Abstract
Background:
This systematic review and meta-analysis aim to assess the effectiveness of Dangguijagyag-san (DJS) for primary dysmenorrhea (PD) and to update the previous reviews.
Methods:
We searched for randomized controlled trials (RCTs) of DJS for PD from inception to April 2019. The search databases were the PubMed, EMBASE, Cochrane Central Register of Controlled Trials, Oriental Medicine Advanced Searching Integrated System, Korean Traditional Knowledge Portal, Korean Medical Database, National Digital Science Library, and the China National Knowledge Infrastructure. The selection of studies, the extraction of data, and the quality assessment with risk of bias tool were performed by 2 authors independently. To analyze the data, the meta-analysis was conducted and qualitative analysis was also performed.
Results:
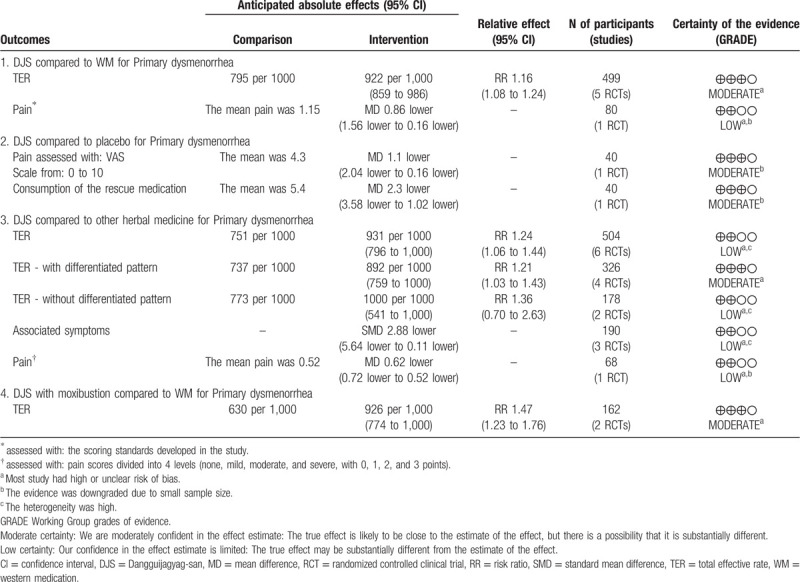
Total 2766 studies were identified, and 14 RCTs were enrolled in this review. According to the type of interventions, the analysis was performed in 4 groups. In comparison to western medication, DJS showed a higher total effective rate (TER) (RR 1.16, 95% CI 1.08–1.24) and a higher effect in reducing the pain (MD = −0.86, 95% CI −1.56–−0.16). Compared with placebo, DJS was superior to placebo in reducing pain (MD = −1.1, 95% CI −2.04 –−0.16) and also in reducing the consumption of the rescue medication during menstrual period (MD = −2.3, 95% CI −3.58–−1.02). Compared with other herbal medicines, the subgroup analysis showed that DJS applied with PD of differentiated patterns had a higher total effective rate (TER) (RR 1.21, 95% CI 1.03–1.43, P=.02). DJS with moxibustion as an adjuvant therapy was also more effective than western medication (RR 1.47, 95% CI 1.23–1.76).
Conclusion:
DJS may be effective for the treatment of PD. However, the quality of the evidence is relatively low, so larger-scale and well-designed RCTs are needed to confirm the effects of DJS.
Systematic review registration:
PROSPERO registration number is CRD42019130768.
Keywords: Danggui Shaoyao San, Dangguijagyag-san, herbal medicine, primary dysmenorrhea, systematic review
1. Introduction
Primary dysmenorrhea (PD) is a common gynecological disorder characterized by spasmodic cramps and pain in the lower abdomen, often accompanied by symptoms such as headache, fatigue, nausea, vomiting, diarrhea or insomnia.[1] The pain usually starts shortly before menstruation and lasts for 8 to 72 hours, and accompanying symptoms also occur just before and/or during menstruation.[1] The prevalence of dysmenorrhea has been estimated in the range between 16% to 91% of women of reproductive age and chronic dysmenorrhea has negative impact on quality of life.[2,3] In the case of PD, unlike secondary dysmenorrhea, identifiable pathological pelvic findings are not identified.[4] PD remains unclear from a scientific standpoint although it is often regarded as a normal symptom of menstruation and the most common disorder among women of reproductive age.[5]
Pathophysiology of PD has been known to be closely associated with overproduction of uterine prostaglandins.[6] High levels of prostaglandin lead to myometrial hypercontractility, resulting in ischemia of the uterine muscle, eventually leading to dysmenorrhea.[6] Exogenous prostaglandin can also cause nausea and diarrhea, which are the common accompanying symptoms of dysmenorrhea. Based on this prostaglandin pathology, the most frequent treatment drugs for PD are nonsteroidal anti-inflammatory drugs (NSAIDs) as prostaglandin synthetase inhibitors.[7,8] When not responding to prostaglandin inhibitors, synthetic hormonal contraceptives may be administered to reduce prostaglandin synthesis and suppress dysmenorrhea.[4] However, such conventional treatments have disadvantages in terms of safety. In the case of NSAIDS, various side effects on liver, kidney, and digestive tract have been reported, and oral contraceptives have also a risk of venous thromboembolism in long-term use based on recent meta-analysis study.[9,10] Thus, further research is needed to develop non-drug treatments that can be widely used.[11] In this situation, traditional East Asian herbal medicine can be a significant alternative treatment for PD.
