Abstract
Background:
The standard treatment for diffuse intrinsic pontine glioma (DIPG) is radiotherapy, although conventional fractionated radiotherapy (CFRT) may not be in the best interest of the patient. Instead, hypofractionated radiotherapy (HFRT) may shorten the treatment period and reduce related costs for this treatment, which is typically palliative in nature.
Methods:
This systematic review and meta-analysis evaluated survival outcomes among patients who received HFRT or CFRT for DIPG. The PubMed, Medline, EMBASE, Cochrane Central Register, and Scopus databases were searched to identify relevant studies. Overall survival was the primary outcome of interest and progression-free survival was the secondary outcome of interest.
Results:
The search identified a total of 2376 reports, although only 4 reports were ultimately included in the meta-analysis. The studies included 88 patients who underwent HFRT and 96 patients who underwent CFRT. Relative to CFRT, HFRT provided comparable outcomes in terms of overall survival (hazard ratio [HR]: 1.07, 95% confidence interval [CI]: 0.77–1.47) and progression-free survival (HR: 1.04, 95% CI: 0.75–1.45).
Conclusions:
The results of this meta-analysis suggest that CFRT and HFRT provide similar survival outcomes for patients with DIPG.
Keywords: diffuse intrinsic pontine glioma, hypofractionated radiotherapy, meta-analysis
1. Introduction
Diffuse intrinsic pontine gliomas (DIPG) have a poor prognosis and are the leading cause of brain tumor-related deaths among pediatric patients.[1] The median overall survival (OS) in these cases is <12 months, with OS rates of only 30% at 1 year and 10% at 2 years.[1–3] Patients with DIPG may present with neurologic symptoms that include cranial nerve disorders, cerebellar ataxia, and long-tract signs.[4] Unfortunately, given the vital nature of the brainstem and the infiltrative growth of DIPG, gross total resection is generally impossible, although stereotactic biopsy can be performed in select cases.[5] Based on these factors, the standard treatment for DIPG is radiotherapy, which can improve or stabilize the patient's neurologic symptoms and decrease the need for corticosteroids.[4] Conventional fractionated radiotherapy (CFRT) is typically used in this setting and takes approximately 6 weeks (generally 54 Gy in 30 fractions). However, given the median survival of <12 months, this treatment is considered palliative, and prolonged treatment periods reduce the patient's quality of life. Thus, hypofractionated radiotherapy (HFRT) may be useful in this setting, based on its shorter treatment time, fewer hospital visits, and decreased use of treatment resources.[6]
Janssens et al[7] performed a matched cohort analysis to compare outcomes between HFRT (44.8 Gy in 16 fractions or 39 Gy in 13 fractions) and CFRT, which revealed that HFRT provided equivalent survival outcomes with a lower treatment-related burden. Zaghloul et al[8] performed the first prospective randomized controlled trial to confirm this result, which revealed no significant differences in the median values for OS and progression-free survival (PFS) between the HFRT (39 Gy in 13 fractions) and CFRT groups. However, the small sample size was insufficient to conclusively determine non-inferiority, which is related to the low incidence of DIPG. Therefore, this systematic review and meta-analysis aimed to confirm whether HFRT and CFRT provided similar survival outcomes among patients with DIPG.
2. Methods
2.1. Search strategy
The systematic review aimed to identify reports that compared survival outcomes between HFRT and CFRT among patients with DIPG. The PubMed, Medline, EMBASE, Cochrane Central Register, and Scopus databases were searched without any language restrictions. The search terms were designed to provide maximum sensitivity: (diffuse intrinsic pontine glioma) or (diffuse brainstem glioma) or (midline glioma) and (radioth∗ or radia∗). Ethical approval are not necessary because this study was not direct enrollment of human.
