Background:
Short-term surgical missions (STSMs) enable visiting surgeons to help address inequalities in the provision of surgical care in resource-limited settings. One criticism of STSMs is a failure to obtain informed consent from patients before major surgical interventions. We aim to use collective evidence to establish the barriers to obtaining informed consent on STSMs and in resource-limited settings and suggest practical solutions to overcome them.
Methods:
A systematic review was performed using PubMed and Web of Science databases and following Preferred Reporting Items for Systematic Review and Meta-Analysis guidelines. In addition to the data synthesized from the systematic review, we also include pertinent data from a recent long-term follow-up study in Ethiopia.
Results:
Of the 72 records screened, 11 studies were included in our review. The most common barrier to obtaining informed consent was a paternalistic approach to medicine and patient education. Other common barriers were a lack of ethics education among surgeons in low-income and middle-income countries, cultural beliefs toward healthcare, and language barriers between the surgeons and patients. Our experience of a decade of reconstructive surgery missions in Ethiopia corroborates this. In a long-term follow-up study of our head-and-neck patients, informed consent was obtained for 85% (n = 68) of patients over a 14-year period.
Conclusions:
This study highlights the main barriers to obtaining informed consent on STSMs and in the resource-limited setting. We propose a checklist that incorporates practical solutions to the most common barriers surgeons will experience, aimed to improve the process of informed consent on STSMs.
INTRODUCTION
The delivery of safe and affordable surgical care plays a vital role in improving and maintaining population health and constitutes a crucial component of a properly functioning healthcare system.1 Unfortunately, there is a significant discrepancy between surgical need and the availability of safe surgical care. Approximately 5 billion people worldwide lack access to safe and affordable surgical care.2 The majority of these people live in low-income and middle-income countries (LMICs), where 77.2 million disability-adjusted life years could be saved by the availability of effective surgical care.3
In response to this well-established need, short-term surgical missions (STSMs) are one method by which international teams can assist local surgeons in the provision of essential surgical and anesthetic care in LMICs.4 Approximately 400 nongovernmental organizations co-ordinate up to 20 surgical missions every year in LMICs.5,6 However, STSMs are not without ethical issues; one of them is obtaining informed consent,7 a process that nurtures 2 fundamental moral values: patient well-being and patient autonomy.8
To obtain valid informed consent, a surgeon must explain the details of a procedure to the patient, including possible and serious complications, ensure that the patients understand this information, and can subsequently recall it, and finally the patient needs to voluntarily authorize the procedure.9 In high-income countries, this legal framework has gained universal acceptance and is applied in a surgeon’s everyday practice.10 Medical and surgical associations provide guidelines that practitioners need to adhere to when obtaining consent; furthermore, they also provide guidelines for decision-making in difficult situations.11 The Royal College of Surgeons of England has recently also issued a set of guidelines for working overseas.12
Unfortunately, in the high-pressure environment of an STSM in a resource-limited setting, the same standards for obtaining informed consent are not always met. This may lead to patient distress and confusion, mismatch of expectations, and ultimately a lack of trust in the visiting surgical team.
Understanding the barriers to informed consent on STSMs is essential for any visiting surgical team, so they can build a robust informed consent process in line with local cultural expectations and guidance from local authorities.
The aim of this systematic review is to establish the main barriers to obtaining informed consent on STSMs abroad. This has been augmented with personal experience of improving consent over a decade of surgical missions treating complex facial deformity in Ethiopia. The authors also outline a checklist of practical solutions to the most common barriers encountered.
METHODS
A systematic review was performed in accordance with the Preferred Reporting Items for Systematic Review and Meta-Analysis recommendations.13
Search Strategy
The Web of Science and PubMed electronic databases were searched for full-text English articles. Both databases were searched on October 10, 2019, and included all articles from the beginning of each database to that date (PubMed, 1996, and Web of Science, 1997). The references for each publication were hand searched by authors U.Č. and C.S.H. to identify further relevant publications. References were combined and organized and duplicates removed. A Preferred Reporting Items for Systematic Review and Meta-Analysis flow diagram was created to summarize the results of the search strategy and the subsequent screening process (Fig. 1).13
Fig. 1.