In East Asia, herbal medicine has long been used as a safe and effective intervention for the treatment of PD. Through recent studies, it was confirmed that herbal medicines commonly used to treat PD have analgesic effects by mechanisms such as anti-inflammation, reducing prostaglandin, and vasorelaxation.[12,13] Among these herbal medicines, Dangguijagyag-san (DJS, also known as Danggui Shaoyao San in China) is one of the most widely known traditional herbal formulas for PD. In the most reliable type of study such as double-blind clinical trials, DJS has also demonstrated analgesic effects on PD.[14]
DJS was used as the main prescription to treat PD with clinical evidence in East Asia. To evaluate the clinical efficacy of DJS that is experimentally evident for PD, a comprehensive review and meta-analysis are required. Although the previous systematic review suggested DJS possible beneficial effect on PD, there were several limitations in that statistical heterogeneity was high and subgroup analysis was not included. In the most recent systematic review, the searching study process was performed in 2015.[15] Several RCTs on treatment of Pd with DJS have been published since 2016[16,17] Thus it is necessary to do systematic review including these studies and additional meta-analysis to update the evidence. Accordingly, this study was conducted to evaluate the efficacy of DJS for the treatment of PD, including the recent study.
2. Methods
2.1. Study registration
We registered the protocol of this study on PROSPERO (registration number: CRD42019130768). The protocol of this study was published in December 2019. We conducted this systematic review according to the previously published protocol.[18] We perform and report this systematic review and meta-analyses according to the preferred reporting items for systematic reviews and meta-analysis (PRISMA).[21]
Ethical approval was not necessary for our study. Our study did not contain any data that requires patients informed consent. This systematic review analyzed data from previous published studies and did not collect personal, sensitive information from participants. Therefore, it is considered that a statement related to institutional review board approval is unnecessary.
2.2. Data search and study selection
We searched the articles in following electronic databases: the PubMed, EMBASE, the Cochrane Central Register of Controlled Trials (CENTRAL), 4 Korean medical databases (Oriental Medicine Advanced Searching Integrated System (OASIS)), Korean Traditional Knowledge Portal, Korean Medical Database and National Digital Science Library (NDSL)), and one Chinese database (the China National Knowledge Infrastructure). The article search was performed from their inception to April 17, 2019 in each database. The searching terms were the terms about PD and DJS. To perform a comprehensive search, we followed the search strategy that was developed using the related term. The details of the search strategies for each database are showed in Supplementary.
Two authors performed study selection independently using the Endnote referencing software. In the first stage of selection, we selected those likely to be of relevance to our review through evaluating the titles and abstracts of studies. Then, we went over the full-text of the first selected studies and confirmed the appropriate studies for this review. We discussed and resolved the different selection results.
2.3. Inclusion and exclusion criteria
2.3.1. Inclusion criteria
-
1.
The type of study were RCTs including quasi-RCTs that were written in English, Korean or Chinese;
-
2.
The participants of the study were the primary dysmenorrhea patients;
-
3.
The experimental interventions were DJS alone or as a combination with other treatments, regardless of the formulation. The control interventions were western medicine, placebo, or the other herbal medicine;
-
4.
Results of the study included the following outcomes: menstrual pain intensity, the total treatment response rate, the use additional analgesics, overall related symptoms, or the quality of life.
Different from our previous systematic review protocol,[18] we also included the studies with DJS plus moxibustion as adjunctive treatment. In previous systematic review study, we detected that the meta-analysis with high heterogeneity included the different type of studies with DJS plus moxibustion as adjunctive treatment. To lower heterogeneity, we included the studies with DJS plus moxibustion as adjunctive treatment and conducted meta-analysis of these studies.
2.3.2. Exclusion criteria
Studies that did not meet the inclusion criteria were excluded. Other exclusion criteria were as follows:
-
1.
The studies that included DJS in both groups as the intervention;
-
2.
The full-text did not available or impossible to extract data.
2.4. Data extraction and Quality assessment
Two independent authors performed the data extraction and the quality assessment using the Excel program. The data extraction and assessment form included the year of study publication, participant characteristics, the number of participants, dropouts, study period, intervention details, outcomes and adverse events. To assess the quality of the studies, we determined the risk of bias using the “Risk of bias” tool from the Cochrane Handbook Version 6.0.[19]
Two authors cross-checked the results of this process and disagreement between the results were resolved with a discussion.
2.5. Data analysis
We used the Cochrane Collaborations software program Review Manager (RevMan version 5.3) for Windows to analyze the extracted data. To conduct the quantitative synthesis, the included studies were divided according to the types of interventions. To synthesize dichotomous outcomes such as the total effective rate (TER), the results were presented by the relative risks (RRs) with 95% confidence intervals (CIs). For continuous outcomes, the effect size was presented by the mean differences (MDs) with 95% CIs. For the homogeneous outcome presented by different scales, we calculated the effect size using the standard mean difference (SMD). The outcomes that were insufficient to meta-analysis were described qualitatively. We included each participants outcome measurement that were measured at the end of the treatment period only once. We used the random effects model to perform the quantitative synthesis with the RevMan program. The heterogeneity between the outcomes was assessed by the I2 statistic value calculated with the RevMan program. If the I2 is more than 70, the heterogeneity was judged high. To reduce the heterogeneity of the studies, we conducted the subgroup analysis. About the study inadequate for quantitative synthesis, we analyzed the data qualitatively.
