision: Shiying Yuan.
Writing – original draft: Xiaoyan Chen.
Writing – review & editing: Jiancheng Zhang.
Footnotes
Abbreviations: DCs = dendritic cells, G-CSF = granulocyte-colony stimulating factor, GM-CSF = granulocyte-macrophage colony-stimulating factor, ICU = intensive care unit, IL = interleukin, TNF-α = tumor necrosis factor-α.
How to cite this article: Chen X, Yuan S, Zhang J. Correlation study between blood cytokines and lymphocytes in early postoperative critical patients with compromised immune function. Medicine. 2020;99:42(e22459).
The authors report no conflicts of interest.
This work was supported by a grant from the China International Medical Foundation: Special Research Fund for Middle-aged and Youth (Z-2018-35-1902).
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].Shavit Y, Fridel K, Beilin B. Postoperative pain management and proinflammatory cytokines: animal and human studies. J Neuroimmune Pharmacol 2006;1:443–51. [DOI] [PubMed] [Google Scholar]
- [2].Takenaka K, Ogawa E, Wada H, et al. Systemic inflammatory response syndrome and surgical stress in thoracic surgery. J Crit Care 2006;21:53–5. [DOI] [PubMed] [Google Scholar]
- [3].Menger MD, Vollmar B. Surgical trauma: hyperinflammation versus immunosuppression? Langenbecks Arch Surg 2004;389:475–84. [DOI] [PubMed] [Google Scholar]
- [4].Torrance H, Longbottom ER, Vivian ME, et al. Post-operative immune suppression is mediated via reversible. Interleukin-10 dependent pathways in circulating monocytes following major abdominal surgery. PLoS One 2018;13:e203795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bartal I, Melamed R, Greenfeld K, et al. Immune perturbations in patients along the perioperative period: alterations in cell surface markers and leukocyte subtypes before and after surgery. Brain Behav Immun 2010;24:376–86. [DOI] [PubMed] [Google Scholar]
- [6].Angele MK, Faist E. Clinical review: immunodepression in the surgical patient and increased susceptibility to infection. Crit Care 2002;6:298–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Hogan BV, Peter MB, Shenoy HG, et al. Surgery induced immunosuppression. Surgeon 2011;9:38–43. [DOI] [PubMed] [Google Scholar]
- [8].Shakhar G, Ben-Eliyahu S. Potential prophylactic measures against postoperative immunosuppression: could they reduce recurrence rates in oncological patients? Ann Surg Oncol 2003;10:972–92. [DOI] [PubMed] [Google Scholar]
- [9].Dupont G, Flory L, Morel J, et al. Postoperative lymphopenia: An independent risk factor for postoperative pneumonia after lung cancer surgery, results of a case-control study. PLoS One 2018;13:e205237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Drewry AM, Samra N, Skrupky LP, et al. Persistent lymphopenia after diagnosis of sepsis predicts mortality. Shock 2014;42:383–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Neeman E, Ben-Eliyahu S. Surgery and stress promote cancer metastasis: new outlooks on perioperative mediating mechanisms and immune involvement. Brain Behav Immun 2013;30 suppl:S32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Smith JW, Gamelli RL, Jones SB, et al. Immunologic responses to critical injury and sepsis. J Intensive Care Med 2006;21:160–72. [DOI] [PubMed] [Google Scholar]
- [13].Pol JG, Caudana P, Paillet J, et al. Effects of interleukin-2 in immunostimulation and immunosuppression. J Exp Med 2020;217:e20191247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hosseinian H, Mahnam K, Shakhsi-Niaei M. The c.305del3 in IL-2 gene in Homonoidea theoretically affects IL-2/IL-2Ralpha interaction as well as lymphocyte homeostasis. Cytokine 2018;108:232–8. [DOI] [PubMed] [Google Scholar]
- [15].Reem GH, Yeh NH. Interleukin 2 regulates expression of its receptor and synthesis of gamma interferon by human T lymphocytes. Science 1984;225:429–30. [DOI] [PubMed] [Google Scholar]
- [16].Shi M, Lin TH, Appell KC, et al. Janus-kinase-3-dependent signals induce chromatin remodeling at the Ifng locus during T helper 1 cell differentiation. Immunity 2008;28:763–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Liao W, Lin JX, Wang L, et al. Modulation of cytokine receptors by IL-2 broadly regulates differentiation into helper T cell lineages. Nat Immunol 2011;12:551–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Waldmann TA. The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nat Rev Immunol 2006;6:595–601. [DOI] [PubMed] [Google Scholar]
