Abstract
Background
The treatment of proximal humerus tumors with endoprostheses is associated with a high risk of implant-related surgical complications. Because of extensive soft-tissue resection and muscular detachment during surgery, instability is the most common serious complication. A reverse total shoulder arthroplasty with a highly constrained design is one option to mitigate instability, but few studies have reported the results of this prosthesis for proximal humerus tumor resections.
Questions/purposes
(1) What are the short-term functional results of the constrained reverse total shoulder prosthesis in terms of Musculoskeletal Tumor Society (MSTS), DASH, and Constant-Murley scores and ROM values? (2) What is the frequency of revision, using a competing-risks estimator to assess implant survival, and what were the causes of the revisions that occurred? (3) What proportion of patients experienced dislocations at short-term follow-up?
Methods
Between January 2014 and June 2017, we treated 55 patients with proximal humeral resections and reconstructions for malignant tumors. Of those, 33% (18) of patients were treated with the constrained, reverse total shoulder arthroplasty implant under study here. During that period, no other constrained reverse total shoulder implant was used; however, 13% (seven) of patients were treated with conventional (unconstrained) reverse total shoulder implants, 27% (15) had hemiarthroplasties, 15% (eight) of patients had biologic reconstructions with auto- or allografts and 13% (seven) underwent amputation. During the period in question, our general indications for use of the constrained device under study here were resection of the deltoid muscle/axillary nerve or the deltoid insertion on the humerus due to tumor invasion, or extensive rotator cuff and surrounding soft tissue resection that might result in shoulder instability. During this period, these indications were adhered to consistently. Four of 18 patients treated with the study implant died (three died with the implant intact) and none were lost to follow-up before 2 years, leaving 14 patients (seven women and seven men) for study at a median (range) follow-up of 35 months (25 to 65). Two authors evaluated the clinical and functional status of each patient with ROM (flexion, extension, internal and external rotation, abduction, and adduction) and MSTS, (range 0% to 100%), Constant-Murley (range 0% to 100%), and DASH (range 0 points to 100 points) scores. For the MSTS and Constant-Murley scores, higher percentage scores mean better functional outcome; and for the DASH score, a higher score means more severe disability. Radiographs were obtained at each visit and were used to look for signs of loosening, which we defined as progressive radiolucencies between visits, prosthetic component migration, and fragmentation/fracture of the cement. The Sirveaux classification was used to determine scapular notching. A competing risks analysis with 95% confidence intervals was performed to estimate the cumulative incidence of revision surgery, which we defined as any reoperation in which the implant was removed or changed for any reason, with patient mortality as a competing event.
Results
At the most recent follow-up, the median (range) MSTS score was 78% (50 to 90), the DASH score was 20 (8 to 65), and the Constant-Murley score was 53% (26 to 83). The median ROM was 75° in forward flexion (40 to 160), 78° in abduction (30 to 150), 35° in internal rotation (10 to 80), and 33° in external rotation (0 to 55). Postoperatively, two of 14 patients underwent or were supposed to undergo revision surgery, and the cumulative incidence of revision surgery was 18% for both 30 and 48 months (95% CI 2 to 45). During the study period, no patients reported instability, and no dislocations occurred.
Conclusions
Our findings are concerning because the revision risk with this constrained reverse total shoulder implant was higher than has been reported by others for other proximal humerus prostheses. The highly constrained design that helps prevent instability might also transmit increased stresses to the humeral component-bone interface, therefore making it susceptible to loosening. We believe that any other implant with a similar degree of constraint will have the same problem, and changing the indications for patient selection may not solve this issue. These theories need to be tested biomechanically, but our desire is to warn surgeons that while trying to prevent instability, one might trade one complication (instability) for another: aseptic loosening.
Level of Evidence
Level IV, therapeutic study.
Introduction
The proximal humerus is a common site for primary bone tumors [7, 22] and metastases [17]. Surgical treatment of the tumor with limb-salvage procedures usually results in a large bone defect and insufficient soft-tissue support, which poses a challenge for the surgeon in preserving function [24]. There are multiple options for reconstruction after resection, including endoprosthetic reconstruction with anatomic [5] or reverse [4, 13, 16] shoulder systems, osteoarticular allografts [28], allograft-prosthesis composites [1, 18, 20], arthrodesis [3, 11, 23], and the clavicula pro humeri procedure [2, 29]. Endoprosthetic reconstruction with reverse total shoulder systems are seeing wider use, but complication rates remain high, ranging between 20% and 45% [24, 26]. Surgery for the tumor usually involves resection of the rotator cuff and detachment of the teres major, latissimus dorsi, and pectoralis major tendons, and occasionally the deltoid muscle. Loss of these stabilizing structures leaves the prosthesis prone to instability and dislocations, which is the most frequently encountered complication after reverse total shoulder reconstruction [4, 8, 12], and the second most common cause of revision [14].
