Abstract
Background:
Central poststroke pain (CPSP) is a neuropathic pain syndrome that can occur after a cerebrovascular accident. It has negative effects on mood, sleep, rehabilitation, and quality of life in stroke patients. This systematic review assessed the efficacy and safety of nonpharmacological therapies for treating CPSP.
Methods:
The Cochrane, PubMed, Embase, and Web of Science databases were systematically searched for studies from inception to August 2020. Two authors worked independently and in duplicate to identify suitable studies.
Results:
Eleven studies were identified. Pain related to CPSP was ameliorated by precentral gyrus stimulation (P = .01), caloric vestibular stimulation (P = 0.004), transcranial direct current stimulation (P < .05), and bee venom acupuncture point injection (P = .009). Acupuncture (P = .72) and electroacupuncture therapies (P > .05) were as effective for thalamic pain as oral carbamazepine treatment. Motor cortex stimulation, but not deep brain stimulation (DBS), was effective for treating refractory CPSP, and appeared to be more effective than thalamic stimulation for controlling bulbar pain secondary to Wallenberg syndrome. However, DBS in the ventral striatum or anterior limb of the internal capsule improved depression (P = .020) and anxiety in patients with refractory CPSP. Some serious adverse events were reported in response to invasive electrical brain stimulation, but most of these effects recovered with treatment.
Conclusions:
Nonpharmacological therapies appear to be effective in CPSP, but the evidence is relatively weak. Invasive electrical brain stimulation can be accompanied by serious adverse events, but most patients recover from these effects.
Keywords: central poststroke pain (CPSP), controlled trial, nonpharmacological therapies, systematic review
1. Introduction
Central poststroke pain (CPSP) is a neuropathic pain syndrome that occurs after a cerebrovascular accident and corresponding vascular lesion. It mostly develops within 1 to 6 months of stroke.[1–3] It is commonly characterized by spontaneous or evoked pain and sensory abnormalities, associated with the disruption of somatic sensations.[4,5] The pain can be continuous or intermittent[1] and usually varies in intensity, increasing with external stimuli such as cold, movement, touching, or stress, and decreasing with relaxation and distraction.[1,6,7] In most cases, the pain is described as burning, throbbing, pricking, aching, allodynia, lacerating, freezing, squeezing, or penetrating.[1,6] It has also been proposed that CPSP has a negative impact on mood, quality of sleep, rehabilitation, and quality of life in patients with stroke.[5,8] The underlying mechanisms of CPSP have not yet been elucidated.[8] Generally, CPSP is diagnosed by exclusion, when other causes of nociceptive, psychogenic, or peripheral neuropathic origin have been excluded.[1,5] Patients with CPSP may also have aphasia and severe depression, which can make pain assessment more difficult, and delay the start of treatment.
Treatment of CPSP is aimed at pain alleviation rather than complete pain relief, and no standard treatment has yet been established.[6,8,9] The current mainstream treatments are pharmacological in nature.[10] Several nonpharmacological therapies have also been demonstrated to be effective for patients with CPSP in clinical trials;[9,11,12] however, few systematic reviews have summarized their effectiveness and safety.[10,13] Hence, this systematic review was conducted to evaluate the efficacy and safety of nonpharmacological therapies for controlling CPSP, based on scientific evidence.
2. Methods
2.1. Data sources and search strategy
Cochrane, PubMed, Embase, and Web of Science databases were searched from inception to August 2020 to identify available data sources using the chosen search strategies (Supplemental Table). Both free-text words and Medical Subject Headings were used to search PubMed. Language was restricted to English. Conference abstracts and the reference lists of all identified related publications were also scanned to ensure the inclusion of relevant randomized controlled trials (RCTs).
2.2. Selection criteria
To screen studies, the inclusion criteria were as follows: RCTs involving individuals with a diagnosis of CPSP; RCTs evaluating all nonpharmacological therapies for the treatment of CPSP; RCTs comparing nonpharmacological therapies with placebo or other therapeutic interventions (pharmacological or nonpharmacological); and RCTs reporting efficacy outcome(s).
The exclusion criteria were as follows: reviews, animal studies, case reports, dissertations, and duplicate or secondary analyses; studies that were unavailable in English; or CPSP data were unable to be separately extracted.
2.3. Data extraction and analysis
Two authors worked independently and in duplicate to identify suitable studies that met the selection criteria, by screening the title and abstract of each study and then verifying all potential articles in full. Data extracted from the RCTs included study design, participant characteristics, and outcomes. Each study's methodological quality was assessed using the “risk of bias” assessment tool developed by the Cochrane Collaboration.[14] Disagreements and discrepancies were resolved by discussion, or with the help of an adjudicator if necessary. All statistical analyses were conducted in RevMan 5.3 software. We calculated mean differences (MDs) with 95% confidence intervals (CIs) for continuous outcomes and odds ratios with 95% CIs for dichotomous data. Statistical significance was defined as P < .05. Due to the small number of participants included in the paper, if the paper directly provides data analysis results, we choose to directly extract the analysis results rather than reanalysis.
