Abstract
Introduction
Early studies suggest that acute cerebrovascular events may be common in patients with coronavirus disease 2019 (COVID-19) and may be associated with a high mortality rate. Most cerebrovascular events described have been ischemic strokes, but both intracerebral hemorrhage and rarely cerebral venous sinus thrombosis (CVST) have also been reported. The diagnosis of CVST can be elusive, with wide-ranging and nonspecific presenting symptoms that can include headache or altered sensorium alone.
Objective
To describe the presentation, barriers to diagnosis, treatment, and outcome of CVST in patients with COVID-19.
Methods
We abstracted data on all patients diagnosed with CVST and COVID-19 from March 1 to August 9, 2020 at Boston Medical Center. Subsequently, we reviewed the literature and extracted all published cases of CVST in patients with COVID-19 from January 1, 2020 through August 9, 2020 and included all studies with case descriptions.
Results
We describe the clinical features and management of CVST in 3 women with COVID-19 who developed CVST days to months after initial COVID-19 symptoms. Two patients presented with encephalopathy and without focal neurologic deficits, while one presented with visual symptoms. All patients were treated with intravenous hydration and anticoagulation. None suffered hemorrhagic complications, and all were discharged home. We identified 12 other patients with CVST in the setting of COVID-19 via literature search. There was a female predominance (54.5%), most patients presented with altered sensorium (54.5%), and there was a high mortality rate (36.4%).
Conclusions
During this pandemic, clinicians should maintain a high index of suspicion for CVST in patients with a recent history of COVID-19 presenting with non-specific neurological symptoms such as headache to provide expedient management and prevent complications. The limited data suggests that CVST in COVID-19 is more prevalent in females and may be associated with high mortality.
Key Words: COVID-19, SARS-CoV-2, Stroke, Cerebral venous sinus thromboses
Introduction
Early reports suggest that neurologic complications are frequent in patients with coronavirus disease 19 (COVID-19), occurring in 36% to 65% of hospitalized patients.1 , 2 While early reports described stroke as a complication seen primarily in critically ill patients, up to 5% in a cohort from Wuhan,1 other studies have described many cases of stroke in COVID-19 patients from the community.3 Cerebral venous sinus thrombosis (CVST) is a rare form of stroke (<1%), caused by occlusion of the dural venous sinuses and/or cerebral veins.4 Clinical presentation may be highly variable, with symptoms ranging from headache, visual complaints, or nausea to focal deficits or seizure. Though death with CVST is uncommon in patients without COVID-19 (3–15%),5 neurologic deterioration is common (23%).6 Risk factors for CVST are similar to those for other forms of venous thromboembolism, including pro-thrombotic state and trauma. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is thought to cause endothelial injury specifically through binding with angiotensin converting enzyme 2 (ACE-2) receptors, activating a cytokine cascade and leading to a hypercoagulable state.7 Though systemic venous thromboembolism has been commonly reported in COVID-19 infection, cases of CVST are less frequently described in the literature.8, 9, 10, 11, 12, 13, 14, 15, 16, 17
Here, we describe the presentation of 3 patients with COVID-19 diagnosed with CVST, discuss management considerations, and review published literature on patients with COVID-19 and CVST.
Material and methods
This was a single-center retrospective study at an urban, safety-net, academic hospital in Massachusetts, approved by the Boston Medical Center Institutional Review Board. Data on CVST in patients with COVID-19 were abstracted from the electronic medical record system from March 1 to August 9, 2020. The literature on CVST in patients with COVID-19 was reviewed on August 9, 2020 by searching the terms “cerebral venous sinus thrombosis AND COVID-19” or “CVST AND COVID-19” or “Stroke AND COVID-19.” We manually searched the bibliography of studies obtained to extract additional published cases. Only studies with case descriptions were included.
