Abstract
The coronavirus disease 2019 (COVID-19) pandemic exposes unexpected cardiovascular vulnerabilities and the need to improve cardiometabolic health. Four cardiometabolic drivers—abnormal adiposity, dysglycemia, dyslipidemia, and hypertension—are examined in the context of COVID-19. Specific recommendations are provided for lifestyle change, despite social distancing restrictions, and pharmacotherapy, particularly for those with diabetes. Inpatient recommendations emphasize diligent and exclusive use of insulin to avert hyperglycemia in the face of hypercytokinemia and potential islet cell injury. Continuation of statins is advised, but initiating statin therapy to treat COVID-19 is as yet unsubstantiated by the evidence. The central role of the renin-angiotensin system is discussed. Research, knowledge, and practice gaps are analyzed with the intent to motivate prompt action. An emerging model of COVID-related cardiometabolic syndrome encompassing events before, during the acute phase, and subsequently in the chronic phase is presented to guide preventive measures and improve overall cardiometabolic health so future viral pandemics confer less threat.
Key Words: adiposity, angiotensin-converting enzyme 2, cardiometabolic, cardiovascular disease, chronic disease, COVID-19, diabetes, dysglycemia, dyslipidemia, hypertension, lifestyle, lipids, obesity, prevention, SARS-CoV-2, statin
Abbreviations and Acronyms: BMI, body mass index; CIRCS, coronavirus disease–related cardiometabolic syndrome; CMBCD, cardiometabolic-based chronic disease; COVID-19, coronavirus disease 2019; HCP, health care professional; ICU, intensive care unit; RAS, renin-angiotensin system; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2
Central Illustration
Highlights
-
•
COVID-19 exposes epidemiological and mechanistic relationships with cardiometabolic links (abnormal adiposity, dysglycemia, dyslipidemia, and hypertension).
-
•
Lifestyle, glycemic control, and regulation of the RAS have important implications for management of patients with COVID-19.
-
•
CIRCS applies to all stages of COVID-19 illness, including prevention, acute management, and long-term outcomes.
-
•
Further research should address gaps in current knowledge and clinical implementation of available strategies to mitigate the adverse consequences of CIRCS.
Cardiometabolic-based chronic disease (CMBCD) results from primary (genetic, environmental, behavioral) and metabolic drivers (abnormal adiposity, dysglycemia, metabolic syndrome traits) (1,2). Epidemiological/mechanistic associations of CMBCD with coronavirus disease 2019 (COVID-19) substantiate a postulated coronavirus disease–related cardiometabolic syndrome (CIRCS) (Table 1 ). The role of healthy lifestyles and pharmacotherapy targeting metabolic drivers to reduce cardiovascular risk is well established (1,2). However, lessons learned from the COVID-19 pandemic support shorter-term benefits of these interventions, similar to observed benefits on acute cardiovascular disease (CVD) outcomes (3). Therefore, a prevention program for patients of all ages should be developed to create a healthy culture, reduce chronic disease risks, and mitigate unforeseen acute insults, such as COVID-19.
Table 1.
Components of a Postulated COVID-Related Cardiometabolic Syndrome
| Pre-CIRCS | Acute CIRCS | Chronic CIRCS |
|---|---|---|
| Prior to COVID-19 diagnosis | From COVID-19 diagnosis to resolution or 3 months | ≥ 3 months from COVID-19 diagnosis |
| Unhealthy lifestyle | Hypercytokinemia and inflammation | Preparedness for theoretical post-viral syndrome |
| Unfavorable social determinants of health | Severe acute respiratory syndrome | Implement chronic care model |
| Transcultural factors | Severe insulin resistance and hyperglycemia | Intensive lifestyle change |
| Cardiometabolic-based chronic disease | Abnormal adiposity | Address social determinants of health |
| Preventive care | Cardiovascular disease | Novel therapies for preventive care |
| Hypercoagulable state and thromboemboli | Infrastructural change in healthcare system | |
| High insensible water losses and hypernatremia | ||
| Acute kidney injury | ||
| Hyperphosphatemia and hypocalcemia | ||
| High nutritional risk | ||
| Encephalopathy | ||
| Prolonged acute and chronic critical illness | ||
| Intensive metabolic support |
This postulated model will require validation (research gap), with particular focus on whether and how chronic CIRCS (with or without antecedent critical illness) differs from chronic critical illness.
CIRCS = coronavirus disease–related cardiometabolic syndrome; COVID-19 = coronavirus disease 2019.
Cardiometabolic Targets
Abnormal adiposity
Obesity is a major global problem. According to the World Health Organization, over 1.9 billion people age ≥18 years were overweight (39%) or obese (13%) in 2016, with 40 million children age <5 years overweight/obese in 2018 (4). Obesity is defined by the body mass index (BMI), which despite adjustments based on ethnicity, remains inadequate as a cardiovascular risk stratifier (5). Consequently, adiposity-based chronic disease has been developed as a new framework incorporating abnormal adiposity amount, distribution, and function, along with clinical complication severity (5). Not surprisingly, abnormal adiposity is a key driver, not only for obesity-related complications, but also insulin resistance, inflammation, type 2 diabetes (T2D), and CVD (1,2,5,6). A principal mechanism of abnormal adiposity leading to CVD is the accumulation of inflammatory pericardial/epicardial fat (1). This ectopic adipose tissue secretes more type II phospholipase A2 with ischemia, leading to more phospholipid hydrolysis, local free fatty acids, nerve impulse disruption, and arrhythmia; vascular inflammation, atherosclerosis, and arterial stiffness; cardiomyocyte fibrosis/apoptosis and left ventricular hypertrophy; and aortic valve sclerosis (1,7). Because this pathological adiposity results, in part, from unhealthy lifestyles, and is poorly detected with conventional anthropometrics, many people are at increased risk for cardiac injury.
Initial reports of COVID-19 in Italy (8) and China (9, 10, 11, 12, 13, 14) point to those age >50 years at higher risk for infection and hospitalization, and >80 years at higher risk for mortality. As of June 2020 in the United States, those age >85 years accounted for the highest mortality numbers (31,778), but there was a disproportionately younger age associated with mortality among those with obesity (652 [ages 55 to 64 years] and 605 [65 to 74 years], compared with only 109 [≥85 years]) (15). One explanation relates to abnormal adiposity and its sequelae, based on prior experiences with various respiratory viruses (16). The 2017 to 2018 U.S. obesity prevalence rate was 42.4%, with 40.0% age 20 to 39 years (17). Moreover, 71.6% of adults age ≥20 years were overweight/obese in 2015 to 2016 (18). In several studies (19, 20, 21, 22), patients with COVID-19 and obesity were more likely to be admitted to the intensive care unit (ICU) and have higher mortality rates than those without obesity. In 1 study of 3,615 patients with COVID-19, hospitalization and ICU admissions of patients with obesity were higher in those age <60 years, compared with those age ≥60 years (20). Possible mechanisms for this observation include dysregulated immunity with high leptin/adiponectin ratios (23) and sedentariness (24), increased angiotensin-converting enzyme 2 (ACE2) expression in epicardial adipose tissue (25), concurrent cardiopulmonary disease (1,26), and lipotoxic adiposity (1). This phenomenon not only applies to Americans who have greater BMIs and risk for visceral/ectopic fat, but also Asians who are more prone to visceral/ectopic fat accumulation and dysglycemia at milder BMI elevations (1,5,6). A common link is unhealthy “Western” lifestyles. Taken together, the increasingly “sick” nature of populations with increased cardiometabolic risk factors, particularly at younger ages and below detection thresholds, amplifies effects of any acute insult, particularly one that subverts the immune-cardiopulmonary system, such as COVID-19 (27).