We conducted meta-analysis according to the different combinations of interventions. In case that the heterogeneity was high, we performed a subgroup analysis. After investigating the possible causes of high heterogeneity, the subgroup analyses were conducted considering the differentiation of syndromes, types of DJS or the other factors affecting the outcomes.
To estimate the strength of the evidence, the results of the meta-analysis were assessed with the Grading of Recommendations Assessment, Development and Evaluation (GRADE).[20]
A plan to measure the reporting biases was reported in the protocol, but not measured because the number of studies in each meta-analysis group was not enough.
3. Results
3.1. Results of the search and description of the included studies
The result of the study search and selection was presented in the PRISMA flow chart (Fig. 1).[21]
Figure 1.
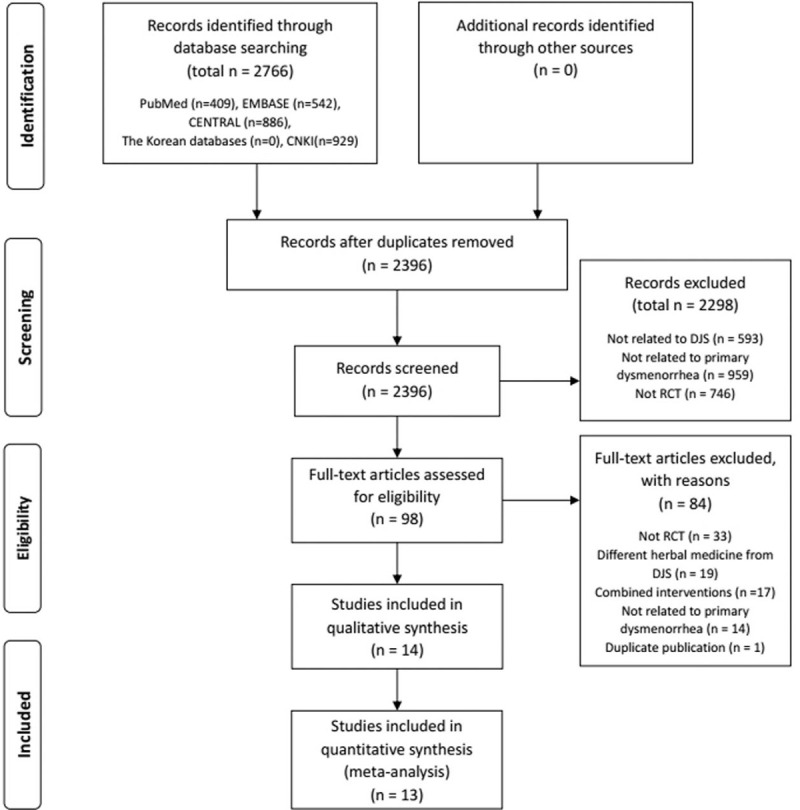
The flow chart of the study selection process. CENTRAL = Cochrane Central Register of Controlled Trials, CNKI = Chinese National Knowledge Infrastructure, DJS= Dangguijagyag-san, RCT=randomized controlled trial.
Total 2766 articles were identified after search of 8 electronic databases. The number of duplicated studies was 370. Through the selection process, 14 RCTs were selected.[14,16,22–33] We conducted a quantitative analysis in 13 RCTs[16,22–33] and a qualitative analysis in 1 RCT.[14] The selection process and reasons for exclusion are noted in Figure 1. All included studies were parallel group RCTs, and only 1 study[24] was quasi-RCT. 13 RCTs[16,22–33] were conducted in China and 1 RCT[14] was in Japan. The total number of participants was 1215 and the sample size ranged from 40 to 203. The included participants of 13 RCTs[14,16,22–27,29–33] were diagnosed with PD. 1 RCT[28] included participants diagnosed with primary and secondary dysmenorrhea, but we included only participants diagnosed with PD in the outcome analysis. In 8 RCTs,[14,22,23,25,26,29,32,33] the differentiated pattern that is considering related symptoms was applied to the participants inclusion criteria. The differentiated patterns were qi stagnation and blood stasis, cold coagulation, spleen deficiency and liver depression or deficiency of both qi and blood. The details of the included are shown in Table 1.
Table 1.
Summary of the included studies.
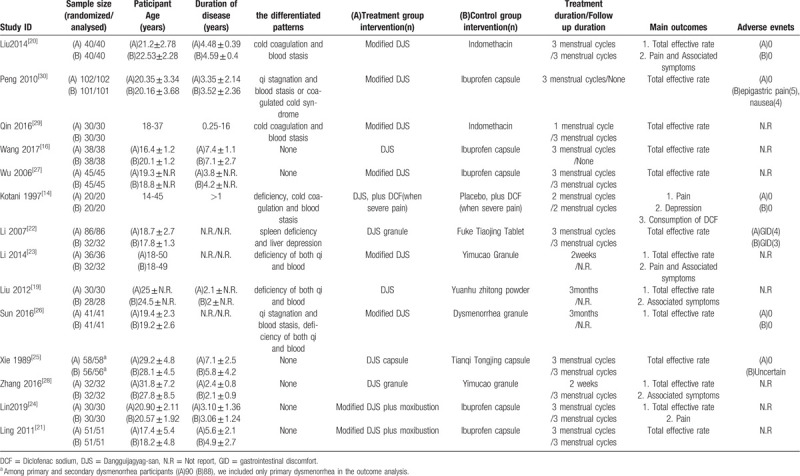
Most treatment durations were 3 menstrual cycles or 3 months, except that 1[14] was 2 menstrual cycles and 2[26,31] were 2 weeks. Most formulation type of DJS as experiment intervention was the form of a decoction. The granule or capsule of DJS was used in 3 RCTs[25,28,31] and 1 RCT[14] did not inform about formulation type. DJS or modified DJS was used for experiment intervention, and the details of the compositions are presented in Table 2.