3. Results
3.1. Study selection and characteristics
From the initial database search, 6499 papers were identified. Of these, only 11 studies[4,8,9,11,12,15–21] fulfilled the inclusion criteria and were included in this systematic review (Fig. 1). Table 1 summarizes the general characteristics and outcomes of each trial. The included trials were published between 1993 and 2017. Sample sizes ranged from 3 to 60, with 166 participants in total.
Figure 1.
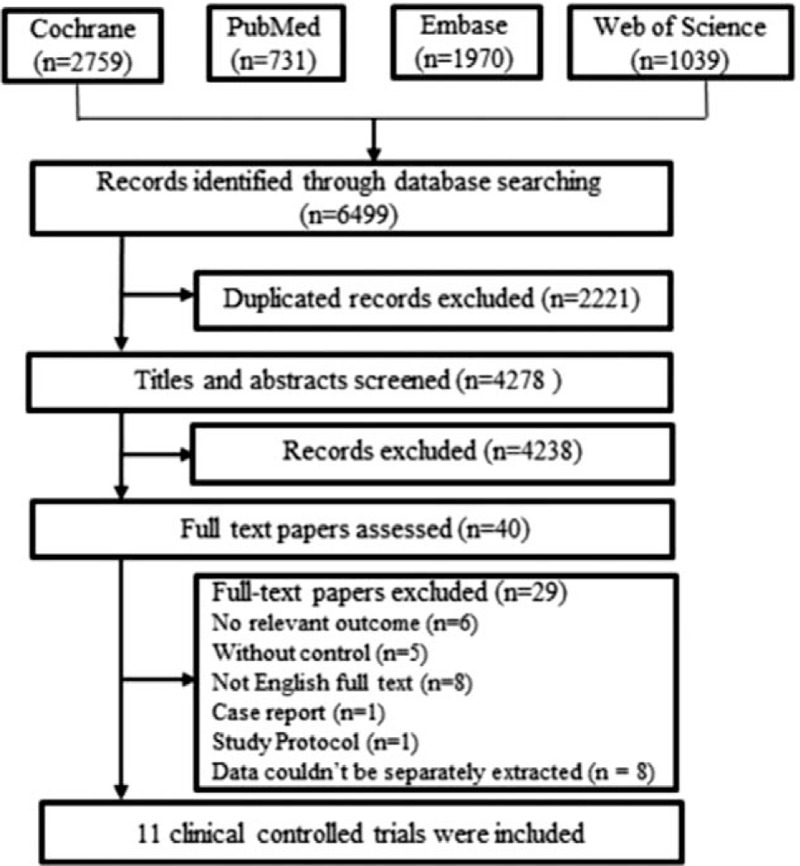
Flowchart of study selection.
Table 1.
The outcomes of nonpharmacological therapies for CPSP.
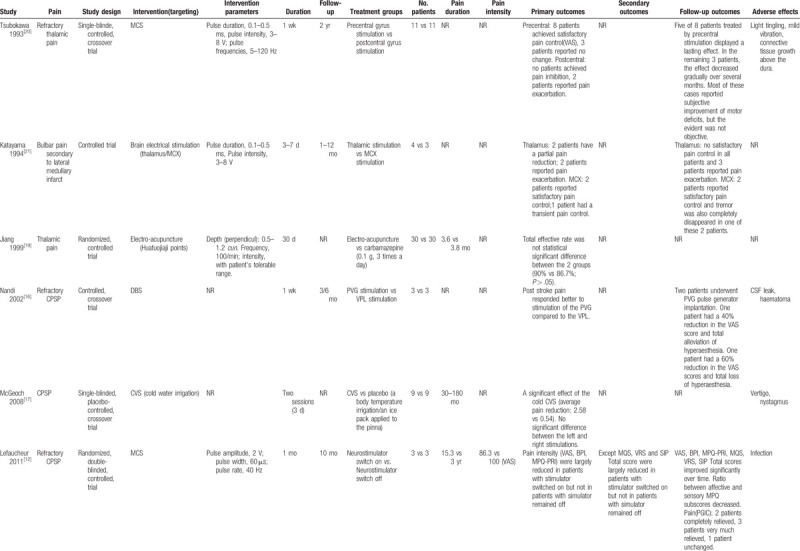
Table 1 (Continued).
The outcomes of nonpharmacological therapies for CPSP.