Dysglycemia
Diabetes is characterized by abnormally high blood glucose levels sufficient to cause end-organ damage (Table 2 ). Diabetes is a global problem affecting approximately 463 million people worldwide in 2019, expected to increase 51% to 700 million by 2045, with about 90% having T2D (28). T2D falls within a “dysglycemia-based chronic disease” spectrum consisting of insulin resistance, pre-diabetes, T2D, and vascular complications (1,6). There are higher prevalence rates of obesity, hypertension (HTN), and CVD with diabetes, intensifying risks for CMBCD. Of note, 50.1% of those with diabetes and 88.4% with pre-diabetes are unaware of their condition (28,29), necessitating case finding for dysglycemia in all hospitalized patients.
Table 2.
Diabetes Types, Cardiometabolic Context, and COVID-Related Cardiometabolic Syndrome Relevance
| Diabetes Type | Description | Cardiometabolic Context | CIRCS Relevance |
|---|---|---|---|
| T1D | |||
| Generally younger age at onset∗ | Increased CVD risk | Insulin only while in hospital† | |
| Primary destruction of ß-cells | Increased CRD risk | Consider using CGM technology | |
| Autoimmune | Increased hypoglycemia risk | Avoid SGLT2 during acute COVID-19 and consider stopping SGLT2i in patients at risk for COVID-19 | |
| Increased risk for DKA | Consider SGLT2i, especially with HF | Arrange telemedicine contacts | |
| Lifestyle and insulin treatment‡ | Reassure about prescriptions/supplies | ||
| Diabetes complications | Avoid overprescribing | ||
| Management per guidelines | Restructure routines at home | ||
| Insulin on-board at all times | |||
| T2D | |||
| Generally older age at onset | Metabolic driver in CMBCD | Insulin only while in hospital† | |
| Primary insulin resistance | Hypoglycemia risk | Avoid SGLT2 during acute COVID-19 and consider stopping SGLT2i in patients at risk for COVID-19 | |
| Subsequent destruction of ß-cells | Consider SGLT2i and/or GLP1ra with CMBCD | Arrange telemedicine contacts | |
| Associated with CVD risk factors | Emphasis on healthy weight | Reassure about prescriptions/supplies | |
| Preceded by milder hyperglycemia | Avoid overprescribing | ||
| May develop DKA | Restructure routines at home | ||
| Multimodality treatments§ | Greater emphasis on healthy weight | ||
| Diabetes complications | |||
| Management per guidelines |
CIRCS = coronavirus disease–related cardiometabolic syndrome; CKD = chronic kidney disease; CMBCD = cardiometabolic-based chronic disease; CVD = cardiovascular disease; DKA = diabetic ketoacidosis; GLP1ra = glucagon-like peptide-1 receptor agonist; SGLT2i = sodium-glucose co-transporter 2 inhibitor; T1D = type 1 diabetes; T2D = type 2 diabetes.
Some adults may develop autoimmune ß-cell destruction and a T1D picture, possibly with DKA, at later ages (latent autoimmune diabetes in adults); this state may persist as T1D, revert to T2D, or resolve.
Insulin drips should be used judiciously to avoid unnecessary exposures of personnel to COVID+ patients. Alternatives include more aggressive subcutaneous insulin dosing (e.g., q6h NPH + correction rapid-acting insulin), use of intravenous chromium, and permissive underfeeding until glycemic target achieved (140 to 180 mg/dl).
Some patients with T1D are treated with SGLT2i.
Patients with T2D may be treated with lifestyle change, oral medications, noninsulin injectables, insulin, and/or metabolic procedures.
COVID-19 is associated with worse outcomes in patients with T2D, but less so when the hyperglycemia is better controlled (30). Specifically, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection rates and disease severity markers are increased in patients with diabetes (14,31, 32, 33, 34, 35, 36, 37, 38). Potential mechanisms include: cytokine-mediated aggravation of insulin resistance (39) and hypercoagulability (40); increased expression of the SARS-CoV-2 receptor (ACE2) with renin-angiotensin system (RAS) agents (41); effects of SARS-CoV-2 on pancreatic ACE2 with decreased β-cell insulin reserve (42); immunosuppression; glycosylation of viral spike protein and ACE2 with increased viral binding/entry (43); decreased viral clearance and increased viral replication (44,45); and comorbidities (38). Adverse outcomes with diabetes have also been reported with pandemic influenza A 2009 and Middle-East respiratory syndrome coronavirus (46).
Dyslipidemia
In 2015 to 2016, over 12% of adults age ≥20 years and about 7% of children/adolescents ages 6 to 19 years had high cholesterol (47). Statin therapy is indicated as CVD preventive therapy for high-risk patients. Statins have cholesterol-lowering and anti-inflammatory properties that mitigate the risk of CVD events. Multiple prospective population studies and randomized clinical trials involving patients at risk, and with established atherosclerotic CVD, have attributed risk reduction with statins to both low-density lipoprotein cholesterol and high-sensitivity C-reactive protein lowering (48). Statins also diminish inflammatory responses and possibly improve survival in a hyperinflammatory subphenotype of acute respiratory distress syndrome (49).
COVID-19 associated cardiac injury and mortality is higher in patients with older age and certain comorbidities: HTN, diabetes, chronic kidney disease, atherosclerotic cardiovascular disease, and chronic heart failure (38,50). Thus, most patients with cardiac injury are identified as very high risk for CVD events (51). Viral illnesses incite a profound systemic inflammatory response leading to tissue injury and organ failure. Among patients with COVID-19, cardiac injury is associated with higher leukocyte counts and elevated procalcitonin and C-reactive protein levels (50). This immune response involves activation/proliferation of lymphocytes/macrophages, with increased pro-inflammatory cytokines: monocyte chemoattractant protein-1; macrophage inflammatory protein-1α; tumor necrosis factor-α; and interleukin-2, -7, and -10 (52). Although many patients with COVID-19 take a statin, the use of statins to specifically manage viral illnesses remains unclear. In cell culture studies with human alveolar epithelial cells, H5N1 (influenza A) infection induced cytokine production (53). Moreover, suppression of sterol biosynthesis with simvastatin reduced viral replication and cytokine production at a farnesylation step (54). Nevertheless, combined treatment with simvastatin did not enhance the efficacy of oseltamivir in mice (55). Also, atorvastatin mediates epigenetic histone modifications and ACE2 up-regulation in a rabbit atherosclerosis model (56). Patients with familial hypercholesterolemia represent another challenge with COVID-19 due to increased risk for premature coronary heart disease, low-density lipoprotein receptor variants modulating the immune response to SARS-CoV-2, and higher lipoprotein(a) and risk for atherothrombosis (57,58).
Hypertension
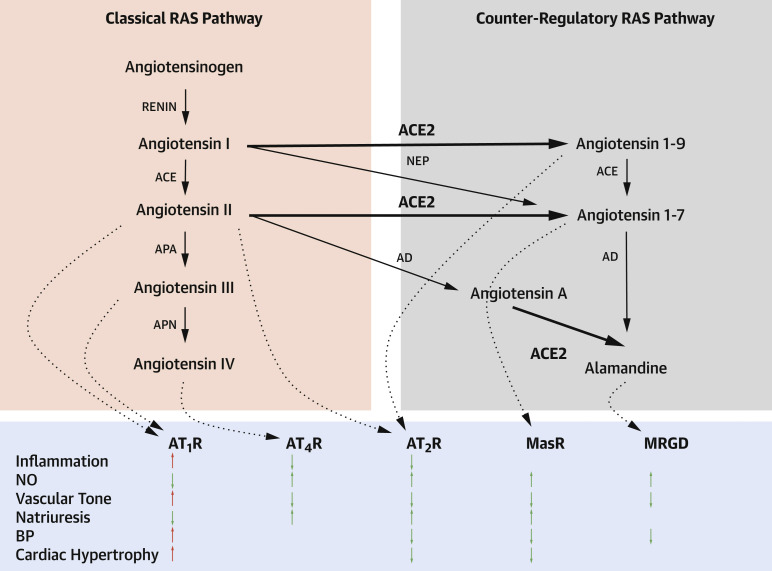
The age-adjusted prevalence of HTN among U.S. adults in 2015 to 2016 was 44.1% based on 2017 American College of Cardiology/American Heart Association criteria (compared with 29.0% [JNC7] and 32.4% [self-reported]) (59, 60, 61), with high population-attributable risks of coronary heart disease, heart failure, stroke, chronic renal disease, and dementia, as well as increasing HTN-related CVD mortality from 2007 to 2017 (62, 63, 64). Among patients with HTN, 49.5% had obesity, 63.2% hypercholesterolemia, and 27.2% diabetes (63). There are multiple drivers of HTN: genetic; environmental; behavioral; and abnormalities in the cardiovascular, autonomic nervous, renal, and endocrine systems. Central to the RAS is ACE2, a master regulator that converts angiotensin I and II into angiotensin 1-9 and 1-7, respectively (Figure 1 ) (65). Based on this schematic, down-regulation of ACE2 with SARS-CoV-2 binding impairs cardioprotection via the counter-regulatory RAS pathway.