2.2. Selection criteria
Two authors (JWP, JP) independently reviewed the search results to identify studies that compared HFRT to CFRT and enrolled >20 patients with DIPG. Eligible reports were required to include specific data regarding the OS and PFS outcomes. However, reports were considered ineligible if they were letters, conference papers, review articles, case reports, or part of a book. In the event that the search identified both a pilot study and a definitive study, the definitive study was selected for inclusion.
2.3. Data extraction
The 2 reviewers independently extracted data regarding the patient eligibility criteria, interventions, and survival outcomes. The primary outcome of interest was OS and the secondary outcome of interest was PFS. Data from all included studies were re-reviewed by another reviewer, and any discrepancies were resolved via discussion until all reviewers achieved complete agreement regarding the extracted data.
2.4. Statistical analysis
The data analysis was performed using Cochrane's Review manger (version 5.3). Treatment efficacy was judged based on the hazard ratios (HRs) and 95% confidence intervals (CIs) for OS and PFS. If the HR or CI values were not reported, these values were calculated based on the number of events and median survival. The standard error (SE) values were calculated based on the CI values.[9] Heterogeneity testing was performed, and a fixed-effect model was used in instances with an I2 of <50% or a P-value of >0.1. A funnel plot and Egger test were used to identify publication bias. Differences were considered statistically significant at P-values of <0.05.
3. Results
3.1. Search results and study characteristics
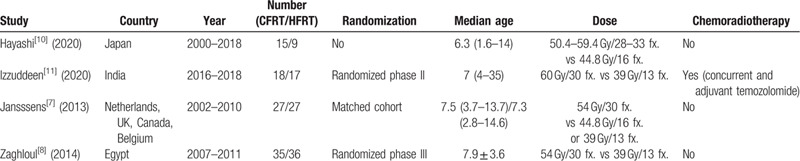
The review identified 2376 reports, although only 1629 reports remained after duplicate publications were removed. A further 1615 reports were excluded after the titles and abstracts were screened, and 10 additional reports were excluded after a full-text review (Fig. 1). All screened abstracts were written in English, and the reports to be read in full text were also written in English. Thus, the meta-analysis ultimately included data from 4 articles (Table 1).[10,11,7,8] Two studies were randomized controlled trials[11,8] and 2 were retrospective studies,[10,7] that included one matched cohort analysis.[7] The eligible reports were published during 2013 to 2020. The studies evaluated 194 patients, including 88 patients who underwent HFRT (39 Gy in 13 fractions or 44.8 Gy in 16 fractions) and 96 patients who underwent CFRT (total dose: 50.4–60 Gy). Adjuvant temozolomide was concurrently administered with the radiotherapy in one trial.[11]
Figure 1.
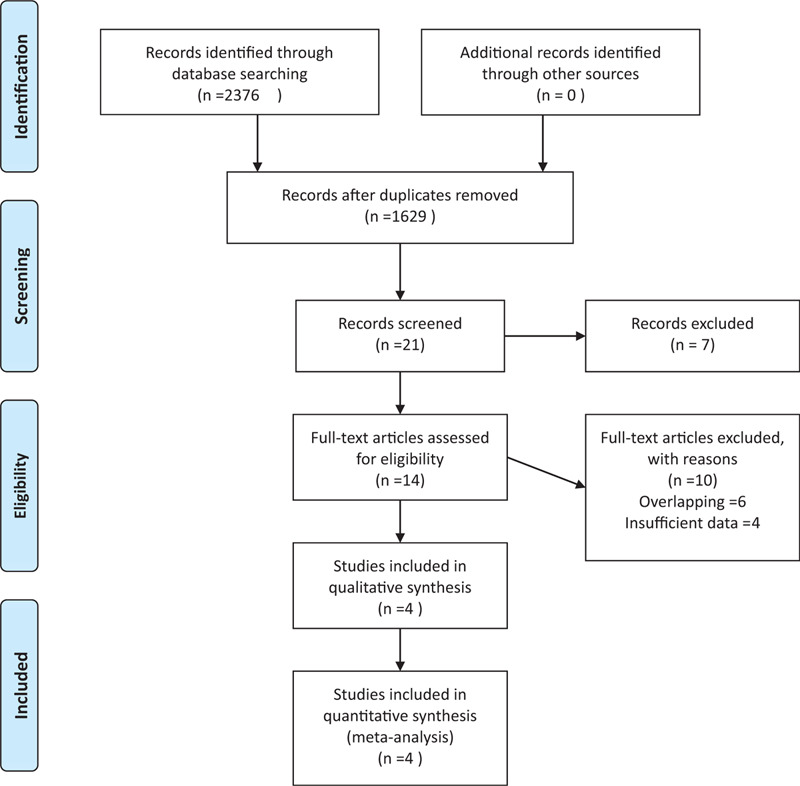
PRISMA flow chart.