Results
Patient 1
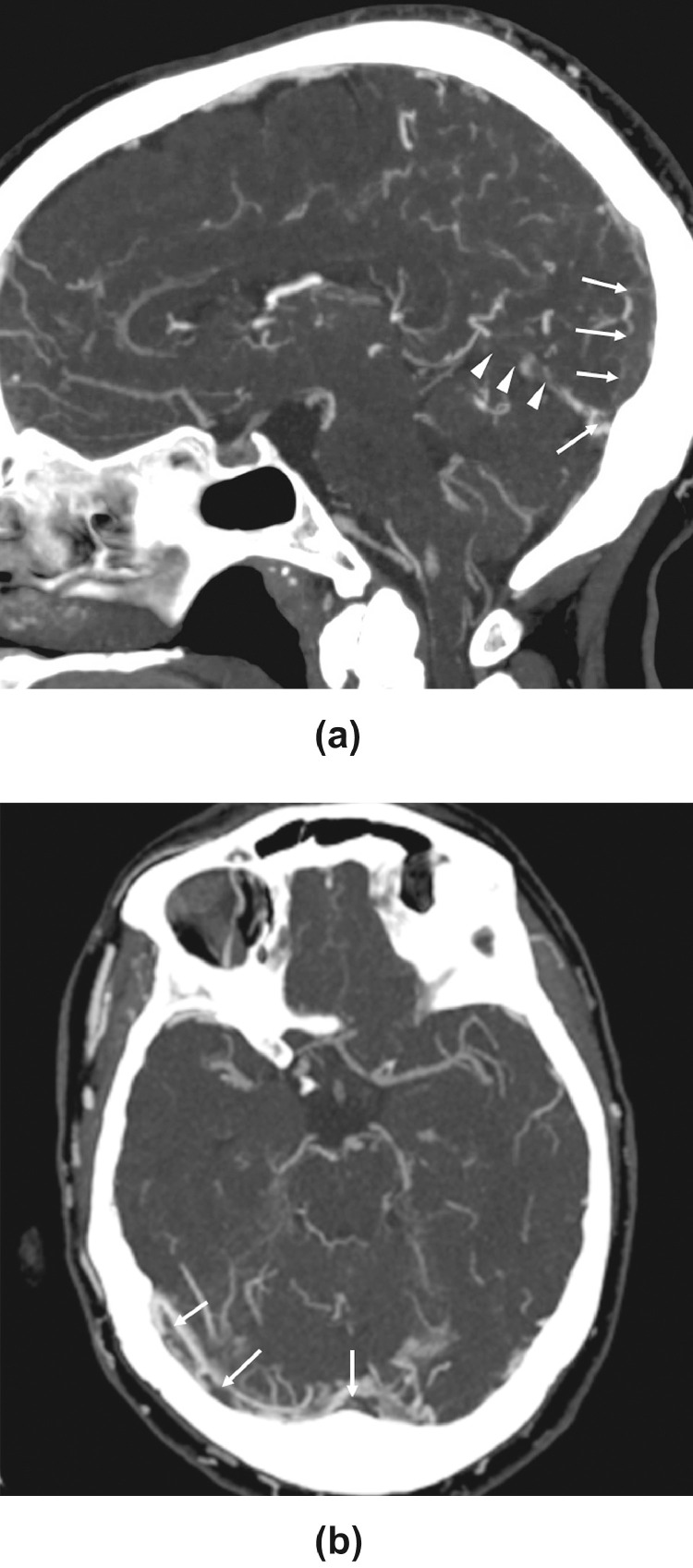
A healthy 68-year-old woman presented with fever, cough, and shortness of breath. A nasopharyngeal swab RT-PCR test resulted positive for SARS-CoV-2. She was hospitalized for two days, then discharged home. Three weeks later, she returned with four days of nausea, vomiting, generalized weakness, and headache (Table 1 ). Her neurologic exam revealed only disorientation to place and time, and her laboratory workup showed increased inflammatory markers. CT venogram (Fig. 1 ) revealed filling defects within major venous structures including posterior superior sagittal sinus and torcula, straight sinus, the vein of Galen, inferior sagittal sinus, the internal cerebral veins, and bilateral transverse sinuses. MRI demonstrated abnormal susceptibility artifact interdigitating within the sulci of the bilateral posterior frontal, parietal, and occipital lobes suggestive of cortical vein thrombosis, and T2/FLAIR hyperintensity within the same regions suggestive of venous congestion. Repeat nasopharyngeal swab RT-PCR test for SARS-CoV-2 was negative.
Table 1.