Table 2.
Composition of Dangguijagyag-san (DJS) of included studies.

According to experiment and control interventions, the included studies were divided into 4 groups:
- 1.
-
2.
DJS vs placebo (1 study);[14]
-
3.
DJS vs other herbal medicine (6 studies);[22,25,26,28,29,31]
- 4.
Drugs such as NSIADs used as control interventions are expressed in the term “western medicine (WM)”. To distinguish our experimental therapy of traditional herbal medicine, we used in the term western medicine.
All RCTs except 1[14] used the total treatment effective rate judged by the practitioner. The treatment effective was generally divided into 4 categories or 3 categories and TER was the percentage of patients whose PD improved after treatment. 4 RCTs[14,23,26,27] measured the pain intensity using the visual analogue scale (VAS) or the degree score of pain. Associated menstrual symptoms were measured in the way of score the symptoms severity in 4 RCTs.[22,23,26,31] 1 RCT[14] reported the degree of depression and the consumption of the rescue medication. Adverse events (AEs) were reported in 2 RCTs.[25,33]
3.2. Risk of bias in the included studies
Most of the bias items were evaluated unclear or high. In random sequence generation, most of studies did not describe the specific method of random sequence generation. Three studies[22,31,33] were evaluated low, while 2 studies[24,30] were evaluated high. In allocation concealment, all studies did not report the allocation concealment. In the included studies, the participants could not be blinded and the blinding of outcome assessment was unclear. All patients of every included study completed the study and there were no losses to follow up. We evaluated the items of incomplete outcome data as low. Although none of the studies published study protocols, 4 studies[14,27,29,31] that intended to describe methods for measuring outcomes were evaluated low. The result of each evaluation is shown in Figure 2.
Figure 2.
Summary of the risk of bias.
3.3. Effects of DJS
3.3.1. DJS vs WM
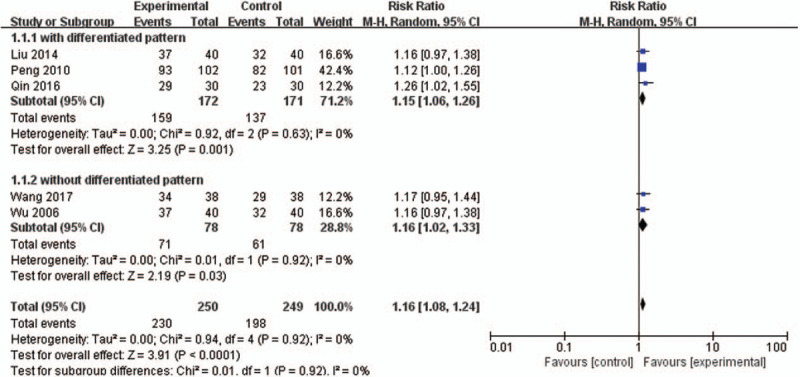
Five studies[16,23,30,32,33] (499 women) compared DJS or modified DJS with NSAIDs (Ibuprofen or Indomethacin). All study in this group reported TER. In TER, the result of meta-analysis revealed that DJS was more effective than NSAIDs (RR 1.16, 95% CI 1.08–1.24, P < .0001) The statistic I2 for heterogeneity was no heterogeneity (I2 = 0%) (Fig. 3).
Figure 3.
Forest plot of DJS vs WM, outcome: TER. CI = confidence interval, DJS = Dangguijagyag-san, RR = relative risk, TER = total effective rate, WM = Western medication.
Subgroup analyses were performed depending on whether the differentiated patterns are used in participants inclusion criteria. With the differentiated patterns, the result of meta-analysis revealed that DJS was more effective in TER than NSAIDs (RR 1.15, 95% CI 1.06 to 1.26, P = .001). Without the differentiated patterns, the result also revealed that DJS was more effective in TER than NSAIDs (RR 1.16, 95% CI 1.02–1.33, P = .03). In both analyses, there was low heterogeneity (I2 = 0%) (Fig. 3).
Only 1 study[23] reported the outcomes associated with menstrual symptoms including pain. In this study, it was found that the DJS could significantly improve the symptoms of abdominal pain, oligomenorrhea, chills and cold (P < .05), and menstrual clots and vomiting (P < .01).
3.3.2. DJS vs placebo
One study[14] (40 women) compared DJS against placebo. The results were reported VAS and reduction of rescue medicine, it was found that the DJS could significantly improve the dysmenorrhea compared to the placebo (P < .05). The depression symptom was also observed, but there were no statistically significant differences in the scores of self-rating depression scale between groups.
