Abstract
Background:
To evaluate the effectiveness of neuromuscular electrical stimulation (NMES) in early rehabilitation of patients with postoperative complications after cardiovascular surgery.
Methods:
37 patients (25 men and 12 women) aged 45 to 70 years with postoperative complications after cardiovascular surgery were included in the study. Eighteen patients underwent NMES daily since postoperative day 3 until discharge in addition to standard rehabilitation program (NMES group), and 19 patients underwent standard rehabilitation program only (non-NMES group). The primary outcome was the knee extensors strength at discharge in NMES group and in control. Secondary outcomes were the handgrip strength, knee flexor strength, and cross-sectional area (CSA) of the quadriceps femoris in groups at discharge.
Results:
Baseline characteristics were not different between the groups. Knee extensors strength at discharge was significantly higher in the NMES group (28.1 [23.8; 36.2] kg on the right and 27.45 [22.3; 33.1] kg on the left) than in the non-NMES group (22.3 [20.1; 27.1] and 22.5 [20.1; 25.9] kg, respectively; P < .001). Handgrip strength, knee flexor strength, quadriceps CSA, and 6 minute walk distance at discharge in the groups had no significant difference.
Conclusions:
This pilot study shows a beneficial effect of NMES on muscle strength in patients with complications after cardiovascular surgery. The use of NMES showed no effect on strength of non-stimulated muscle, quadriceps CSA, and distance of 6-minute walk test at discharge.
Further blind randomized controlled trials should be performed with emphasis on the effectiveness of NEMS in increasing muscle strength and structure in these patients.
Keywords: cardiovascular surgery, early rehabilitation, neuromuscular electrical stimulation, postoperative complications
1. Introduction
Even a successful cardiac surgery is not a clear guarantee of success and restoration of the patient's lost function and improvement in quality of life. There are a number of factors that facilitate development of perioperative complications, such as advanced age, comorbidity, severity of the underlying disease, etc. One of the significant factors contributing to deterioration of the rehabilitation outcome is “secondary sarcopenia,” which develops as a result of limited physical activity in patients, worsens the immediate results of rehabilitation, and increases the length of hospitalization.[1] The production of myostatin and pro-inflammatory cytokines is activated after cardiovascular surgery, which leads to development of myodystrophy and postoperative proteolysis of skeletal muscles.[2,3] Taking all of physiological manifestations of the underlying disease, catabolism of muscle tissue proteins with formation of sarcopenia and early postoperative complications into consideration, postoperative rehabilitation is mainly aimed at preventing muscle loss and its weakness. Accordingly, developing effective methods of rehabilitation is especially crucial in the early stages after surgery.
In this regard, the use of neuromuscular electrostimulation (NMES), which is usually used in chronic diseases with secondary sarcopenia, looks promising.[4,5] It has been shown that the use of NMES is not only safe in patients immediately after cardiovascular surgery,[6,7] but also reduces skeletal muscle proteolysis and weakness.[8] In patients with early activation after cardiovascular surgery, the use of NMES did not lead to additional improvement in muscle strength, which could be due to uncontrolled physical activity in the control.[9] This was the basis for the present study, the purpose of which was to evaluate the effectiveness of NMES in patients with complications after cardiac surgery limiting their physical activity.
2. Methods
2.1. Study design and participants
This single-center, clinical randomized study was performed at the Federal State Budgetary Institution's Research Institute for Complex Issues of Cardiovascular Diseases. Consecutive patients who had significant postoperative complications after cardiovascular surgery (prolonging their stay in the intensive care unit [ICU] for at least 3 days) from March 2017 to June 2019 were approached. Exclusion criteria for the study included: the need for a repeated surgery; postoperative delirium; need for hemodialysis after surgery; or refusal to participate in the study. Participants were randomly assigned to undergo NMES or to receive the usual postoperative rehabilitation program. Considering the intervention protocol, it was not possible to blind patients and/or the investigator who performed the NMES. However, the investigator who performed an ultrasound scan and CSA measurement quadriceps were blind to patients’ group of assignment. The study protocol was approved by the Local Ethics Committee of the Institution. This work was supported by exploratory scientific research “Rehabilitation of patients with complicated postoperative period of cardiac surgery” (№ 0546-2017-0007).