Figure 1.
The Role of ACE2 in the RAS
ACE2 figures prominently in the RAS, mediates cardioprotection, and is a receptor for SARS-CoV-2. Loss of ACE2 function with SARS-CoV-2 binding can lead to CIRCS. Bold arrows correspond to ACE2 pathways. In the bottom panel, red arrows correspond to adverse and green arrows to beneficial effects on cardioprotection. Adapted from Paz Ocaranza et al. (65). ACE = angiotensin-converting enzyme; AD = aspartate decarboxylase; APA = aminopeptidase A; APN = aminopeptidase N; AT1R = type 1 angiotensin II receptor; AT2R = type II angiotensin II receptor; AT4R = angiotensin IV receptor; BP = blood pressure; MasR = proto-oncogene Massey receptor; MRGD = Mas-related G protein-coupled receptor member D; NEP = neprilysin; NO = nitric oxide; RAS = renin-angiotensin system.
Widely expressed, ACE2 mediates SARS-CoV-2 entry in epithelium. The viral spike protein is primed for viral entry by different host proteases, including transmembrane protease serine 2 (TMPRSS2) (45). The risk of SARS-CoV-2 infection does not appear affected with RAS inhibitors (66,67). Among patients with COVID-19, HTN was associated with frequent cardiovascular morbidities (24.3%), diabetes (15.2%), cardiac disease (6.2%), and high mortality risk (68). In another study, HTN was associated with a ∼2.5-fold increased risk of COVID-19 severity and mortality, mainly in those patients over age >60 years (69).
Cardiometabolic Intervention
Abnormal adiposity
Prudent recommendations can be made based on the high prevalence of overweight/obesity and unhealthy lifestyle in the general population. Formal lifestyle medicine programs should be implemented in the context of social distancing. Specifically, with school and lunch program closings (70), and new work or stay-at-home routines (71), the risks of undernutrition, weight gain, and deconditioning in children/adults need to be countered by planned home physical activities. Social distancing foments a culture of ordering in and over-consuming “comfort foods.” Simple instructions should be provided to promote a healthy lifestyle (Table 3 ) (72). Health care professionals (HCPs) should be trained in lifestyle medicine using printed materials, web-based learning tools, and practical experiences. At a systems level, a network of lifestyle medicine programs forges a new culture of healthy living.
Table 3.
Healthy Lifestyle During and After Social Distancing
| Healthy Lifestyle Component | During Social Distancing | After Social Distancing |
|---|---|---|
| Nutrition | Arrange home delivery of healthy foods/meals | Continue same healthy eating recommendations |
| Consume >5–7 daily servings of plants | When food shopping, adhere with lists, created after meal consumption | |
| Minimize starchy, sugary, salty, and fried foods | In restaurants, order healthy dishes without a menu | |
| Prepare and consume high-fiber breakfasts | Create healthy eating culture at home, work, and school | |
| Have high fiber between meal snacks Avoid fad diets/supplements unless advised by your HCP Learn/practice how to cook/prepare healthy foods |
||
| Physical activity | Consider ordering home exercise equipment | Increase exercise time at home |
| Engage in home aerobic and strength training | Increase physical activity at work and school | |
| Judicious outdoor walking/running | Engage in organized sports | |
| Create/execute realistic daily exercise program | ||
| Behavior | Innovate new home routines for healthy lifestyle∗ | Adapt routines to “normal” schedules |
| Reassure family members about preparedness | Continue positive attitudes | |
| Re-message healthy lifestyle and short-term protection | Increase outdoor fun activities with family/friends | |
| Continue message healthy lifestyle and long-term benefits | ||
| Sleep | Restructure routines for about 7 h sleep per night | Adapt sleep routines to “normal” schedule |
| Implement new routines for better quality of sleep | ||
| Alcohol | Abstinence preferred | Continue same recommendation |
| Otherwise limit to <1 (women), <2 (men) drinks/day | ||
| Use telemedicine for counseling | ||
| Tobacco | No tobacco products | Continue same recommendation |
| Use telemedicine for counseling | ||
| Community engagement | Contact local charities, houses or worship, schools, and so on Engage to help others remotely (phone, internet, and so on) |
Continue same recommendation |
| Technology | Prepare for telemedicine visits with your HCP | Continue same recommendation |
| Download smartphone lifestyle applications | ||
| Consider purchase of wearable technologies |
HCP = health care professional.
Innovation results from organizational change at the micro (individuals), meso (guidelines), and macro levels (organizations, government, policies) (72).
The “gaps” in research evidence, knowledge dissemination, and practical implementation are wide with respect to obesity and COVID-19. Research gaps correspond to unanswered scientific questions (73). Epidemiological studies are needed to capture associations of COVID-19 risks with adiposity and short-term benefits of lifestyle change on body composition. Subsequent basic research studies, clinical trials, and targeted interventions should be designed. Simple technologies that detect abnormal adiposity should be developed. Knowledge gaps correspond to the nonuniform dissemination of information to HCPs, patients, and policymakers (74). New information should be well-communicated to HCPs in live educational activities and in printed and web-based materials. Practice gaps correspond to failures converting information into action (75). The identification of champions and team members, adequate funding, administrative support from sponsoring organizations, and innovative leveraging of technology can optimize lifestyle medicine programs.
Dysglycemia
Because many patients with CVD have T2D, healthy lifestyle changes are encouraged in the context of social distancing. Patients should restructure their routines due to potential disruptions in work, sleep, and meal times. Diabetes practice preparedness includes patient access to telemedicine technology. Overprescribing is dissuaded to avoid hoarding, and patients are reassured about ready accessibility to medications and supplies. Continuous glucose monitoring should be considered in patients checking levels multiple times a day, especially with type 1 diabetes (T1D), to alleviate burdens of maintaining supplies. Notwithstanding secondary CVD prevention goals, HCPs should: 1) avoid sodium-glucose cotransporter-2 inhibitors (SGLT2i) in patients with acute COVID-19; and 2) consider holding outpatient SGLT2i in patients at risk for COVID-19, especially with poor or variable oral intake, to lower the risk for diabetic ketoacidosis (euglycemic and hyperglycemic) and avoidable non-COVID hospitalizations. Of note, the SGT2i dapagliflozin is being evaluated as a potential treatment of COVID-19 for organ protection (NCT04350593 and NCT04393246). Urinary glucose losses resulting from SGLT2i create a physiological state mimicking starvation, with elevated glucagon/insulin ratios, ketone production and reabsorption, and risk for ketoacidosis at lower-than-anticipated glucose levels (76).
