Abstract
Introduction:
Proton pump inhibitors (PPIs) are widely prescribed and generally well tolerated but can rarely cause severe allergic reactions, such as drug rash with eosinophilia and systemic symptoms (DRESS). We report a case of DRESS and renal injury induced by PPIs, and describe the therapeutic process.
Patient concerns:
The patient was a 66-year-old female who complained of fever, pruritus, desquamation, erythema multiforme, and anuria caused by omeprazole taken for 2 weeks to treat abdominal distention.
Diagnosis:
The clinical history revealed a similar episode of PPI-induced fever, eosinophilia, and acute kidney injury more than 1 year ago. The present laboratory tests revealed eosinophilia and oliguric renal failure. The renal biopsy was performed subsequently and proved the diagnosis of PPI-induced DRESS.
Interventions:
After the suspected diagnosis of PPI-induced DRESS, omeprazole was discontinued and methylprednisolone infusion (40 mg qd) was initiated. Because of oliguric renal failure, the patient received intermittent hemodialysis.
Outcomes:
The patient initially responded to omeprazole discontinuation, hemodialysis, and glucocorticoids but later died from severe infection during the tapering of glucocorticoid therapy.
Conclusion:
Clinicians should remain on high alert for potential life-threatening complications when prescribing PPIs. If unexplained renal injury develops in a patient taking a PPI, renal biopsy may help in identifying the pathogenesis and might facilitate timely intervention.
Keywords: DRESS, severe renal injury, PPI
1. Introduction
Drug rash with eosinophilia and systemic symptoms (DRESS) is a severe delayed-type hypersensitivity syndrome associated with erythema multiforme-type drug eruption and life-threatening systemic manifestations that primarily involve the liver, kidneys, lungs, and pancreas.[1] DRESS is clinically divided into immediate and delayed types. Immediate-type DRESS is considered an immunoglobulin E (IgE)-mediated type-I hypersensitivity reaction,[2–4] whereas delayed-type DRESS is thought to involve a T cell-mediated hypersensitivity reaction.[5] Although proton pump inhibitors (PPIs) rarely cause DRESS,[6] these drugs have been reported to cause hypersensitivity reactions in 0.2% to 3% of cases,[7] with a mortality rate of 10%.[8] Previous studies have reported DRESS syndrome induced by a variety of PPIs, including rabeprazole, pantoprazole, esomeprazole, lansoprazole, and omeprazole.[5,9–12]
Here, we present the case of a woman who died following the repeat occurrence of DRESS syndrome and renal failure caused by a 2-week course of omeprazole taken to treat symptoms of abdominal distention. The patient had developed DRESS syndrome in response to PPI therapy the previous year but had not received a renal biopsy or regular follow-up after the first episode. This case highlights the need for clinicians to remain on high alert for potential life-threatening complications when prescribing PPIs and to consider renal biopsy in cases of unexplained renal injury during therapy with a PPI.
2. Case presentation
On August 28, 2015, a 66-year-old Chinese woman was admitted to Beilun People's Hospital (Ningbo, China) with symptoms of fever, rash, chest tightness, and anuria, and a provisional diagnosis of renal failure was made. The patient had started a course of oral omeprazole (20 mg qd; purchased from a drug store) 2 weeks before admission due to symptoms of abdominal distension. In the week prior to admission, the patient had gradually developed pruritus and a rash over her whole body (including the limbs), with features that included desquamation, papules, macules, partially integrated blisters, and scabs (Fig. 1).
Figure 1.