3.3.3. DJS vs other herbal medicine
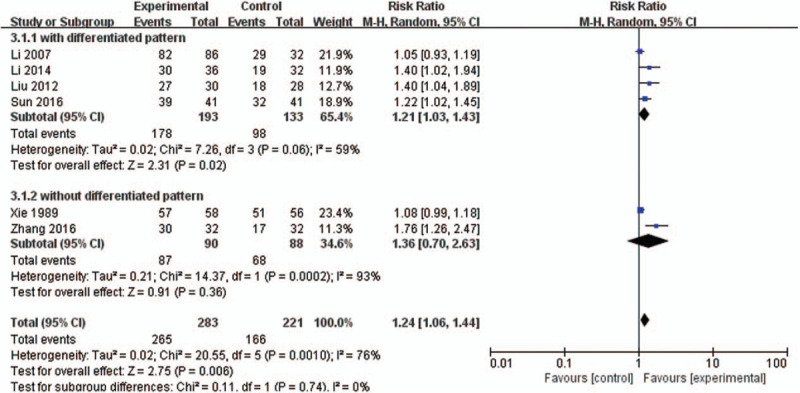
Six studies[22,25,26,28,29,31] (504 women) compared DJS or modified DJS with other herbal medicines. All study reported TER and the TER result of meta-analysis revealed that DJS was more effective than other herbal medicines (RR 1.24, 95% CI 1.06–1.44, P = .006) with high heterogeneity (I2 = 76%) (Fig. 4).
Figure 4.
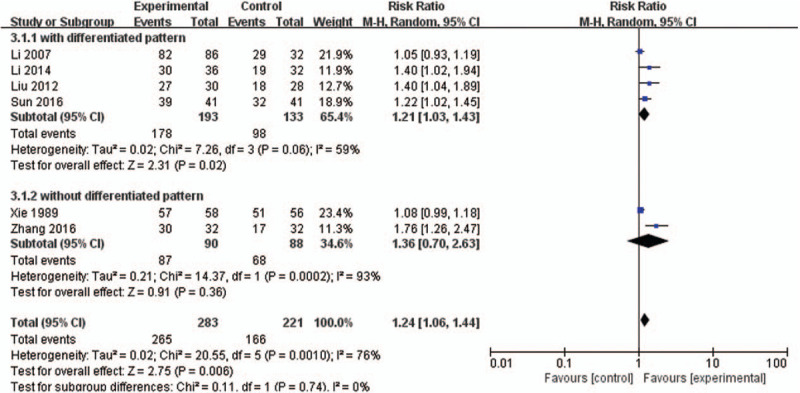
Forest plot of DJS vs other herbal medicine, outcome: TER. CI = confidence interval, DJS = Dangguijagyag-san, RR = relative risk, TER = total effective rate.
In 4[22,25,26,29] (326 women) out of 6 studies, the participants with the differentiated patterns. For sensitivity analyses, we performed meta-analysis except 2 studies[28,31] without the differentiated patterns and the TER result revealed that DJS was more effective than other herbal medicines (RR 1.21, 95% CI 1.03–1.43, P = .02) with lower heterogeneity (I2 = 59%) (Fig. 4).
Subgroup analysis were also performed depending on the types of DJS. When conducting this subgroup analysis, we excluded 2 studies[28,31] without the differentiated patterns because of high heterogeneity. In the group of DJS decoction, the result of meta-analysis revealed that DJS was more effective in TER than other herbal medicines (RR 1.29, 95% CI 1.12–1.48, P = .0003) with low heterogeneity (I2 = 0%) (Fig. 5). In 1 study[25] with DJS granule, the result also revealed that DJS was more effective in TER than other herbal medicine (RR 1.05, 95% CI 0.93–1.19, P = .83), but there was no statistically significant difference (Fig. 5).
Figure 5.
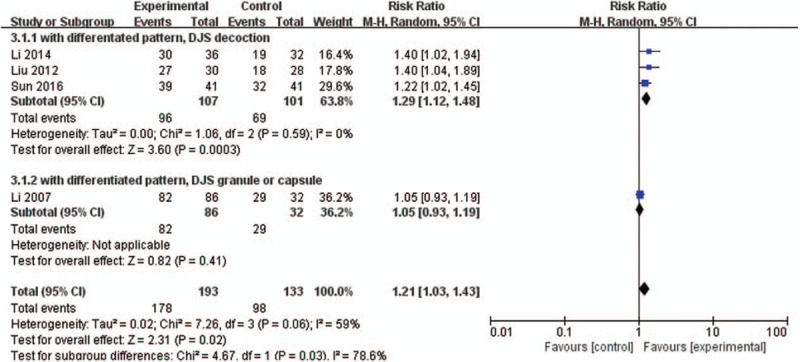
Forest plot of DJS vs other herbal medicine with subgroup analysis, outcome: TER. CI = confidence interval, DJS = Dangguijagyag-san, RR = relative risk, TER = total effective rate.
Three studies[22,26,31] (190 women) reported the associated symptoms. The results of associated symptoms were presented by different scales, so we performed meta-analysis using the SMD. DJS significantly reduced the severity of associated symptoms than other herbal medicines (SMD −2.88, 95% CI −5.64 –−0.11, P = .04) with high heterogeneity (I2 = 98%) (Fig. 6).
Figure 6.
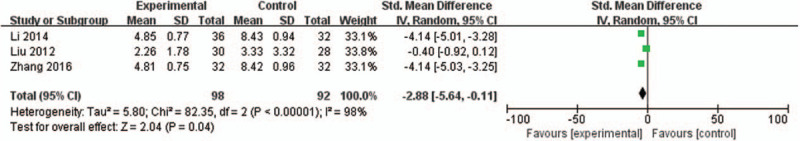
Forest plot of DJS vs other herbal medicine, outcome: associated symptoms. CI = confidence interval, DJS = Dangguijagyag-san, SMD = standard mean difference.
One study[26] reported the outcome about pain. In this study, it was found that the DJS could significantly improve the symptoms of pain (MD −0.62, 95% CI −0.72 –−0.52, P < .00001).
