An 18 year-old female patient with no medical history, no recent travel or exposure to animals, presented with fever and dyspnea to the Emergency Department on April 1st, 2020. She had been exposed to COVID-19 as her grandmother had tested positive two weeks earlier. As the symptoms were perfectly tolerated and there was no risk factor of severe infection, she was discharged without any laboratory testing.
On Day 8 from the beginning of symptoms, as dyspnea worsened and she complained of chest pain, she was re-admitted to the Emergency Department. She had moderate fever of 38.3 °C. On clinical examination, the respiratory rate was 26/min, saturation 98% without oxygen, and pulmonary auscultation was normal. She presented with shock (low blood pressure at 70/42 mmHg, heart rate at 124/min, and elevated lactate at 2.9 mmol/mL). Cardiac auscultation was normal. Abdominal palpation showed non-specific pain.
Lab tests showed: Troponin above 10,000 ng/ml, elevated NT-pro-BNP, high d-dimers and procalcitonin. She exhibited severe KDIGO 3 acute kidney injury (AKI) with anuria developing within the first 24 h. Baseline characteristics are outlined in Table 1 . A computed tomography performed on the day of admission showed moderate pulmonary lesions evocative of SARS-CoV2 infection (Fig 1 ).
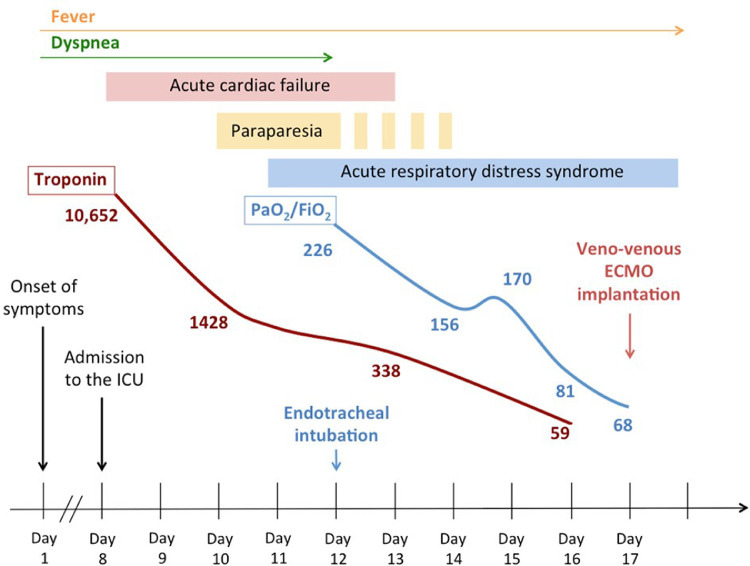
Fig. 2.
Time course for clinical evolution from ICU admission to Day 17.
Table 1.
Baseline characteristics of the patient on the day of admission to the ICU unit (Day 8 from the beginning of symptoms).
| Variable | Value | Reference range |
|---|---|---|
| Age | 19 | – |
| Troponin (ng/mL) | 10,652 | < 12 |
| NT-pro BNP (pg/mL) | 2585 | < 450 |
| White cells count (per mm3) | 22,880 | 3900–10,200 |
| Hemoglobin (g/dL) | 11.7 | 12–16 |
| Hematocrit (%) | 32.2 | 38.0 – 50.0 |
| Platelet count (per mm3) | 191,000 | 150,000 - 450,000 |
| Sodium (mmol/liter) | 123 | 135 – 145 |
| Potassium (mmol/liter) | 3.8 | 3.5 – 5 |
| Chloride (mmol/liter) | 84 | 95 – 105 |
| Total proteins (g/liter) | 65 | 58 – 80 |
| Urea (mmol/liter) | 13.6 | 2.5 – 7.6 |
| Creatinine (µmol/liter) | 272 | 65 – 110 |
| Alanine aminotransferase (U/liter) | 82 | 10 – 40 |
| Aspartate aminotransferase (U/liter) | 62 | 10 – 45 |
| Procalcitonin (ng/ml) | 67.6 | < 0.1 |
| D-dimers (ng/mL) | 4235 | < 450 |
| Lactic acid (mmol/mL) | 2.9 | 0.5 – 1.9 |
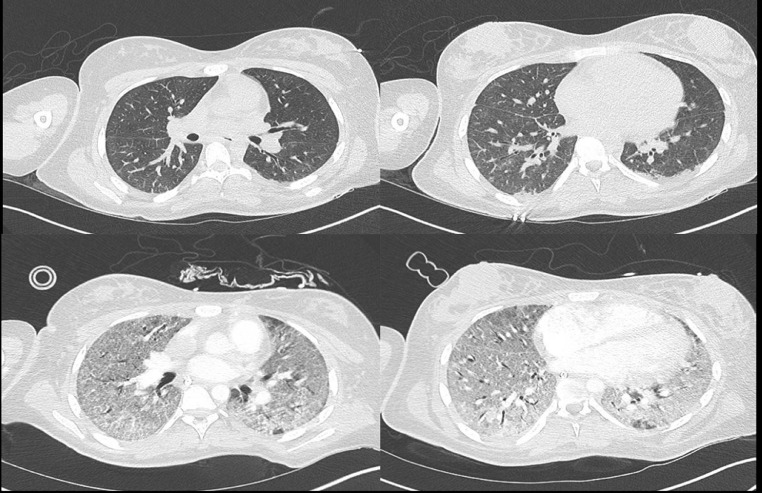
Fig. 1.