2.2. Data collection
The following preoperative clinical data were collected from the patient's clinical record: age, sex, body mass index, and standard biochemical parameters of a blood sample. All patients underwent transthoracic echocardiography with assessment of the heart chambers structure, valvular pathology, left ventricle systolic function. We also took perioperative parameters into account: type of surgical intervention, cardiopulmonary bypass time, and cross clamping time.
2.3. Intervention
The patients underwent NMES on the bilateral quadriceps femoris muscle daily from postoperative day (POD) 3 until discharge from the hospital (12 sessions or more). NMES was delivered to each patient by a specially trained postgraduate in the intensive care unit and then in the cardiovascular surgery department using the device “Beurer EM80” (Germany). Self-adhesive electrodes were located above the points of attachment of the quadriceps femoris, the duration of each session was at least 90 minutes, including a 5-minute warm-up period. During the session, rectangular pulses with a frequency of 45 Hz were modulated. As a result, a tonic contraction of M.quadriceps femoris was induced for 12 seconds, followed by a pause of 5 seconds. Amplitude of the electric impulse was selected separately for each of the 4 channels of the stimulator, until muscle contraction identified visually or by palpation was achieved, taking into account the individual characteristics and the level of pain threshold of each patient.
2.4. Outcomes
The primary result of this study was the isometric knee extensors strength (KES) at discharge in groups. Secondary outcomes were the handgrip strength (HS), knee flexor strength (KFS), and cross-sectional area (CSA) of the quadriceps femoris in groups at discharge. The indicators were measured upon POD 3 and upon discharge from the hospital. Dynamometry of the lower extremities muscles was carried out using an isokinetic dynamometer “Lafayette MMT 01165” (USA), assessing of the results (maximum and average muscle strength) directly on the screen of the device. Four exercises were performed in pairs for various muscle groups: knee extensors, knee flexors, ankle joint extensors, and flexors. HS was measured with a dynamometer “DK-100” (RF) sequentially, paired measurements of the right and left upper limb were performed. Before discharge from the hospital, a 6-minute walk test (6MWT) was performed in a 75 meters long corridor with measurement of walk distance.
Quadriceps femoris CSA was measured at two-third distance from the anterior superior iliac spine to the superior border of the patella. The measurements were carried out in a standardized manner – the thickness of subcutaneous fat and muscle thickness were assessed using real-time longitudinal scanning (Aloka SSD-280 LS, 7.5 MHz sensor), while quadriceps CSA was evaluated using ultrasound (Aloka SSD-190b) equipped with a 5 MHz probe. The mean value of 3 consecutive measurements of CSA using each ultrasound probe was recorded.[10]
2.5. Statistical analysis
All statistical analyses were calculated with STATISTICA 10.0 (Dell Software, Inc., Round Rock, TX). The Shapiro–Wilk test was used to test data for normal distribution. Since the distribution for all quantitative variables differed from normal, they are presented as median, lower, and upper quartile. Categorical data were reported as percentages. Differences in quantitative data between NMES groups and controls were evaluated using the Mann–Whitney test. Nominal and binary signs were compared using the χ2 criterion with Yates correction for small samples. The Wilcoxon test was used to assess the change of muscle status indicators in groups. A difference with P-value < .05 was considered statistically significant.