- [19].Alva A, Daniels GA, Wong MK, et al. Contemporary experience with high-dose interleukin-2 therapy and impact on survival in patients with metastatic melanoma and metastatic renal cell carcinoma. Cancer Immunol Immunother 2016;65:1533–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Nelms K, Keegan AD, Zamorano J, et al. The IL-4 receptor: signaling mechanisms and biologic functions. Annu Rev Immunol 1999;17:701–38. [DOI] [PubMed] [Google Scholar]
- [21].Giamarellos-Bourboulis EJ, Netea MG, Rovina N, et al. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe 2020;27: 992-1000.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol 2015;15:486–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Sonoda Y. Interleukin-4—a dual regulatory factor in hematopoiesis. Leuk Lymphoma 1994;14:231–40. [DOI] [PubMed] [Google Scholar]
- [24].Barik S, Cattin-Roy AN, Miller MM, et al. IL-4 and IL-13 guide early thymic progenitors to mature toward dendritic cells. J Immunol 2018;201:2947–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Shi Y, Liu CH, Roberts AI, et al. Granulocyte-macrophage colony-stimulating factor (GM-CSF) and T-cell responses: what we do and don’t know. Cell Res 2006;16:126–33. [DOI] [PubMed] [Google Scholar]
- [26].Burgess AW, Camakaris J, Metcalf D. Purification and properties of colony-stimulating factor from mouse lung-conditioned medium. J Biol Chem 1977;252:1998–2003. [PubMed] [Google Scholar]
- [27].Shiomi A, Usui T. Pivotal roles of GM-CSF in autoimmunity and inflammation. Mediators Inflamm 2015;2015:568543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Francisco-Cruz A, Aguilar-Santelises M, Ramos-Espinosa O, et al. Granulocyte-macrophage colony-stimulating factor: not just another haematopoietic growth factor. Med Oncol 2014;31:774. [DOI] [PubMed] [Google Scholar]
- [29].Meisel C, Schefold JC, Pschowski R, et al. Granulocyte-macrophage colony-stimulating factor to reverse sepsis-associated immunosuppression: a double-blind, randomized, placebo-controlled multicenter trial. Am J Respir Crit Care Med 2009;180:640–8. [DOI] [PubMed] [Google Scholar]
- [30].Bronte V, Chappell DB, Apolloni E, et al. Unopposed production of granulocyte-macrophage colony-stimulating factor by tumors inhibits CD8 + T cell responses by dysregulating antigen-presenting cell maturation. J Immunol 1999;162:5728–37. [PMC free article] [PubMed] [Google Scholar]
- [31].Marchingo JM, Kan A, Sutherland RM, et al. T cell signaling, antigen affinity, costimulation, and cytokine inputs sum linearly to amplify T cell expansion. Science 2014;346:1123–7. [DOI] [PubMed] [Google Scholar]
- [32].Shortman K, Liu YJ. Mouse and human dendritic cell subtypes. Nat Rev Immunol 2002;2:151–61. [DOI] [PubMed] [Google Scholar]
- [33].Lutz MB, Schuler G. Immature, semi-mature and fully mature dendritic cells: which signals induce tolerance or immunity? Trends Immunol 2002;23:445–9. [DOI] [PubMed] [Google Scholar]
- [34].Loving CL, Brockmeier SL, Sacco RE. Differential type I interferon activation and susceptibility of dendritic cell populations to porcine arterivirus. Immunology 2007;120:217–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Posch W, Lass-Florl C, Wilflingseder D. Generation of human monocyte-derived dendritic cells from whole blood. J Vis Exp 2016;e54968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Chen QF, Kwang J, He F, et al. GM-CSF and IL-4 stimulate antibody responses in humanized mice by promoting T, B, and dendritic cell maturation. J Immunol 2012;189:5223–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Briseno CG, Haldar M, Kretzer NM, et al. Distinct transcriptional programs control cross-priming in classical and monocyte-derived dendritic cells. Cell Rep 2016;15:2462–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Estrada-Capetillo L, Hernandez-Castro B, Monsivais-Urenda A, et al. Induction of Th17 lymphocytes and Treg cells by monocyte-derived dendritic cells in patients with rheumatoid arthritis and systemic lupus erythematosus. Clin Dev Immunol 2013;2013:584303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Lachmann G, von Haefen C, Kurth J, et al. Innate immunity recovers earlier than acquired immunity during severe postoperative immunosuppression. Int J Med Sci 2018;15:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