Summary of Patients with COVID-19 and CVST
| Patient 1 (present study) | Patient 2(present study) | Patient 3(present study) | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | Patient 8 | Patient 9 | Patient 10 | Patient 11 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (years) | 68 | 79 | 25 | 59 | 65 | 38 | 41 | 29 | 72 | 44 | 62 | 54 | 63 | 30 |
| Gender | Female | Female | Female | Male | Male | Male | Female | Female | Male | Female | Female | Female | Female | Male |
| Significant past medical history | Unknown | Hypertension | steroid/IVIG refractory ITP, lupus anticoagulant, right parietal subdural hematoma and right frontal intraparenchymal hemorrhage, von-Willebrand disease, Evans Syndrome | NIDDM, hypertension | N/A | Mild autism | Unknown | Unknown | Unknown | Unknown | Morbid obesity | Breast cancer |
N/A | Unknown |
| Medications prior to admission | None | Amlodipine, hydrochlorothiazide, gabapentin | avatrombopag and fostamatinib | N/A | N/A | N/A | Hydroxychloroquine and azithromycin, estrogen oral contraceptives | N/A | N/A | N/A | N/A | Hormone therapy | N/A | N/A |
| Neurologic Signs and symptoms | Nausea, vomiting, generalized weakness, headache, and disorientation | Headaches, dizziness, somnolence, disorientation, and inattentiveness | Right eye blurriness, horizontal diplopia, bilateral papilledema, and tingling in her right hand | Worsening headache, weakness and numbness in right upper and lower limbs, slurred speech and expressive dysphasia. | Loss of consciousness, upward gaze, tongue biting | Fever, headache, vomiting and diarrhea, and altered mental status, dehydration | Confusion and aphasia, extensor posturing | Tonic-clonic seizures, post-ictal confusion, decreased arousal, global aphasia, right facial palsy | Left hemiparesis, altered mental status, and refractory status epilepticus | Dyspnea, headache, altered mental status, aphasia, and right hemiparesis | Headache, altered vision, right hemicorporeal deficit and altered consciousness | Severe headache | Aphasia, right hemiplegia | Generalized tonic-clonic seizures |
| Vessels involved | Posterior aspect of the superior sagittal sinus, the inferior sagittal sinus, the internal cerebral veins, vein of Galen, bilateral transverse sinuses, proximal right sigmoid sinus, and left sigmoid sinus extending into the upper left internal jugular vein | Right transverse sinus | superior sagittal sinus and right transverse sinus | Right sigmoid and transverse sinus involving the torcula. | hemorrhagic infarct in right temporal and right sigmoid and transverse sinus thrombosis | Straight sinus, distal superior sagittal sinus, torcula, and right transverse sinus, and several cortical veins adjacent to the superior sagittal sinus | Internal cerebral veins, vein of Galen and distal straight sinus. | Distal left transverse and sigmoid sinus, and left internal jugular vein | Internal cerebral veins and vein of Galen, medullary veins | Vein of Galen, straight sinus, torcular herophili, and left internal cerebral vein | Left transverse sinus, straight vein, vein of Galen and internal cerebral veins | Left transverse sinus | Straight sinus and left lateral sinus | Right sphenoparietal venous sinus, the torcula, left transverse sinus, and sigmoid sinus extending to the proximal part of the left internal jugular vein |
| Diagnostic imaging | CT, CTV, MRI | CT, CTA, CTV | CT, CTV, MRI/MRV | CT/CTV | CT/MRI/MRV | CT/CTV and DSA | CT/CTV | CT and MRI/MRV | CT, CTV, MRI | CTA | CT, CTV, MRI | CT, CTV, MRA | CTV, MRI | CT, MRI/MRV |
| Treatment for CVST | Unfractionated high-dose intravenous heparin | Therapeutic low-molecular-weight heparin | Unfractionated high-dose intravenous heparin | LMWH | Anticoagulant (not specified) | Percutaneous venous mechanical thrombectomy, tPA via in situ microcatheter in the SSS, | Heparin infusion | Intravenous heparin | Curative anticoagulation (not specified) | LMWH | N/A | N/A | Intravenous heparin | LMWH |
| COVID-19 symptoms | Fever, cough, chest tightness, and shortness of breath | Fever, nausea, vomiting, and diarrhea | Fever, chills, headache, nausea, and cough | Worsening headache | N/A | N/A | N/A | Cough, low grade fever, mild shortness of breath, and mild headache | mild respiratory symptoms a few days earlier | Worsening respiratory status, fever, and cough | Fever, cough, dyspnea | Fever and asthenia, cough | Fever, cough, and anosmia | N/A |
