Abstract
There are still no unified guidelines of surgical treatment and timing for human immunodeficiency virus (HIV)-negative patients with cryptococcal meningitis (CM).
The clinical data and follow-up data were collected from HIV-negative CM patients in Xiangya Hospital of Central South University from January 2009 to November 2018, and 42 patients who were treated with surgical intervention were enrolled in the present study. These 42 patients were divided into ventriculoatrial (VA) group, ventriculoperitoneal group, external ventricle drainage (EVD) group, hydrocephalus (HYC) group, non-HYC group, EVD group, and non-EVD group (VA/ ventriculoperitoneal) according to different surgical procedures. Statistical analyses were conducted using SPSS (version 19.0, Chicago, IL).
Signs of headache, fever, and loss of consciousness in the VA group were significantly improved compared with the EVD group at 1 week after operation (P < .05). The mortality rate of the VA group was significantly lower than that of the EVD group (P < .05). Moreover, male patients were more prone to have HYC (P < .05). Younger patients tended to develop HYC (P < .05). Cerebrospinal fluid sugar in the non-HYC group was significantly lower compared with the HYC group (P < .05). Time of CM-to-operation in the non-HYC group was markedly shorter compared with the HYC group (P < .01).
VA procedure could be one of the first choices for the treatment of uncontrollable intracranial hypertension caused by CM. Severe uncontrollable headache, loss of consciousness, and cerebral hernia were indications of emergency surgery. Repeated headache, hearing impairment, and especially progressive loss of vision were indications of early surgery to avoid permanent damage to nerve functions of HIV-negative CM patients.
Keywords: cryptococcal meningitis, hydrocephalus, intracranial hypertension, shunt
1. Introduction
As a known opportunistic pathogen, Cryptococcus neoformans is frequently detected in patients with human immunodeficiency virus (HIV) infection or other immunodeficiency diseases.[1,2] Cryptococcal meningitis (CM) can lead to higher rates of mortality and disability if not treated in a timely manner.[3] A recent study has documented that the CD4 expression is correlated with the prognosis of CM patients.[4] HIV is the most important risk factor for CM patients.[5–7] However, infection of Cryptococcus has also been reported in an HIV-negative CM patient recently.[8] It is now certain that uncontrollable intracranial hypertension and hydrocephalus (HYC) are the 2 major complications affecting the prognosis of CM patients.[9,10] The pathogenesis of HYC and uncontrollable intracranial hypertension remains largely unexplored in CM patients. Some reports have proposed the possible pathways as follows:
-
(1)
surface polysaccharides of Cryptococcus block cerebrospinal fluid (CSF) circulation pathway, or
-
(2)
inflammation blocks the CSF circulation pathway.[11]
Early diagnosis and timely treatment can effectively improve the prognosis of CM patients, such as visual and hearing impairment, severe headache, loss of consciousness, and even death. As a surgical intervention, ventriculoperitoneal (VP) operation can effectively relieve the intracerebral hypertension (ICH) in CM patients.[10,12] Recently, it has been reported that VP surgery has achieved positive results in the treatment of ICH and HYC in HIV-negative CM patients.[13] However, VP surgery has its unique procedure-related complications, such as CSF overdrainage, shunt infection, or shunt malfunction.[14] If there is a VP procedure-related complication in a CM patient with ICH and HYC, or due to individual reasons, including prior abdominal surgery, other operational procedures should be premeditated. Ventriculoatrial (VA) operation can be used as an alternative at this time. However, very few investigations have documented VA shunt in HIV-negative CM patients. In the treatment of CM, only few reports have studied the criteria, timing, and curative effect of VA operation. In the present study, we recorded clinical symptoms, CSF opening pressure and test results, choice of surgical methods, and imaging findings of 42 HIV-negative CM patients before and after operation, and a comprehensive statistical analysis was conducted. We aimed to assess the effects of different surgical treatment and operation time on HIV-negative CM patients.
2. Methods
2.1. Clinical data collection
Patients in the Xiangya Hospital of Central South University, Changsha, China, were enrolled in this retrospective study from January 2009 to November 2018. The clinical data of 212 HIV-negative CM patients were reviewed, while 170 cases without surgery were precluded from our current work. Finally, forty-two patients who met the diagnostic criteria were enrolled in our study. Figure 1 presents the details of the enrollment process.
Figure 1.