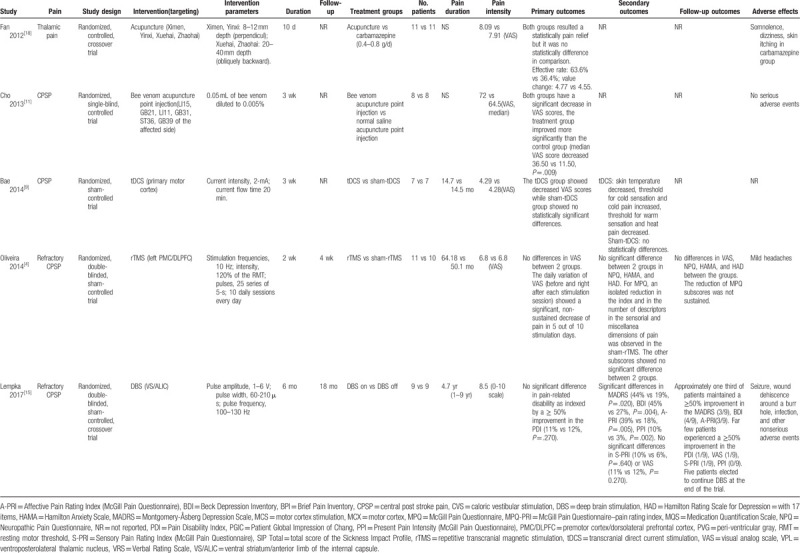
Nonpharmacological therapies for CPSP involved implanted electrical brain stimulation (n = 5),[12,15,16,20,21] repetitive transcranial magnetic stimulation (rTMS; n = 1),[4] caloric vestibular stimulation (CVS; n = 1),[17] acupuncture (n = 3),[11,18,19] and transcranial direct current stimulation (tDCS; n = 1).[9] Secondary outcomes, such as depression, were reported in 4 trials,[4,9,15,20] and adverse effects were reported in 8 trials.[4,11,12,15–18,20] Of the 11 trials included in our study, 6 used parallel group designs[4,9,11,12,16,19,21] and 5 used crossover designs.[15,20]
3.2. Quality assessment
We assessed the quality of the included studies using the “risk of bias,” shown in the summary figure (Fig. 2). The quality of the trials varied widely, and limitations in study designs were a major concern. Overall, the quality of the trial methodology in the included studies was relatively poor. We judged only 1 study[15] to have an overall low risk of bias.
Figure 2.
Results of nonpharmacological therapies for CPSP. CPSP = central poststroke pain.
3.3. Effects of nonpharmacological therapies on aspects of pain
3.3.1. Invasive electrical brain stimulation
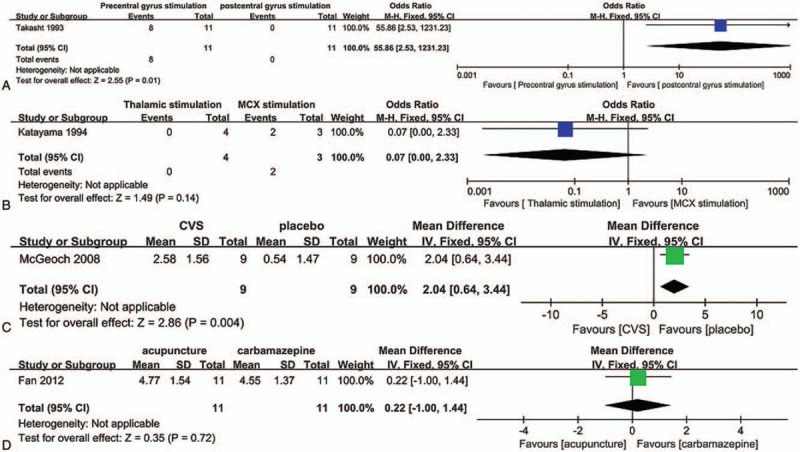
Tsubokawa et al[20] were the first to assess electrical brain stimulation to ameliorate pain in patients with CPSP. In their study, 4 plate electrodes (0.5 cm in diameter, each separated by 1 cm) were inserted into the epidural space of 11 patients with thalamic pain. Stimuli, delivered by monophasic square wave pulses, were applied continuously for 5 to 10 minutes on each occasion. Although the application of precentral gyrus stimulation for l week achieved satisfactory pain control, the application of postcentral gyrus stimulation had no clear effect on pain, or may have even exacerbated the original pain (P = 0.01) (Fig. 3A). The following year, Katayama et al[21] performed a clinical study to assess whether thalamic stimulation and/or motor cortex stimulation (MCS) were effective in bulbar pain secondary to Wallenberg syndrome. The results of this small trial revealed that MCS appeared to be more effective than thalamic stimulation for controlling bulbar pain, although this result was not significant (P = .14) (Fig. 3B). Through the aforementioned studies, the authors hypothesized that better control of deafferentation pain secondary to central nervous system lesions may be provided by stimulation at a level more rostral to the site of deafferentation.
Figure 3.