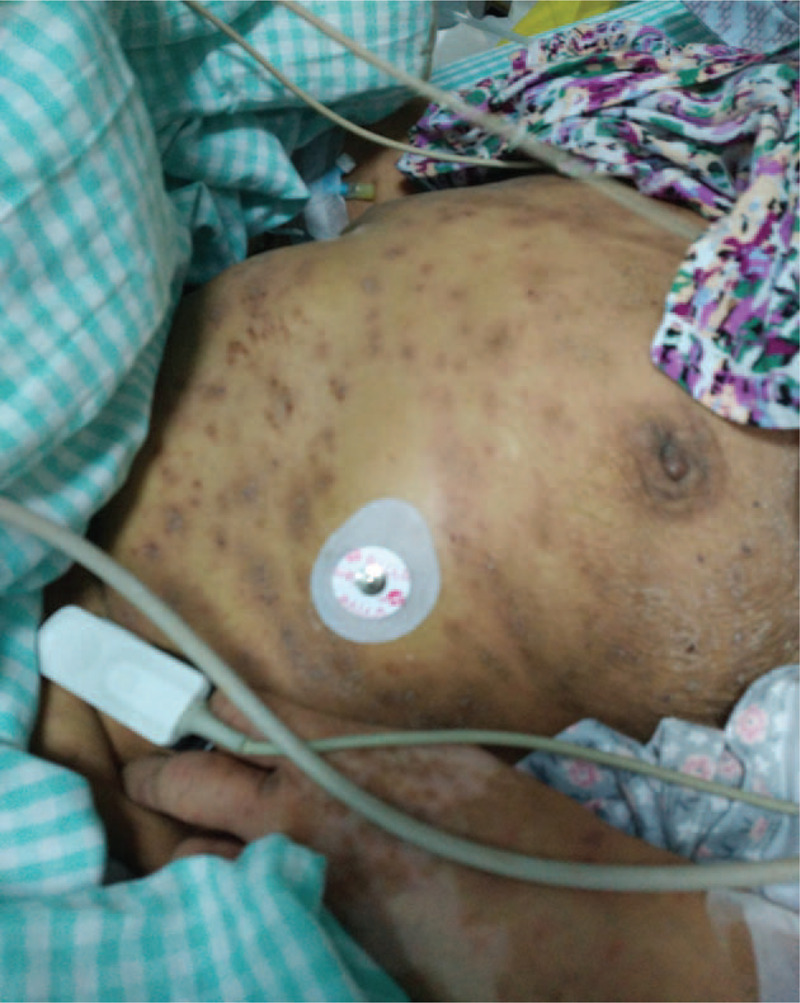
Pruritus and rash over the whole body (including the limbs) of the patient, with features that include desquamation, papules, macules, partially integrated blisters, and scabs.
The patient denied a history of chronic diseases, such as hypertension or diabetes, or the long-term use of traditional Chinese medicines (a potential cause of renal injury). However, the patient had developed DRESS and acute renal failure after PPI therapy more than 1 year before the current admission. Specific details of the previous episode of DRESS are as follows: On July 1, 2014, the patient was admitted to a local hospital because of cough and gastrointestinal symptoms, such as nausea and vomiting. No abnormalities were detected in basic renal function tests (serum creatinine concentration, 75 μmol/L) or in a routine urine test. Three days later, the patient developed fever, cough, abdominal pain, nausea, and vomiting. She was treated initially with omeprazole infusion (40 mg qd) and then with long-term (>1 month) PPI therapy that included omeprazole, pantoprazole, and esomeprazole, in succession. Subsequently, the patient developed a fever (>38.5 °C) without any rash. Routine blood tests revealed an increase in eosinophil count (as high as 2.55 × 109/L; normal range, 0.1–0.4 × 109/L) and eosinophil proportion (as high as 20.1%; normal range, 0.4%–3%). A routine urine test was positive for leukocytes (an eosinophil granulocyte test was not conducted) but negative for proteinuria and microscopic hematuria. The patient received continuous renal replacement therapy (CRRT) for oliguria and acute renal failure for more than 2 weeks, which restored her serum creatinine level to 100 μmol/L. However, the patient did not undergo a renal biopsy after the completion of CRRT and was not followed-up during the subsequent year.
The blood hematology and biochemistry findings on admission of the patient to our hospital were as follows: leukocyte count, 8.7 × 109/L (normal range, 3.6–11.0 × 109/L), eosinophil count, 0.83 × 109/L (normal range, 0.1–0.4 × 109/L), eosinophil proportion, 9.6% (normal range, 0.4%–3%), hemoglobin concentration, 56 g/L (normal range, 115–165 g/L), platelet count, 108 × 109/L (normal range, 140–400 × 109/L), serum creatinine concentration, 1181 μmol/L (normal range, 45–84 μmol/L), blood urea nitrogen, 57.9 mmol/L (normal range, 2.5–7.0 mmol/L), and K+ concentration 7.17 mmol/L (normal range, 3.5–5.5 mmol/L). Erythrocyte sedimentation rate was normal. Arterial blood gas analysis showed severe metabolic acidosis (pH, 7.03 [normal range, 7.36–7.44]; HCO3−, 2 mmol/L [normal range, 22–28 mmol/L]; base excess, 28 mmol/L [normal range, ± 2 mmol/L]); and hyponatremia (Na+, 123 mmol/L; normal range, 133–146 mmol/L). Color Doppler ultrasonography of the urinary system showed that the sizes of the two kidneys were 102 × 48 mm and 98 × 47 mm, respectively, and that the bilateral renal cortex was about 4 mm in thickness. Routine urine tests were positive for glucose (2+), protein (+), and red blood cells (+ or 2+). The concentration of urinary α1-microglobulin was 21.9 mg/dL, and that of urinary micro-albumin was 36 mg/dL. Tests for the presence of anti-myeloperoxidase (MPO) antibody in peripheral blood produced weak positive results on two occasions. Tests for perinuclear and cytoplasmic anti-neutrophil cytoplasmic antibodies (p-ANCA and c-ANCA) were negative. Blood culture and echocardiography showed no abnormalities, and there was no swelling of the superficial lymph nodes. The patient subsequently received intermittent hemodialysis.