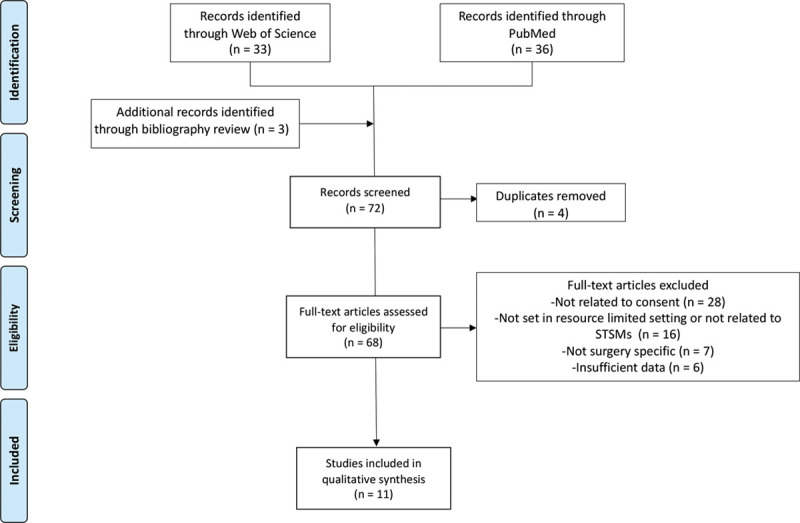
Our search strategy shown as a Preferred Reporting Items for Systematic Review and Meta-Analysis flow diagram.
Study Eligibility
Studies were included if they contained data relating to barriers to obtaining informed consent on STSMs or in the LMIC setting. Studies were excluded if they did not relate to informed consent for surgical procedures (eg, medical procedures or medical research), did not occur in resource-limited settings, and did not contain sufficient data to facilitate comparative analysis.
Data Extraction
Two authors (U.Č., C.S.H.) independently screened studies by title and abstract to identify eligible articles for review. A purpose-designed checklist was used.
Additional Data
In addition to the data synthesized from the systematic review, we included pertinent data from a recent retrospective cohort study to establish long-term patient outcomes following surgery to treat complex facial disfigurement in Ethiopia.
Between February 16, 2017, and March 2, 2017, a team of 4 medical volunteers followed up 80 patients over a 2-week period. All patients were operated on in Addis Ababa by the charity Project Harar between 2002 and 2016. As part of the follow-up, all adult patients were asked if they felt they had been appropriately consented for their surgical procedure and for any feedback relating to their experience of the consent process.
Bias Review
All studies included in the review were assessed at the study level using an adaptation of the Cochrane Risk of Bias Tool to identify potential biases, and the results were taken into account when interpreting the results.14 We looked at selection bias, recall bias, and observer bias summarized in Table 1. We also assessed our own study for any risk of bias.
Table 1.
Definitions of Potential Biases Encountered in the Review
| Bias | Definition |
|---|---|
| Selection bias | When the study population is different from that of the general population or of the population of interest, leading to a systematic error in an association or outcome.16 |
| Recall bias | Systematic error due to differences in accuracy or completeness of recall of memories pertaining to past events or experiences.17 |
| Observer bias | The difference between the actual, true value and the value noted due to observer variation.18 |
| Perception bias | The tendency of observers to be subjective about people and events, causing biased information to be collected in a study or the biased interpretation of the study’s results.19 |
RESULTS
Of the 72 records screened (Fig. 1), 11 studies were included in our review, and their main characteristics are summarized in Table 2. Four studies related specifically to informed consent practice on STSMs, while the others dealt with informed consent in resource-limited settings outside STSMs. In 9 articles, consent formed the primary focus of the study, 1 article focused on the patients understanding of the surgical procedure, and 1 dealt with patient satisfaction and social impact of the surgeries.
Table 2.