Table 1.
Summary of the included studies.
3.2. Survival outcomes
The fixed-effect model was selected based on the findings for OS (I2 = 0 and P = .97) and for PFS (I2 = 0 and P = .56). Relative to CFRT, HFRT provided comparable outcomes in terms of OS (HR: 1.07, 95% CI: 0.77–1.47) and PFS (HR: 1.04, 95% CI: 0.75–1.45). The results are shown in Figs. 2 and 3.
Figure 2.
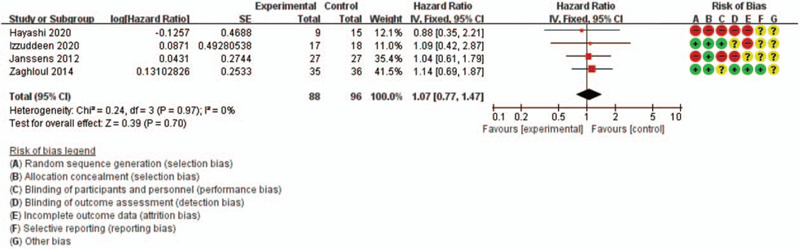
Forest plot of overall survival.
Figure 3.
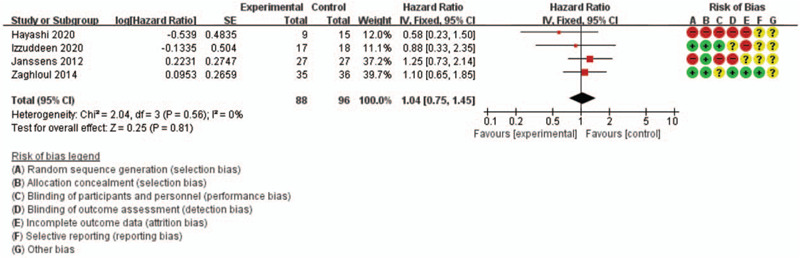
Forest plot of progression-free survival.
3.3. Toxicity
Janssens et al[7] reported that HFRT had a significant shorter median treatment time than CFRT (20 days vs 41 days, P < .01). Three reports described toxicity-related data,[11,7,8] albeit with very different criteria. The only reported grade 3 toxicity involved a single case of subdural hemorrhage in the CFRT plus temozolomide arm from the study by Izzuddeen et al.[11] There were no statistically significant differences in grades 1 and 2 toxicities from the other 2 studies.[7,8]
3.4. Sensitivity analysis and publication bias
Table 2 shows the results of the sensitivity analysis for OS, which failed to detect significant heterogeneity. Figures 4 and 5 show the funnel plot results for OS and PFS, which failed to detect significant publication bias.
Table 2.
Sensitivity analysis for overall survival.

Figure 4.
Funnel plot of overall survival.
Figure 5.
Funnel plot of progression-free survival.