3.3.4. DJS with moxibustion vs WM
In 2 studies[24,27] (162 women), experimental group of modified DJS combined with moxibustion was compared with control group of NSAIDs (Ibuprofen). Both studies reported TER and the result of meta-analysis revealed that DJS with moxibustion was more effective than NSAIDs (RR 1.47, 95% CI 1.23–1.76, P < .0001) with low heterogeneity (I2 = 0%) (Fig. 7). In 1 study,[27] DJS with moxibustion had a therapeutic effect on the score of pain, but no significant differences from control treatment.
Figure 7.
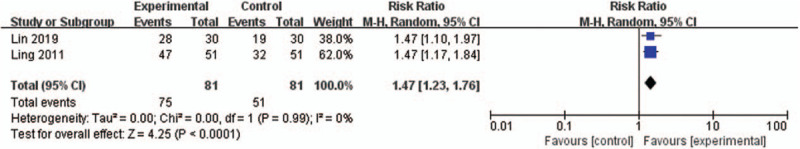
Forest plot of DJS with moxibustion vs WM, outcome: TER. CI = confidence interval, DJS = Dangguijagyag-san, RR = relative risk, TER = total effective rate, WM = Western medication.
3.3.5. Adverse events
In studies of DJS vs WM, 1 study[23] reported AEs in both groups, and 1 study[33] reported no AEs in DJS, 9 AEs in WM. And the other 3 studies[16,30,32] did not reported about AEs. No AEs were reported in DJS group and 9 cases of AEs were reported in WM group. AEs were epigastric pain (5 cases) and nausea (4 cases). RR of AEs was 0.05 (95% CI 0.00–0.88) in the DJS group.
In studies of DJS vs other herbal medicine, 1 study[29] reported no AEs, 1 study[25] reported 4 cases of AEs in DJS, 3 cases in other herbal medicine. The other studies did not specifically report AEs. AEs in the both groups were mainly gastrointestinal discomfort. RR of AEs was 0.50 (95% CI 0.12 –2.10) in the DJS group, but there was no significantly difference between the 2 groups.
One study[14] compared DJS against placebo reported no AEs in the DJS group. Two studies[24,27] compared DJS with moxibustion vs WM did not reported AEs.
3.4. Publication bias
Publication bias was not measured because there were not enough studies included in each meta-analysis group.
3.5. Quality of the evidence
The quality of evidence for each outcome was assessed with the GRADE method. The levels of evidences were moderate for TER in DJS vs WM group and DJS with moxibustion vs WM group. For the other outcomes, the GRADE level of evidence was moderate to low. The results of the GRADE assessment and the reasons for a deceasing level were shown in Table 3.
Table 3.
Summary of findings for each comparison group.
4. Discussion
This systematic review analyzed a total of 14 RCTs with 1215 participants dealing with the effects of DJS on PD. Since the results show that DJS was effective for PD, the main objective of this study was achieved. DJS was more effective than NSAIDs for treatment of PD, as was placebo or other herbal medicines. This result is also significant in that it is derived from the addition of new evidence published after 2016. Compared to previous reviews,[15] our results updated the level of evidence of TER in DJS vs WM group from low to moderate.
DJS and NSAIDs were compared in 5 RCTs.[16,23,30,32,33] DJS showed a superior effect on PD compared to NSAIDs (RR 1.16, 95% CI 1.08–1.24, P < .0001). The heterogeneity in meta-analysis of studies in which NSAIDs were used as control intervention was very low, with I2 = 0%. The results were similar in the 2 RCTs[24,27] comparing DJS with moxibustion and NSAIDs (RR 1.47, 95% CI 1.23–1.76, P < .0001), with low heterogeneity. There was only 1 study[14] that compared DJS with placebo. The results indicated DJS was superior to placebo in reducing pain of dysmenorrhea (MD = −1.1, 95% CI −2.04–−0.16) and also in reducing the consumption of the rescue medication during menstrual period (MD = −2.3, 95% CI −3.58–−1.02). All of these can be interpreted to support that DJS has significant effects on PD.
According to the theories of traditional medicine, DJS is more effective in the patients with deficiency of both qi and blood symptoms than the other herbal medicine formulas. So, we included the studies that compared DJS to other herbal medicines in this review. Therefore, this study compared the effects of other herbal medicine and DJS based on 6 RCTs.[22,25,26,28,29,31] This analysis also showed that DJS was more effective than other herbal medicine in terms of TER and PD associated symptom. Generally, this study could identify the effects of DJS on primary dysmenorrhea better than previous studies in that it is based on an extended analysis of RCTs. It is noteworthy that conclusions reinforce existing findings although this study is designed more extensively.
Previous study did not perform subgroup analysis on the included RCTs and showed high heterogeneity.[15] To complement this, the meta-analysis was performed in 4 groups according to the type of interventions. In addition, this study sets differentiated patterns, which are the criteria for the administration of Traditional Korean medicine, as the main conditions of subgroup analysis. In both meta-analysis groups of RCTs using WM or using other herbal medicine as comparison, DJS's effectiveness advantage was generally similar. With the GRADE method, the levels of evidences were assessed moderate for TER in DJS vs WM and DJS vs other herbal medicine with differentiated pattern. Particularly in DJS vs other herbal medicine group, for sensitivity analyses, meta-analysis was conducted on RCTs including the differentiated patterns PD. In further the subgroup analysis of DJS decoction, heterogeneity was markedly reduced to 0%. However, in the subgroup analysis of DJS capsule or granule, heterogeneity was decreased, but no significant effect advantage was observed. Therefore, the high heterogeneity observed in 6 RCTs[22,25,26,28,29,31] comparing the effects of other herbal medicine and DJS may be influenced by the drug form of the DJS as well as the differentiated patterns included in the study. However, the number of studies analyzed on this topic is still small, so it is only possible to draw further conclusions if additional RCTs are included.