Main Characteristics of the Studies Included in the Systematic Review
| Article | Country | Type of Study | STSM Specific | Study Participants | No. Participants |
|---|---|---|---|---|---|
| Agu et al20 | Nigeria | Survey study | No | Patients | 2,545 |
| Irabor et al21 | Nigeria | Survey study | No | Doctors | 47 |
| Nnabugwu et al22 | Nigeria | Survey study | No | Patients | 369 |
| Ochieng et al23 | Uganda | Semistructured interview | No | Doctors | 133 |
| Ochieng et al24 | Uganda | Semistructured interview | No | Patients | 371 |
| Sanwal et al25 | India | Survey study | No | Patients | 100 |
| Sceats et al26 | Guatemala | Mixed methods | Yes | Patients | 13 |
| Sutton et al27 | Haiti | Survey study | Yes | Patients | 55 |
| Teshome et al28 | Ethiopia | Survey study | No | Patients | 229 |
| Walker et al29 | Honduras | Survey study | Yes | Patients | 71 |
| White et al30 | Benin | Semistructured interview | Yes | Patients | 71 |
Survey studies were defined as those relying on questionnaires and surveys. The mixed methods study was conducted for the patients, while the process of obtaining informed consent was taped and analyzed using descriptive statistics.
Doctors were the subjects of 2 studies, totaling 180 participants, while patients were the subjects of the remaining 9, totaling 3824 participants.
The majority of the studies were based in African countries (n = 7), followed by Central America and the Caribbean (n = 3), and lastly 1 study was based in India (Fig. 2).
Fig. 2.
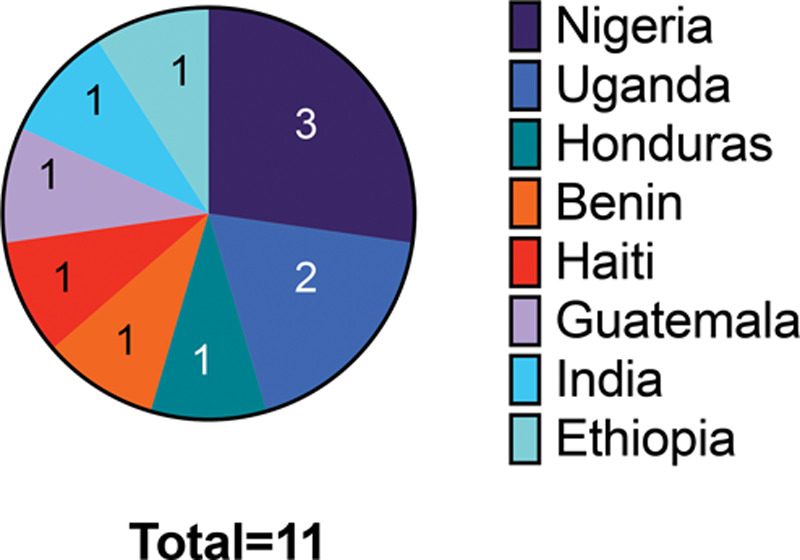
The countries in which the studies took place, and the number of studies that took place in each country.
Eight of the studies specified a surgical subspecialty. These included general surgery (n = 5), orthopedics (n = 4), and obstetrics and gynecology (n = 3) (Fig. 3).
Fig. 3.
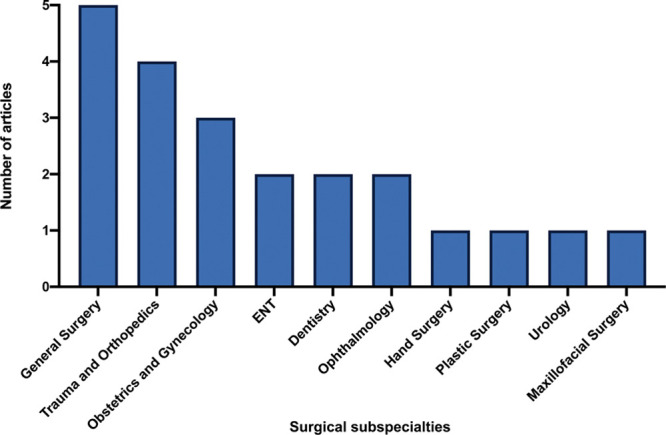
The surgical subspecialties that were included in 8 of the studies, and the number of times each subspecialty appears in the studies. The remaining studies did not specify a surgical subspecialty.