4. Discussion
There is a growing interest for the use of HFRT for glioblastoma in elderly patients,[12–16] and to treat breast cancer,[17–19] and prostate cancer.[20–24] This strategy aims to reduce treatment costs and hospital visits, while providing similar treatment outcomes.[25–27] Treatment costs and patient convenience are important factors for breast cancer cases with a good prognosis, and the HFRT strategy may even improve the quality of life for elderly patients with glioblastoma who have a poor prognosis.[27] Similar to glioblastoma, DIPG has a very poor prognosis and most patients die within 1 year, which generally makes the treatment palliative in nature.[1,2,4] Radiotherapy is the treatment of choice for DIPG because it may help improve symptoms, reduce steroid dependency, and improve quality of life. Moreover, a treatment period of approximately 6 weeks is not ideal for patients who are expected to survive for <1 year, and Negretti et al[6] have reported that HFRT was effective and required a shorter treatment duration for DIPG.
Haas-Kogan et al[28] estimated that the α/β ratio of p53 mutated glioblastoma was 2 Gy. According to a linear-quadratic model, a relatively low α/β ratio may be sensitive to high fraction sizes.[29] The biologically effective dose (BED) can be calculated using the following formula:
n is a number of fractions and d is the dose per fraction.[10]
The BEDs from the 2 schedules of 44.8 Gy per 16 fractions and 39 Gy per 13 fractions were calculated to be 107.52 and 97.5 Gy, respectively; these values were similar to 102.6 Gy, the BED from the conventional fractionation schedule. Therefore, the biological effects of HFRT and CFRT might be similar.
This meta-analysis revealed no significant differences in OS and PFS between the CFRT and HFRT groups. Zaghloul et al[8] performed the first phase III randomized controlled trial that compared HFRT (39 Gy in 13 fractions) to CFRT (54 Gy in 30 fractions) in pediatric patients with DIPG. The study enrolled 80 patients, but only randomized 71 patients, and ultimately failed to detect significant differences in OS and PFS. Moreover, the differences in the 18-month rates were only 2.2% for OS and 1.2% for PFS, with CI values (–21–25% for OS and –20–22% for PFS) that substantially exceeded the required non-inferiority margin (20%). A second phase II randomized controlled trial was performed by Izzuddeen et al,[11] which only included 35 patients. This is likely related to the difficulty in enrolling a large sample of patients, given the low incidence of DIPG. Therefore, a meta-analysis is likely necessary to determine whether HFRT and CFRT provide equivalent outcomes for DIPG cases.
Several systematic reviews have evaluated the role of HFRT to treat DIPG. Hu et al[30] performed a systematic review of the Cochrane database to identify phase III randomized controlled trials, although their review only identified the Zaghloul et al[8] study. Gallitto et al[31] also performed a systematic review that compared CFRT, HFRT, and hyperfractionated radiotherapy for DIPG. Their search identified 49 CFRT, 7 hyperfractionated radiotherapy, and 3 HFRT studies, although they failed to detect significant differences between HFRT and CFRT in terms of the median OS (9.0 months vs 9.4 months) and time to progression (5.0 months vs 7.6 months). However, that review did not employ a meta-analysis to compare HFRT and CFRT, and only included 2 studies that were published before 2019.[7,8] However, 2 related reports were published in 2020, which facilitated our meta-analysis of the outcomes after HFRT and CFRT.[10,11]
The HFRT and CFRT strategies appear to have similar toxicities. Zaghloul et al[8] reported various acute toxicities in the 2 groups, which included skin reactions, hearing issues, decreased appetite, dysphagia, fatigue, insomnia, nightmares, and seizure, although there were no significant differences between the HFRT and CFRT groups. Other studies[10,11,7] failed to identify any grade 3–4 radiotherapy-related acute toxicities in patients who underwent HFRT, and the HFRT and CFRT strategies appeared to provide a similar efficacy. Zaghloul et al[8] evaluated steroid discontinuation, which occurred in 6 HFRT cases and 5 CFRT cases, and also noted that the HFRT group exhibited significant improvements in neurologic conditions (cranial nerve disorders and ataxia). Janssens et al[7] reported that steroid treatment could be temporarily discontinued for 78% of patients in the HFRT group, and that the treatment duration was significantly shorter for HFRT than for CFRT (20 days vs 41 days, P < .01).