The Bayley-Walker (Stanmore Implants, Elstree, UK) prosthesis is a highly constrained, linked-glenoid reverse shoulder system (Fig. 1). Its unique design is believed to resist subluxation and dislocation, which are important issues after wide tumor resections, especially when the deltoid muscle and/or tendon is totally or partially resected because of tumoral invasion. Several studies with promising results have been published [12, 13, 21]. All the same, the global evidence base on this device is scanty, and because of the potential utility of this approach, we thought it important to assess our results with it.
Fig. 1.

Photograph of the Bayley-Walker (Stanmore Implants, Elstree, UK) prosthesis.
We therefore asked (1) What are the short-term functional results of the constrained reverse total shoulder prosthesis in terms of Musculoskeletal Tumor Society (MSTS), DASH, and Constant-Murley scores and ROM values? (2) What is the frequency of revision, using a competing-risks estimator to assess implant survival, and what were the causes of the revisions that occurred? (3) What proportion of patients experienced dislocations at short-term follow-up?
Patients and Methods
Our study was designed as a retrospective case series and was conducted at the Department of Orthopedics and Traumatology at Hacettepe University Hospital. With the approval of our institutional ethical committee, we searched our hospital’s database for patients who underwent resection and endoprosthetic reconstruction for a malignant tumor of the proximal humerus.
Patients and Study Flow
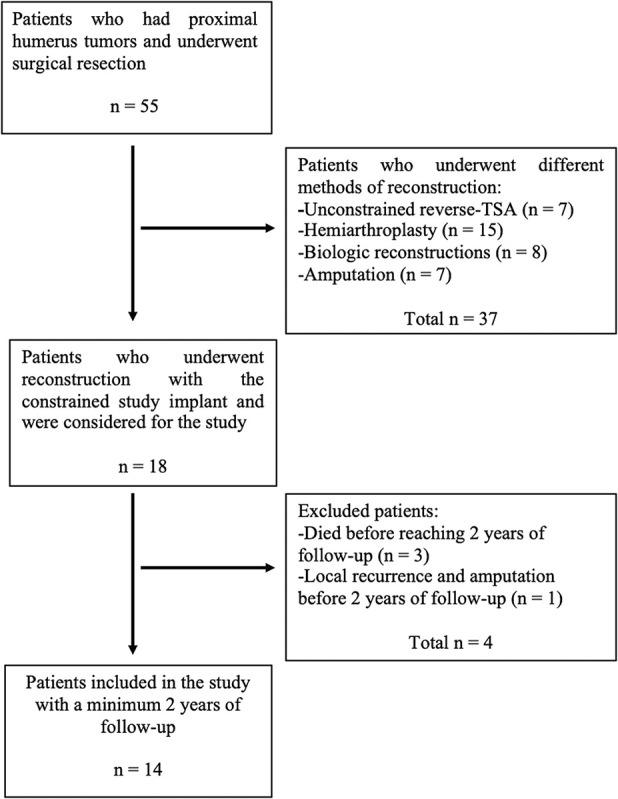
Between January 2014 and June 2017, 55 patients with proximal humeral resections and reconstructions for malignant tumors were treated. Of those, 33% (18) of patients were treated with the constrained, reverse total shoulder arthroplasty implant under study here (Bayley-Walker, Stanmore Implants, Elstree, UK). During that period, no other constrained reverse total shoulder implants were used; however, 13% (seven) of patients were treated with conventional (unconstrained) reverse total shoulder implants, 27% (15) of patients had hemiarthroplasties, 15% (eight) of patients had biologic reconstructions with auto- or allografts and 13% (seven) of patients underwent amputation.
During the period in question, our general indications for the use of the constrained device under study here were deltoid muscle/axillary nerve resection because of tumor invasion and resection of the deltoid insertion on humerus (completely or near completely). We also used this prosthesis when the rotator cuff/surrounding soft tissue resection was so extensive that in spite of deltoid preservation, we anticipated an unstable prosthetic shoulder after reconstruction. This decision had to be made intraoperatively at times. During that time, unconstrained reverse total shoulder arthroplasty was mainly used on patients who did not meet the criteria mentioned above and consequently, in whom instability was not anticipated. Hemiarthroplasty was preferred when the tumor was a minor lesion and limited to the proximal humerus. Our indications for the use of biologic reconstructions were younger age (younger than 18 years) and adequate remaining soft tissue for joint reconstruction. Forequarter amputation was performed when the axillary artery and brachial plexus were invaded by the tumor. During the period of this study, we adhered to these criteria for patient selection, but for a small number of patients, the decision was not clear, especially when choosing between constrained and unconstrained reverse total shoulder systems. Intraoperative stability assessment played an important role for these few patients.