Seven of the studies consisted of surveys, 3 were semistructured interviews, and the last study adopted a mixed methods approach.
The identified barriers to informed consent can be divided into 3 main categories: barriers related to the medical practice, process-centered barriers, and patient-centered barriers, as defined by Taylor.15
Barriers Related to Medical Practice
A paternalistic approach to medicine in LMICs was the most commonly identified barrier relating to medical practice (n = 6). Other barriers included a lack of expertise of the medical practitioners responsible for gaining informed consent (n = 3) and a lack of ethics education received in LMIC medical schools (n = 2) (Fig. 4).
Fig. 4.
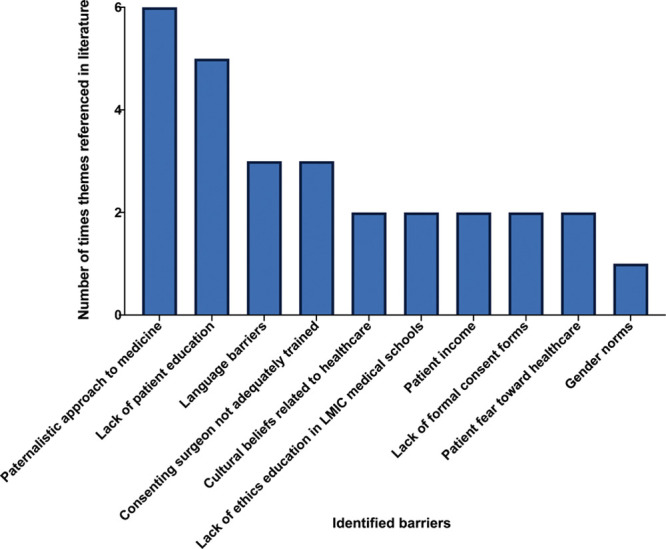
The barriers to obtaining informed consent identified from the articles included in this review, and the total number of times they were mentioned.
Process-centered Barriers
Process-centered barriers consisted of language barriers between patients and doctors (n = 3) and a lack of appropriate consent forms (n = 2) (Fig. 4).
Patient-related Barriers
Lack of education in patients was a clear obstacle to informed consent (n = 5) followed by cultural beliefs related to healthcare (n = 2), patients’ fear toward healthcare (n = 2), patients’ income and socioeconomic status (n = 2), and gender norms present in the patient’s culture (n = 1) (Fig. 4).
Strategies that Improved the Consent Process
Three studies recommended strategies to improve the process of obtaining informed consent.26–28 The 4 recommended strategies were using visual aids such as videos, providing a context for the surgery based on the patient’s everyday life (eg, explaining how will the recovery affect a patient’s ability to work or how long they will need to stay away from their family), making the patient repeat the most important points, and pausing after each point.
Patient Experience of Consent after Discharge from a Short-term Reconstructive Mission
During the follow-up mission, 80 patients were directly asked about their experience of being consented. Mean follow-up time after surgery was 48 months (range 12–180 months). Fifty-seven patients were men and 33 women, and the average age was 26 (±12) years. Informed consent was obtained for 85% (n = 68) patients.
Five patients did not consent for the surgery, but only 1 explained that they would have liked more information about the procedure. In this group, 3 patients were satisfied with the results; for 1 patient, the results exceeded expectations; and lastly, one patient’s expectations were not met.
Bias
The number of publications on the topic of informed consent in a resource-poor setting was low, increasing the risk of publication bias. As the gathered data was mainly qualitative, the authors might have been subject to perception bias while extracting data. Furthermore, as we only included studies in English, there is a risk of selection bias. Table 3 details the risk of recall, selection, and observer bias for each study included in this review.
Table 3.