A diagnosis of PPI-induced DRESS was suspected based on the rapid development of fever, rash, and acute renal failure after the patient had started taking omeprazole. The skin lesions were also considered to be a drug rash upon consultation with dermatologists.
The criteria suggested by the Registry of Severe Cutaneous Adverse Reaction (RegiSCAR) are widely used for assessing the probability of an adverse drug reaction.[13] According to this scoring system, a case for DRESS can be estimated as follows: fever ≥38.5 °C (0 point), enlarged lymph nodes (0 point), eosinophilia >1.5 × 109/L (2 point), atypical lymphocytes (0 point), organ involvement (2 points), resolution ≥15 days (0 point), skin rash >50% of the body surface area (1 point), skin rash suggesting DRESS (1 point), organ involvement including the kidneys and digestive system (2 point), and negative evaluation of other potential causes (1 point). The RegiSCAR score of 9 points indicates that the case presented here was definitely of DRESS.
A renal biopsy on day 18 of hospitalization revealed severe and chronic injury to the renal tubules and interstitium, accompanied by glomerular ischemia and shrinkage. Immunohistochemical analysis showed that most renal interstitial lymphocytes were CD3+/MPO+ neutrophils with a small number of CD20+ lymphocytes and CD38+ interstitial cells. Furthermore, there was diffuse atrophy of kidney tubules, proliferation of interstitial collagen fibers, and infiltration of lymphocytes, and occasional presence of eosinophils (see Figs. 2 and 3 for details). Electron microscopy showed vacuolar degeneration of the capillary endothelium and narrowing of the lumen of capillaries.
Figure 2.
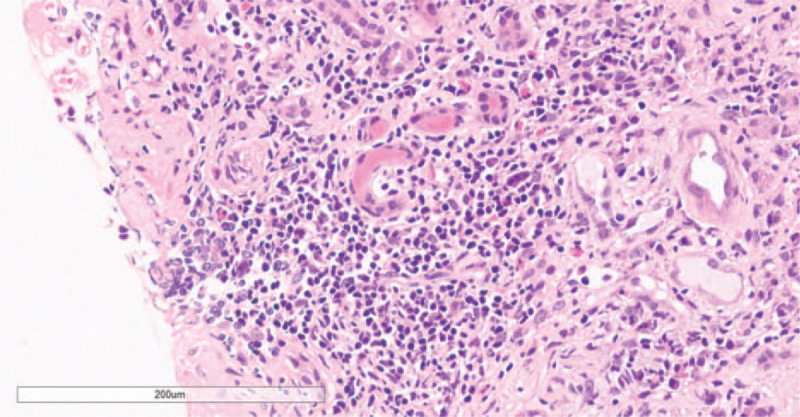
Hematoxylin and eosin staining of renal biopsy specimens showing chronic tubular-interstitial inflammation, diffuse atrophy of the renal tubular epithelium, extensive lymphocytic infiltration of the renal interstitial, and occasionally eosinophils (200×).
Figure 3.
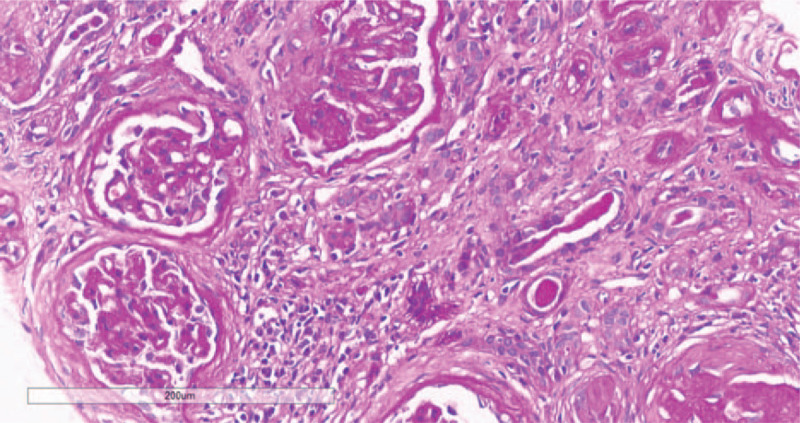
Periodic acid–Schiff staining showing glomerular ischemic changes; fibrosis can be seen around the balloon, and the capillary vasospasm is contracted by ischemia. Renal tubular epithelial cells showing vacuoles, granule degeneration, and a small amount of protein casts and diffuse atrophy. Renal interstitial showing lymphocytes and mononuclear cell infiltration with fibrosis, vitreous changed in the wall of the arterioles, increased thickness of the wall of small arteries, intimal hyperplasia, stenosis of the lumen, no cellulose-like necrosis (200×).