In our study, we included patients who were treated with the study implant and who had a minimum follow-up of 2 years (Table 1). Four of 18 patients treated with the study implant died (three with the implant intact) and none were lost to follow-up before 2 years, leaving 14 patients (seven women and seven men) for study at a median (range) follow-up of 35 months (25 to 65) (Fig. 2). The median (range) age at the time of surgery was 51 years (16 to 79). The histologic diagnoses of the tumors were as follows: five metastases, four chondrosarcomas, two osteosarcomas, one multiple myeloma, one plasmacytoma, and one angiosarcoma.
Table 1.
Patients’ demographic, diagnostic, and surgical information
| Patient | Age (years) | Diagnosis | Follow-up period (months) | Resection length (cm) | MSTS resection level | Deltoid or axillary nerve preserved? | Attachment tube used? | DASH score | Constant-Murley score | MSTS score |
| 1 | 22 | Osteosarcoma | 65 | 16 | S3-4-5 | No | No | 52 | 28 | 53 |
| 2 | 79 | Metastatic (prostate cancer) | 26 | 15 | S3-4-5 | Yes | No | 13 | 66 | 87 |
| 3 | 54 | Chondrosarcoma | 44 | 11 | S3-4 | Yes | No | 13 | 83 | 90 |
| 4 | 48 | Chondrosarcoma | 58 | Total humerus | S1-2-3-4-5 | Yes | Yes | 65 | 53 | 50 |
| 5 | 16 | Chondrosarcoma | 54 | 16 | S3-4-5 | Yes | Yes | 10 | 33 | 83 |
| 6 | 56 | Multiple myeloma | 50 | 15 | S3-4-5 | Yes | No | 27 | 38 | 83 |
| 7 | 31 | Angiosarcoma | 26 | 13 | S3-4-5 | Yes | No | 8 | 59 | 73 |
| 8 | 55 | Metastatic (lung cancer) | 26 | 10 | S3-4 | Yes | No | 11 | 66 | 90 |
| 9 | 29 | Chondrosarcoma | 32 | 7 | S1-2-3 | No | No | 46 | 26 | 60 |
| 10 | 57 | Metastatic (gastric cancer) | 26 | 16 | S3-4-5 | Yes | Yes | 9 | 53 | 87 |
| 11 | 29 | Metastatic (breast cancer) | 40 | 12 | S3-4 | Yes | Yes | 34 | 58 | 73 |
| 12 | 16 | Osteosarcoma | 37 | 17 | S3-4-5 | No | No | 36 | 34 | 60 |
| 13 | 77 | Metastatic (esophageal cancer) | 25 | 10 | S3-4 | Yes | No | 29 | 34 | 67 |
| 14 | 57 | Plasmacytoma | 28 | 11 | S3-4 | Yes | Yes | 9 | 70 | 90 |
Fig. 2.
The study flow chart according to the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) statement.
Two authors (RMC, SB), who helped to care for the patients, retrospectively obtained the included patients’ data from the hospital’s database. The data included the patients’ demographic information, detailed preoperative and postoperative orthopaedic examination findings, as well as detailed information on the surgical process, histologic diagnosis, and subsequent complications.
All patients were evaluated preoperatively in the oncology department (or the pediatric oncology department, based on age), and adjuvant or neoadjuvant therapies were administered accordingly.
Surgical Treatment and Aftercare
All surgical procedures were performed by one of two surgeons (AMT, MA) who specialize in oncologic surgery. The surgeons used the deltopectoral approach or its extended variants in each patient. The deltoid was retracted laterally and the cephalic vein was mobilized and protected. The muscular insertions on the proximal humerus were identified, released, and tagged. The axillary nerve was identified and protected if wide surgical margins could be achieved by doing so. The humerus was resected at the level determined preoperatively (Fig. 3A-C). All patients received a Bayley-Walker linked-glenoid reverse shoulder prosthesis, and, if necessary for muscular reattachment, a MUTARS® attachment tube (Implantcast GmbH, Buxtehude, Germany). The MUTARS® attachment tube was wrapped around the diaphyseal portion of the prosthesis to aid in the reinsertion of detached muscles, mainly the latissimus dorsi and the pectoralis major. It was not used around the articulating part of the prosthesis, therefore, we did not consider it as an additional constraining device. The resected bone length was documented and the resection margin was classified according to the MSTS system [10]. After one patient with total humerus resection was excluded (Patient 4), the median (range) resection length was 13 cm (7 to 17) (Table 1).
Fig. 3 A-C.