Physicians should determine if patients have diabetes or take a “sugar medicine.” The glucose and hemoglobin A1c levels should be checked in all patients upon presentation. All oral and noninsulin injectable diabetes medication are stopped in the hospital, and only insulin is used. Inpatient monitoring and therapeutics must be protocolized. Because all patients with COVID-19 and significant hyperglycemia will require insulinization, a correction insulin protocol must be started immediately. In addition, a guideline-directed, standing subcutaneous insulin protocol must be started based on the nutritional regimen and in all patients with T1D. Endocrinologists should be consulted for all patients with T1D or those with recalcitrant hyperglycemia. In the ICU, hyperglycemia is managed with guidelines-directed insulin protocols. Both ICU and non-ICU glycemic targets are 140 to 180 mg/dl, prioritizing the avoidance of severe hyperglycemia and hypoglycemia. Despite the adverse effects of undernutrition in COVID-19 (77), nutrition support should not be started until severe hyperglycemia is controlled, especially with concurrent or planned glucocorticoid use. If hyperglycemia is recalcitrant on nutrition support, then hold (or significantly reduce) the nutrition support until glycemic control is re-established. Also, to limit HCP exposures, it may be necessary to manage patients without intravenous insulin and/or with less frequent monitoring; in this case, subcutaneous NPH was administered every 6 to 8 h for basal insulinization, and rapid-acting insulin every 3 to 6 h for correction depending on when personnel are already in the room. Last, insulin requirements need to be preemptively increased when steroids are administered, and decreased as steroids are tapered.
Research gaps exist in epidemiology, mechanisms, and clinical trials. Knowledge gaps are related to decreased awareness about the impact of diabetes and hyperglycemia on COVID-19 outcomes, nuances in diabetes and nutrition management in the ICU, and adaptive protocols with resource limitations. Finally, practice gaps involve optimal use of telemedicine to prevent hospitalizations and early insulinization once in hospitals.
Dyslipidemia
Continuation of statin therapy is recommended in high-/very high-risk patients who have increased susceptibility to a CVD event with hypercytokinemia. The safety of continued statin therapy must be considered as 5% to 20% of patients taking a statin report adverse muscle events (78), mimicking viral-induced muscle symptoms. In addition, drug interactions and tissue organ failure may adversely impair statin elimination, increasing the risk of muscle injury.
Research is needed on the anti-inflammatory effects of statins in patients with COVID-19. Before considering a clinical trial, this multidimensional effort requires: 1) establishing the efficacy of statins in reducing viral replication and inflammatory responses in human alveolar cells and experimental COVID-19 models; and 2) assessment of inflammatory responses, myocardial damage, and event rates in patients with COVID-19 treated with/without statin therapy. Certain immunomodulatory bioactive lipids (arachidonic acid and other unsaturated fatty acids) may confer anti-SARS-CoV-2 activity and should be investigated (79). Ongoing clinical trials are investigating anti-inflammatory effects of statins. Ruxo-Sim-20 is an open-label, randomized trial investigating whether combined ruxolitinimb with simvastatin has a synergistic effect on viral entry and reduced inflammation with confirmed SARS-Cov-2 (NCT04348695). C-19 ACS is a prospective, multicenter clinical trial investigating multiple cardioprotective therapies on all-cause mortality at 30 days after admission with acute coronary syndrome (NCT04333407). The intervention includes aspirin 75 mg or clopidogrel 75 mg, rivaroxaban 2.5 mg for patients not receiving an anticoagulant, atorvastatin 40 mg if not taking a statin, and omeprazole 20 mg daily. Knowledge gaps include the evidence-based role of lipid-lowering therapies in patients with COVID-19. Practice gaps include algorithms and policies to implement relevant clinical protocols.
Hypertension
Manipulation of ACE2 activity in patients with COVID-19 decreases pulmonary and vascular injury, pulmonary hypertension, lung fibrosis, and systemic inflammation, with arterial remodeling, improved right ventricular function, and cardioprotective effects (80, 81, 82). Although still controversial, there is consensus among professional medical societies to continue RAS antagonists in those currently prescribed these agents (83,84). Of note, inpatient use of angiotensin-converting enzyme (ACE) inhibitor/angiotensin receptor blocker (ARB) in patients with hypertension and COVID-19 was associated with lower disease severity and interleukin-6 levels, peak viral load, and increased CD3 and CD8 T-cell counts in peripheral blood, compared with other antihypertensive drugs (85). In another inpatient study, there was lower mortality with ACE inhibitor/ARB use compared with those not on ACE inhibitor/ARB therapy (86). Spironolactone has also been proposed as an alternative therapy due to theoretical advantages of avoiding ACE inhibitor/ARB withdraw (87).
Research gaps focus on the role of RAS antagonists in patients with COVID-19, including the role of dual receptor (androgen and mineralocorticoid) antagonism with spironolactone. Besides the role of intensive lifestyle change in the routine management of HTN, knowledge gaps in the context of COVID-19 are focused on guideline-directed use of RAS antagonists. Practice gaps for HTN can be addressed with greater use of home BP monitoring, telemedicine visits with HCPs, and wearable technologies to increase physical activity.
CIRCS and beyond
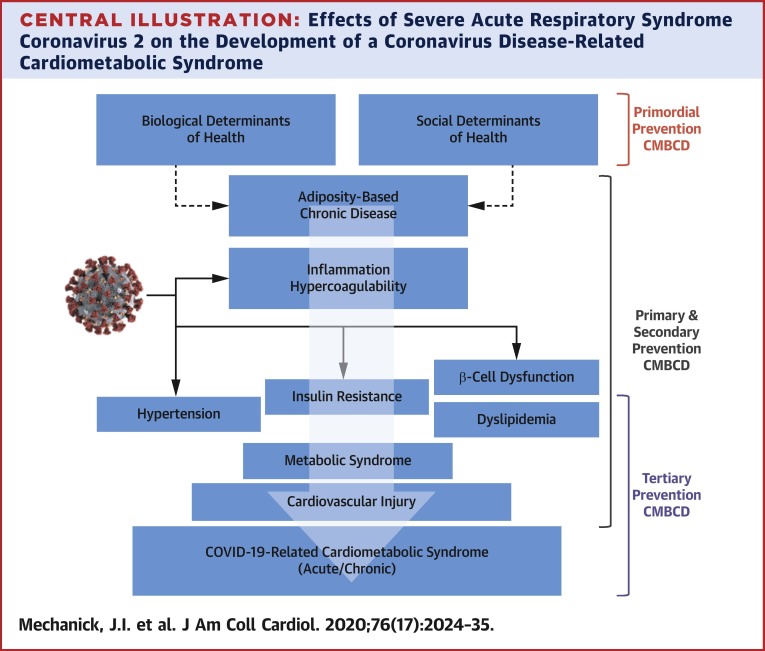
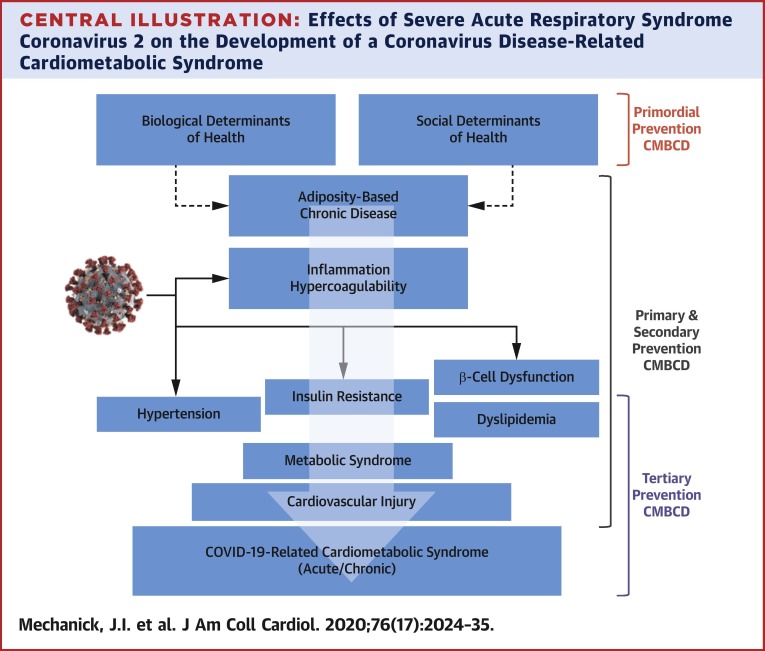
The current imperative to aggressively address key metabolic drivers of CVD in patients with COVID-19 is supported by epidemiological evidence implicating a nexus of cardiometabolic risks with disease severity (Table 4 ) (4,8,19,20,29,32, 33, 34,37,38,40,47,59,61,88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103, 104, 105, 106, 107, 108, 109, 110, 111). For example, in a case series of 5,700 patients admitted to 12 hospitals in the New York metropolitan area, the most common comorbidities were hypertension (56.6%), obesity (41.7%), and diabetes (33.8%) (109). In a meta-analysis, CVD (odds ratio [OR]: 2.93; p < 0.001), diabetes (OR: 2.47; p < 0.001), and hypertension (OR: 2.29; p < 0.001) were identified as predictors of COVID-19 symptoms or ICU admission (112). Many patients and HCPs are unaware of this interconnection among a cluster of metabolic drivers, CVD, and COVID-19, representing a CIRCS framework, and the downstream risks, representing research, knowledge, and practice gaps that must be expeditiously addressed (Central Illustration , Table 5 ). Clinical actions range from preventive care before CIRCS, to acute care during CIRCS, to chronic care after CIRCS (Table 1). The anticipation of a chronic CIRCS should alert the health care system to avoid another wave of acute and chronic illnesses. Starting points include addressing research, knowledge, and practice gaps now: planning and implementing lifestyle medicine and cardiometabolic programs for people of all ages; improving infrastructure for comprehensive prevention plans to reduce CMBCD burden before, during, and after CIRCS; addressing potential post-CIRCS phenomena; and improving preparedness for future pandemics.