Risk of Bias of Each of the Studies
| Article | Selection Bias | Recall Bias | Observer Bias | Perception Bias |
|---|---|---|---|---|
| Agu et al20 | Low; 2,545 participants selected from the population of Enugu, Nigeria | Not applicable | High; several residents travelled to different locations to administer questionnaires | Low; survey contained multiple choice questions only |
| Irabor et al21 | Low; participants recruited among surgeons from various departments in a hospital | Not applicable | Unclear | Low; survey contained multiple choice questions only |
| Nnabugwu et al22 | Low; 369 patients recruited from various surgical departments in a hospital | Low; patients were given questionnaire on day before surgery | High; 3 interns administered the questionnaires | High; questionnaire contained mostly free-hand answers |
| Ochieng et al23 | Low; participants recruited among surgeons from 3 different hospitals | Not applicable | Unclear | High; semistructured interviews used mainly qualitative approach |
| Ochieng et al24 | Low; participants recruited among patients in various departments of a hospital | Low; participants interviewed within 2 weeks of surgery | Unclear | High; authors reviewed semistructured interviews |
| Sanwal et al25 | Moderate/high; 100 patients selected from the general surgery department | Low; questionnaire administered within a week of surgery | Unclear | Unclear |
| Sceats et al26 | High; participants selected among patients who underwent hernia repair during an STSM | Low; interviews conducted in the same session as the informed consent session | Moderate; 3 researchers interpreted the data | High; authors reviewed video interviews |
| Sutton et al27 | High; participant cohort selected from patients undergoing surgery on a STSM | Low; patients were given the survey on the day of surgery | Unclear | Low; survey contained multiple choice questions only |
| Teshome et al28 | High; participants selected among patients in an Obstetrics and Gynecology department | Low; interviews conducted immediately after discharge | High; questionnaire administered by several nurses | High; questionnaires were interview based |
| Walker et al29 | High; patients were recruited if received hand surgery on STSM | Low; 2 surveys given, one preoperative and one immediately postoperative | Unclear | Moderate; most of the survey was multiple choice; however, free-hand answers were also part of the assessment |
| White et al30 | High; patients were recruited if received plastic/maxfax/ortho interventions on Mercy Ships | High; patients were asked to recall experiences from several years before the interviews | High; interviews were conducted by several members of staff | High; semistructured interviews were conducted |
DISCUSSION
This article summarizes all published data relating to informed consent in LMICs, focusing on STSM, as well as our experience from over a decade of reconstructive surgery missions in Ethiopia. This review identified several recurring barriers to obtaining informed consent on surgical missions abroad and some key strategies to improve this process.
The most frequently cited barrier to obtaining informed consent was a paternalistic approach to medicine in LMICs. Medical paternalism occurs when a doctor determines a patient’s treatment plan with little respect for patient autonomy. This approach is usually undertaken with the intention of benefiting the patient.31 This physician-centered model of practicing medicine is still very common in many LMICs and is often expected by patients.10 This is exacerbated by a lack of ethics education in medical schools in low-income countries. Ochieng et al23 found that 34% of the study participants, who were doctors from 3 Ugandan teaching hospitals, did not know the definition of informed consent. Teshome et al28 found that doctors at a university hospital in Ethiopia did not include the majority of essential information required to obtain informed consent for obstetric and gynecological surgeries. On average, they only managed to consent patients for 4.5 out of 13 essential domains necessary to gain valid informed consent.28 Updating the medical school curriculum in LMICs to include more ethics teaching would provide a permanent solution to improving LMIC doctors’ understanding of the consent process and helping them create a more patient-centered approach to medicine. However, this solution would most likely need to be adopted at a governmental level.
In several studies, the person obtaining consent before surgical procedures did not have the appropriate level of expertise do so.21,23,25 Furthermore, 2 of the studies highlight the lack of appropriate consent forms in Nigerian and Ugandan hospitals, as the existing ones did not contain sufficient information.21,23 In practical terms, visiting doctors must ascertain whether the local hospitals have appropriate consent forms and guidelines. If they do not have them, then the visiting surgeons should plan accordingly and create their own with input from the local team and translators.