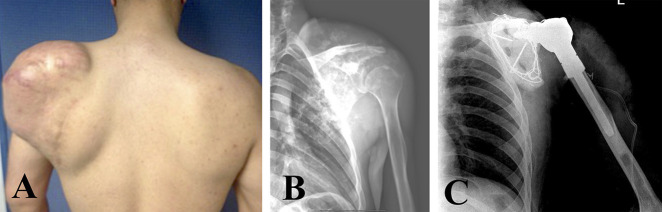
Patient 9 was diagnosed with and subsequently treated for chondrosarcoma as shown in this (A) a clinical photograph, (B) preoperative radiograph and (C) postoperative radiograph. A color image accompanies the online version of this article.
After surgery, all patients were asked to keep their shoulders in a sling for 6 weeks. Passive ROM exercises were initiated as soon as the surgical incision healed, and active ROM exercises combined with strengthening exercises were initiated at the sixth week. Postoperative follow-up visits were scheduled at the 3 weeks, 6 weeks, 3 months, 6 months, 12 months, and yearly thereafter. The clinical and functional status of each patient was evaluated at each follow-up visit, using active and passive ROM (flexion, extension, internal and external rotation, abduction, and adduction; clinically measured as a combination of glenohumeral and scapulothoracic motions), the MSTS score [10], the Constant-Murley score [6], and the DASH score [15]. The MSTS is a questionnaire designed to measure functional outcome and quality of life after treatment of musculoskeletal tumors. Each patient is assigned a score between 0 to 5 in six different categories: pain, function, emotional acceptance, hand positioning, dexterity, and lifting ability. Each patient can obtain a maximum of 30 points; we transformed this into a percentage of the maximum (0% to 100%), with higher scores indicating a better functional status. The Constant-Murley score has four domains: including pain (15 points), activities of daily living (20 points), mobility (40 points), and strength (25 points); the total score ranges between 0 to 100, and a patient’s score is reported as a percentage of the total with a range of 0% to 100%. As with the MSTS, higher scores mean better functional status. DASH is a self-report questionnaire that assesses the ability to perform certain activities with the upper extremity. It has 30 questions, and higher scores indicate a greater level of disability. Multiple radiographs were obtained (AP, true AP, and scapular Y views). We used the radiographs to look for signs of loosening, which we defined as progressive radiolucencies between visits, prosthetic component migration, and fragmentation/fracture of the cement. We used the Sirveaux classification [27], which uses a true AP view of the shoulder to evaluate the bone defect in the inferior part of the glenoid component, to determine scapular notching. The bone defect, which is usually called the scapular notch, is graded between 1 to 4. Increasing grades mean more severe notching, Grade 1 refers to a defect that only involves the inferior pillar of the scapular neck while in Grade 4, the notch extends under the glenoid baseplate.
Study Outcomes
The primary study outcome was functional status, as measured by the MSTS, DASH, and Constant-Murley scores as well as ROM values. The secondary study outcomes were prosthesis survival and the reasons for revision surgery, which we defined as any reoperation in which the implant was removed, for any reason.
Statistical Analysis
The statistical analysis was performed in “R: A language and environment for statistical computing, v3.6.2” (R Foundation for Statistical Computing, Vienna, Austria). Continuous parameters such as functional scores and ROM in each direction are presented with their descriptive statistical values (median and range). Considering the fact that the incidence of mortality is high in our cohort, we performed a competing risks analysis to estimate the cumulative incidence of revision surgery (which, again, we defined as any reoperation in which the implant was removed, for any reason) with patient mortality as a competing event [30]. Results are presented as cumulative incidences (%) with 95% confidence intervals.
Results
The median (range) MSTS score at most-recent follow-up was 78% (50 to 90), the DASH score was 20 (8 to 65), and the Constant-Murley score was 53% (26 to 83) (Table 1). The median (range) active ROM was 75° in forward flexion (40 to 160), 78° in abduction (30 to 150), 35° in internal rotation (10 to 80), and 33° in external rotation (0 to 55).
Postoperatively, two of 14 patients underwent revision surgery. Cumulative incidence of revision surgery was 18% for both 30 and 48 months (95% CI 2 to 45) (Fig. 4). Patient 1 underwent revision of the humeral component because of aseptic loosening at 20 months postoperatively. Patient 12 had a diagnosis of symptomatic loosening of the humeral component at 30 months, but the patient decided not to undergo revision surgery (Fig. 5).
Fig. 4.
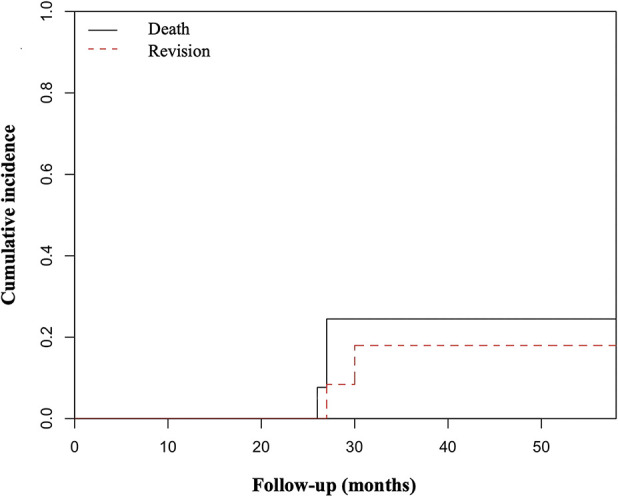
The competing risks analysis graphic, showing the cumulative incidence of revision surgery with patient mortality as a competing event.