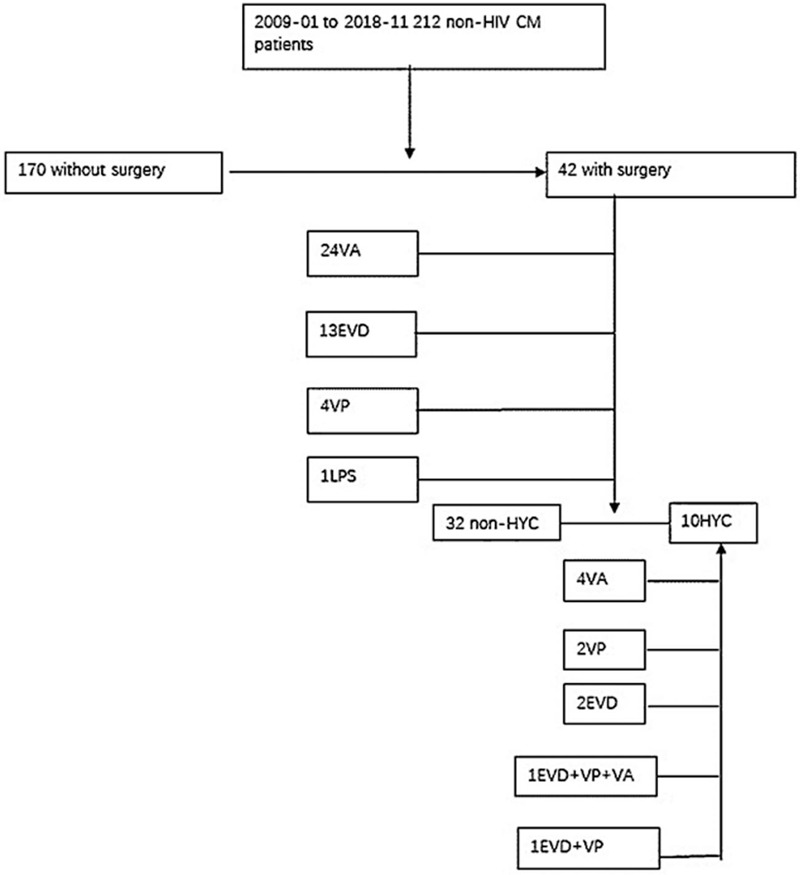
Enrolment process of patients. CM = cryptococcal meningitis, EVD = external ventricular drainage, HYC = hydrocephalus, LPS = lumboperitoneal shunt, non-HYC = non- hydrocephalus, VA = ventriculoatrial shunt, VP = ventriculoperitoneal shunt.
Brain computed tomography (CT) and/or magnetic resonance imaging (MRI) were conducted before and after surgery. The typical brain images of patients with or without HYC are shown in Figure 2. All images were examined by experienced neuroradiologists.
Figure 2.
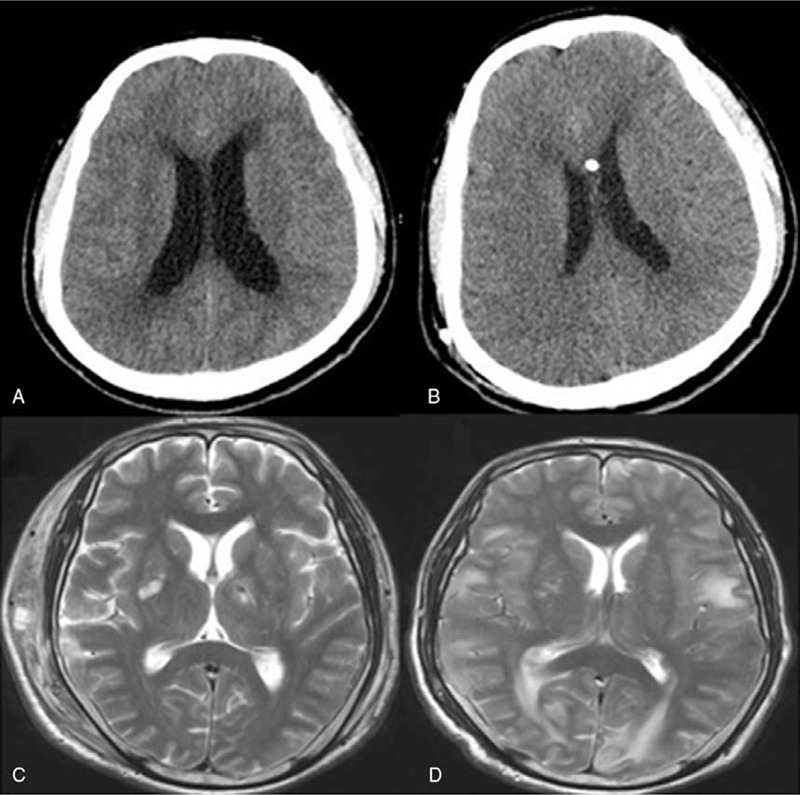
Head CT or MR of 2 patients. (A and B) Head CT of a HYC patient before and after operation. (C and D) Head MR of a non-HYC patient before and after operation. CT = computed tomography, HYC = hydrocephalus, MRI = magnetic resonance imaging.