3. Results
3.1. Study participants
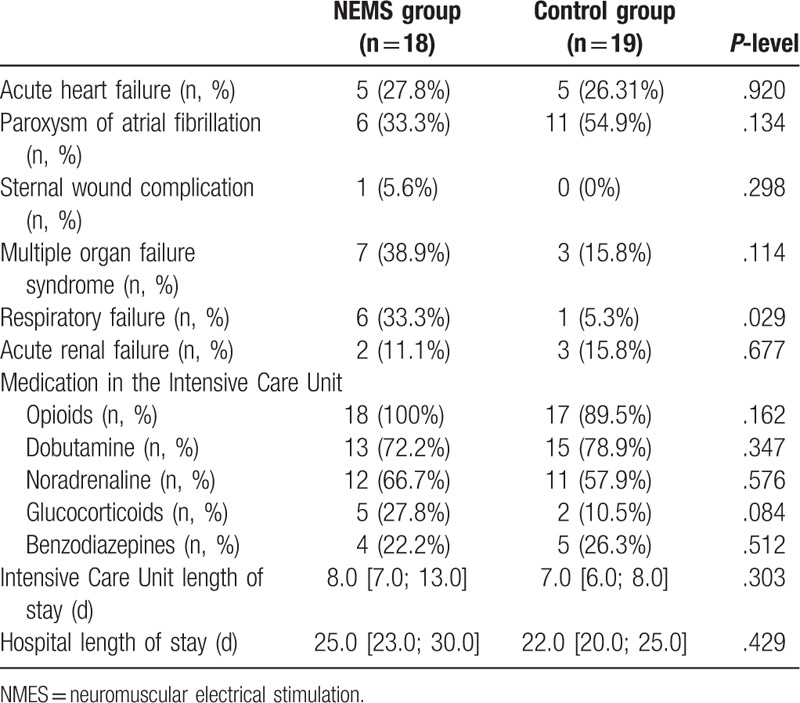
Flow diagram of study participants is presented in Figure 1. Of the 61 patients with postoperative complications after cardiovascular surgery during the study period, 17 were excluded. As a result, 44 patients were included in this study and were randomized: 21 patients received NMES along with the standard postoperative rehabilitation program, and 23 in the control group received only standard postoperative rehabilitation. After randomization 7 patients dropped out. Finally, 37 patients (NMES group, n = 18; control, n = 19) were enrolled in the analysis after excluding patients lost to follow-up. No differences were found in the baseline characteristics of the groups (Table 1), including the results of laboratory tests and instrumental studies (Suppl. Table 1, Suppl. Table 2). Both groups were comparable in the surgery types, the cardiopulmonary bypass duration, and the cross-clamp time (Table 2), the groups also did not differ in types of postoperative complications, with the exception of more frequent respiratory failure (P = .029) in the NEMS group (Table 3). The initial strength indicators of the studied muscles and the quadriceps CSA also had no significant differences in the groups (Suppl. Table 3, Suppl. Table 4).
Figure 1.
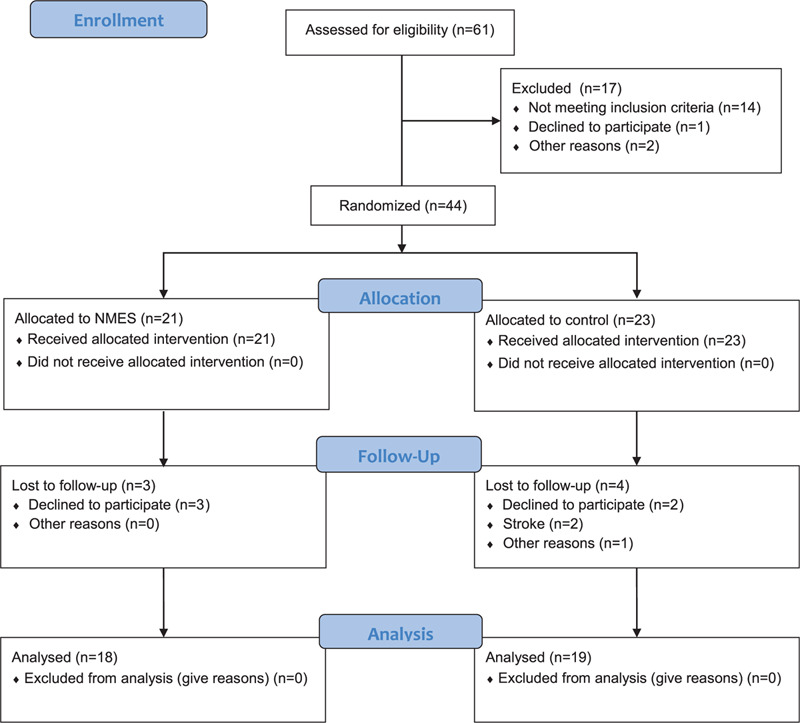
Flow diagram of study participants; NMES = neuromuscular electrical stimulation.
Table 1.
Baseline characteristics of patients.