| Time from onset of COVID-19 symptoms to onset of neurological symptoms (days) | 18 | 3 | 4 months | 4 | N/A | N/A | Recent admission for COVID19 (time from onset of COVID-19 symptoms N/A) | Diagnosed during present admission | Diagnosed during present admission with mild respiratory symptoms a few days earlier | 2 weeks | N/A | N/A | 12 | Diagnosed during present admission |
| White-cell count [4.0 – 11.0 K/UL], minimum - maximum | 8.6 | 4 | 11.8 | 6.3 | Reported as increased (value N/A) | 16.7 | 10 | 8.8 | N/A | 9.6 | 20.2 | 18.3 | N/A | N/A |
| Platelet count [150 – 400 K/UL] | 196 | 113 | 154 | 234 | 141 | 239 | 335 | N/A | 42 | N/A | N/A | N/A | N/A | |
| Fibrinogen (180–460 mg/dL) | 507 | 393 | 289 | 4.9 g/L | N/A | 121 | Not reported | N/A | N/A | N/A | N/A | N/A | 7.2 g/L | N/A |
| D-dimer (<243 ng/dL), maximum value | 6,714 | 8,457 | 241 | N/A | N/A | >55,000 | 2032 | 2876 | N/A | 5975 | 14.2mg/L | N/A | N/A | 0.75 mg/L |
| Ferritin (10 – 109 ng/mL) | 516 | 812 | N/A | N/A | N/A | N/A | N/A | 10.4 | N/A | N/A | N/A | N/A | 1427 ug/L | 91 ug/L |
| C-reactive protein [0 – 5 mg/L] | 121.5 | 96.4 | N/A | 20 | Reported as normal (value N/A) | N/A | N/A | 37 | N/A | N/A | N/A | N/A | N/A | N/A |
| Sedimentation rate [0 – 30 mm/hr] | 60 | 23 | N/A | 31 | Reported as normal (value N/A) | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Lactate dehydrogenase [171 – 308 U/L] | 348 | 434 | N/A | N/A | Reported as normal (value N/A) | N/A | N/A | 287 | N/A | N/A | N/A | N/A | N/V | N/A |
| Outcomes | Discharged home | Discharged home | Discharged home | Discharged home | N/A | Death secondary to respirator complication leading to cardiac arrest | Death secondary to venous infarction in the left basal ganglia, thalamus, and mesial temporal lobe with hemorrhagic transformation, intraventricular hemorrhage, and obstructive hydrocephalus, and loss of brainstem reflexes | Intensive care unit | Brain death secondary to cerebral venous infarction, mass effect of midline structures leading to brain death | N/A | N/A | N/A | Death after intraparenchymal hemorrhage and cerebral infarction and secondary to therapeutic limitations after ethical consultation | Discharged to quarantine center |
| References | Present study | Present study | Present study | Christopher Hughes et al (2020)(8) | H. Hemasian et al (2020)(10) | D.D. Cavalcanti et al (2020)(11) | D.D. Cavalcanti et al (2020)(11) | David E. Klein et al (2020)(12) | L. Chougar et al (2020)(13) | Francesco Garaci et al (2020)(14) | Guillaume Poillon et al (2020)(15) | Guillaume Poillon et al (2020)(15) | Fabian Roy-Gash et al (2020)(17) | Hussain S. et al (2020)(16) |
Fig. 1.
Sagittal and axial CT venogram images demonstrate filling defects (arrows) within major venous structures including posterior superior sagittal sinus and torcular Herophili (arrows), straight sinus (arrowheads), the vein of Galen, inferior sagittal sinus, the internal cerebral veins, bilateral transverse sinuses, proximal right sigmoid sinus and left sigmoid sinus extending into the upper left internal jugular vein.
She was started on dose-adjusted unfractionated intravenous heparin, later transitioned to enoxaparin, followed by dabigatran. On the second night of hospitalization, she had a focal seizure confirmed on electroencephalography and was treated with levetiracetam and lacosamide. Her mental status improved, and she was discharged home.
Patient 2
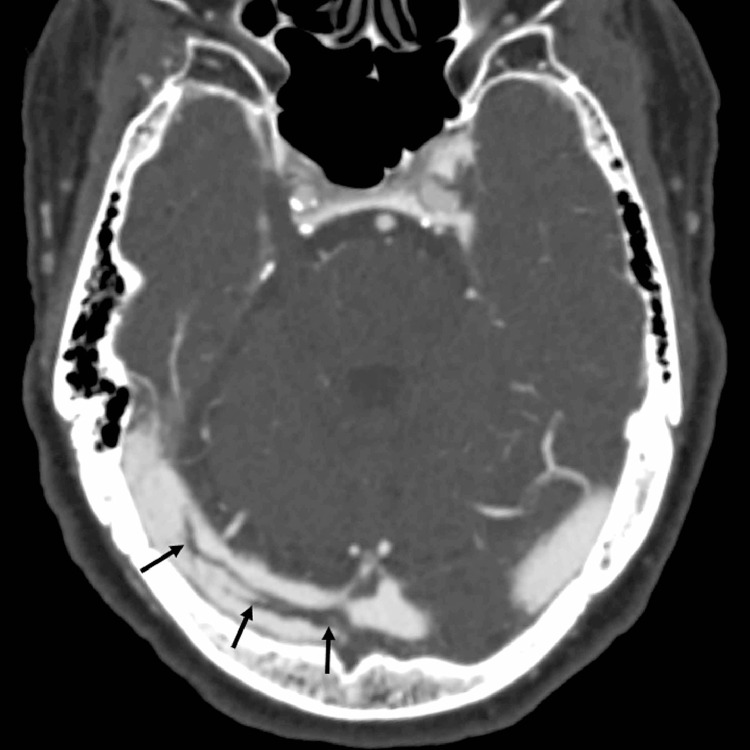
A 79-year-old woman with hypertension presented with fever, nausea, vomiting, and diarrhea (Table 1). She tested positive for SARS-CoV-2 via nasopharyngeal swab RT-PCR and was started on azithromycin and hydroxychloroquine. On day 3 of hospitalization, she was disoriented and reported headaches, but had no focal neurologic deficits. CT venogram revealed a curvilinear thrombus within the right transverse sinus (Fig. 2 ). She was started on therapeutic low-molecular-weight heparin. Her mental status improved with correction of her metabolic derangements, and she was discharged home on enoxaparin.
Fig. 2.