2.2. Patients’ definitions
The inclusion criteria for CM patients included comprehensive evaluation of clinical symptoms, laboratory tests (including CSF stress, biochemical routine culture results, and CSF ink staining results), and imaging examinations. CT or MRI was used for the diagnosis of HYC, which was made based on enlargement of the temporal horn of the lateral ventricle if no obvious brain atrophy was observed during the entire therapeutic time window. The demographic data, risk factors, clinical symptoms before and after surgery, CSF characteristics, CT/MR findings, antifungal therapy, and outcomes are listed.
2.3. Laboratory measurement
Repeated lumbar puncture (LP) was performed in all patients, and CSF opening pressure, differential counts, glucose, protein, chloride, India ink smear, and CSF culture were recorded. The CSF sample was subjected to India ink test.
2.4. Therapeutic methods
All groups were administered with antifungal agents as previously described.[15] LP was conducted within 1 week of admission and before discharge from the hospital. After discharge from hospital, all patients were followed up for at least 4 months.
2.5. Statistical analysis
Statistical analyses were performed by SPSS (version 19.0, Chicago, IL). Data were represented as the mean ± standard deviation or median, and categorical variables were expressed as a percentage. P < .05 was considered as statistically significant. Variables of normal distribution were analyzed using Student t test, while categorical variables were assessed by Chi-square or Fisher exact test.
3. Results
3.1. Clinical characteristics of all patients
A total of 42 HIV-negative CM patients who underwent the VA or VP shunt or EVD or LPS procedure, were enrolled in this study. Twenty-four VA shunts, 4 VP shunts, 13 EVD, and 1 LPS were performed, including 10 cases with HYC and 32 cases without HYC. All patients received non-programmable shunts, the valves were set to the highest pressure, and the follow-up adjustment of shunt pressure was made according to patient's symptoms. The postoperative period could be divided into 2 sections as follows: 1 week after operation and phone follow-up at least 4 months after discharged from the hospital. There was only 1 patient who was given LPS. Therefore, such case was not statistically analyzed in the surgery group.
3.2. Surgery groups
3.2.1. Clinical characteristics
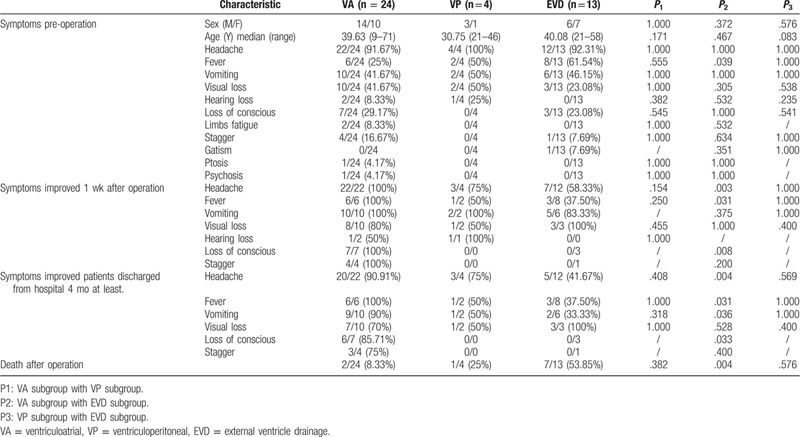
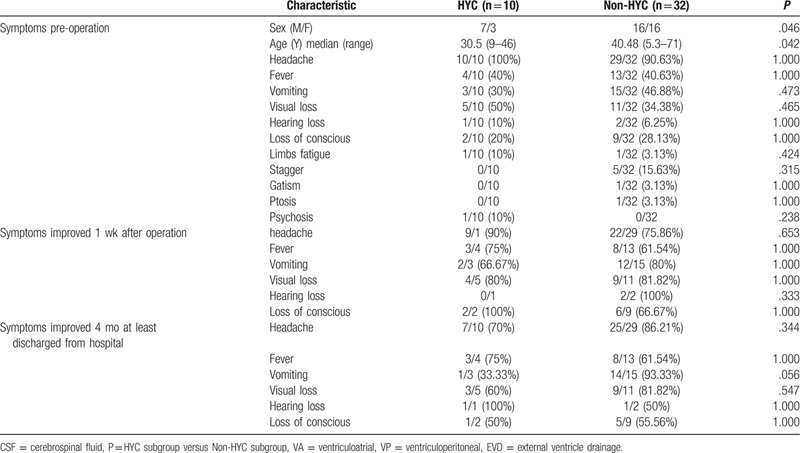