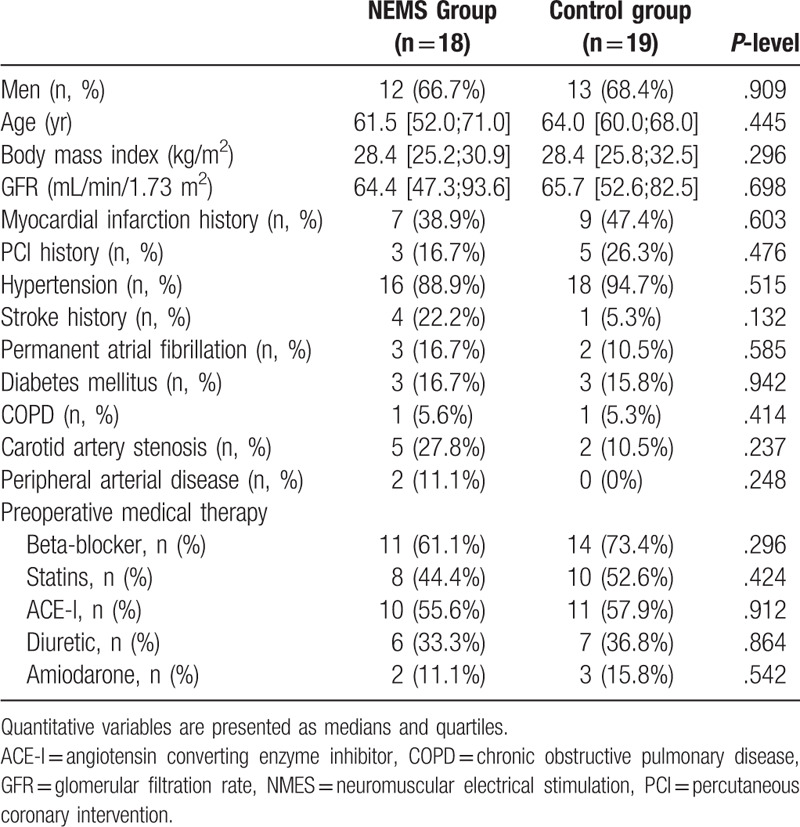
Table 2.
Operative procedure.
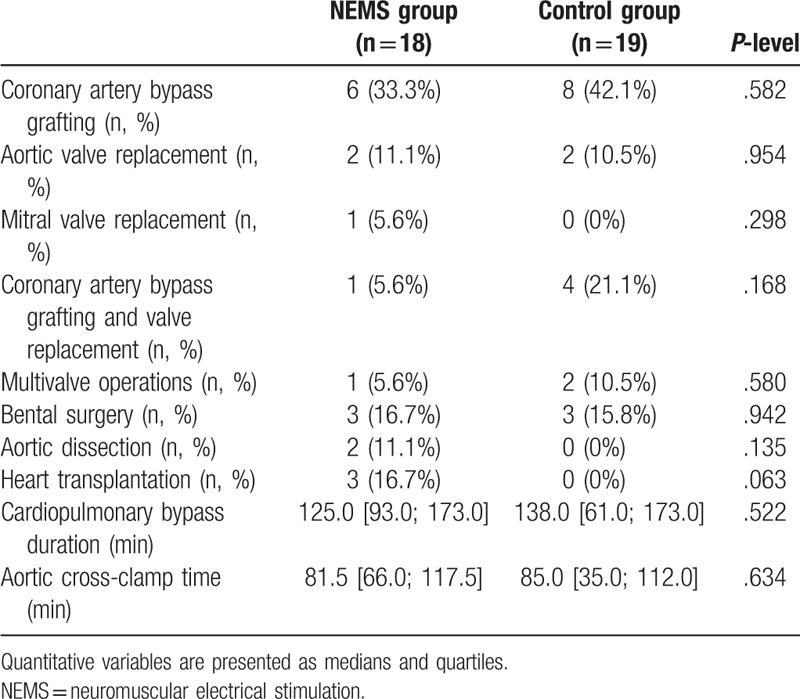
Table 3.
Types of postoperative complications and duration of inpatient treatment.
3.2. Effects of NMES on primary and secondary outcomes
The change in the KES from baseline to discharge is shown in Figure 2. Although it increased significant in both group, in the NMES group the increase was more pronounced (P < .001), in contrast to the control group (P = .001). Accordingly KES at discharge was significantly higher in the NMES group than in the non-NMES group (P = .004 on the right and P = .017 on the left).
Figure 2.