Several treatment strategies have been attempted for DIPG. Unfortunately, chemotherapy failed to improve survival in these cases.[32] Reirradiation is an attractive option to improve the prognosis with minimal toxicity for patients who experience disease progression after radiation therapy.[33] However, future strategies should be based on our increasing understanding of the radiological, molecular, and clinical characteristics of DIPG, such as mutations that affect the H3 histone variants H3F3A and HIST1H3B.[34,35]
The present study has several limitations. For example, 2 of the 4 studies used a retrospective design, which might have introduced significant bias. However, Janssens et al[7] used a matched cohort design with well-balanced treatment arms. Hayashi et al[10] involved a retrospective review of a small sample of patients, although the sensitivity analysis revealed that study had a minimal effect on the heterogeneity in our findings. There is an ongoing phase III randomized trial that is comparing HFRT (39 Gy in 13 fractions and 45 Gy in 15 fractions) to CFRT (54 Gy in 30 fractions) among a larger sample of patients (NCT01878266: https://clinicaltrials.gov/ct2/show/study/NCT01878266).
In conclusion, this systematic review and meta-analysis revealed that HFRT and CFRT provided similar survival outcomes for patients with DIPG. Furthermore, HFRT and CFRT may have similar treatment toxicities. Thus, HFRT may be considered a standard treatment given its benefits in terms of decreased treatment burden, fewer hospital visits, and increased quality of life. Nevertheless, the small sample size and retrospective design of some studies highlights the need for larger randomized controlled trials to confirm this benefit.
Author contributions
Conceptualization: Jae Won Park.
Data curation: Ji Woon Yea, Jaehyeon Park.
Funding acquisition: Jae Won Park.
Methodology: Jae Won Park.
Project administration: Jae Won Park.
Software: Ji Woon Yea, Jae Won Park.
Writing – original draft: Jaehyeon Park, Jae Won Park.
Writing – review & editing: Jae Won Park, Jaehyeon Park, Ji Woon Yea.
Footnotes
Abbreviations: CFRT = conventional fractionated radiotherapy, CI = confidence interval, DIPG = diffuse intrinsic pontine glioma, HFRT = hypofractionated radiotherapy, HR = hazard ratio, OS = overall survival, PFS = progression-free survival, SE = standard error.
How to cite this article: Park J, Yea JW, Park JW. Hypofractionated radiotherapy versus conventional radiotherapy for diffuse intrinsic pontine glioma: a systematic review and meta-analysis. Medicine. 2020;99:42(e22721).
This work was supported by a grant from the National Research Foundation of Korea [NRF-2019M3E5D1A02068143], which is funded by the Korean government (MSIT).