In recent laboratory studies, DJS has been shown to have effects such as suppression of uterus contraction, prostaglandin level reduction, and correction of luteal insufficiency, demonstrating the potential of promising drugs for PD.[34–36] Meanwhile, an experimental study conducted in 2014 also reported that DJS administration to ovariectomized rats improved blood flow by inhibiting platelet aggregation and thrombus formation.[37] The mechanisms of DJS identified through these previous studies specifically support the beneficial effects seen in the treatment of PD.
Although more RCTs were included in the analysis, the overall quality of the study was still not high and AEs were not specifically reported in most studies. In addition to these issues, this systematic review has several limitations: first, most included studies do not describe randomization methods, allocation concealment and blinding in detail. This means that the conclusions of this study are exposed to a high risk of bias. Second, many studies have used TER as an evaluation variable. TER lacks in terms of reliability and validity, so further research is required to adopt more internationally available assessment tools. Third, since the sample size of most studies is less than 100, the accuracy of the result has some limitation. Fourth, it is difficult to generalize the results because all the studies involved have been conducted in East Asia.
5. Conclusions
In conclusion, the 14 RCTs included in this meta-analysis support the finding that DJS may be effective for the treatment of primary dysmenorrhea. However, this conclusion is not free from risk of bias because of the poor quality of the studies and the small sample size. Therefore, it is necessary to confirm the effects of DJS on primary dysmenorrhea based on better designed RCTs.
Author contributions
DL: revised the manuscript.
HGJ: performed the search, selection, quality assessment, extraction and analysis of data, and revised the manuscript.
JS, HL: performed the search, selection, quality assessment, extraction and analysis of data, and prepared the manuscript draft.
All authors have read and approved the final manuscript.
Supplementary Material
Footnotes
Abbreviations: AE = adverse event, CI = confidence interval, DE = development and evaluation, DJS = Dangguijagyag-san, GRADE = Grading of Recommendations Assessment, MD = mean difference, NSAIDs = nonsteroidal anti-inflammatory drugs, PD = primary dysmenorrhea, PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analyses, RCT = randomized controlled clinical trial, RR = relative risk, SMD = standard mean difference, TER = total effective rate, VAS = visual analogue scale, WM = western medication.
How to cite this article: Seo J, Lee H, Lee D, Jo HG. Dangguijagyag-san for primary dysmenorrhea: a PRISMA-compliant systematic review and meta-analysis of randomized-controlled trials. Medicine. 2020;99:42(e22761).
JS and HL are co-first authors and contributed equally.
The authors declare that they have no conflicts of interest.
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].Osayande AS, Mehulic S. Diagnosis and initial management of dysmenorrhea. Am Fam Physician 2014;89:341–6. [PubMed] [Google Scholar]
- [2].Ju H, Jones M, Mishra G. The prevalence and risk factors of dysmenorrhea. Epidemiol Rev 2014;36:104–13. [DOI] [PubMed] [Google Scholar]
- [3].Iacovides S, Avidon I, Baker FC. What we know about primary dysmenorrhea today: a critical review. Hum Reprod Update 2015;21:762–78. [DOI] [PubMed] [Google Scholar]
- [4].Burnett M, Lemyre M. No. 345-primary dysmenorrhea consensus guideline. J Obstet Gynaecol Can 2017;39:585–95. [DOI] [PubMed] [Google Scholar]
- [5].Wong LP, Khoo EM. Dysmenorrhea in a multiethnic population of adolescent Asian girls. Int J Gynaecol Obstet 2010;108:139–42. [DOI] [PubMed] [Google Scholar]
- [6].Dawood MY. Primary dysmenorrhea: advances in pathogenesis and management. J Obstet Gynaecol Res 2006;108:428–41. [DOI] [PubMed] [Google Scholar]
- [7].Zahradnik HP, Hanjalic-Beck A, Groth K. Nonsteroidal anti-inflammatory drugs and hormonal contraceptives for pain relief from dysmenorrhea: a review. Contraception 2010;81:185–96. [DOI] [PubMed] [Google Scholar]
- [8].Armour M, Parry K, Al-Dabbas MA, et al. Self-care strategies and sources of knowledge on menstruation in 12,526 young women with dysmenorrhea: a systematic review and meta-analysis. PLoS One 2019;14:e0220103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Harirforoosh S, Asghar W, Jamali F. Adverse effects of nonsteroidal antiinflammatory drugs: an update of gastrointestinal, cardiovascular and renal complications. J Pharm Pharm Sci 2013;16:821–47. [DOI] [PubMed] [Google Scholar]
- [10].Manzoli L, De Vito C, Marzuillo C, et al. Oral contraceptives and venous thromboembolism: a systematic review and meta-analysis. Drug Saf 2012;35:191–205. [DOI] [PubMed] [Google Scholar]