Axial CT venogram image demonstrates a curvilinear filling defect (arrows) within the right transverse sinus extending from the torcular Herophili, consistent with non-flow limiting thrombus.
Patient 3
A 25-year-old woman with Evans Syndrome, idiopathic thrombocytopenic purpura on avatrombopag, von-Willebrand Disease, and a history of multiple intracerebral hemorrhages presented with intractable headache, blurry vision, and tingling of the right upper extremity (Table 1). She tested positive for SARS-CoV-2 via nasopharyngeal swab RT-PCR 4 months prior but was negative upon repeat testing. MR venogram demonstrated superior sagittal sinus thrombosis and bilateral transverse sinus thrombosis. She was started on dose-adjusted unfractionated intravenous heparin and transitioned to apixaban. Her immunotherapy was also changed to fostamatinib for its lower risk of prothrombotic events.
Literature review
We identified 12 patients with COVID-19 and CVST in the published literature and extracted data for 11 patients for whom detailed case descriptions were available.8 , 10, 11, 12, 13, 14, 15, 16, 17 Clinical data are summarized in Table 1. Patients ranged between 29 and 72 years old, with 54.5% female (n = 6). The majority (7, 63.6%) of patients did not have any underlying co-morbidities, and 9 (81.8%) were not taking any prescribed medications prior to presentation. The most common presenting symptom was altered sensorium (6, 54.5%). The COVID diagnosis preceded identification of CVST diagnoses by 4 days to 2 weeks. Thromboses were visualized most frequently in the straight and left transverse sinuses (5, 45.5%). Of the reported patient outcomes, 4 (36.4%) died during admission and treatment for CVST.
Discussion
We describe three cases of CVST in women with COVID-19, all of whom survived with favorable neurologic outcomes. We additionally identified detailed case descriptions of 11 other patients in the literature (6 women) including 4 deaths. Though we cannot attribute causality, we suspect COVID-19 infection may increase the risk for development of CVST, especially in those with other predisposing risk factors.
Patients who developed CVST in this series all exhibited symptoms of COVID-19. These included a febrile illness with respiratory symptoms, although one patient initially had gastrointestinal complaints, also commonly seen with COVID-19. It is unclear whether symptoms of nausea, vomiting, and resultant dehydration contributed to or resulted from CVST in this patient.
At least 2 patients from our 3 had other major contributing factors for the development of CVST. One patient had an underlying malignancy, breast cancer, and another had Evans syndrome. Few cases of COVID-19 have been reported to occur in the setting of Evans syndrome, complicating the interpretation of CVST in this patient.18 , 19 Our patient had been diagnosed with Evans syndrome approximately 8 years prior, with no interval thromboembolic events despite additional therapeutic predisposing factors, including avatrombopag. Therefore, we think it is possible that COVID-19 decreased the threshold for the development of CVST in this patient in the setting of her underlying predisposition.
Though two of the patients included in our case series had initial SARS-CoV-2 testing which was positive during initial infection and negative at the time of presentation for CVST, we suspect COVID-19 infection may still have been a provoking factor. Given the myriad of reports of persistent symptoms weeks to months after initial infection, it is plausible that a pro-thrombotic state also lingers after acute infection.20, 21, 22 Consistent with this hypothesis, studies during prior coronavirus pandemics have found that patients have been shown to harbor leukocytes that are persistently infected two months after initial infection with coronavirus that could potentially be pro-inflammatory.21 , 23 The majority of patients in our series were found to have elevated inflammatory markers. Given that abundant evidence has demonstrated increased rates of thromboembolic events in the setting of SARS-CoV-2,24 , 25 we posit that SARS-CoV-2 infection itself predisposes patients to the development of CVST. The presence of a pro-thrombotic state in the post-acute phase of infection is not unique to COVID-19, as the majority of venous thromboembolic events (VTEs) post-acute illness occurs in the first 30 days following hospital discharge.26 As such, current guidance suggests a continuation of thromboprophylaxis up to 45 days following discharge for some COVID-19 patients.27, 28, 29
Reports from China and Europe suggest a similar prevalence of SARS-CoV-2 infection between men and women but differences in disease severity, with a higher global rate of hospitalization and deaths among male patients.30 , 31 In our study and the published literature, women with COVID-19 appeared to be at higher risk for CVST, similar to data in non-COVID-19 patient populations.32 It is important to highlight, as women overall may experience a headache more frequently as a symptom of COVID-19 or have a history of migraine disorder, which may lower clinician's suspicion for this critical pathology.33 Gender-specific risk factors including oral contraceptives, pregnancy, puerperium, and hormone replacement therapy are thought to facilitate the development of CVST.32 During a prior coronavirus pandemic, estrogen was shown to protect against SARS by activating an immune response and inhibiting SARS-CoV replication.34 It is hypothesized that a similar mechanism could occur in patients with COVID-19.35 We hypothesize that the same strong and protective immune response in females could worsen central and systemic inflammation and contribute to the development of venous thromboembolism including CVST.