Table 4.
Association of Cardiometabolic Risk Factors With Degrees of COVID-19 Severity
| General Population (% With Risk Factor) | COVID-19 Positive Total (% With Risk Factor) | COVID-19 Positive Not Severe (% With Risk Factor) | COVID-19 Positive Severe (% With Risk Factor) |
||
|---|---|---|---|---|---|
| Hospitalization | Intensive Care Unit | Mortality | |||
| Obesity∗ | |||||
| China (6.2) | ND | ND | 22 | 25.5–27.0 | 88.2 |
| France (21.6–25.8) | ND | ND | ND | 47.6 | ND |
| United States (34.0–42.4) | ND | 14.4 | 14–53.7 | 19.0–45.7† | ND |
| Diabetes | |||||
| China (9.2–10.9) | 2–22 | 4.5–11 | 7.4–19 | 13.8–34.6 | 7.3–31‡ |
| Italy (5-9) | 33.9–35.5 | ND | ND | 17 | 33.9–35.5 |
| Spain (6.9) | ND | ND | ND | ND | 12 |
| United States (9.8-10.8) | 5.4–10.9 | 5.3–24.0 | 15.0–37.8 | 58 | ND |
| Dyslipidemia | |||||
| United States (12.0) | ND | 10.5 | 25.9 | 26.6 | ND |
| Hypertension | |||||
| China (15.0–44.7) | 9.5–34 | ND | 23.7–40.8 | 58 | 37.6 |
| Italy (30) | ND | ND | ND | ND | 73.8 |
| United States (32.4–44.1) | ND | 11.5 | 37.1–63.0 | 39.5–66.9 | 73.5 |
| CVD | |||||
| China (43) | 1.6–40.0 | ND | 15.7 | 9.6–25.0 | 9.4–11.8 |
| Italy (36) | 36.0–42.5 | ND | ND | ND | 24.5–30.1 |
| United States (30–37.4) | ND | 16.3 | 27.8–44.6 | 30.6–47.1 | 45.6 |
Percentage ranges correspond to the proportion of patients in a country’s general population and at varying levels of COVID-19 severity (column), with a particular cardiometabolic risk factor (row). These percentages are synthesized based on existing published data covering a wide range of surveillance dates, denominators, definitions, and populations, limiting the validity of comparisons and representing research gaps. However, the pattern of increased proportions of these cardiometabolic risk factors with COVID-19 severity compared with the respective general population support a COVID-Related Cardiometabolic Syndrome (figures with increased proportions in bold). See references: China (9,32, 33, 34,37,40,88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98); France (19); Italy (8,99,100); Total (4,31,101, 102, 103, 104); United States (15,20,29,38,47,59,61,105, 106, 107, 108, 109, 110, 111).
COVID-19 = coronavirus disease 2019; CVD = cardiovascular disease; ND = no data (represents epidemiological research gap).
Obesity defined by body mass index >30 kg/m2.
Increased risk for intensive care unit with increased BMI >35 kg/m2 with age <60 years (20).
In largest retrospective study to date in China (n = 72,314), only 0.9% of mortality without any comorbidities, compared with 7.3% with diabetes (66).
Central Illustration.
Effects of Severe Acute Respiratory Syndrome Coronavirus 2 on the Development of a Coronavirus Disease-Related Cardiometabolic Syndrome
The coronavirus disease 2019 (COVID-19)–related cardiometabolic syndrome is triggered by severe acute respiratory syndrome coronavirus 2 infection in susceptible hosts. Primordial prevention focuses on societal/infrastructural changes and social determinants of cardiometabolic health to decrease cardiometabolic-based chronic disease (CMBCD) risk and COVID-19 severity in a population. Primary prevention focuses on specific CMBCD risk factors to decrease severity of abnormal adiposity, dysglycemia, dyslipidemia, and hypertension. Secondary prevention focuses on specific CMBCD components that have progressed to early disease states to decrease COVID-19 severity. Tertiary prevention focuses on advanced CMBCD states associated with severe COVID-19. Severe acute respiratory syndrome coronavirus 2 image adapted from the Centers for Disease Control and Prevention (113).Courtesy of CDC/Alissa Eckert, MS; Dan Higgins, MAMS.
Table 5.
Actions to Address Research, Knowledge, and Practice Gaps in COVID-Related Cardiometabolic Syndrome
| Research Gaps | Knowledge Gaps | Practice Gaps |
|---|---|---|
| Optimize cardiac imaging for epi/pericardial adiposity | Educate about CIRCS | Formulate and implement clinical practice algorithms and protocols |
| Clarify roles of DPP4i, GLP1ra, RAS antagonists, and TZD | Update on abnormal adiposity, dysglycemia, hypertension, and prior CVD effects on risk | Address social determinants of health, including structural racism |
| Clarify glucocorticoid and hydroxychloroquine roles | Emphasize importance of prevention and lifestyle change | Plan, build, and operate a lifestyle medicine program |
| Formalize management in children, adolescents, and pregnancy (including gestational diabetes) | Increase use of webinars, teleconferences, and rapid publication of position papers and guidelines for education | Identify champions, team members, funding sources, administrative allies, and appropriate technologies |
| Design and implement clinical trials on molecular/metabolic targeting (e.g., ACE2) | Communicate effectively with media and policy-makers | Optimize telemedicine and use of wearable technologies |
| Clarify roles of specific nutrients (e.g., vitamins B, C, D; chromium, zinc; and fatty acids) | Develop and distribute public service announcements | Create formal preventive care plans to apply before, during, and after COVID-19 infection |
| Continue epidemiological studies on associations of metabolic syndrome traits with COVID-19 | Collaborate with public and private entities to create a culture of awareness | Use a chronic care model for post-COVID-19 follow-up |
See text for definitions of gaps. Gaps need to be addressed promptly to create successful prevention plans.
ACE2 = angiotensin-converting enzyme 2; CIRCS = COVID-related cardiometabolic syndrome; COVID-19 = coronavirus disease 2019; CVD = cardiovascular disease; DPP4i = dipeptidyl peptidase-4 inhibitor; GLP1ra = glucagon-like peptide-1 receptor agonist; TZD = thiazolidinedione.
Footnotes
Dr. Mechanick has received honoraria for lectures and program development from Abbott Nutrition. Dr. Rosenson has received research grants to institution from Amgen, The Medicines Company, Novartis, and Regeneron; has served on consulting/advisory boards for Amgen, C5, Corvidia, CVS Caremark, and The Medicines Company; has received honoraria from Angen, Kowa, Pfizer, and Regeneron; has received royalties from UpToDate; and has stock holdings in MediMergent, LLC. Dr. Pinney has received consulting fees from Abbott, CareDx, Medtronic, and Procyrion. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose. Muthiah Vaduganathan, MD, served as Guest Editor for this paper. Deepak L. Bhatt, MD, MPH, served as Guest Editor-in-Chief for this paper.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACCauthor instructions page.