Patient education and beliefs were often cited as barriers to informed consent.20–23,25 Cultural beliefs and tradition can have a big impact on the choices patients make in relation to their health.10 For example, in Ethiopia, where we run our annual surgical mission, it is commonplace for patients to seek medical help from traditional healers before seeking the help of trained healthcare professionals. Surgeons embarking on STSMs are often unaware of the cultural norms that might make obtaining informed consent in a particular region challenging. It is always advisable to discuss local belief systems and cultural expectations with local surgeons to understand how they may impact the consent process and treatment in general. In our experience, working closely with the local team throughout the surgical mission is essential.
Two papers included in this study highlight that less-educated patients have a harder time recollecting and understanding diagnoses, treatment plans, and complications.20,25 Sceats et al26 found that practical steps like pausing after key points and stopping regularly for patients to repeat information can be beneficial and aids patient recall. Alternative means of communication such as drawings, medical photographs, and videos can also be helpful.27 On our missions, pre- and postoperative photographs of previous patients (with appropriate consent) and of equipment (eg, drains and NG tubes) are used to aid understanding and recall, while wound care and dressing changes can be effectively taught with practical demonstrations in the preoperative period. As this process takes time, we allow 2 full preoperative weeks and plan to consent each patient over 2 separate sessions.
Our article highlights that the language barrier between visiting doctors and patients is an important obstacle to obtaining informed consent on STSMs. It is uncommon for the visiting team to speak the local language, and patients from rural areas often do not speak English.27 This is particularly notable on our missions, as Ethiopia has over 50 languages, making obtaining an appropriate translator challenging. In practical terms, this means that more than one translator is often required to consent patients from remote areas with less common dialects. It is essential to brief all translators at the start of the mission with information about the different surgeries, possible complications, and what patients should expect from the postoperative recovery period. When possible, local surgeons’ help with translation is very helpful.
The last decade has seen significant refinements in our consent practice in Ethiopia. Early missions had few doctors and limited resources and as such relied on verbal consent for the majority of our surgical procedures. Few of these early patients were seen in our follow-up study, and as such the 6% rate of no consent may reflect changes to our consent practice over the last 5 years. More funding, better staffing (including junior doctors), and a longer preoperative phase have transformed our informed consent process. Our experience and the outcomes from this systematic review have been summarized into a practical checklist (Fig. 5), which incorporates solutions to the most common barriers encountered by surgeons working on missions in resource-limited settings. The checklist is aimed to improve the process of informed consent on STSMs.
Fig. 5.
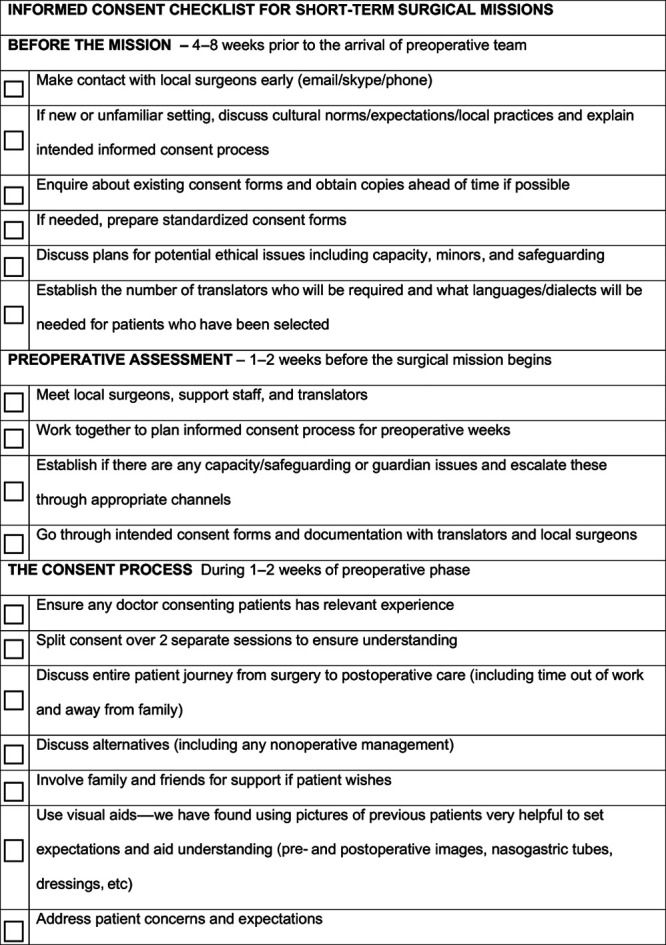
The checklist we created on the basis of our experience from STSMs in Ethiopia and from the systematic review findings. This can be used as a basis for surgeons who are planning an STSM.