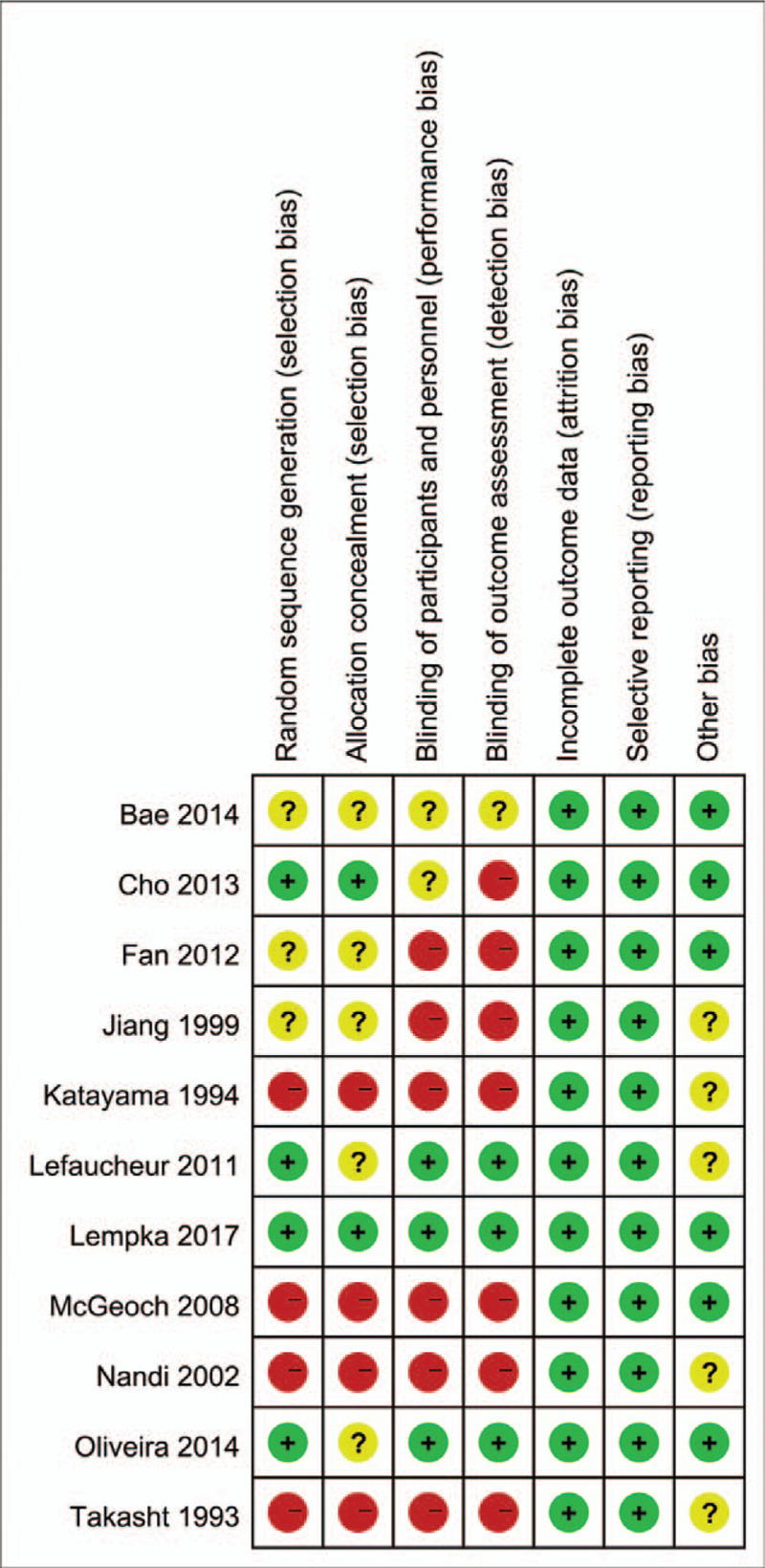
“Risk of bias” summary: review authors’ judgements about each risk of bias item for each included trails.
In recent years, there have been 2 randomized, double-blind, placebo-controlled trials evaluating epidural MCS[12] and ventral striatum/anterior limb of the internal capsule (VS/ALIC) deep brain stimulation (DBS)[15] to treat refractory CPSP. Eight-electrode epidural MCS therapy led to good clinical outcomes,[12] whereas DBS targeting the VS/ALIC resulted in negative outcomes[15] as evaluated by the visual analog scale (11% DBS ON vs 12% DBS OFF, odds ratio = 1.05, 95% CI 0.96– 1.15, P = .270). These results seemingly confirm the previous findings by Katayama et al.[21]
In another preliminary study,[16] 4 patients with CPSP were implanted with DBS electrodes in the ventral posterolateral thalamic nucleus and/or periventricular gray (PVG) contralateral to the pain. After 1 week of trial stimulation, poststroke pain appeared to be improved, and there was a better response to PVG stimulation than to stimulation of the ventral posterolateral thalamic nucleus. These results suggest that DBS may be effective in some conditions in CPSP patients.