Fig. 5.
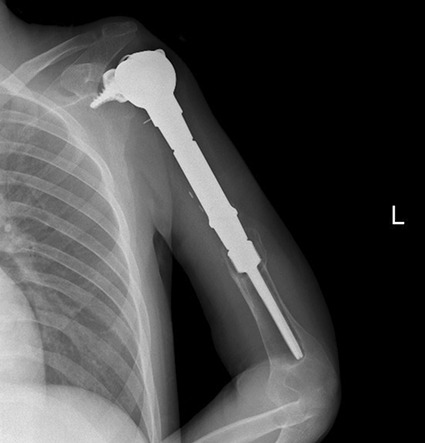
This radiograph shows symptomatic humeral component loosening in Patient 12 at 30 months of follow-up.
No subluxations, dislocations, or periprosthetic joint infections occurred. One patient had Grade 2 and another had Grade 1 scapular notching on the Sirveaux classification.
Discussion
Reconstruction after proximal humerus resections for malignant tumors can be performed in several ways, and reverse total shoulder arthroplasty is seeing wider use more recently [19, 24, 25]. Despite the fact that reverse total shoulder arthroplasty allows patients to achieve shoulder function in situations where anatomic shoulder arthroplasty implants would not deliver this, such as in the presence of large resections [4, 8], complications occur with disturbing frequency; major complications occur in 20% to 45% of patients [24, 26]. The most frequent complication is instability (subluxations and dislocations) [9, 21, 24, 26]. Henderson et al. [14] analyzed the failure modes of tumor endoprostheses in a large number of patients, and they found that soft tissue-related failures, including instability, were the second most common reason for revision of proximal humeral prostheses. The Bayley-Walker prosthesis, with its highly constrained linked-glenoid design, was designed to try to mitigate instability-related complications. Although in the short term, we found that most patients in our small series generally achieved fair/good shoulder scores, the proportion of patients who developed aseptic loosening was higher than has been reported by others [4, 5, 8, 14].
There are several limitations to this study. Most important is selection bias associated with a retrospective, non-randomized study design. We used the device studied here only in a minority of patients who underwent proximal humerus resections, and generally we used this device in patients who underwent larger resections. Even though the patient group is heterogenous, we tried to apply consistent criteria for patient selection, and we hope that gives readers a better sense of how they can interpret our results, and to which patients our findings might best apply. Another limitation is the lack of a control group; without one, it is impossible to know whether the results of this device would be better or worse than, for example, an unconstrained reverse total shoulder implant in these patients. However, we believe that in our patient group and with our criteria, an unconstrained reverse total shoulder prosthesis would not prevent instability because of the wide resection and soft tissue compromise. We hope that future studies will compare the constrained and unconstrained implants with larger, randomized groups. Another limitation here is the small study size; although this report is the largest of which we are aware, it still was too small to ascertain the frequency of a number of less common but very important complications. For example, there were no infections in this study; unquestionably they occur, and a larger study would better be able to characterize their frequency. Related to this is that our follow-up duration was short, and so we expect that as time passes, further complications are likely to appear, and more patients may develop loosening or undergo revision for other causes. We plan to follow this group closely because of the high risk of loosening we observed early.
Our patients generally achieved good scores on the MSTS, DASH and Constant-Murley scales relative to other similar reports (Table 2) [4, 12, 13, 16, 21]. Anecdotally, we observed that the patients with resection of the axillary nerve/deltoid muscle seemed to do more poorly, which is not surprising. Two prior studies [12, 13] report heterogenous cohorts including patients with non-constrained and constrained implants, and as would be expected, the subgroups with constrained implants had worse functional outcomes. Two series [4, 16] reported their results with non-constrained implants, and the mean ROM values were higher than our study. Functional scores seemed comparable between those two series [4, 16] and ours; but we must take care in interpreting these results because the patient populations in these studies include some patients with very large resections who might have been considered appropriate for constrained reverse shoulder prosthesis if they had been treated by our team.
Table 2.