References
- 1.Mechanick J.I., Farkouh M.E., Newman J.D. Cardiometabolic-based chronic disease, adiposity and dysglycemia drivers: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75:525–538. doi: 10.1016/j.jacc.2019.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mechanick J.I., Farkouh M.E., Newman J.D. Cardiometabolic-based chronic disease, addressing knowledge and clinical practice gaps: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75:539–555. doi: 10.1016/j.jacc.2019.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee Y.M., Kim R.B., Lee H.J. Relationships among medication adherence, lifestyle modification, and health-related quality of life in patients with acute myocardial infarction: a cross-sectional study. Health Qual Life Outcomes. 2018;16:100. doi: 10.1186/s12955-018-0921-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization Obesity and overweight. March 3, 2020. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight Available at:
- 5.Mechanick J.I., Hurley D.L., Garvey W.T. Adiposity-based chronic disease as a new diagnostic term: American Association of Clinical Endocrinologists and the American College of Endocrinology position statement. Endocr Pract. 2017;23:372–378. doi: 10.4158/EP161688.PS. [DOI] [PubMed] [Google Scholar]
- 6.Mechanick J.I., Garber A.J., Grunberger G. Dysglycemia-based chronic disease: An American Association of Clinical Endocrinologists position statement. Endocr Pract. 2018;24:995–1011. doi: 10.4158/PS-2018-0139. [DOI] [PubMed] [Google Scholar]
- 7.Dutour A., Achard V., Sell H. Secretory type II phospholipase A2 is produced and secreted by epicardial adipose tissue and overexpressed in patients with coronary artery disease. J Clin Endocrinol Metab. 2010;95:963–967. doi: 10.1210/jc.2009-1222. [DOI] [PubMed] [Google Scholar]
- 8.Onder G., Rezza G., Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020 Mar 23 doi: 10.1001/jama.2020.4683. [E-pub ahead of print] [DOI] [PubMed] [Google Scholar]
- 9.Novel Coronavirus Pneumonia Emergency Response Epidemiology Team Vital surveillances: the epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19)—China, 2020. China CDC Weekly. 2020;2:113–122. [PMC free article] [PubMed] [Google Scholar]
- 10.Chen N., Zhou M., Dong X. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Q., Guan X., Wu P. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. N Engl J Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wan S., Xiang Y., Fang W. Clinical features and treatment of COVID-19 patients in northeast Chongqing. Med Virol. 2020;92:797–806. doi: 10.1002/jmv.25783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tian S., Hu N., Lou J. Characteristics of COVID-19 infection in Beijing. J Infect. 2020;80:401–406. doi: 10.1016/j.jinf.2020.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang J.J., Dong X., Cao Y.Y. Clinical characteristics of 140 patients infected by SARS-CoV-2 in Wuhan, China. Allergy. 2020;75:1730–1741. doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention Provisional death counts for coronavirus disease (COVID-19), updated June 10, 2020. https://www.cdc.gov/nchs/nvss/vsrr/covid_weekly/index.htm#AgeAndSex Available at:
- 16.Moser J.A.S., Galindo-Fraga A., Ortiz-Hernandez A.A. Underweight, overweight, and obesity as independent risk factors for hospitalization in adults and children from influenza and other respiratory viruses. Influenza Other Respi Viruses. 2019;13:3–9. doi: 10.1111/irv.12618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention Overweight & obesity. https://www.cdc.gov/obesity/index.html Available at:
- 18.Centers for Disease Control and Prevention National Center for Health Statistics, Obesity and overweight. https://www.cdc.gov/nchs/fastats/obesity-overweight.htm Available at:
- 19.Simonnet A., Chetboun M., Poissy J. High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARSCoV-2) requiring invasive mechanical ventilation. Obesity. 2020;28:1195–1199. doi: 10.1002/oby.22831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lighter J., Phillips M., Hochman S. Obesity in patients younger than 60 years is a risk factor for Covid-19 hospital admission. Clin Infect Dis. 2020;71:896–897. doi: 10.1093/cid/ciaa415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Intensive Care National Audit & Research Centre . Intensive Care National Audit & Research Centre; London: 2020. ICNARC Report on COVID-19 in Critical Care. April 10, 2020. [Google Scholar]
- 22.Wu J., Li W., Shi X. Early antiviral treatment contributes to alleviate the severity and improve the prognosis of patients with novel coronavirus disease (COVID-19) J Intern Med. 2020;288:128–138. doi: 10.1111/joim.13063. [DOI] [PubMed] [Google Scholar]
- 23.Richard C., Wadowski M., Goruk S. Individuals with obesity and type 2 diabetes have additional immune dysfunction compared with obese individuals who are metabolically healthy. BMJ Open Diabetes Res Care. 2017;5 doi: 10.1136/bmjdrc-2016-000379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zheng Q., Cui G., Chen J. Regular exercise enhances the immune response against microbial antigens through upregulation of toll-like receptor signaling pathways. Cell Physiol Biochem. 2015;37:735–746. doi: 10.1159/000430391. [DOI] [PubMed] [Google Scholar]
- 25.Patel V.B., Mori J., McLean B.A. ACE2 deficiency worsens epicardial adipose tissue inflammation and cardiac dysfunction in response to diet-induced obesity. Diabetes. 2016;65:85–95. doi: 10.2337/db15-0399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jose R.J., Manuel A. Does COVID-19 disprove the obesity paradox in ARDS? Obesity. 2020;28:1007. doi: 10.1002/oby.22835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li B., Yang J., Zhao F. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol. 2020;27:1320–1324. doi: 10.1007/s00392-020-01626-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.International Diabetes Federation . 9th Edition. 2019. IDF Diabetes Atlas.https://www.diabetesatlas.org/en/ Available at: [Google Scholar]
- 29.Centers for Disease Control and Prevention 2020 National Diabetes Statistics Report. https://webcache.googleusercontent.com/search?q=cache:46NJDjtRNTkJhttps://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf+&cd=1&hl=en&ct=clnk&gl=us Available at:
- 30.Zhu L., She Z.G., Cheng X. Association of blood glucose control and outcomes in patients with COVID-19 and pre-existing type 2 diabetes. Cell Metab. 2020;31:1068–1077. doi: 10.1016/j.cmet.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bode B., Garrett V., Messler J. Glycemic characteristics and clinical outcomes of COVID-19 patients hospitalized in the United States. J Diabetes Sci Technol. 2020;14:813–821. doi: 10.1177/1932296820924469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang X., Yu Y., Xu J. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guan W., Ni Z., Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese center for disease control and prevention. J Am Med Assoc. 2020 Feb 24 doi: 10.1001/jama.2020.2648. [E-pub ahead of print] [DOI] [PubMed] [Google Scholar]