This case series illustrates the challenges in recognition of symptoms of CVST in COVID-19 patients. Only one of our patients had neurologic deficits suggesting a focal lesion, but two patients had alterations in mental status, potentially due to reduced venous outflow and consequent elevated intracranial pressure with reduced cerebral perfusion. The first case highlights the spectrum of complications of CVST, including seizures, which can occur due to cortical irritation, edema, or subarachnoid hemorrhage, all of which may result from venous outflow obstruction. Our patients were all discharged to home after treatment but at least 4 patients in the reported literature died.
Diagnosis of CVST in this patient population may be particularly challenging given that preliminary reports suggest that neurological symptoms are quite common, including headache in 5.6% to 70.3% and encephalopathy in 7.5% to 84.3%.1 , 36, 37, 38 These non-specific neurological symptoms may obscure the early presenting findings of CVST in patients with COVID-19, particularly in those with a critical illness where toxic-metabolic derangement is common. Seizure may also be a common presenting symptom in patients with COVID-19, even in those without prior history of epilepsy.39 In patients with COVID-19 presenting with headache, mental status deterioration, or seizure, CVST should be suspected even in the absence of focal neurological deficits. In our series, symptoms suggestive of CVST occurred days to months after the initial diagnosis of COVID-19, highlighting the variability of symptom onset, and similar to the timeline of presentation of other cerebrovascular events in some patients with COVID-19.40 Increasing reports suggest a longer duration of illness in some patients than initially described. The delay of weeks to months between typical COVID-19 symptoms and the development of CVST observed in our study suggests the possibility of a persistent hypercoagulable state.41 Multiple additional factors may prevent the early diagnosis of CVST in patients with COVID-19 and limit the use of diagnostic imaging in this patient population, including concerns regarding viral transmission to healthcare workers and other patients.
Treatment for CVST typically includes aggressive hydration and anticoagulation, even in the presence of cerebral hemorrhage, to decrease further propagation of clot and pulmonary embolism. There is no literature to guide whether this tenet of treatment of CVST holds in those with SARS-CoV2 infection, as hemorrhagic complications have also been reported, including acute hemorrhagic necrotizing encephalopathy42 and increased rates of in intracerebral hemorrhage in patients on therapeutic anticoagulation for systemic VTE.43 Furthermore, patients with acute respiratory distress syndrome or refractory hypoxemia may also be unable to tolerate aggressive hydration.44, 45, 46
Conclusion
The diagnosis, monitoring, and treatment of CVST may present unique challenges in patients with COVID-19. Given the hypercoagulability associated with SARS-CoV-2 infection, CVST should be considered within the differential diagnosis in patients with COVID-19 with headache, encephalopathy, seizure, or focal neurologic deficit. Women may be at higher risk for development of CVST associated with SARS-CoV-2 infection. Larger studies are needed to guide therapy in this population.
Grant Support
Pria Anand is supported by a Simon Grinspoon Research Grant.
References
- 1.Mao L, Jin H, Wang M, Hu Y, Chen S, He Q. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020 doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fan S, Xiao M, Han F, Xia P, Bai X, Chen H. Neurological manifestations in Critically Ill patients with COVID-19: a retrospective study. Front Neurol. 2020;11:806. doi: 10.3389/fneur.2020.00806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carneiro T DJ, Leung LY, Nobleza COS, Marulanda-Londono E, Hathidara M. Intravenous tPA for acute ischemic stroke in patients with COVID-19. J Stroke Cerebrovasc Dis. 2020;2020(105201) doi: 10.1016/j.jstrokecerebrovasdis.2020.105201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stam J. Thrombosis of the cerebral veins and sinuses. New Engl J Med. 2005;352(17):1791–1798. doi: 10.1056/NEJMra042354. [DOI] [PubMed] [Google Scholar]
- 5.Dentali F, Crowther M, Ageno W. Thrombophilic abnormalities, oral contraceptives, and risk of cerebral vein thrombosis: a meta-analysis. Blood. 2006;107(7):2766–2773. doi: 10.1182/blood-2005-09-3578. [DOI] [PubMed] [Google Scholar]
- 6.Gameiro J, Ferro JM, Canhao P, Stam J, Barinagarrementeria F, Lindgren A. Prognosis of cerebral vein thrombosis presenting as isolated headache: early vs. late diagnosis. Cephalalgia: Int J Headache. 2012;32(5):407–412. doi: 10.1177/0333102412439353. [DOI] [PubMed] [Google Scholar]