3.3.2. Repetitive transcranial magnetic stimulation
rTMS is a noninvasive technique that improves cortical plasticity, thus altering brain functions. It has been used in a range of neurological disorders.[22,23] To date, 3 sham-controlled studies have examined the analgesic effects of rTMS on neuropathic pain;[4,24,25] however, the data in 2 of these trials were unable to be separately extracted for CPSP, and were thus excluded.[24,25] In the 1 included trial,[4] patients randomly received 10 daily sessions of active rTMS (n = 11) or sham rTMS (n = 10) over the premotor cortex/dorsolateral prefrontal cortex (PMC/DLPFC) (10 Hz, 1250 pulses/d). There was a significant reduction in pain intensity immediately after active rTMS sessions, but this effect was not sustained. The study was terminated because of a consistent lack of analgesic effects. Thus, rTMS of the left PMC/DLPFC appears not to be effective for relieving CPSP, and new targets and other stimulation parameters should be trialed.
3.3.3. Transcranial direct current stimulation
tDCS is another noninvasive brain stimulation technique, and has been used in various neurological diseases based on its mechanism of altering the excitability of the cerebral cortex.[26,27] To date, only 1 sham-controlled study has examined the analgesic effects of tDCS on CPSP.[9] The results indicated that 3 weeks of tDCS (2 mA) of the primary motor cortex exerted a significant analgesic effect (P < .05) and improved sensory identification in patients with CPSP. However, further research into its mechanisms of pain control is necessary.
3.3.4. Caloric vestibular stimulation
In a crossover study published in 2008,[17] 9 patients with CPSP underwent cold water irrigation and placebo intervention, either by the application of an ice pack to the pinna or by body temperature irrigation. Cold CVS had a significant immediate treatment effect for the control of CPSP compared with placebo (MD = 2.04, 95% CI 0.64–3.44, P = .004) (Fig. 3C). The authors of this study also revealed that patients with stroke that spared the dominant parieto-insular vestibular cortex, thus permitting its activation, responded best to CVS. This finding indicates that CVS may alleviate CPSP via cross-activation between the parieto-insular vestibular cortex and the thermosensory cortex in the adjacent dorsal posterior insula.
3.3.5. Acupuncture
Acupuncture has been an integral part of traditional Chinese medicine for more than 2000 years.[28,29] Fan et al[18] conducted a crossover trial to assess acupuncture in the treatment of CPSP. In this study, 11 patients with thalamic pain were treated with acupuncture or carbamazepine in a randomized order. Both acupuncture therapy and oral carbamazepine provided comparable benefits for reducing thalamic pain, and the analgesic effects of acupuncture were potentially superior to those of carbamazepine (MD = 0.22, 95% CI −1.00–1.44, P = .72) (Fig. 3D). The acupoints used in the patients in this study were PC 4, HT 6, SP 10, and KI 6.
Before this study by Fan et al, Jiang et al[19] assessed the effectiveness of electroacupuncture for treating postapoplectic thalamic spontaneous pain. The electroacupuncture treatment was performed as follows: stainless steel filiform needles (No. 28–30, 1.5-cun) were inserted perpendicularly into Huatuojiaji points, to a depth of 0.5 to 1.2 cun. The needles were then connected to an electroacupuncture apparatus for 30 minutes at a frequency of 100/min, once daily. The results from 30 days of treatment indicated that electroacupuncture at Huatuojiaji points is as effective for postapoplectic thalamic spontaneous pain as the oral administration of carbamazepine (P > .05).
In another small preliminary study, conducted by Cho et al,[11] patients were randomly injected with 0.05 mL of diluted bee venom or normal saline into acupoints (LI15, GB21, LI11, GB31, ST36, and GB39) twice a week for 3 weeks. Acupoint injection with bee venom (also known as apipuncture) significantly improved CPSP (P = .009), although the underlying mechanisms remain unclear.
3.4. Effects of nonpharmacological therapies on aspects of mood disorders
Only 2 RCTs investigated the role of nonpharmacological therapies on mood disorders in individuals with CPSP. In a 6-month RCT published in 2017,[15] active VS/ALIC DBS produced greater improvements in indices of depression (44% DBS ON vs 19% DBS OFF, odds ratio = 0.30, 95% CI 0.11–0.83, P = .020) and anxiety than sham stimulation, although the analgesic effects were negative. In contrast, in a trial by de Oliveira et al,[4] 10 days of rTMS in the left PMC/DLPFC area did not have a significant effect on depression or anxiety in patients with CPSP.