- 35.Deng S.Q., Peng H.J. Characteristics of and public health responses to the coronavirus disease 2019 outbreak in China. J Clin Med. 2020;9:575. doi: 10.3390/jcm9020575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ruan Q., Yang K., Wang W. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guan W.J., Liang W.H., Zhao Y. Comorbidity and its impact on 1590 patients with Covid-19 in China: a nationwide analysis. Eur Respir J. 2020 May 14 doi: 10.1183/13993003.00547-2020. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bhatraju P.K., Ghassemieh B.J., Nichols M. Covid-19 in critically ill patients in the Seattle region – case series. N Engl J Med. 2020 May 21 doi: 10.1056/NEJMoa2004500. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rehman K., Akash M.S. Mechanisms of inflammatory responses and development of insulin resistance: how are they linked? J Biomed Sci. 2016;23:87. doi: 10.1186/s12929-016-0303-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guo W., Li M., Dong Y. Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes Metab Res Rev. 2020 Mar 31 doi: 10.1002/dmrr.3319. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wan Y., Shang J., Graham R. Receptor recognition by novel coronavirus from Wuhan: an analysis based on decade long structural studies of SARS. J Virology. 2020 Mar 17 doi: 10.1128/JVI.00127-20. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu F., Long X., Zou W. Highly ACE2 expression in pancreas may cause pancreas damage after SARS-CoV-2 infection. medRxiv. 2020 Mar 3 doi: 10.1016/j.cgh.2020.04.040. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fernandez C., Rysa J., Almgren P. Plasma levels of the proprotein convertase furin and incidence of diabetes and mortality. J Intern Med. 2018;284:377–387. doi: 10.1111/joim.12783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Philips B.J., Meguer J.X., Redman J. Factors determining the appearance of glucose in upper and lower respiratory tract secretions. Intensive Care Med. 2003;12:2204–2210. doi: 10.1007/s00134-003-1961-2. [DOI] [PubMed] [Google Scholar]
- 45.Walls A.C., Park Y.-J., Tortorici M.A. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181:281–292.e6. doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Badawi A., Ryoo S.G. Prevalence of diabetes in the 2009 influenza A (H1N1) and the Middle East respiratory syndrome coronavirus: a systematic review and meta-analysis. J Public Health Res. 2016;5:733. doi: 10.4081/jphr.2016.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Centers for Disease Control and Prevention High cholesterol facts. https://www.cdc.gov/cholesterol/facts.htm Available at:
- 48.Ridker P.M., Kastelein J.J., Genest J. C-reactive protein and cholesterol are equally strong predictors of cardiovascular risk and both are important for quality clinical care. Eur Heart J. 2013;34:1258–1261. doi: 10.1093/eurheartj/eht022. [DOI] [PubMed] [Google Scholar]
- 49.Calfee C.S., Delucchi K.L., Sinha P. ARDS subphenotypes and differential response to simvastatin: secondary analysis of a randomized controlled trial. Lancet Respir Med. 2018;6:691–698. doi: 10.1016/S2213-2600(18)30177-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shi S., Qin M., Shen B. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5:802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Colantonio L.D., Shannon E.D., Orroth K.K. Ischemic event rates in very-high-risk adults. J Am Coll Cardiol. 2019;74:2496–2507. doi: 10.1016/j.jacc.2019.09.025. [DOI] [PubMed] [Google Scholar]
- 52.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jui K.P., Kuok D.I., Kang S.S. Modulation of sterol biosynthesis regulates viral replication and cytokine production in influenza A virus infected human alveolar epithelial cells. Antiviral Res. 2015;119:1–7. doi: 10.1016/j.antiviral.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 54.Mehrbod P., Hair-Bejo M., Ibrahim T.A.T. Simvastatin modulates cellular components in influenza A virus-infected cells. Int J Mol Med. 2014;34:61–73. doi: 10.3892/ijmm.2014.1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Belser J.A., Szretter K.J., Katz J.M. Simvastatin and oseltamivir combination therapy does not improve the effectiveness of oseltamivir alone following highly pathogenic avian H5N1 influenza virus infection in mice. Virology. 2013;439:42–46. doi: 10.1016/j.virol.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 56.Tikoo K., Patel G., Kuman S. Tissue specific up regulation of ACE2 in rabbit model of atherosclerosis by atorvastatin: role of epigenetic histone modifications. Biochem Pharmacol. 2015;93:343–351. doi: 10.1016/j.bcp.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 57.Hennig B.J.W., Hellier S., Frodsham A.J. Association of low-density lipoprotein receptor polymorphisms and outcome of hepatitis C infection [published correction in Genes Immun 2007;8:707] Genes Immun. 2002;3:359–367. doi: 10.1038/sj.gene.6363883. [DOI] [PubMed] [Google Scholar]
- 58.Vuorio A., Watts G.F., Schneider W.J., Tsimikas S., Kovanen P.T. Familial hypercholesterolemia and elevated lipoprotein(a): double heritable risk and new therapeutic opportunities. J Int Med. 2020;287:2–18. doi: 10.1111/joim.12981. [DOI] [PubMed] [Google Scholar]
- 59.Liu B., Sun Y., Xu G. Long-term trends in hypertension and elevated blood pressure among U.S. adults, 1999–2016. J Am Coll Cardiol. 2018;72:2089–2090. doi: 10.1016/j.jacc.2018.07.086. [DOI] [PubMed] [Google Scholar]
- 60.Fryar C.D., Ostchega Y., Hales C.M. Hypertension prevalence and control among adults: United States, 205-2016. NCHS Data Brief. 2017;289:1–8. [PubMed] [Google Scholar]
- 61.Samanic C.M., Barbour K.E., Liu Y. Prevalence of self-reported hypertension and antihypertensive medication use among adults – United States, 2017. MMWR Morb Mortal Wkly Rep. 2020;69:393–398. doi: 10.15585/mmwr.mm6914a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nambiar L., LeWinter M.M., VanBuren P.C. Decade long temporal trends in U.S. hypertension related cardiovascular mortality. J Am Coll Cardiol. 2020;75:2644–2646. doi: 10.1016/j.jacc.2020.03.009. [DOI] [PubMed] [Google Scholar]
- 63.Whelton P.K., Carey R.M., Aranow W.S. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/AphA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults. J Am Coll Cardiol. 2018;71:e127–e248. doi: 10.1016/j.jacc.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 64.Carey R.M., Muntner P., Bosworth H.B. Prevention and control of hypertension: JACC health promotion series. J Am Coll Cardiol. 2018;72:1278–1293. doi: 10.1016/j.jacc.2018.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Paz Ocaranza M., Riquelme J.A., Garcia L. Counter-regulatory renin-angiotensin system in cardiovascular disease. Nat Rev Cardiol. 2020;17:116–129. doi: 10.1038/s41569-019-0244-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mancia G., Rea F., Ludergnani M. Renin-angiotensin-aldosterone system blockers and the risk of Covid-19. N Engl J Med. 2020;382:2431–2440. doi: 10.1056/NEJMoa2006923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Reynolds H.R., Adhikari S., Pulgarin C. Renin-angiotensin-aldosterone system inhibitors and risk of COVID-19. N Engl J Med. 2020;382:2441–2448. doi: 10.1056/NEJMoa2008975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zuin M., Rigatelli G., Zuliani G. Arterial hypertension and risk of death in patients with COVID-19 infection: systematic review and meta-analysis. J Infect. 2020;81:e84–e86. doi: 10.1016/j.jinf.2020.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lippi G., Wong J., Henry B.M. Hypertension in patients with coronavirus disease 2019 (COVID-19): a pooled analysis. Pol Arch Intern Med. 2020;130:304–309. doi: 10.20452/pamw.15272. [DOI] [PubMed] [Google Scholar]
- 70.Rundle A.G., Park Y., Herbstman J.B. COVID-19 related school closings and risk of weight gain among children. Obesity. 2020;28:1008–1009. doi: 10.1002/oby.22813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen P., Mao L., Nassis G.P. Coronavirus disease (COVID-19): the need to maintain regular physical activity while taking precautions. J Sport Health Sci. 2020;9:103–104. doi: 10.1016/j.jshs.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tate M. Your colleagues are not dinosaurs – it’s workplace routines that make innovation difficult. The Conversation. 2018 https://theconversation.com/your-colleagues-are-not-dinosaurs-its-workplace-routines-that-make-innovation-difficult-100889 Available at: [Google Scholar]
- 73.Philibert I. Review article: closing the research gap at the interface of learning and clinical practice. Can J Anesth. 2012;59:203–212. doi: 10.1007/s12630-011-9639-7. [DOI] [PubMed] [Google Scholar]
- 74.Zielinski C. Causes of the knowledge gap. Lancet Global Health. 2019;7:e842. doi: 10.1016/S2214-109X(19)30206-2. [DOI] [PubMed] [Google Scholar]