Functional results and complications reported by different studies
| Study | Implant | Number of patients | Mean follow-up period (months) | Mean ROM | Mean MSTS score | Mean TESS score | Component loosening | Instability | Other complications |
| Griffiths et al. [12]a | Bayley-Walker Constrained (Stanmore Implants, Elstree, UK) | 4 a | 15 months (12-18 months) | Not specified | 78% (73 to 87) | 80% (range not specified) | Not specified | None (compared with 26% for unconstrained prosthesis) | Not specified |
| Kaa et al. [16] | Standard RSR (DePuy Synthes, Warsaw, IN, USA) | 10 | 46 months (12 to 136) | Abduction 78° (30 to 150) | 77% (60 to 90) | 70% (30 to 91) | Two patients | One patient (dislocated twice) | Two superficial infections |
| Flexion: 98° (45 to 180) | One deep infection | ||||||||
| Internal rotation 51° (10 to 80) | Two perioperative pathologic fractures | ||||||||
| Bonnevialle et al. [4] | Seven Delta Xtend™ and 1 Delta3 (DePuy Synthes) | 10 | 42 months (24 to 84) | Flexion: 122° (40 to 170) | 20 points (7 to 29) | Not specified | One patient | None | Four scapular notching |
| Two Aequalis™ Reversed (Tornier, Montbonnot, France) | External rotation: -2° (-20 to 30) | Two radiolucency | |||||||
| Internal rotation: L4 (Greater Troch.-T7) | |||||||||
| Guven et al. [13]a | Bayley-Walker Constrained (Stanmore Implants) | 3a | 19 months (14 to 22) | Flexion: 43° (30 to 70) | 75% (60 to 93) | Not specified | None | None (compared with two subluxations and one inferior instability in the non-constrained group) | Not specified |
| Abduction: 45° (30 to 65) | |||||||||
| External rotation: 7° (0 to 20) | |||||||||
| Maclean et al. [21] | Bayley-Walker Constrained (Stanmore Implants) | 8 | 49 months (36 to 90) | Abduction: 62° | 60% (43 to 73) | 63% (42 to 74) | One patient (asymptomatic loosening) | None | One neuropathic pain |
| Flexion: 71° | |||||||||
| External rotation: 50° | |||||||||
| Internal rotation:50° | |||||||||
| Current Study | Bayley-Walker Constrained (Stanmore Implants) | 14 | Median: 36 months (25 to 65) | Median: | Median: 78% (50 to 90) |
Not specified | Two patients | None | Two scapular notching |
| Flexion:75o (40 to 160) | |||||||||
| Abduction:78° (30 to 50) | |||||||||
| -Internal rotation: 35° (10 to 80) |
These two studies include patients with constrained and unconstrained prostheses. We included the results of the subgroups with a constrained implant.
Two of our 14 patients underwent revision surgery during the follow-up period, both for aseptic loosening of the humeral stem (Patient 1 at 27 months, and Patient 12 at 30 months). Among the three small series that included constrained implants [12, 13, 21], only one [21] reported component loosening among the patients, and it was reported to be asymptomatic. This difference probably is because different indications were applied in the different studies, and all the studies reported only short-term results. Similar to our study, no instability-related events occurred with constrained implants in those studies [12, 13, 21]. Considering the fact that lower functional scores were obtained with constrained implants by many different studies, as we discussed in the previous paragraph, it appears that with this approach, we may sacrifice some function as we seek to prevent instability. The cumulative incidence of revision surgery was 18% for both 30 and 48 months (95% CI 2 to 45). To our knowledge, no other study has performed a survival analysis for the Bayley-Walker prosthesis; however, our mean follow-up period was not long enough to predict the long-term prosthesis survival. A concerning outcome of our study that should be strongly emphasized is that 14% of patients underwent revision for aseptic loosening; this incidence is notably higher than that reported by a study on the failure modes of tumor endoprostheses [14] (Henderson Type 2 failures [14] were responsible for revision in 2.6% of all patients and constituted 15.3% of all revisions). The study also reported a total revision rate of 17% for proximal humerus prostheses; soft-tissue related failures (including instability or dislocations, identified as Henderson Type 1 failure [14]) were the second most common reason, accounting for 24% of all revisions. Biomechanical studies are needed to prove this, but we believe that the highly constrained design of this implant exerts increased stresses on the component-bone interface, making it susceptible to aseptic loosening. We also believe this likely is not a manufacturer-related issue, and that this same problem may arise with different implants that employ a similar degree of constraint. For this reason, we caution surgeons that by attempting to prevent instability with more constrained implants, one might trade one complication for another.
We observed no instability-related complications in our patients during the follow-up period Instability tends to occur earlier rather than later after proximal humerus arthroplasty [14], and our median (range) follow-up period of 35 months (25-65) without any dislocations shows that this implant might reduce instability-related events, at least in the short term.