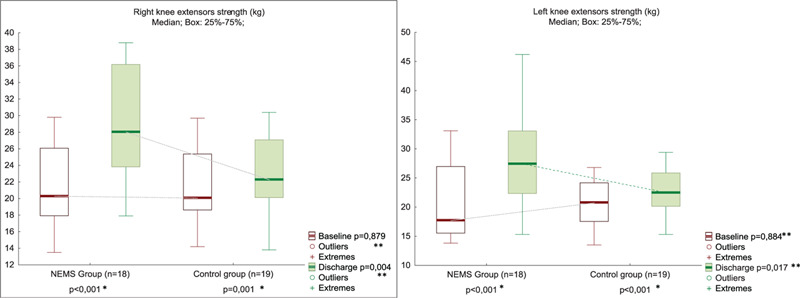
Knee extensor strength at POD 3 and discharge in NMES (n = 18) and control (n = 19) groups. ∗Between Baseline and Discharge in the Group; ∗∗Between Groups in Baseline or in Discharge. NMES = neuromuscular electrical stimulation, POD = postoperative day.
The KFS and handgrip strength increased from baseline to discharge to the same extent in both groups (Figs. 3 and 4, Suppl. Table 3). The quadriceps CSA muscle increased more in the NEMS group than in the control from the POD 3 to the time of discharge (Fig. 5, Suppl. Table 4). However, the quadriceps CSA muscle at discharge, as well as HS, and KFS in the NMES group and control group had no significant difference.
Figure 3.
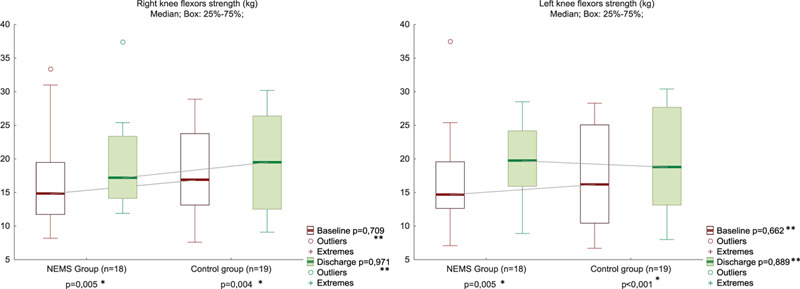
Knee flexor strength at POD 3 and discharge in NMES (n = 18) and control (n = 19) groups. ∗Between Baseline and Discharge in the Group; ∗∗Between Groups in Baseline or in Discharge. NMES = neuromuscular electrical stimulation, POD = postoperative day.
Figure 4.
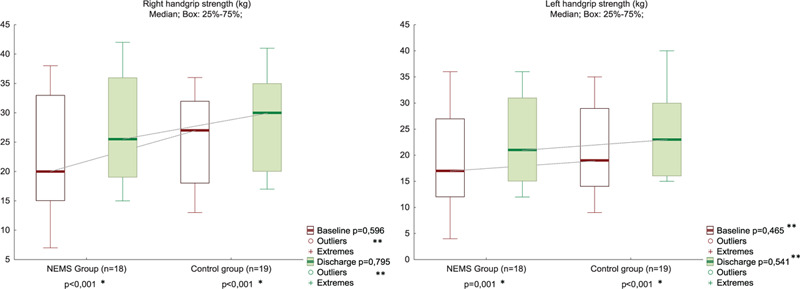
Handgrip strength at POD 3 and discharge in NMES (n = 18) and control (n = 19) groups. ∗Between Baseline and Discharge in the Group; ∗∗Between Groups in Baseline or in Discharge. NMES = neuromuscular electrical stimulation, POD = postoperative day.
Figure 5.
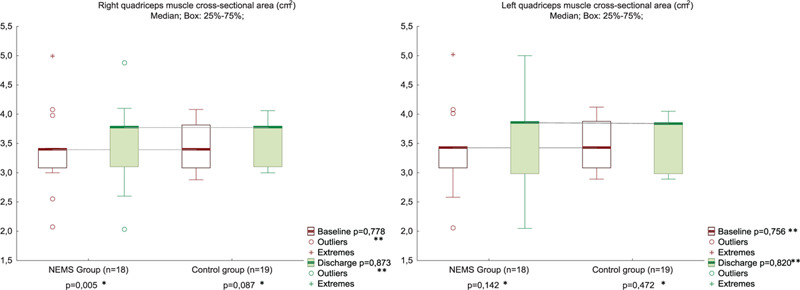
Quadriceps muscle cross-sectional area at POD 3 and discharge in NMES (n = 18) and control (n = 19) groups. ∗Between Baseline and Discharge in the Group; ∗∗Between Groups in Baseline or in Discharge. NMES = neuromuscular electrical stimulation, POD = postoperative day.