Pulmonary CT-Scan evolution. Upper panels (Day 8) showing mild abnormalities compatible with SARS-CoV2 infections. Lower panels (Day 15) showing diffuse aveolo-interstitial edema compatible with ARDS COVID-19.
Echocardiography revealed severe dysfunction (ejection fraction 30%, aortic time velocity integral 9.5 cm for a cardiac index of 2.0 L/min/m2) with global hypokinesia. The electrocardiogram showed a sinusal rhythm without repolarization or conduction abnormalities (Supplementary Fig. 1).
A perfusion of 750 mL of 0.9% saline serum did not improve hemodynamics. Norepinephrine was therefore introduced at a rate of 0.7 µg/kg/min, stabilizing the mean arterial pressure at 65 mmHg. Dobutamine was introduced up to 12.5 µg/kg/min. Control echocardiography showed a moderate improvement of cardiac function, with an aortic time velocity integral of 14 cm giving an estimated cardiac index of 3.5 L/min/m2 (Supplementary Videos & Supplementary Fig. 2).
On Day 10 from the onset of symptoms, the patient displayed bilateral proximal paraparesia of the lower limbs, without any sphincter disorder, medullary level or deglutition difficulty. Tendon reflexes were abolished at the lower limbs. A brain and spinal MRI revealed no abnormality, whilst a lumbar puncture found no elevated proteins or cellular aberration. No microbial agent was found in the cerebrospinal fluid. The SARS-CoV2 RT-PCR in the cerebrospinal fluid was negative.
Evolution was rapidly favorable for cardiogenic shock. On Day 12, the ejection fraction reached 50%, allowing a decrease of dobutamine dose (Fig. 2). AKI stabilized with a creatinine level of 230 µmol per liter, with slow resumption of diuresis. There was no associated hematuria or proteinuria. Repeated control of the electrocardiogram revealed no abnormality at any time.
Upon admission to the intensive care unit, dyspnea and mild desaturation required oxygen at 2 liter per minute. Probabilistic antibiotherapy was introduced with cefotaxime, but was discontinued after three days when no microbial agent was found. Thereafter, on Day 12, fever rose to 41 °C, and despite the improvement of cardiogenic shock, respiratory function quickly deteriorated with worsening oxygen dependency and the necessity for endotracheal intubation. On the same day, the computed tomography found major images compatible with severe COVID-19 lesions (Fig. 1). There was no evidence of cardiac impairment in the worsening of the respiratory condition. The patient was treated with protective ventilation, neuromuscular blocking agents, prone ventilation and administration of nitric oxide. Intravenous corticotherapy by dexamethasone was initiated at Day 14 at a dose of 20 mg according to the protocol proposed by Villar et al.1 Because respiratory function did not improve after two sessions of prone ventilation at Day 17, the patient was transferred for veno-venous extra-corporeal membrane oxygenation.