3.5. Adverse effects
Eight of the 11 included trials reported information about side effects.[4,11,12,15–18,20] Serious adverse events mainly occurred in the invasive electrical brain stimulation studies. Serious events included infection with epidural MCS therapy,[12] cerebrospinal leakage and hematoma with PVG stimulation therapy,[16] and seizure, wound dehiscence around the burr hole, and infection with DBS therapy.[15] However, most patients recovered from the adverse events following treatment. Mild adverse events were also reported, including paresthesia and tissue reactions (MCS),[20] vertigo (CVS),[17] and mild headaches (rTMS).[4]
3.6. Discussion and conclusions
CPSP is proposed to be associated with fatigue, mood lability, sleep disturbances, stress, lack of fitness,[30] and poor physical function related to quality of life.[5] There is thus an urgent need to identify effective treatments for CPSP patients. The recommended pharmacological treatments for CPSP are tricyclic antidepressants and anticonvulsants.[31–33] However, a recent systematic review found no evidence that anticonvulsants or tricyclic antidepressants are effective for CPSP.[13] In addition, evidence-based studies have indicated that, for most CPSP patients, pharmacological treatment is insufficient to relieve pain, even with high doses of different drugs.[2,34–37] Thus, the aim of the present study was to assess all evidence-based nonpharmacological therapies, in the hope that an effective treatment option for CPSP would be identified.
However, because of the heterogeneity and paucity of identified studies, our review is not a comprehensive guide to nonpharmacological methods of analgesia management in CPSP; additional work is required. We identified 11 published trials involving a total of 166 patients with CPSP. The trials compared nonpharmacological therapies with placebo, other nonpharmacological therapies, or any other therapies. The effects of nonpharmacological therapies on related mood disorders were also discussed, because CPSP can cause depression and anxiety in patients, thereby increasing the difficulty of pain analgesia.
Overall, there was low-certainty evidence in our systematic review, providing minimal support for the benefits of any nonpharmacological therapies for managing CPSP. Our results revealed that CPSP-related pain was able to be ameliorated by CVS, tDCS, and bee venom acupuncture point injection. Furthermore, acupuncture therapy and electroacupuncture at Huatuojiaji were as effective for thalamic pain as the oral administration of carbamazepine. Because these therapies are easy to operate and have no apparent side effects, they seem to be promising complementary and alternative therapies in the management of CPSP. Another widely used technique, rTMS, was considered in the present review, but 2 placebo-controlled trials included participants with neuropathic pain other than CPSP, and were thus excluded because of the strict selection criteria that we used. The results thus need be further confirmed in a large sample of CPSP patients only.
Invasive electrical brain stimulation is currently the most commonly investigated therapy for CPSP, and is almost always applied in refractory CPSP. Based on the results of the current review, MCS, but not VS/ALIC DBS, is effective for treating refractory CPSP. Moreover, MCS was also proposed to be more effective than thalamic stimulation for controlling bulbar pain. However, VS/ALIC DBS unexpectedly produced improvements in depression and anxiety in patients with refractory CPSP, even though the analgesic effects were negative. This result suggests the relevance of fibers projecting through the ALIC for the control of emotion.
Notably, some serious adverse events were reported in these invasive electrical brain stimulation therapies, such as infection, seizure, and cerebrospinal fluid leakage. However, considering the low incidence of these serious complications, and because most of these side effects recovered following treatment, invasive electrical brain stimulation seems to be a relatively safe therapy. Thus, for patients with refractory CPSP, invasive electrical brain stimulation such as MCS may be a good choice.
Several limitations of our study should be noted. First, because of the limited research available, very few trials were included, and most of the included trials contained a relatively small number of CPSP patients. This limited our ability to draw conclusions about these nonpharmacological approaches for the treatment of CPSP. Second, most of the included trials had methodological and/or reporting deficiencies. Information about randomization and allocation concealment was only described in a few studies, and participant–observer blindness was implemented in only a few articles. As a result, the reliability of the outcomes may be compromised. Third, because of the heterogeneity of the included trials and the limited reporting of data, it was not possible to pool data or make any further assessments. Our ability to reach a comprehensive conclusion regarding treatment options for CPSP was therefore very limited.
In conclusion, although the preliminary data on nonpharmacological therapies are promising, larger, high-quality trials with longer therapy times are needed before these approaches can be routinely prescribed for CPSP. We hope that this study encourages clinicians to pay more attention to nonpharmacological therapies for CPSP patients, especially those resistant to various medicines.
4. Ethics
As this is a review article, the ethical approval was not necessary.
Author contributions
Conceptualization: Xiao-Min Xu, Zuo-xiao Li.
Data curation: Xiao-Min Xu, Hua Luo.
Formal analysis: Hua Luo, Xiao-Mei Zheng.
Funding acquisition: Xiao-Min Xu, Zuo-xiao Li.
Investigation: Feng-tao Wang.
Methodology: Xiao-Min Xu, Hua Luo, Ben-bing Rong, Shu-Jiang Zhang.