- 7.Connors JM, Levy JH. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135(23):2033–2040. doi: 10.1182/blood.2020006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hughes C, Nichols T, Pike M, Subbe C, Elghenzai S. Cerebral venous sinus thrombosis as a presentation of COVID-19. EJCRIM. 2020:7. doi: 10.12890/2020_001691. doi:10.128902020_001691.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Porfidia A, Pola R. Venous thromboembolism in COVID-19 patients. J Thromb Haemost: JTH. 2020 doi: 10.1111/jth.14842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hemasian H, Ansari B. First case of Covid-19 presented with cerebral venous thrombosis: a rare and dreaded case. Rev Neurol (Paris) 2020;176(6):521–523. doi: 10.1016/j.neurol.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cavalcanti DD, Raz E, Shapiro M, Dehkharghani S, Yaghi S, Lillemoe K. Cerebral venous thrombosis associated with COVID-19. AJNR Am J Neuroradiol. 2020 doi: 10.3174/ajnr.A6644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klein DE, Libman R, Kirsch C, Arora R. Cerebral venous thrombosis: A typical presentation of COVID-19 in the young. J Stroke Cerebrovasc Dis. 2020;29(8) doi: 10.1016/j.jstrokecerebrovasdis.2020.104989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chougar L, Mathon B, Weiss N, Degos V, Shor N. Atypical deep cerebral vein thrombosis with hemorrhagic venous infarction in a patient positive for COVID-19. AJNR Am J Neuroradiol. 2020 doi: 10.3174/ajnr.A6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garaci F, Di Giuliano F, Picchi E, Da Ros V, Floris R. Venous cerebral thrombosis in COVID-19 patient. J Neurol Sci. 2020;414 doi: 10.1016/j.jns.2020.116871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poillon G, Obadia M, Perrin M, Savatovsky J, Lecler A. Cerebral venous thrombosis associated with COVID-19 infection: causality or coincidence? J Neuroradiol. 2020 doi: 10.1016/j.neurad.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hussain S, Vattoth S, Haroon Khawaja H, Muhammad A. A case of coronavirus disease 2019 presenting with seizures secondary to cerebral venous sinus thrombosis. Case Rep Neurol. 2020;12:260–265. doi: 10.1159/000509505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roy-Gash F, Marine M, Jean-Michel D, Herve V, Raphael B, Nicolas E. COVID-19-associated acute cerebral venous thrombosis: clinical, CT. MRI EEG features. Crit Care. 2020;24(1):419. doi: 10.1186/s13054-020-03131-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li M, Nguyen CB, Yeung Z, Sanchez K, Rosen D, Bushan S. Evans syndrome in a patient with COVID-19. Br J Haematol. 2020;190(2):e59–e61. doi: 10.1111/bjh.16846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zarza J, Von Horoch J, Aguayo N, Baez E. Evans syndrome associated with antiphospholipid antibodies in a patient with SARS-COV-2 infection. Hematol Transfus Cell Ther. 2020 doi: 10.1016/j.htct.2020.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carfi A, Bernabei R, Landi F, Gemelli Against C-P-ACSG. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324(6):603–605. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arbour N, Day R, Newcombe J, Talbot PJ. Neuroinvasion by human respiratory coronaviruses. J Virol. 2000;74(19):8913–8921. doi: 10.1128/jvi.74.19.8913-8921.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu Y, Li X, Geng D, Mei N, Wu PY, Huang CC. Cerebral micro-structural changes in COVID-19 patients - an MRI-based 3-month follow-up study. E Clin Med. 2020;25 doi: 10.1016/j.eclinm.2020.100484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Desforges M, Miletti TC, Gagnon M, Talbot PJ. Activation of human monocytes after infection by human coronavirus 229E. Virus Res. 2007;130(1-2):228–240. doi: 10.1016/j.virusres.2007.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spiezia L, Boscolo A, Poletto F, Cerruti L, Tiberio I, Campello E. COVID-19-related severe hypercoagulability in patients admitted to intensive care unit for acute respiratory failure. Thromb Haemost. 2020 doi: 10.1055/s-0040-1710018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lodigiani C, Iapichino G, Carenzo L, Cecconi M, Ferrazzi P, Sebastian T. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang W, Goldberg RJ, Anderson FA, Kiefe CI, Spencer FA. Secular trends in occurrence of acute venous thromboembolism: the Worcester VTE study (1985-2009) Am J Med. 2014;127(9):829–839. doi: 10.1016/j.amjmed.2014.03.041. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abou-Ismail MY, Diamond A, Kapoor S, Arafah Y, Nayak L. The hypercoagulable state in COVID-19: incidence, pathophysiology, and management. Thromb Res. 2020;194:101–115. doi: 10.1016/j.thromres.2020.