3.3. Other follow-up changes
Other indicators of muscle status are presented in Suppl. Table 3. Average KES increased to a greater extent during treatment in the NMES group. At the same time, average and maximum KFS increased equally in both groups. A 6MWT before discharge did not show a difference between groups (P = .166). The NMES course also did not affect the overall duration of treatment in the hospital (P = .429).
4. Discussion
In the present study, NMES of the quadriceps muscle was associated with a more noticeable increase in the strength of the stimulated muscles compared to the control group in patients with complications after cardiovascular surgery. We did not identify the effect of NEMS on unstimulated muscles (HS and KFS) and quadriceps CSA.
4.1. Muscle strength
There is no consensus regarding the possibility of NMES improving muscle strength in patients after cardiovascular surgery. Iwatsu et al[8] showed that the NMES during the first 5 PODs was able to increase not only the KES, but also the HS. However, this study was nonrandomized, NMES intervention was split between 2 different hospitals. Apparently, this limitation influenced the results of the study. The randomized study in the same clinic did not reveal the effect of NMES on the stimulated muscles strength.[9] Inclusion of patients before surgery made it possible in this study to compare pre-surgery muscle status to its restoration in the postoperative period. However, this way of the patients’ recruitment led to the majority of patients having an uncomplicated postoperative period. This allowed patients to participate in the rapid activation program, therefore, the addition of a short course of NMES, apparently, was not sufficient to provide an additional increase in muscle strength. In other study patients were included before the surgery, they were rapidly activated and the additional NMES during the first 5 PODs did not lead to an increase in the stimulated muscles strength.[11] In contrast, postoperative complications were the inclusion criterion in our study, which forbade the rapid mobilization of patients. Similar, in the Catastim 2 study the median stay of patients in ICU was 6 to 7 days, and in NMES group muscle strength was restored faster.[12] But by the discharge time (after the cessation of the NMES course in the NMES group and after regime expansion and activation of patients in the control), the change in muscle strength was independent of group allocation. In our study, the stay in the ICU was longer, and the NMES course continued until hospital discharge, which, apparently, allowed preserving the favorable effect of NMES.
The training impact of isolated NMES is inferior to active training with voluntary muscle contractions,[13] therefore, it is usually proposed to combine NMES with active exercises,[14] for example, such a combination is used in athletes.[15] This is not surprising, since NMES provides an additional activation of those muscle fibers that are not involved in the voluntary muscle contraction.[16] As a result of NMES, a number of adaptation processes occur in skeletal muscles,[17] and in the function of the nervous system.[18] To maximize the effect of NMES, sufficiently intense exposure regimes[19] and/or a long course of stimulation (at least 4 weeks for athletes) are required. Based on this, a 5-day NMES course with a fairly moderate degree of exposure, as was the case in the above studies in patients after cardiovascular surgery, is quite likely to have a limited effect.[9,11] A longer NMES course in our study is a likely explanation for its ability to furtherly increase the stimulated muscles strength.
Absence of differences between the groups in the strength increase of unstimulated muscles in our study is quite consistent with these considerations. Nevertheless, there are well-known observations of strength gain in contralateral muscles that have not been exposed to stimulation[8,20]; this phenomenon is called cross education and may be explained by changes in the nervous system.[21] The non-detection of this phenomenon in our study may be explained by the short duration of the NMES course (for example, changes in the contralateral limb appeared after 3 weeks of exposure[20]).
4.2. Muscle structural changes
Early course NMES does not cause changes in muscle structure in patients after cardiac surgery, regardless of increased or decreased muscle strength,[12] which was confirmed in the present study (the quadriceps CSA muscle at discharge in groups had no significant difference). It was previously shown that the intensity of NMES in healthy has a positive correlation with muscle CSA.[19] Also NEMS in healthy people, with a course duration of up to 4 weeks, leads to an increase in the strength of stimulated muscles without their hypertrophy.[22] The NMES intensity in patients after cardiac surgery was significantly lower,[8,9] and the course duration was shorter, which explains our results. Nevertheless, the fact that the CSA quadriceps muscle increased more in the NEMS group than in the control from POD 3 until discharge, in our opinion, deserves examination in further randomized trials.