In the present study, all participants displayed some symptoms of neurological deficit before operation, such as headache, nausea, visual and hearing impairment, and loss of consciousness, among which fever, visual impairment, and loss of consciousness were the most frequently observed clinical symptoms (Table 1). At 1 week after operation, most of the symptoms disappeared, the symptoms of headache, fever and loss of consciousness in the VA group were significantly improved compared with the EVD group (P < .05). In our observation period, we made phone follow-up and tracked the CT or MRI after the patients were discharged from hospital for at least 4 months. The signs of headache, fever, vomiting, and loss of conscious were markedly improved in the VA group compared with the EVD group (P < .05). VA shunt could significantly alleviate symptoms caused by uncontrollable intracranial hypertension. Moreover, the mortality rate of the VA group was markedly lower than that of the EVD group (P < .05). Regrettably, 10 patients died due to different reasons after operation, such as irreversible visual impairment, respiratory and circulatory failure, loss of consciousness, and inability to afford the high cost of treatment.
Table 1.
Clinical characteristics in patients with VA, VP or EVD surgery.
3.2.2. CSF features
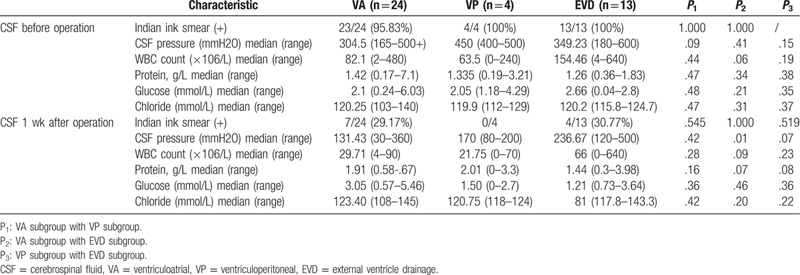
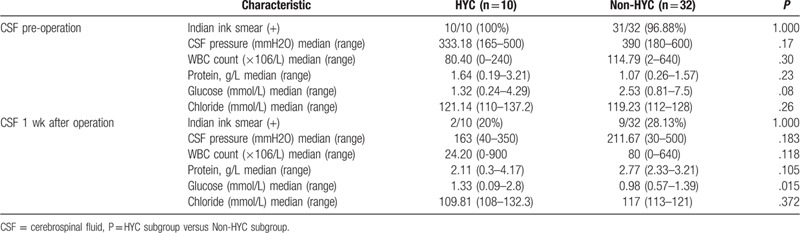
We found VA shunt could significantly decrease ICH (P < .05) (Table 2), we did not find significant differences in preoperative and postoperative data of CSF, which could possibly be attributed to the small sample size of enrolled patients in the group.
Table 2.
CSF characteristics in patients with VA, VP, or EVD surgery.
3.3. HYC group and non-HYC group
3.3.1. Clinical characteristics
Male patients were more prone to have HYC (P < .05). Younger patients tended to develop HYC compared with elders (P < .05). In addition, no significant difference in clinical symptoms was detected between the HYC group and non-HYC group preoperatively and postoperatively (Table 3).
Table 3.
Clinical characteristics in HYC or non-HYC patients.
3.3.2. CSF features
Before the operation, there was no statistical difference in LP's CSF assay between the HYC group and non-HYC group (P > .05). At 1-week post-surgery, the non-HYC group exhibited significantly lower CSF sugar level compared with the HYC group (P < .05). However, there were no statistical difference in the levels of CSF opening pressure, India ink smear, leukocyte count, protein, and chloride between the 2 groups (P > .05) (Table 4).
Table 4.
CSF characteristics in HYC or non-HYC patients.