The authors have no conflicts of interest to disclose.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
References
- [1].Kebudi R, Cakir FB. Management of diffuse pontine gliomas in children: recent developments. Pediatr Drugs 2013;15:351–62. [DOI] [PubMed] [Google Scholar]
- [2].Mathew RK, Rutka JT. Diffuse intrinsic pontine glioma: clinical features, molecular genetics, and novel targeted therapeutics. J Korean Neurosurg Soc 2018;61:343–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Plessier A, Le Dret L, Varlet P, et al. New in vivo avatars of diffuse intrinsic pontine gliomas (DIPG) from stereotactic biopsies performed at diagnosis. Oncotarget 2017;8:52543–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Schroeder KM, Hoeman CM, Becher OJ. Children are not just little adults: recent advances in understanding of diffuse intrinsic pontine glioma biology. Pediatr Res 2014;75:205–9. [DOI] [PubMed] [Google Scholar]
- [5].Cavalheiro S, Yagmurlu K, da Costa MDS, et al. Surgical approaches for brainstem tumors in pediatric patients. Childs Nerv Syst 2015;31:1815–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Negretti L, Bouchireb K, Levy-Piedbois C, et al. Hypofractionated radiotherapy in the treatment of diffuse intrinsic pontine glioma in children: a single institution's experience. J Neurooncol 2011;104:773–7. [DOI] [PubMed] [Google Scholar]
- [7].Janssens GO, Jansen MH, Lauwers SJ, et al. Hypofractionation vs conventional radiation therapy for newly diagnosed diffuse intrinsic pontine glioma: a matched-cohort analysis. Int J Radiat Oncol Biol Phys 2013;85:315–20. [DOI] [PubMed] [Google Scholar]
- [8].Zaghloul MS, Eldebawy E, Ahmed S, et al. Hypofractionated conformal radiotherapy for pediatric diffuse intrinsic pontine glioma (DIPG): a randomized controlled trial. Radiother Oncol 2014;111:35–40. [DOI] [PubMed] [Google Scholar]
- [9].Statistical Formulae for Calculating Some 95% Confidence Intervals. A Concise Guide to Clinical Trials; 205–207. [Google Scholar]
- [10].Hayashi A, Ito E, Omura M, et al. Hypofractionated radiotherapy in children with diffuse intrinsic pontine glioma. Pediatr Int 2020;62:47–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Izzuddeen Y, Gupta S, Haresh KP, et al. Hypofractionated radiotherapy with temozolomide in diffuse intrinsic pontine gliomas: a randomized controlled trial. J Neurooncol 2020;146:91–5. [DOI] [PubMed] [Google Scholar]
- [12].Biau J, Chautard E, De Schlichting E, et al. Radiotherapy plus temozolomide in elderly patients with glioblastoma: a “real-life” report. Radiat Oncol 2017;12:197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Chang-Halpenny CN, Yeh J, Lien WW. Elderly patients with glioblastoma multiforme treated with concurrent temozolomide and standard- versus abbreviated-course radiotherapy. Perm J 2015;19:15–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lombardi G, Pace A, Pasqualetti F, et al. Predictors of survival and effect of short (40 Gy) or standard-course (60 Gy) irradiation plus concomitant temozolomide in elderly patients with glioblastoma: a multicenter retrospective study of AINO (Italian Association of Neuro-Oncology). J Neurooncol 2015;125:359–67. [DOI] [PubMed] [Google Scholar]
- [15].Minniti G, Scaringi C, Lanzetta G, et al. Standard (60 Gy) or short-course (40 Gy) irradiation plus concomitant and adjuvant temozolomide for elderly patients with glioblastoma: a propensity-matched analysis. Int J Radiat Oncol Biol Phys 2015;91:109–15. [DOI] [PubMed] [Google Scholar]
- [16].Wang TJC, Wu CC, Jani A, et al. Hypofractionated radiation therapy versus standard fractionated radiation therapy with concurrent temozolomide in elderly patients with newly diagnosed glioblastoma. Pract Radiat Oncol 2016;6:306–14. [DOI] [PubMed] [Google Scholar]
- [17].Haviland JS, Owen JR, Dewar JA, et al. The UK Standardisation of Breast Radiotherapy (START) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-year follow-up results of two randomised controlled trials. Lancet Oncol 2013;14:1086–94. [DOI] [PubMed] [Google Scholar]