Software: Ben-bing Rong, Xiao-Mei Zheng, Feng-tao Wang, Shu-Jiang Zhang.
Writing – original draft: Xiao-Min Xu, Ben-bing Rong.
Writing – review & editing: Zuo-xiao Li, Hua Luo.
Supplementary Material
Footnotes
Abbreviations: CPSP = central poststroke pain, CVS = caloric vestibular stimulation, DBS = deep brain stimulation, MCS = motor cortex stimulation, PMC/DLPFC = premotor cortex/dorsolateral prefrontal cortex, PVG = periventricular gray, RCT = randomized controlled trial, rTMS = repetitive transcranial magnetic stimulation, tDCS = transcranial direct current stimulation, VS/ALIC = ventral striatum/anterior limb of the internal capsule.
How to cite this article: Xu XM, Luo H, Rong Bb, Zheng XM, Wang Ft, Zhang SJ, Li ZX. Nonpharmacological therapies for central poststroke pain: A systematic review. Medicine. 2020;99:42(e22611).
This work was supported by the Science and Technology Support Project of Science & Technology Department of Sichuan (No.14ZC0063), as well as Research Project of Southwest Medical University (No. 2019ZQN138).
The authors have no conflicts of interest to disclose.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
References
- [1].Akyuz G, Kuru P. Systematic review of central post stroke pain: what is happening in the central nervous system? Am J Phys Med Rehabil 2016;95:618–27. [DOI] [PubMed] [Google Scholar]
- [2].Leijon G, Boivie J, Johansson I. Central post-stroke pain—neurological symptoms and pain characteristics. Pain 1989;36:13–25. [DOI] [PubMed] [Google Scholar]
- [3].Klit H, Finnerup NB, Jensen TS. Central post-stroke pain: clinical characteristics, pathophysiology, and management. Lancet Neurol 2009;8:857–68. [DOI] [PubMed] [Google Scholar]
- [4].de Oliveira RA, de Andrade DC, Mendonca M, et al. Repetitive transcranial magnetic stimulation of the left premotor/dorsolateral prefrontal cortex does not have analgesic effect on central poststroke pain. J Pain 2014;15:1271–81. [DOI] [PubMed] [Google Scholar]
- [5].Sahin-Onat S, Unsal-Delialioglu S, Kulakli F, et al. The effects of central post-stroke pain on quality of life and depression in patients with stroke. J Phys Ther Sci 2016;28:96–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Frese A, Husstedt IW, Ringelstein EB, et al. Pharmacologic treatment of central post-stroke pain. Clin J Pain 2006;22:252–60. [DOI] [PubMed] [Google Scholar]
- [7].Bowsher D. Central pain: clinical and physiological characteristics. J Neurol Neurosurg Psychiatry 1996;61:62–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Oh H, Seo W. A comprehensive review of central post-stroke pain. Pain Manag Nurs 2015;16:804–18. [DOI] [PubMed] [Google Scholar]
- [9].Bae SH, Kim GD, Kim KY. Analgesic effect of transcranial direct current stimulation on central post-stroke pain. Tohoku J Exp Med 2014;234:189–95. [DOI] [PubMed] [Google Scholar]
- [10].Chen CC, Chuang YF, Huang AC, et al. The antalgic effects of non-invasive physical modalities on central post-stroke pain: a systematic review. J Phys Ther Sci 2016;28:1368–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Cho SY, Park JY, Jung WS, et al. Bee venom acupuncture point injection for central post stroke pain: a preliminary single-blind randomized controlled trial. Complement Ther Med 2013;21:155–7. [DOI] [PubMed] [Google Scholar]
- [12].Lefaucheur JP, Keravel Y, Nguyen JP. Treatment of poststroke pain by epidural motor cortex stimulation with a new octopolar lead. Neurosurgery 2011;68:180–7. [DOI] [PubMed] [Google Scholar]
- [13].Mulla SM, Wang L, Khokhar R, et al. Management of central poststroke pain: systematic review of randomized controlled trials. Stroke 2015;46:2853–60. [DOI] [PubMed] [Google Scholar]
- [14].Higgins JPTGS. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. London, UK: The Cochrane Collaboration; 2011. [Google Scholar]
- [15].Lempka SF, Malone DA, Jr, Hu B, et al. Randomized clinical trial of deep brain stimulation for poststroke pain. Ann Neurol 2017;81:653–63. [DOI] [PubMed] [Google Scholar]
- [16].Nandi D, Smith H, Owen S, et al. Peri-ventricular grey stimulation versus motor cortex stimulation for post stroke neuropathic pain. J Clin Neurosci 2002;9:557–61. [DOI] [PubMed] [Google Scholar]