3.4. Operation time and reasons
The time of CM-to-operation in the HYC group was markedly longer compared with the non-HYC group (P < .01), and there were no statistical differences in the time of HYC-to-operation and the time of ICH-to-operation (P > .05), while both median numbers of the non-HYC group were shorter than those of the HYC group. Emergency ratio of the non-HYC group (19/32, 59.38%) was lower than that of in the HYC group (9/10, 90%), although no statistical difference was found (Table 5).
Table 5.
Surgery time in HYC or non-HYC patients.

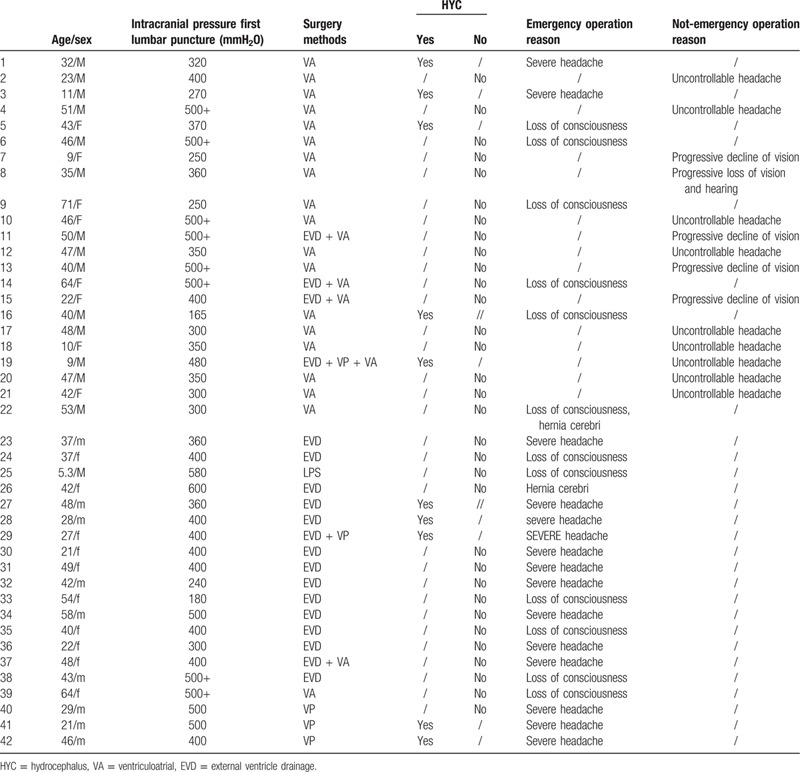
Retrospective analysis found that the main reasons of emergency surgery included severe headache, impaired consciousness, and the onset of cerebral hernia. Besides, although no emergency surgery was required for uncontrollable headache, progressive loss of hearing and vision, early surgery should be performed to rescue the neurological deficit of the patients (Table 6).
Table 6.
Different kind of surgery methods, reason to emergency operation or selective operation of patients.
3.5. Typical case
Case 17 was a 48-year-old man. He presented headache, nausea, visual impairment and staggering when he came to hospital. After he was diagnosed as CM, he was administered with antifungal agents, amphotericin B, and 5-fluorocytosine. Due to persistent headache, he was given VA shunt, after which most symptoms, except for vision loss, were relieved. The visual acuity of both eyes after operation was only light sense. When he returned home, he fell to death because of irreversible visual impairment.
4. Discussion
Cryptococcosis caused by the encapsulated yeasts, Cryptococcus neoformans, and C. gattii, is acquired from the environment, which is one of the most common opportunistic infections and reasons of morbidity and mortality in HIV-infected or immunosuppressed patients, especially in resource-limited settings, such as sub-Saharan Africa and other developing countries.[1,2,16,17] In recent years, CM has also been reported in a small number of patients without immunodeficiency disease.[18–23] Therefore, it is essential to further study the clinical characteristics and effective treatment, especially for intracranial hypertension, to reduce the morbidity and mortality rates of CM patients. In the present study, non-HIV CM patients were divided different surgery group, HYC, and non-HYC group. The clinical symptoms, CSF characteristics before and after operation, surgical procedures, operation time of each group were analyzed.