- [18].Owen JR, Ashton A, Bliss JM, et al. Effect of radiotherapy fraction size on tumour control in patients with early-stage breast cancer after local tumour excision: long-term results of a randomised trial. Lancet Oncol 2006;7:467–71. [DOI] [PubMed] [Google Scholar]
- [19].Yarnold J, Ashton A, Bliss J, et al. Fractionation sensitivity and dose response of late adverse effects in the breast after radiotherapy for early breast cancer: long-term results of a randomised trial. Radiother Oncol 2005;75:9–17. [DOI] [PubMed] [Google Scholar]
- [20].Aluwini S, Pos F, Schimmel E, et al. Hypofractionated versus conventionally fractionated radiotherapy for patients with prostate cancer (HYPRO): late toxicity results from a randomised, non-inferiority, phase 3 trial. Lancet Oncol 2016;17:464–74. [DOI] [PubMed] [Google Scholar]
- [21].Arcangeli G, Fowler J, Gomellini S, et al. Acute and late toxicity in a randomized trial of conventional versus hypofractionated three-dimensional conformal radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys 2011;79:1013–21. [DOI] [PubMed] [Google Scholar]
- [22].Arcangeli G, Saracino B, Arcangeli S, et al. Moderate hypofractionation in high-risk, organ-confined prostate cancer: final results of a phase III randomized trial. J Clin Oncol 2017;35:1891–7. [DOI] [PubMed] [Google Scholar]
- [23].Catton CN, Lukka H, Gu C-S, et al. Randomized trial of a hypofractionated radiation regimen for the treatment of localized prostate cancer. J Clin Oncol 2017;35:1884–90. [DOI] [PubMed] [Google Scholar]
- [24].Pollack A, Walker G, Horwitz EM, et al. Randomized trial of hypofractionated external-beam radiotherapy for prostate cancer. J Clin Oncol 2013;31:3860–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Budach W, Bölke E, Matuschek C. Hypofractionated radiotherapy as adjuvant treatment in early breast cancer. a review and meta-analysis of randomized controlled trials. Breast Care (Basel) 2015;10:240–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Guo W, Sun Y-C, Bi J-Q, et al. Hypofractionated radiotherapy versus conventional radiotherapy in patients with intermediate- to high-risk localized prostate cancer: a meta-analysis of randomized controlled trials. BMC Cancer 2019;19:1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Lu VM, Kerezoudis P, Brown DA, et al. Hypofractionated versus standard radiation therapy in combination with temozolomide for glioblastoma in the elderly: a meta-analysis. J Neurooncol 2019;143:177–85. [DOI] [PubMed] [Google Scholar]
- [28].Haas-Kogan DA, Yount G, Haas M, et al. p53-dependent G1 arrest and p53-independent apoptosis influence the radiobiologic response of glioblastoma. Int J Radiat Oncol Biol Phys 1996;36:95–103. [DOI] [PubMed] [Google Scholar]
- [29].Fowler JF. The linear-quadratic formula and progress in fractionated radiotherapy. Br J Radiol 1989;62:679–94. [DOI] [PubMed] [Google Scholar]
- [30].Hu X, Fang Y, Hui X, et al. Radiotherapy for diffuse brainstem glioma in children and young adults. Cochrane Database System Rev 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Gallitto M, Lazarev S, Wasserman I, et al. Role of radiation therapy in the management of diffuse intrinsic pontine glioma: a systematic review. Adv Radiat Oncol 2019;4:520–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Rashed WM, Maher E, Adel M, et al. Pediatric diffuse intrinsic pontine glioma: where do we stand? Cancer Metastasis Rev 2019;38:759–70. [DOI] [PubMed] [Google Scholar]
- [33].Lu VM, Welby JP, Mahajan A, et al. Reirradiation for diffuse intrinsic pontine glioma: a systematic review and meta-analysis. Child's Nerv Syst 2019;35:739–46. [DOI] [PubMed] [Google Scholar]
- [34].Hoffman LM, van Zanten SEMV, Colditz N, et al. Clinical, radiologic, pathologic, and molecular characteristics of long-term survivors of diffuse intrinsic pontine glioma (dipg): a collaborative report from the International and European Society for Pediatric Oncology DIPG Registries. J Clin Oncol 2018;36:1963–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Mackay A, Burford A, Carvalho D, et al. Integrated molecular meta-analysis of 1,000 pediatric high-grade and diffuse intrinsic pontine glioma. Cancer Cell 2017;32:520.e5–37.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]