Limitations
The study has a number of limitations. First, the number of studies included is low and heterogeneous, which may limit the generalizability of our results and increase the risks of reporting bias. As we have only included English articles, we may have missed relevant studies in different languages and further increased our risk of selection bias. The results of our follow-up study may have been affected by recall bias from patients who underwent surgery many years before the study was carried out.
CONCLUSIONS
This article is the first to summarize all published literature relating to barriers experienced by surgical teams obtaining consent in resource-limited settings. These barriers include a paternalistic approach to medicine common in LMICs, a lack of ethics education in LMIC medical schools, patients’ beliefs toward healthcare, patients’ education, and language barriers. These barriers were similar to those encountered on our missions. We propose a practical checklist that will be of benefit for both established and new surgical teams operating overseas.
ACKNOWLEDGMENT
The authors express their appreciation to Project Harar.
Footnotes
Published online 21 May 2020.
Presented at the ASPS Plastic Surgery The Meeting 2019 in San Diego, CA, and at the BAPRAS Summer Scientific Meeting 2019 in Bournemouth, United Kingdom.
Disclosure: The authors have no financial interest to declare in relation to the content of this article.
REFERENCES
- 1.Meara JG, Hagander LM, Leather AJ.Surgery and global health: a Lancet Commission. Lancet. 2014;383:12. [DOI] [PubMed] [Google Scholar]
- 2.Shrime MG, Sleemi A, Ravilla TD.Charitable platforms in global surgery: a systematic review of their effectiveness, cost-effectiveness, sustainability, and role training. World J Surg. 2015;39:10–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meara JG, Greenberg SL.The Lancet Commission on global surgery global surgery 2030: evidence and solutions for achieving health, welfare and economic development. Surgery 2015;157:834–835. [DOI] [PubMed] [Google Scholar]
- 4.Nolte MT, Maroukis BL, Chung KC, et al. A systematic review of economic analysis of surgical mission trips using the world health organization criteria. World J Surg. 2016;40:1874–1884. [DOI] [PubMed] [Google Scholar]
- 5.Ng-Kamstra JS, Riesel JN, Arya S, et al. Surgical non-governmental organizations: global surgery’s unknown nonprofit sector. World J Surg. 2016;40:1823–1841. [DOI] [PubMed] [Google Scholar]
- 6.Maki J, Qualls M, White B, et al. Health impact assessment and short-term medical missions: a methods study to evaluate quality of care. BMC Health Serv Res. 2008;8:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Howe KL, Malomo AO, Bernstein MA.Ethical challenges in international surgical education, for visitors and hosts. World Neurosurg. 2013;80:751–758. [DOI] [PubMed] [Google Scholar]
- 8.Lin Y, Liu K, Chen C, et al. How to effectively obtain informed consent in trauma patients: a systematic review. BMC Med Ethics. 2019;20:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.GMC. Consent: patients and doctors making decisions together. 2019. Available at http://www.gmc-uk.org/guidance. Accessed October 28, 2019.
- 10.Dunin De Skrzynno SC, Di Maggio F.Surgical consent in sub-Saharan Africa: a modern challenge for the humanitarian surgeon. Trop Doct. 2018;48:217–220. [DOI] [PubMed] [Google Scholar]
- 11.Royal College of Surgeons of England. 2016. Consent: Supported Decision-Making—Royal College of Surgeons. Available at https://www.rcseng.ac.uk/standards-and-research/standards-and-guidance/good-practice-guides/consent/. Accessed October 28, 2019.
- 12.Royal College of Surgeons of England. 2016. Ethical Principles of Working Overseas. Available at https://www.rcseng.ac.uk/standards-and-research/standards-and-guidance/good-practice-guides/consent/. Accessed October 28, 2019.