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cohoon KP, Mahe G, Tafur AJ, Spyropoulos AC. Emergence of institutional antithrombotic protocols for coronavirus 2019. Res Pract Thromb Haemost. 2020;4(4):510–517. doi: 10.1002/rth2.12358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spyropoulos AC, Lipardi C, Xu J, Peluso C, Spiro TE, De Sanctis Y. Modified IMPROVE VTE risk score and elevated D-Dimer identify a high venous thromboembolism risk in Acutely Ill medical population for extended thromboprophylaxis. TH Open. 2020;4(1):e59–e65. doi: 10.1055/s-0040-1705137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gebhard C, Regitz-Zagrosek V, Neuhauser HK, Morgan R, Klein SL. Impact of sex and gender on COVID-19 outcomes in Europe. Biol Sex Differ. 2020;11(1):29. doi: 10.1186/s13293-020-00304-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klein SL, Dhakal S, Ursin RL, Deshpande S, Sandberg K, Mauvais-Jarvis F. Biological sex impacts COVID-19 outcomes. PLoS Pathog. 2020;16(6) doi: 10.1371/journal.ppat.1008570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coutinho JM, Ferro JM, Canhao P, Barinagarrementeria F, Cantu C, Bousser MG. Cerebral venous and sinus thrombosis in women. Stroke. 2009;40(7):2356–2361. doi: 10.1161/STROKEAHA.108.543884. [DOI] [PubMed] [Google Scholar]
- 33.Lechien JR, Chiesa-Estomba CM, Place S, Van Laethem Y, Cabaraux P, Mat Q. Clinical and epidemiological characteristics of 1420 European patients with mild-to-moderate coronavirus disease 2019. J Intern Med. 2020;288(3):335–344. doi: 10.1111/joim.13089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Channappanavar R, Fett C, Mack M, Ten Eyck PP, Meyerholz DK, Perlman S. Sex-based differences in susceptibility to severe acute respiratory syndrome coronavirus infection. J Immunol. 2017;198(10):4046–4053. doi: 10.4049/jimmunol.1601896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gagliardi MC, Tieri P, Ortona E, Ruggieri A. ACE2 expression and sex disparity in COVID-19. Cell Death Discov. 2020;6:37. doi: 10.1038/s41420-020-0276-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Borges do Nascimento IJ, Cacic N, Abdulazeem HM, von Groote TC, Jayarajah U, Weerasekara I. Novel Coronavirus Infection (COVID-19) in humans: a scoping review and meta-analysis. J Clin Med. 2020;9(4) doi: 10.3390/jcm9040941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suzuki K. Rinsho Shinkeigaku; 2020. [Coronavirus disease 2019 (COVID-19) and headaches] [DOI] [PubMed] [Google Scholar]
- 38.Helms J, Kremer S, Merdji H, Schenck M, Severac F, Clere-Jehl R. Delirium and encephalopathy in severe COVID-19: a cohort analysis of ICU patients. Crit Care. 2020;24(1):491. doi: 10.1186/s13054-020-03200-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anand P, Al-Faraj A, Sader E, Dashkoff J, Abdennadher M, Murugesan R. Seizure as the presenting symptom of COVID-19: A retrospective case series. Epilepsy Behav. 2020;112 doi: 10.1016/j.yebeh.2020.107335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li Y, Li M, Wang M, Zhou Y, Chang J, Xian Y. Acute cerebrovascular disease following COVID-19: a single center, retrospective, observational study. Stroke Vasc Neurol. 2020 doi: 10.1136/svn-2020-000431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carfi A, Bernabei R, Landi F, Gemelli Against C-P-ACSG. Persistent symptoms in patients after acute COVID-19. JAMA. 2020 doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Poyiadji N, Shahin G, Noujaim D, Stone M, Patel S, Griffith B. COVID-19-associated acute hemorrhagic necrotizing encephalopathy: CT and MRI features. Radiology. 2020 doi: 10.1148/radiol.2020201187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dogra S, Jain R, Cao M, Bilaloglu S, Zagzag D, Hochman S. Hemorrhagic stroke and anticoagulation in COVID-19. J Stroke Cerebrovasc Dis. 2020;29(8) doi: 10.1016/j.jstrokecerebrovasdis.2020.104984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ilyas A, Chen CJ, Raper DM, Ding D, Buell T, Mastorakos P. Endovascular mechanical thrombectomy for cerebral venous sinus thrombosis: a systematic review. J Neurointerventional Surg. 2017;9(11):1086–1092. doi: 10.1136/neurintsurg-2016-012938. [DOI] [PubMed] [Google Scholar]
- 45.Siddiqui FM, Dandapat S, Banerjee C, Zuurbier SM, Johnson M, Stam J. Mechanical thrombectomy in cerebral venous thrombosis: systematic review of 185 cases. Stroke. 2015;46(5):1263–1268. doi: 10.1161/STROKEAHA.114.007465. [DOI] [PubMed] [Google Scholar]
- 46.Eskey CJ, Meyers PM, Nguyen TN, Ansari SA, Jayaraman M, McDougall CG. Indications for the performance of intracranial endovascular neurointerventional procedures: a scientific statement from the American Heart Association. Circulation. 2018;137(21):e661–ee89. doi: 10.1161/CIR.0000000000000567. [DOI] [PubMed] [Google Scholar]