- 75.Zieber M., Wojtowicz B. To dwell within: bridging the theory-practice gap. Nurs Philosophy. 2020;21 doi: 10.1111/nup.12296. [DOI] [PubMed] [Google Scholar]
- 76.Handelsman Y., Henry R.R., Bloomgarden Z. American Association of Clinical Endocrinologists and American College of Endocrinology position statement on the association of SGLT-2 inhibitors and diabetic ketoacidosis. Endocrine Pract. 2016;22:753–762. doi: 10.4158/EP161292.PS. [DOI] [PubMed] [Google Scholar]
- 77.Caccialanza R., Laviano A., Lobascio F. Early nutritional supplementation in non-critically ill patients hospitalized for the 2019 novel coronavirus disease (COVID-19): rationale and feasibility of a shared pragmatic protocol. Nutrition. 2020;74:110835. doi: 10.1016/j.nut.2020.110835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rosenson R.S., Baker S., Banach M. Optimizing cholesterol treatment in patients with muscle complaints. J Am Coll Cardiol. 2017;70:1290–1301. doi: 10.1016/j.jacc.2017.07.752. [DOI] [PubMed] [Google Scholar]
- 79.Das U.N. Can bioactive lipids inactivate coronavirus (COVID-19)? Arch Med Res. 2020;51:282–286. doi: 10.1016/j.arcmed.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Imai Y., Kuba K., Rao S. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436:112–116. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hemnes A.R., Rathinasabapathy A., Austin E.A. A potential therapeutic role for angiotensin converting enzyme 2 in human pulmonary arterial hypertension. Eur Respir J. 2018;51:1702638. doi: 10.1183/13993003.02638-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Khan A., Benthin C., Zeno B. A pilot clinical trial of recombinant human angiotensin-converting enzyme 2 in acute respiratory distress syndrome. Crit Care. 2017;21:234. doi: 10.1186/s13054-017-1823-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bavishi C., Maddox T.M., Messerli F.H. Coronavirus disease 2019 (COVID-19) infection and renin angiotensin system blockers. JAMA Cardiol. 2020 Apr 3 doi: 10.1001/jamacardio.2020.1282. [E-pub ahead of print] [DOI] [PubMed] [Google Scholar]
- 84.Vaduganathan M., Vardeny O., Michel T. Renin-angiotensin-aldosterone system inhibitors in patients with Covid-19. N Engl J Med. 2020;382:1653–1659. doi: 10.1056/NEJMsr2005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Meng J., Xiao G., Zhang J. Renin-angiotensin system inhibitors improve the clinical outcomes of COVID-19 patients with hypertension. Emerg Microbes Infect. 2020;9:757–760. doi: 10.1080/22221751.2020.1746200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang P., Zhu L., Cai J. Association of inpatient use of angiotensin converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID-19. Circ Res. 2020;126:1671–1681. doi: 10.1161/CIRCRESAHA.120.317134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cadegiani F.A. Can spironolactone be used to prevent COVID-19-induced acute respiratory distress syndrome in patients with hypertension? Am J Physiol Endocrinol Metab. 2020;318:e587–e588. doi: 10.1152/ajpendo.00136.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang L., He W., Yu X. Coronavirus disease 2019 in elderly patients: characteristics and prognostic factors based on 4-week follow-up. J Infection. 2020;80:639–645. doi: 10.1016/j.jinf.2020.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kumar Singh A., Gupta R., Misra A. Comorbidities in COVID-19: outcomes in hypertensive cohort and controversies with renin angiotensin system blockers. Diabet Metab Syndr Clin Res Rev. 2020;14:283–287. doi: 10.1016/j.dsx.2020.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Du Y., Tu L., Zhu P. Clinical features of 85 fatal cases of COVID-19 from Wuhan: a retrospective observational study. Am J Respir Crit Care Med. 2020;201:1372–1379. doi: 10.1164/rccm.202003-0543OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Xie J., Tong Z., Guan X. Clinical characteristics of patients who died of coronavirus disease 2019 in China. JAMA. 2020;3 doi: 10.1001/jamanetworkopen.2020.5619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Liu M., He P., Liu H.G. Clinical characteristics of 30 medical workers infected with new coronavirus pneumonia. Zhonghua Jie He He Hu Xi Za Zhi. 2020;43 doi: 10.3760/cma.j.issn.1001-0939.2020.0016. [DOI] [PubMed] [Google Scholar]
- 95.Peng T.D., Meng K., Guan H.Q. Clinical characteristics and outcomes of 112 cardiovascular disease patients infected by 2019-nCoV. Zhonghua Xin Xue Guan Bing Za Zhi. 2020;48 doi: 10.3760/cma.j.cn112148-20200220-00105. [DOI] [PubMed] [Google Scholar]
- 96.Wang Z., Chen Z., Zhang L. Status of hypertension in China: results from the China hypertension survey, 2012-2015. Circulation. 2018;137:2344–2356. doi: 10.1161/CIRCULATIONAHA.117.032380. [DOI] [PubMed] [Google Scholar]
- 97.Lu J., Lu Y., Wang X. Prevlence, awareness, treatment, and control of hypertension in China: data from 1.7 million adults in a population-based screening study (China PEACE Million Persons Project) Lancet. 2017;390:2549–2558. doi: 10.1016/S0140-6736(17)32478-9. [DOI] [PubMed] [Google Scholar]
- 98.Novel Coronavirus Pneumonia Emergency Response Epidemiology Team The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China. Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41:145–151. [Google Scholar]
- 99.Istituto Superiore Di Sanita. Coronavirus. https://www.epicentro.iss.it/coronavirus/ Available at:
- 100.Grasselli G., Zangrillo A., Zanella A. Baseline Characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region. Italy. JAMA. 2020;323:1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mills K.T., Stefansecu A., He J. The global epidemiology of hypertension. Nature Rev Nephrol. 2020;16:223–237. doi: 10.1038/s41581-019-0244-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.European Association for the Study of Obesity Share of adults with obesity. https://easo.org/share-of-adults-who-are-overweight-2/ Available at:
- 103.Indexmundi Diabetes prevalence (% of population ages 20 to 79) - country ranking. https://www.indexmundi.com/facts/indicators/SH.STA.DIAB.ZS/rankings Available at:
- 104.World Health Organization Non-communicable diseases – country profiles 2018. https://www.who.int/nmh/publications/ncd-profiles-2018/en/ Available at:
- 105.Garg S., Kim L., Whitaker M. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019 – COVID-NET, 14 States, March 1-30, 2020. MMWR. 2020;69:458–464. doi: 10.15585/mmwr.mm6915e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.CDC COVID-19 Response Team Preliminary estimates of the prevalence of selected underlying health conditions among patients with coronavirus disease 2019 - United States, February 12-March 28, 2020. MMWR. 2020;69:382–386. doi: 10.15585/mmwr.mm6913e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Petrilli C.M., Jones S.A., Yang J. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966. doi: 10.1136/bmj.m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Virani S.S., Alonso A., Benjamin E.J. Heart disease and stroke statistics – 2020 update. Circulation. 2020;141:e139–596. doi: 10.1161/CIR.0000000000000757. [DOI] [PubMed] [Google Scholar]
- 109.Richarson S., Hirsch J.S., Narasimhan M. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cummings M.J., Baldwin M.R., Abrams D. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Version 1. medRxiv. 2020 Apr 20 doi: 10.1016/S0140-6736(20)31189-2. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Argenziano M.G., Bruce S.L., Slater C.L. Characterization and clinical course of 1000 patients with COVID-19 in New York: retrospective case series. medRxiv. 2020 May 7 doi: 10.1136/bmj.m1996. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wang B., Li R., Lu Z. Does comorbidity increase the risk of patients with COVID-19: evidence from meta-analysis. Aging. 2020;12:6049–6057. doi: 10.18632/aging.103000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.The Centers for Disease Control and Prevention Public Health Image Library. https://phil.cdc.gov/Details.aspx?pid=23312 Available at: Accessed on April 28, 2020.