In this small series at short-term follow-up, we found that with a constrained reverse total shoulder arthroplasty, our initial results are favorable in terms of function compared with previous similar studies (Table 2). This device is mainly used for preventing instability, and in the short term we did not observe any subluxation or dislocations. However, our study suggested that the decision to use this device comes with an increased revision risk because of aseptic loosening, which is not a risk that is well characterized in prior studies. We believe that this implant still can have a place in orthopaedic tumor surgery practice, especially when the risk of instability is high after wide resection and no other implant is likely to work, but the indications may need to be revised. We suggest that limiting the age group and excluding younger patients may result in lower loosening rates. The high frequency of implant loosening is concerning, and when treating younger, more-active patients, alternative methods of reconstruction should be considered. Studies with longer follow-up periods are needed to define the frequencies of important complications that we did not see in this small series, and to understand better the biomechanical basis of the observed high rate of loosening rate we observed.
Footnotes
Each author certifies that neither he, nor any member of his immediate family, has funding or commercial associations (consultancies, stock ownership, equity interest, patent/licensing arrangements, etc.) that might pose a conflict of interest in connection with the submitted article.
Clinical Orthopaedics and Related Research® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA approval status, of any drug or device before clinical use.
Each author certifies that his institution approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
References
- 1.Abdeen A, Hoang BH, Athanasian EA, Morris CD, Boland PJ, Healey JH. Allograft-prosthesis composite reconstruction of the proximal part of the humerus: functional outcome and survivorship. J Bone Joint Surg Am. 2009;91:2406-2415. [DOI] [PubMed] [Google Scholar]
- 2.Barbier D, De Billy B, Gicquel P, Bourelle S, Journeau P. Is the clavicula pro humero technique of value for reconstruction after resection of the proximal humerus in children? Clin Orthop Relat Res. 2017;475:2550-2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bilgin SS. Reconstruction of proximal humeral defects with shoulder arthrodesis using free vascularized fibular graft. J Bone Joint Surg Am. 2012;94:e94. [DOI] [PubMed] [Google Scholar]
- 4.Bonnevialle N, Mansat P, Lebon J, Laffosse JM, Bonnevialle P. Reverse shoulder arthroplasty for malignant tumors of proximal humerus. J Shoulder Elbow Surg. 2015;24:36-44. [DOI] [PubMed] [Google Scholar]
- 5.Cannon CP, GU Paraliticci, Lin PP, Lewis VO, Yasko AW. Functional outcome following endoprosthetic reconstruction of the proximal humerus. J Shoulder Elbow Surg. 2009;18:705-710. [DOI] [PubMed] [Google Scholar]
- 6.Constant CR, Murley AH. A clinical method of functional assessment of the shoulder. Clin Orthop Relat Res. 1987:160-164. [PubMed] [Google Scholar]
- 7.Dahlin DC, Unni KK. Bone Tumors: General Aspects and Data on 8,542 Cases. Springfield, IL: Thomas; 1986. [Google Scholar]
- 8.De Wilde L, Boileau P, Van der Bracht H. Does reverse shoulder arthroplasty for tumors of the proximal humerus reduce impairment? Clin Orthop Relat Res. 2011;469:2489-2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Wilde LF, Van Ovost E, Uyttendaele D, Verdonk R. [Results of an inverted shoulder prosthesis after resection for tumor of the proximal humerus] [in French]. Rev Chir Orthop Reparatrice Appar Mot. 2002;88:373-378. [PubMed] [Google Scholar]
- 10.Enneking WF, Dunham W, Gebhardt MC, Malawar M, Pritchard DJ. A system for the functional evaluation of reconstructive procedures after surgical treatment of tumors of the musculoskeletal system. Clin Orthop Relat Res. 1993:241-246. [PubMed] [Google Scholar]
- 11.Fuchs B, O'Connor MI, Padgett DJ, Kaufman KR, Sim FH. Arthrodesis of the shoulder after tumor resection. Clin Orthop Relat Res. 2005:202-207. [DOI] [PubMed] [Google Scholar]
- 12.Griffiths D, Gikas PD, Jowett C, Bayliss L, Aston W, Skinner J, Cannon S, Blunn G, Briggs TW, Pollock R. Proximal humeral replacement using a fixed-fulcrum endoprosthesis. J Bone Joint Surg Br. 2011;93:399-403. [DOI] [PubMed] [Google Scholar]