- [11].Khan KS, Champaneria R, Latthe PM. How effective are non-drug, nonsurgical treatments for primary dysmenorrhoea? BMJ 2012;344:e3011. [DOI] [PubMed] [Google Scholar]
- [12].Chen HY, Lin YH, Su IH, et al. Investigation on Chinese herbal medicine for primary dysmenorrhea: implication from a nationwide prescription database in Taiwan. Complement Ther Med 2014;22:116–25. [DOI] [PubMed] [Google Scholar]
- [13].Park KS, Park KI, Hwang DS, et al. A review of in vitro and in vivo studies on the efficacy of herbal medicines for primary dysmenorrhea. Evid Based Complement Alternat Med 2014;2014:296860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kotani N, Oyama T, Sakai I, et al. Analgesic effect of a herbal medicine for treatment of primary dysmenorrhea--a double-blind study. Am J Chin Med 1997;25:205–12. [DOI] [PubMed] [Google Scholar]
- [15].Lee HW, Jun JH, Kil KJ, et al. Herbal medicine (Danggui Shaoyao San) for treating primary dysmenorrhea: a systematic review and metaanalysis of randomized controlled trials. Maturitas 2016;85:19–26. [DOI] [PubMed] [Google Scholar]
- [16].Wang X. Clinical study on treatment of primary dysmenorrhea with Danggui Shaoyao San. Cardiovasc Dis J Integr Tradit Chin Western Med 2017;35:180–1. [Google Scholar]
- [17].Zhang Y. Clinical observation on treatment of primary dysmenorrhea with Danggui Shaoyao San. J North Pharm 2018;15:87. [Google Scholar]
- [18].Seo J, Lee D, Jo HG. Dangguijagyag-san for primary dysmenorrhea: a protocol for systematic review and meta-analysis of randomized controlled trials. Medicine 2019;98:e18345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Higgins JPT, Thomas J, Chandler J, et al. (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook. [Google Scholar]
- [20].Balshem H, Helfand M, Schünemann HJ, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol 2011;64:401–6. [DOI] [PubMed] [Google Scholar]
- [21].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Liu G, Wang W, Sun J. Clinical study of Danggui Shaoyao San in treating dysmenorrhea due to deficiency of qi and blood. Chin J for Clinicians 2012;40:59–60. [Google Scholar]
- [23].Liu C, Chen J, Liu Q, et al. 40 cases of dysmenorrhea of cold coagulation and blood stasis by strengthening the spleen and kidney, removing phlegm and activating blood circulation. J Jiangxi Univ TCM 2014;26:35–8. [Google Scholar]
- [24].Ling GM. Efficacy of Modified Danggui Shaoyao San with moxibustiontreatment on primary dysmenorrhea. Guangxi TCM 2011;34:19–20. [Google Scholar]
- [25].Li Q, Wang Y, Ni J. Danggui Shaoyao Granules for treatment of 86 cases of primary dysmenorrhea. Herald of Med 2007;11:1321–3. [Google Scholar]
- [26].Li A, Lou Y. Clinical application of Modified Danggui Shaoyao San in treating dysmenorrhea. Chin Naturo 2014;22:30–1. [Google Scholar]
- [27].Lin Y, Ma Z, Zhang H. Observation ofn therapeutic efficacy of Danggui Shaoyao Powder combined with Zhuang nationality thread moxibustion on primary dysmenorrhea and its effect on prostaglandins. J Youjiang Med Univ Nationalitie 2019;41:83–5. [Google Scholar]
- [28].Xie C, Wang X, Jiang D, et al. Clinical observation of Danggui Shaoyao Powder in treating dysmenorrhea. J TCM 1989;08:33–5. [Google Scholar]
- [29].Sun Q. Clinical effect observation of Modified Danggui Shaoyao Powder in treating primary dysmenorrhea. Clin J TCM 2016;28:675–7. [Google Scholar]
- [30].Wu XM, Li HJ. Modified Danggui Shaoyao San for treatment of 45 cases with primary dysmenorrhea. J Liaoning Univ TCM 2006;8:91. [Google Scholar]
- [31].Zhang C. Clinical analysis of the treatment of primary dysmenorrhea with Chinese Peony Granule. J Math Med 2016;29:1036–7. [Google Scholar]
- [32].Qin X. Effect of Danggui Shaoyao Decoction on dysmenorrhea and TCM nursing. For all Health 2016;10:50–1. [Google Scholar]
- [33].Peng Y. Cinical observation on dysmenorrhea of female college students withmodified Danggui Shaoyao San. J Lianoning Univ TCM 2010;12:169–70. [Google Scholar]
- [34].Hsu CS, Yang JK, Yang LL. Effect of “Dang-Qui-Shao-Yao-San” a Chinese medicinal prescription for dysmenorrhea on uterus contractility in vitro. Phytomedicine 2006;13:94–100. [DOI] [PubMed] [Google Scholar]
- [35].Hua YQ, Su SL, Duan JA, et al. Danggui-Shaoyao-San, a traditional Chinese prescription, suppresses PGF2alpha production in endometrial epithelial cells by inhibiting COX-2 expression and activity. Phytomedicine 2008;15:1046–52. [DOI] [PubMed] [Google Scholar]
- [36].Usuki S, Higa TN, Soreya K. The improvement of luteal insufficiency in fecund women by tokishakuyakusan treatment. Am J Chin Med 2002;30:327–38. [DOI] [PubMed] [Google Scholar]
- [37].Park IS, Lee HW, Ryuk JA, et al. Effects of an aqueous extract of dangguijagyagsan on serum lipid levels and blood flow improvement in ovariectomized rats. Evid Based Complement Alternat Med 2014;2014:497836. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