We found that the most common clinical symptoms included headache, nausea, fever, visual impairment, and loss of consciousness (Table 1). However, no significant difference in clinical symptoms was found between the patients with HYC and those without HYC (P > .05) (Table 3). In the surgery group, we found that VA shunt could markedly alleviate headache, fever, nausea, and loss of conscious caused by uncontrollable intracranial hypertension (P < .05). Nevertheless, there was no significant difference in postoperative symptoms between the VA group and VP group (P > .05). The efficacy of VP shunt in the treatment of intracranial hypertension in HIV-positive and HIV-negative CM patients has been widely recognized by neurosurgeons and neurophysicians worldwide.[10,13,24,25] We did not find significant differences in pre-operative and post-operative data between the VP group and EVD group, which could possibly be attributed to the small sample size of enrolled patients in the VP group.
The India ink test, microscopy, and culture methods are widely used for diagnosis of cryptococcal infection in many laboratories in Asian countries.[26–27] With the development of medical test methods, the positive rate of cryptococcal infection in our group was above 96.87%. Except that a few patients received antifungal therapy before positive results were available, most of the cases received standardized antifungal therapy after diagnosis of CM with amphotericin B + 5-fluorocytosine ± Fluc. Most patients underwent shunt operation during antifungal therapy. VP shunt is a classical surgery for HYC.[28] In recent years, VA operation is more and more used as an approach to treat HYC and intracranial hypertension. Compared with the VP shunt, VA operation can dramatically diminish complications, such as obstruction of the abdominal end of shunt tube, abdominal infection, ascites formation, and so on. VA shunts have specific complications, such as postoperative neck hematomas, revision in lower end of the tube for growing children, shunt nephritis, and migration of the distal segment.[29] No above-mentioned complications were found in all the patients who underwent VA shunt after operation. In our study, we think it is easier to control Cryptococcus with high concentration of blood drug and avoid the infection of VP in abdominal cavity. Besides, the distance of VA shunt is shorter than that of VP shunt, which may reduce the occurrence of fissured ventricular syndrome in ICP patients. Comparison of the VA group and VP group revealed that there were no significant differences in postoperative complications, clinical symptoms, and mortality, while the morbidity and mortality rates of the VA group were remarkably lower compared with the EVD group (P < .05) (Table 1). For patients with rapid increase of intracranial pressure in a short period of time, the symptoms worsen rapidly. The effect of shunt operation is more lasting, stable, effective, and safe than that of LP and EVD. The mortality of EVD patients is higher than that of shunt group (P < .05, Table 1), so we think VA/VP is more conducive to improve the prognosis of such critical patients. Therefore, our findings suggested that VA procedure was one of the first choices for the treatment of uncontrollable intracranial hypertension caused by CM. But more case studies are still needed to further verify.
In our current investigation, our data indicated that male patients were more prone to have HYC (P < .05), and younger patients tended to develop HYC compared with older patients (P < .05) (Table 3) This result is different from Liu's study.[13] Our case number is small, and there may be bias. We need further large samples to verify the correlation between gender, age, and HYC. More importantly, the time of CM-to-operation in the non-HYC group was shorter compared with the HYC group (P < .01) (Table 5), indicating that non-HYC HIV-negative CM patients tended to have shorter duration and earlier surgery, which was consistent with Liu's study.[13] Besides, in our research, there was no difficulty in puncture of ventricles in all non-HYC patients, and there was no fissured ventricular syndrome in these patients. Before further statistical analysis, we assumed that HYC was one of the emergency surgical indications for CM patients with intracranial hypertension. Final results revealed that the emergency ratio in the HYC group (9/10, 90%) was higher than that in non-HYC group (19/32, 59.38%), although there was no statistical significance (Table 5). The results indicated that HYC was not a predictor of rapid deterioration of CM. Moreover, the postoperative morbidity and mortality rates were not significantly different between the HYC group and non-HYC group (P > .05) (Table 3), suggesting that HYC was not associated with the prognosis of CM patients. The content of glucose in CSF of the HYC group was dramatically higher compared with the non-HYC group (P < .05) (Table 4), which was consistent with Xu's study.[18]
We found that severe uncontrollable headache, loss of consciousness, and the onset of cerebral hernia were the main reasons of emergency surgery. Besides, although no emergency surgery was required for uncontrollable headache, progressive loss of vision and hearing impairment, early surgery should be performed to rescue the neurological deficit of the patients (Table 6). At present, there is no unified guide to determine the specific operation timing of CM patients. In this study, we reviewed the efficacy of different operation modes and preliminarily explored the selection of operation timing. Besides, the heterogeneity of patients, such as different age and underlying diseases, may indeed lead to different immune responses and outcomes. This needs further study.