- 13.Guven MF, Aslan L, Botanlioglu H, Kaynak G, Kesmezacar H, Babacan M. Functional outcome of reverse shoulder tumor prosthesis in the treatment of proximal humerus tumors. J Shoulder Elbow Surg. 2016;25:e1-6. [DOI] [PubMed] [Google Scholar]
- 14.Henderson ER, Groundland JS, Pala E, Dennis JA, Wooten R, Cheong D, Windhager R, Kotz RI, Mercuri M, Funovics PT, Hornicek FJ, Temple HT, Ruggieri P, Letson GD. Failure mode classification for tumor endoprostheses: retrospective review of five institutions and a literature review. J Bone Joint Surg Am. 2011;93:418-429. [DOI] [PubMed] [Google Scholar]
- 15.Hudak PL, Amadio PC, Bombardier C. Development of an upper extremity outcome measure: the DASH (disabilities of the arm, shoulder and hand) [corrected]. The Upper Extremity Collaborative Group (UECG). Am J Ind Med. 1996;29:602-608. [DOI] [PubMed] [Google Scholar]
- 16.Kaa AK, Jorgensen PH, Sojbjerg JO, Johannsen HV. Reverse shoulder replacement after resection of the proximal humerus for bone tumours. Bone Joint J. 2013;95:1551-1555. [DOI] [PubMed] [Google Scholar]
- 17.Kakhki VR, Anvari K, Sadeghi R, Mahmoudian AS, Torabian-Kakhki M. Pattern and distribution of bone metastases in common malignant tumors. Nucl Med Rev Cent East Eur. 2013;16:66-69. [DOI] [PubMed] [Google Scholar]
- 18.King JJ, Nystrom LM, Reimer NB, Gibbs CP, Jr., Scarborough MT, Wright TW. Allograft-prosthetic composite reverse total shoulder arthroplasty for reconstruction of proximal humerus tumor resections. J Shoulder Elbow Surg. 2016;25:45-54. [DOI] [PubMed] [Google Scholar]
- 19.Kitagawa Y, Thai DM, Choong PF. Reconstructions of the shoulder following tumour resection. J Orthop Surg (Hong Kong). 2007;15:201-206. [DOI] [PubMed] [Google Scholar]
- 20.Lozano-Calderon SA, Chen N. Proximal humerus allograft prosthetic composites: technique, outcomes, and pearls and pitfalls. Curr Rev Musculoskelet Med. 2015;8:324-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maclean S, Malik SS, Evans S, Gregory J, Jeys L. Reverse shoulder endoprosthesis for pathologic lesions of the proximal humerus: a minimum 3-year follow-up. J Shoulder Elbow Surg. 2017;26:1990-1994. [DOI] [PubMed] [Google Scholar]
- 22.Niu X, Xu H, Inwards CY, Li Y, Ding Y, Letson GD, Bui MM. Primary bone tumors: Epidemiologic comparison of 9200 patients treated at Beijing Ji Shui Tan Hospital, Beijing, China, with 10 165 patients at Mayo Clinic, Rochester, Minnesota. Arch Pathol Lab Med. 2015;139:1149-1155. [DOI] [PubMed] [Google Scholar]
- 23.Padiolleau G, Marchand JB, Odri GA, Hamel A, Gouin F. Scapulo-humeral arthrodesis using a pedicled scapular pillar graft following resection of the proximal humerus. Orthop Traumatol Surg Res. 2014;100:177-181. [DOI] [PubMed] [Google Scholar]
- 24.Potter BK, Adams SC, Pitcher JD, Jr., Malinin TI, Temple HT. Proximal humerus reconstructions for tumors. Clin Orthop Relat Res. 2009;467:1035-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodl RW, Gosheger G, Gebert C, Lindner N, Ozaki T, Winkelmann W. Reconstruction of the proximal humerus after wide resection of tumours. J Bone Joint Surg Br. 2002;84:1004-1008. [DOI] [PubMed] [Google Scholar]
- 26.Sirveaux F. Reconstruction techniques after proximal humerus tumour resection. Orthop Traumatol Surg Res. 2019;105:S153-S164. [DOI] [PubMed] [Google Scholar]
- 27.Sirveaux F, Favard L, Oudet D, Huquet D, Walch G, Mole D. Grammont inverted total shoulder arthroplasty in the treatment of glenohumeral osteoarthritis with massive rupture of the cuff. Results of a multicentre study of 80 shoulders. J Bone Joint Surg Br. 2004;86:388-395. [DOI] [PubMed] [Google Scholar]
- 28.Squire G, Grundy TJ, Ferran NA, Harper WM, Ashford RU. Long-term survival of proximal humerus allografts for reconstruction following resection of malignant bone tumours. Acta Orthop Belg. 2013;79:260-265. [PubMed] [Google Scholar]
- 29.Tsukushi S, Nishida Y, Takahashi M, Ishiguro N. Clavicula pro humero reconstruction after wide resection of the proximal humerus. Clin Orthop Relat Res. 2006;447:132-137. [DOI] [PubMed] [Google Scholar]
- 30.Wongworawat MD, Dobbs MB, Gebhardt MC, Gioe TJ, Leopold SS, Manner PA, Rimnac CM, Porcher R. Editorial: Estimating survivorship in the face of competing risks. Clin Orthop Relat Res. 2015;473:1173-1176. [DOI] [PMC free article] [PubMed] [Google Scholar]