- 13.Moher D, Altman DG, Liberati A, et al. PRISMA statement. Epidemiology. 2011;22:128; author reply 128. [DOI] [PubMed] [Google Scholar]
- 14.Bossuyt P, Davenport C, Deeks J, et al. Deeks JJ, Bossuyt PM, Gatsonis C.Chapter 11: Interpreting results and drawing conclusions. In: Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy Version 0.9. 2013London, UK: The Cochrane Collaboration; [Google Scholar]
- 15.Taylor HA.Barriers to informed consent. Semin Oncol Nurs. 1999;15:89–95. [DOI] [PubMed] [Google Scholar]
- 16.Nunan D, Bankhead C, Aronson JCatalogue of Bias Collaboration. Selection bias.catalogue of bias 2017. Available at http://www.catalogofbias.org/biases/selection-bias/. Accessed October 28, 2019.
- 17.Spencer EA, Brassey J, Mahtani KCatalogue of Bias Collaboration. Recall bias. in: catalogue of bias 2017. Available at https://catalogofbias.org/biases/recall-bias/. Accessed October 28, 2019.
- 18.Mahtani K, Spencer EA, Brassey JCatalogue of Bias Collaboration. Observer bias. in: catalogue of bias 2017. Available at https://www.catalogofbias.org/biases/observer-bias. Accessed October 28, 2019. [DOI] [PubMed]
- 19.Spencer EA, Brassey JCatalogue of Bias Collaboration. Perception bias. in: catalogue of bias 2017. Available at https://www.catalogofbias.org/biases/perception-bias. Accessed October28, 2019.
- 20.Agu KA, Obi EI, Eze BI, et al. Attitude towards informed consent practice in a developing country: a community-based assessment of the role of educational status. BMC Med Ethics. 2014;15:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Irabor DO, Omonzejele P.Local attitudes, moral obligation, customary obedience and other cultural practices: their influence on the process of gaining informed consent for surgery in a tertiary institution in a developing country. Dev World Bioeth. 2009;9:34–42. [DOI] [PubMed] [Google Scholar]
- 22.Nnabugwu II, Ugwumba FO, Udeh EI, et al. Informed consent for clinical treatment in low-income setting: evaluating the relationship between satisfying consent and extent of recall of consent information. BMC Med Ethics. 2017;18:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ochieng J, Ibingira C, Buwembo W, et al. Informed consent practices for surgical care at university teaching hospitals: a case in a low resource setting. BMC Med Ethics. 2014;15:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ochieng J, Buwembo W, Munabi I, et al. Informed consent in clinical practice: patients’ experiences and perspectives following surgery. BMC Res Notes. 2015;8:765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sanwal AK, Kumar S, Sahni P, et al. Informed consent in indian patients. J R Soc Med. 1996;89:196–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sceats LA, Morris AM, Narayan RR, et al. Lost in translation: informed consent in the medical mission setting. Surgery. 2019;165:438–443. [DOI] [PubMed] [Google Scholar]
- 27.Sutton CD, Lynde GC.A survey of Haitian attitudes towards informed consent. Clin Ethics. 2017;12:197–204. [Google Scholar]
- 28.Teshome M, Wolde Z, Gedefaw A, et al. Surgical informed consent in obstetric and gynecologic surgeries: experience from a comprehensive teaching hospital in southern ethiopia. BMC Med Ethics. 2018;19:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walker ME, Chuang C, Moores CR, et al. The hand surgeon consultation improves patient knowledge in a hand surgery mission to honduras. J Hand Surg. 2018;23:11–17. [DOI] [PubMed] [Google Scholar]
- 30.White MC, Randall K, Avara E, et al. Clinical outcome, social impact and patient expectation: a purposive sampling pilot evaluation of patients in Benin seven years after surgery. World J Surg. 2018;42:1254–1261. [DOI] [PubMed] [Google Scholar]
- 31.Brennan T.Just Doctoring: Medical Ethics in the Liberal State. 1991:Oakland, CA: University of California Press; 51–53. [Google Scholar]