4.3. Study limitations
The main limitation of this study was non-blind muscle strength testers, which can lead to biased measurements. Although identical stimuli were used in the initial and final measurements, we suggest that the results of muscle strength assessment in this study still have the potential to overestimate the effect of NEMS. To clarify this, a blind randomized controlled trial focused on the effectiveness of NEMS in increasing muscle strength in patients with complications after cardiovascular surgery will be required. However, the results of this study provide data on indications for NMES based on patient characteristics and information on dose-response relationships in this category of patients.
Another limitation was the lack of a preoperative assessment of muscle strength, quadriceps muscle CSA, and the distance in 6MWT, since patients were included in the postoperative period after the development of complications. This did not allow us to evaluate the change of these parameters compared to the preoperative period. However, the assessment of the dynamics of these parameters in the postoperative period seems to us important in itself.
5. Conclusions
This pilot study show beneficial effects of NMES on muscle strength in patients with complications after cardiovascular surgery. The use of NMES showed no effect on strength in non-stimulated muscle, quadriceps CSA, and 6MWT distance at discharge. Further blind randomized controlled trials should be performed with emphasis on the effectiveness of NEMS in increasing muscle strength and structure in these patients.
Author contributions
Conceptualization: Alexey N. Sumin, Andrey V. Bezdenezhnykh.
Data curation: Pavel A. Oleinik, Andrey V. Bezdenezhnykh, Anna V. Ivanova.
Formal Analysis: Alexey N. Sumin, Andrey V. Bezdenezhnykh.
Investigation: Pavel A. Oleinik, Andrey V. Bezdenezhnykh, Anna V. Ivanova.
Methodology: Pavel A. Oleinik, Andrey V. Bezdenezhnykh, Anna V. Ivanova.
Project administration: Alexey N. Sumin, Andrey V. Bezdenezhnykh.
Resources: Pavel A. Oleinik, Andrey V. Bezdenezhnykh, Anna V. Ivanova.
Supervision: Alexey N. Sumin, Andrey V. Bezdenezhnykh.
Writing – original draft: Alexey N. Sumin, Pavel A. Oleinik, Andrey V. Bezdenezhnykh. Writing – review & editing: Alexey N. Sumin. Alexey N. Sumin.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Footnotes
Abbreviations: 6MWT = 6-minute walk test, CSA = cross-sectional area, HS = handgrip strength, ICU = intensive care unit, KES = knee extensors strength, KFS = knee flexor strength, NMES = neuromuscular electrical stimulation, POD = postoperative day.
How to cite this article: Sumin AN, Oleinik PA, Bezdenezhnykh AV, Ivanova AV. Neuromuscular electrical stimulation in early rehabilitation of patients with postoperative complications after cardiovascular surgery: A randomized controlled trial. Medicine. 2020;99:42(e22769).
The authors have no conflicts of interest to disclose.
The research was financed by exploratory scientific research “Rehabilitation of patients with complicated postoperative period of cardiac surgery” (0546-2017-0007).