- [17].McGeoch PD, Williams LE, Lee RR, et al. Behavioural evidence for vestibular stimulation as a treatment for central post-stroke pain. J Neurol Neurosur Psychiatry 2008;79:1298–301. [DOI] [PubMed] [Google Scholar]
- [18].Fan X-n, Zhang X, Wu L-z, et al. Clinical efficacy observation of thalamic pain treated with acupuncture under the guidance of evidence-based medicine. World J Acupuncture Moxibustion 2012;22:1–7. [Google Scholar]
- [19].Jiang Z, Li C, Li Y. Treatment of postapoplectic thalamic spontaneous pain by electroacupuncture at huatuojiaji points. J Tradit Chin Med 1999;19:195–9. [PubMed] [Google Scholar]
- [20].Tsubokawa T, Katayama Y, Yamamoto T, et al. Chronic motor cortex stimulation in patients with thalamic pain. J Neurosurg 1993;78:393–401. [DOI] [PubMed] [Google Scholar]
- [21].Katayama Y, Tsubokawa T, Yamamoto T. Chronic motor cortex stimulation for central deafferentation pain: experience with bulbar pain secondary to Wallenberg syndrome. Stereotact Funct Neurosurg 1994;62:295–9. [DOI] [PubMed] [Google Scholar]
- [22].Altunrende B, Yildiz S, Cevik A, et al. Repetitive transcranial magnetic stimulation in restless legs syndrome: preliminary results. Neurol Sci 2014;35:1083–8. [DOI] [PubMed] [Google Scholar]
- [23].Strafella AP, Ko JH, Monchi O. Therapeutic application of transcranial magnetic stimulation in Parkinson's disease: the contribution of expectation. Neuroimage 2006;31:1666–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hosomi K, Shimokawa T, Ikoma K, et al. Daily repetitive transcranial magnetic stimulation of primary motor cortex for neuropathic pain: a randomized, multicenter, double-blind, crossover, sham-controlled trial. Pain 2013;154:1065–72. [DOI] [PubMed] [Google Scholar]
- [25].Andre-Obadia N, Peyron R, Mertens P, et al. Transcranial magnetic stimulation for pain control. Double-blind study of different frequencies against placebo, and correlation with motor cortex stimulation efficacy. Clin Neurophysiol 2006;117:1536–44. [DOI] [PubMed] [Google Scholar]
- [26].Webster BR, Celnik PA, Cohen LG. Noninvasive brain stimulation in stroke rehabilitation. NeuroRx 2006;3:474–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Antal A, Terney D, Poreisz C, et al. Towards unravelling task-related modulations of neuroplastic changes induced in the human motor cortex. Eur J Neurosci 2007;26:2687–91. [DOI] [PubMed] [Google Scholar]
- [28].Chen L, Houghton M, Seefeld L, et al. A survey of selected physician views on acupuncture in pain management. Pain Med 2010;11:530–4. [DOI] [PubMed] [Google Scholar]
- [29].Pan W, Wang M, Li M, et al. Actigraph evaluation of acupuncture for treating restless legs syndrome. Evid Based Complement Alternat Med 2015;2015:343201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Widar M, Ek AC, Ahlstrom G. Coping with long-term pain after a stroke. J Pain Symptom Manage 2004;27:215–25. [DOI] [PubMed] [Google Scholar]
- [31].Attal N, Cruccu G, Baron R, et al. European Federation of Neurological S. EFNS guidelines on the pharmacological treatment of neuropathic pain: 2010 revision. Eur J Neurol 2010;17:1113–88. [DOI] [PubMed] [Google Scholar]
- [32].Moulin D, Boulanger A, Clark AJ, et al. Pharmacological management of chronic neuropathic pain: revised consensus statement from the Canadian Pain Society. Pain Res Manag 2014;19:328–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Dworkin RH, O’Connor AB, Backonja M, et al. Pharmacologic management of neuropathic pain: evidence-based recommendations. Pain 2007;132:237–51. [DOI] [PubMed] [Google Scholar]
- [34].Haanpaa M, Attal N, Backonja M, et al. NeuPSIG guidelines on neuropathic pain assessment. Pain 2011;152:14–27. [DOI] [PubMed] [Google Scholar]
- [35].Vestergaard K, Andersen G, Gottrup H, et al. Lamotrigine for central poststroke pain: a randomized controlled trial. Neurology 2001;56:184–90. [DOI] [PubMed] [Google Scholar]
- [36].Vranken JH, Dijkgraaf MG, Kruis MR, et al. Pregabalin in patients with central neuropathic pain: a randomized, double-blind, placebo-controlled trial of a flexible-dose regimen. Pain 2008;136:150–7. [DOI] [PubMed] [Google Scholar]
- [37].Vranken JH, Hollmann MW, van der Vegt MH, et al. Duloxetine in patients with central neuropathic pain caused by spinal cord injury or stroke: a randomized, double-blind, placebo-controlled trial. Pain 2011;152:267–73. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