It could be an avoidable tragedy that case 17 fell to death because of permanent visual loss. Therefore, we suggested that severe uncontrollable headache, loss of consciousness, and cerebral hernia were indications of emergency surgery. Repeated headache, loss of hearing, and especially progressive visual impairment were indications of early surgery to avoid permanent damage to nerve functions.
5. Conclusions
In the present study, we found that VA procedure could be one of the first choices for the treatment of uncontrollable intracranial hypertension caused by CM. HYC was not associated with the prognosis of HIV-negative CM patients. HIV-negative non-HYC CM patients tended to have shorter duration, earlier surgery. CSF glucose might be related to higher mortality in HIV-negative CM patients, and low CSF glucose was an indication for early surgery. We suggested that severe uncontrollable headache, loss of consciousness and cerebral hernia were indications of emergency surgery. Repeated headache, loss of hearing, and especially progressive visual impairment were indications of early surgery to avoid permanent damage to nerve functions in HIV-negative CM patients.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 81571880, 81373147, 30901555, 30972870) and the Natural Science Foundation of Hunan Province (No. 2016JJ2157). All the funding bodies funded in the study design, collection, analysis, interpretation of data, and writing the manuscript.
Author contributions
Jie Zhao were responsible for the data integrity and the accuracy of the data analysis. Xiang Zhao analyzed the data. Jie Zhao, Ying Liu, and Xiang Zhao prepared the manuscript. Jie Zhao and Ying Liu revised the manuscript and the whole paper, including the figures and legends. All authors read and approved the final manuscript.
Footnotes
Abbreviations: CM = cryptococcal meningitis, CSF = cerebrospinal fluid, CT = computed tomography, EVD = external ventricle drainage, HIV = human immunodeficiency virus, HYC = hydrocephalus, ICH = intracerebral hypertension, LP = lumbar puncture, LPS = lumboperitoneal shunt, MRI = magnetic resonance imaging, VA shunt = ventriculoatrial shunt, VP shunt = ventriculoperitoneal shunt.
How to cite this article: Zhao J, Zhao X, Yang S, Miao S, Liu Y. Surgical treatment and operation time in human immunodeficiency virus-negative cryptococcal meningitis. Medicine. 2020;99:42(e22546).
JZ and XZ contribute equally to this work.
Most of the data in this study were clinical manifestations and CSF characteristics, and the data can be obtained from the corresponding author if necessary.
This research was approved by the ethics committee of the Xiangya Hospital of Central South University. All participants involved in this study provided written informed consent.
The authors report no conflicts of interest.
The datasets generated during and/or analyzed during the current study are publicly available.
References
- [1].Srichatrapimuk S, Sungkanuparph S. Integrated therapy for HIV and cryptococcosis. AIDS Res Ther 2016;13:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Lofgren S, Abassi M, Rhein J, et al. Recent advances in AIDS-related cryptococcal meningitis treatment with an emphasis on resource limited settings. Expert Rev Anti Infect Ther 2017;15:331–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Henao-Martinez AF, Gross L, Mcnair B, et al. Risk factors for cryptococcal meningitis: a single United States center experience. Mycopathologia 2016;181:807–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ndayishimiye E, Ross AJ. An audit of the screen-and-treat intervention to reduce cryptococcal meningitis in HIV-positive patients with low CD4 count. Afr J Prim Health Care Fam Med 2018;10:e1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Abassi M, Boulware DR, Rhein J. Cryptococcal meningitis: diagnosis and management update. Curr Trop Med Rep 2015;2:90–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Gaskell KM, Rothe C, Gnanadurai R, et al. A prospective study of mortality from cryptococcal meningitis following treatment induction with 1200 mg oral fluconazole in Blantyre, Malawi. PLoS One 2014;9:e110285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Adeyemi BO, Ross A. Profile and acute mortality outcome of patients admitted with cryptococcal meningitis to an urban district hospital in KwaZulu-Natal, South Africa. Off J South Afr Acad Fam Pract Prim Care 2015;57:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Xu L, Zhang X, Guo Y, et al. Unique clinical features of cryptococcal meningitis among Chinese patients without predisposing diseases against patients with predisposing diseases. Medical Mycology 2019;57:944–53. [DOI] [PubMed] [Google Scholar]