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].Iida Y, Yamazaki T, Arima H, et al. Predictors of surgery-induced muscle proteolysis in patients undergoing cardiac surgery. J Cardiol 2016;68:536–41. [DOI] [PubMed] [Google Scholar]
- [2].Bloch SA, Lee JY, Wort SJ, et al. Sustained elevation of circulating growth and differentiation factor-15 and a dynamic imbalance in mediators of muscle homeostasis are associated with the development of acute muscle wasting following cardiac surgery. Crit Care Med 2013;41:982–9. [DOI] [PubMed] [Google Scholar]
- [3].Iida Y, Yamazaki T, Kawabe T, et al. Postoperative muscle proteolysis affects systemic muscle weakness in patients undergoing cardiac surgery. Int J Cardiol 2014;172:595–7. [DOI] [PubMed] [Google Scholar]
- [4].Gomes Neto M, Oliveira FA, Reis HF, et al. Effects of neuromuscular electrical stimulation on physiologic and functional measurements in patients with heart failure: a systematic review with meta-analysis. J Cardiopulm Rehabil Prev 2016;36:157–66. [DOI] [PubMed] [Google Scholar]
- [5].Paillard T. Muscle plasticity of aged subjects in response to electrical stimulation training and inversion and/or limitation of the sarcopenic process. Ageing Res Rev 2018;46:1–3. [DOI] [PubMed] [Google Scholar]
- [6].Iwatsu K, Yamada S, Iida Y, et al. Feasibility of neuromuscular electrical stimulation immediately after cardiovascular surgery. Arch Phys Med Rehabil 2015;96:63–8. [DOI] [PubMed] [Google Scholar]
- [7].Bezdenezhnykh AV, Sumin AN, Olejnik PA. The first experience of electrical myostimulation for early rehabilitation of the heart transplant recipient with complicated postoperative period. Complex Issues of Cardiovascular Diseases 2018;7(4S):146–50. [Google Scholar]
- [8].Iwatsu K, Iida Y, Kono Y, et al. Neuromuscular electrical stimulation may attenuate muscle proteolysis after cardiovascular surgery: a preliminary study. J Thorac Cardiovasc Surg 2017;153:373–9.e1. [DOI] [PubMed] [Google Scholar]
- [9].Kitamura H, Yamada S, Adachi T, et al. Effect of perioperative neuromuscular electrical stimulation in patients undergoing cardiovascular surgery: a pilot randomized controlled trial. Semin Thorac Cardiovasc Surg 2019;31:361–7. [DOI] [PubMed] [Google Scholar]
- [10].Mandal S, Suh E, Thompson A, et al. Comparative study of linear and curvilinear ultrasound probes to assess quadriceps rectus femoris muscle mass in healthy subjects and in patients with chronic respiratory disease. BMJ Open Resp Res 2016;3:e000103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Fontes Cerqueira TC, Cerqueira Neto ML, Cacau LAP, et al. Ambulation capacity and functional outcome in patients undergoing neuromuscular electrical stimulation after cardiac valve surgery: a randomised clinical trial. Medicine (Baltimore) 2018;97:e13012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Fischer A, Spiegl M, Altmann K, et al. Muscle mass, strength and functional outcomes in critically ill patients after cardiothoracic surgery: does neuromuscular electrical stimulation help? The Catastim 2 randomized controlled trial. Crit Care 2016;20:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Medrinal C, Combret Y, Prieur G, et al. Comparison of exercise intensity during four early rehabilitation techniques in sedated and ventilated patients in ICU: a randomised cross-over trial. Crit Care 2018;22:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Paillard T. Training based on electrical stimulation superimposed onto voluntary contraction would be relevant only as part of submaximal contractions in healthy subjects. Front Physiol 2018;9:1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].da Cunha RA, Pinfildi CE, de Castro Pochini A, et al. Photobiomodulation therapy and NMES improve muscle strength and jumping performance in young volleyball athletes: a randomized controlled trial study in Brazil. Lasers Med Sci 2020;35:621–31. [DOI] [PubMed] [Google Scholar]
- [16].Adams GR, Harris RT, Woodard D, et al. Mapping of electrical muscle stimulation using MRI. J Appl Physiol 1993;74:532–7. [DOI] [PubMed] [Google Scholar]
- [17].Mancinelli R, Toniolo L, Di Filippo ES, et al. Neuromuscular electrical stimulation induces skeletal muscle fiber remodeling and specific gene expression profile in healthy elderly. Front Physiol 2019;10:1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Maffiuletti NA, Zory R, Miotti D, et al. Neuromuscular adaptations to electrostimulation resistance training. Am J Phys Med Rehabil 2006;85:167–75. [DOI] [PubMed] [Google Scholar]
- [19].Natsume T, Ozaki H, Kakigi R, et al. Effects of training intensity in electromyostimulation on human skeletal muscle. Eur J Appl Physiol 2018;118:1339–47. [DOI] [PubMed] [Google Scholar]
- [20].Song Y, Forsgren S, Yu J, et al. Effects on contralateral muscles after unilateral electrical muscle stimulation and exercise. PLoS One 2012;7:e52230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Frazer AK, Pearce AJ, Howatson G, et al. Determining the potential sites of neural adaptation to cross-education: implications for the cross-education of muscle strength. Eur J Appl Physiol 2018;118:1751–72. [DOI] [PubMed] [Google Scholar]
- [22].Gondin J, Guette M, Ballay Y, et al. Electromyostimulation training effects on neural drive and muscle architecture. Med Sci Sports Exerc 2005;37:1291–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