In search of the cause of this multi-organ failure, a series of serologic tests (HIV, HBV, HBC, parvovirus B19, Borrelia burgdorferi, TPHA-VDRL, Chlamydiae, Mycoplasma) and PCR (including HSV, EBV, CMV, respiratory viruses panel, HHV8), were performed. All returned negative. Several blood cultures and bronchoalveolar fluid remained sterile. Rheumatoid factor and mumps serology turned positive for IgG and IgM at a low titer (1.9 UI/liter with a detection threshold of 1.5 UI/liter), compatible with non-specific immune response. Anti-nuclear antibodies and anti-neutrophil cytoplasmic antibodies were negative and, apart from moderate hyperferritinemia, no evidence was found of Adult Still disease. Several PCR samples for SARS-CoV2 were negative but were repeated as recommended2 and with consideration to differing sensitivity depending on the sample site: three times in the nasopharynx, and once each in the serum, bronchoalveolar lavage fluid and urine. COVID-19 testing was finally positive on bronchoalveolar lavage fluid the day veno-venous extracorporeal membrane oxygenation was started. All investigations are summarized in Supplementary Table 1.
We describe a complex case of multi-organ failure evolution with myocarditis usually associated with a very unfavorable prognosis,3 and subsequently followed by respiratory failure. Pulmonary images quickly evolving from one CT-scan to the next and major hypoxemia upon endotracheal intubation were evocative of COVID-19, even though several PCR tests were initially negative. Interestingly, the onset of the disease did not include respiratory distress, which appeared at a later timepoint during evolution, after favorable evolution of myocarditis. To our knowledge, cases of non-coronary myocardial injury with ST segment elevation have been previously reported,4 but it is the first described case of myocarditis subsequently followed by an acute distress respiratory syndrome secondary to SARS-CoV-2 infection. In this case, the implication of SARS-CoV-2 being responsible for acute inflammatory myocarditis seems more plausible than septic myocarditis, regarding elevated BNP and troponin, and echocardiography showing altered left ventricle ejection fraction and a low cardiac index. In addition, no other pathogen was found despite numerous blood cultures, serologies and PCR (Supplementary Table 1). Unfortunately, the course of clinical events did not allow us to perform electromyograms to confirm the suspected diagnosis of Guillain-Barré, as urgent transfer of the patient was necessary in order to implement veno-venous extracorporeal membrane oxygenation.
A recent correspondence examined a series of five patients presenting with Guillain-Barré syndrome associated with COVID-19,5 a clinical presentation compatible with our patient, even though the subsequent clinical evolution prevented us from performing any electrophysiological examination. In a report studying neurologic features during SARS-CoV2 infection, no RT-PCR assay was found positive in the cerebrospinal fluid.6 As of April 20th, 2020, the patient remains hospitalized under veno-venous extracorporeal membrane oxygenation. This case suggests that there is a wide clinical spectrum of SARS-CoV2 infection, encompassing multi-organ failure and not necessarily always starting with acute respiratory syndrome.
Declaration of Competing Interest
None
Acknowledgments
Acknowledgment
The authors sincerely thank Mr. Didier Patte and Ms. Felicity Kay for his help in preparing the manuscript.
Declarations
Ethics approval and consent to participate: the need for approval was waived
Consent for publication
Obtained from the patient
Funding
None
Authors' contributions
All authors participated to diagnosis and treatment of the patient, Lina Jeantin and Matthieu Jamme wrote the article.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.hrtlng.2020.10.008.
Appendix. Supplementary materials
References
- 1.Villar J., Ferrando C., Martínez D. Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial. Lancet Respir Med. 2020;8(3):267–276. doi: 10.1016/S2213-2600(19)30417-5. [DOI] [PubMed] [Google Scholar]
- 2.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li J.-.W., Han T.-.W., Woodward M. The impact of 2019 novel coronavirus on heart injury: a systemic review and meta-analysis. Prog Cardiovasc Dis. 2020 doi: 10.1016/j.pcad.2020.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bangalore S., Sharma A., Slotwiner A. ST-segment elevation in patients with Covid-19 - a case series. N Engl J Med. 2020 doi: 10.1056/NEJMc2009020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Helms J., Kremer S., Merdji H. Neurologic features in severe SARS-CoV-2 infection. N Engl J Med. 2020 doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Toscano G., Palmerini F., Ravaglia S. Guillain-Barré syndrome associated with SARS-CoV-2. N Engl J Med. 2020 doi: 10.1056/NEJMc2009191. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.