- [9].Liu ZY, Wang GQ, Zhu LP, et al. Expert consensus on the diagnosis and treatment of cryptococcal meningitis. Zhonghua Nei Ke Za Zhi 2018;57:317–23. [DOI] [PubMed] [Google Scholar]
- [10].Liu L, Zhang R, Tang Y, et al. The use of ventriculoperitoneal shunts for uncontrollable intracranial hypertension in patients with HIV-associated cryptococcal meningitis with or without hydrocephalus. Bioscience Trends 2014;8:327–32. [DOI] [PubMed] [Google Scholar]
- [11].Stevens DA, Denning DW, Shatsky S, et al. Cryptococcal meningitis in the immunocompromised host: intracranial hypertension and other complications. Mycopathologia 1999;146:1–8. [DOI] [PubMed] [Google Scholar]
- [12].Moritz D, Mena Lora AJ, Blumer B, et al. Recovery of Cryptococcus gattii from an infected ventriculo-peritoneal shunt, Illinois, USA. Emerg Infect Dis 2018;24:1382–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Liu J, Chen ZL, Li M, et al. Ventriculoperitoneal shunts in non-HIV cryptococcal meningitis. BMC Neurol 2018;18:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Merkler AE, Ch’ang J, Parker WE, et al. The rate of complications after ventriculoperitoneal shunt surgery. World Neurosurg 2017;98:654–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Perfect JR, Dismukes WE, Dromer F, et al. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the infectious diseases society of America. Clin Infect Dis 2010;50:291–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Rajasingham R, Smith RM, Park BJ, et al. Global burden of disease of HIV-associated cryptococcal meningitis: an updated analysis. Lancet Infect Dis 2017;17:873–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Firacative C, Lizarazo J, Illnait-Zaragozí MT, et al. The status of cryptococcosis in Latin America. Mem Inst Oswaldo Cruz 2018;113:e170554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Gassiep I, Aye C, Armstrong M, et al. Correlation between serum cryptococcal antigen titre and meningitis in immunocompetent patients. J Med Microbiol 2018;67:1515–8. [DOI] [PubMed] [Google Scholar]
- [19].Tan ZR, Long XY, Li LG, et al. Spectrum of neuroimaging findings in cryptococcal meningitis in immunocompetent patients in China - a series of 18 cases. J Neurol Sci 2016;368:132–7. [DOI] [PubMed] [Google Scholar]
- [20].Shapiro BB, Hedrick R, Vanle BC, et al. Cryptococcal meningitis in a daily cannabis smoker without evidence of immunodeficiency. BMJ Case Rep 2018;2018:bcr-2017-221435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Niknam N, Niknam N, Dushaj K, et al. A case of recurrent cryptococcal meningoencephalitis in an immunocompetent female. Case Rep Infect Dis 2014;2014:407348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Li J, Wang P, Ye L, et al. Cryptococcal meningitis initially presenting with eye symptoms in an immunocompetent patient: a case report. Exp Ther Med 2016;12:1119–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ito M, Hinata T, Tamura K, et al. Disseminated cryptococcosis with adrenal insufficiency and meningitis in an immunocompetent individual. Intern Med 2017;56:1259–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Liliang PC, Liang CL, Chang WN, et al. Shunt surgery for hydrocephalus complicating cryptococcal meningitis in human immunodeficiency virus-negative patients. Clin Infect Dis 2003;37:673–8. [DOI] [PubMed] [Google Scholar]
- [25].Tunkel AR, Hasbun R, Bhimraj A, et al. 2017 infectious diseases society of America's clinical practice guidelines for healthcare-associated ventriculitis and meningitis. Clin Infect Dis 2017;64:e34–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Escandon P, Lizarazo J, Agudelo CI, et al. Evaluation of a rapid lateral flow immunoassay for the detection of cryptococcal antigen for the early diagnosis of cryptococcosis in HIV patients in Colombia. Med Mycol 2013;51:765–8. [DOI] [PubMed] [Google Scholar]
- [27].Chindamporn A, Chakrabarti A, Li R, et al. Survey of laboratory practices for diagnosis of fungal infection in seven Asian countries: an Asia Fungal Working Group (AFWG) initiative. Med Mycol 2018;56:416–25. [DOI] [PubMed] [Google Scholar]
- [28].Abstracts from hydrocephalus 2018: the tenth meeting of the International Society for Hydrocephalus and Cerebrospinal Fluid Disorders. Fluids Barriers CNS 2018;15: Suppl 2: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Akhtar N, Khan AA, Yousaf M. Experience and outcome of ventricular-atrial shunt: a multi centre study. J Ayub Med Coll Abbottabad 2015;27:817–20. [PubMed] [Google Scholar]