Treatment with methylprednisolone infusion (40 mg qd) was initiated on day 7 of hospitalization, and the rash resolved after 1 week of therapy. The medication was switched from glucocorticoid to oral prednisone (40 mg qd) after 2 weeks of methylprednisolone therapy. Subsequently, a series of investigations demonstrated a decrease in the count and proportion of eosinophils to normal levels, an increase in urinary volume, a fall in serum creatinine to 262 to 276 μmol/L, and a glomerular filtration rate (GFR; estimated using the Modification of Diet in Renal Disease [MDRD] formula) of 25 mL/min. Dialysis was successfully discontinued after 3 weeks of steroid therapy (hemodialysis was given intermittently for a total of 10 sessions). The patient was discharged on day 28 (September 25, 2015) of hospitalization and prescribed prednisone tablets at an initial dose of 35 mg/day, with tapering of the dose by one tablet (5 mg) every 2 weeks. However, severe pulmonary infection and respiratory failure developed ∼2 months after the initiation of steroid therapy. The patient was admitted to hospital, the trachea was cannulated, and a diagnosis of invasive pulmonary aspergillosis (IPA) was made following bronchial brushing during bronchoscopy. Despite the initiation of anti-fungal therapy with caspofugin (Cancidas) and fluconazol, the patient exhibited progressive deterioration of renal function and died on December 8, 2015.
3. Discussion
While dermatologists usually focus on the varied cutaneous manifestations of DRESS, nephrologists are concerned with the renal injury that results from drug-induced acute interstitial nephritis (AIN). DRESS often affects the function of the renal tubules and interstitium and, thus, results in polyuria and glycosuria; however, the syndrome rarely results in kidney failure. Omeprazole-induced AIN was first described in 1992,[14] and all PPIs currently on the market, including pantoprazole,[15] omeprazole,[16] rabeprazole,[17] esomeprazole,[18] and lansoprazole,[19] have been reported to induce AIN. The incidence of PPI-induced AIN is 2 to 20 per 100,000[7,20] and is not related to the drug dosage, suggesting the importance of individual factors, such as an allergic predisposition.[20] The pathogenesis of PPI-induced DRESS is not fully characterized but may involve the deposition of a hapten with the drug (or its metabolite) in the tubulointerstitium, direct stimulation of the abnormal expression of T-cells, or Th1- and Th17-mediated inflammatory processes.[21] Furthermore, genetic polymorphism of CYP2C19 that slows the metabolism of PPIs is thought to increase the risk of acute renal injury.[5,20,22]
The case reported here relates to the recurrence of DRESS syndrome and impaired renal function after the repeated use of PPIs. On both occasions, the patient developed renal failure that required renal replacement therapy. Generally, renal injury occurs within 2 to 6 weeks after exposure to the causative drug. In this case, the patient relapsed 1 year after the initial episode of DRESS due to re-administration of a PPI, which, to the best of our knowledge, has not been described previously. The patient in this case report was found to have chronic interstitial nephritis, whereas the pathologic manifestations of PPI-induced acute renal injury are generally those of acute allergic intestinal nephritis.[7,14–19]
Acute renal injury caused by PPIs is histologically manifested as an infiltration of mixed inflammatory cells (lymphocytes, eosinophils, plasma cells, and isolated groups of neutrophils) in the renal interstitium.[7,17,23] Geevasinga et al identified 18 cases of biopsy proven PPI-induced AIN causing AKI in a retrospective case review.[17] A growing body of literature and case reports confirms that PPI therapy is linked to acute kidney injury, which can potentially lead to chronic kidney disease (CKD) and end-stage renal disease (ESRD).[24–26]
The pathologic changes underlying the development of chronic interstitial nephritis in the present case may have involved the initial occurrence of AIN and edema (during the first episode of PPI-induced DRESS), which then gradually progressed to renal interstitial fibrosis, tubular atrophy, and glomerular sclerosis.[27] The pathological finding in the present case is in line with the role of acute injury in CKD, as reported in literature. The exact pathology of CKD caused by PPIs has not been previously reported for any case.
One of the factors that likely contribute to the development of chronic interstitial nephritis in patients with PPI-induced AIN is a missed or delayed diagnosis due to the atypical clinical manifestations of hypersensitivity reactions caused by PPIs. For example, fewer than half of the cases have fever, <10% develop a rash, about one-third manifest hypereosinophilia, and only 5% to 10% present with typical symptoms of a hypersensitivity reaction.[28] Because it can take several weeks or even months to confirm a diagnosis of AIN after the development of symptoms, some patients inevitably develop chronic renal interstitial fibrosis before treatment can be initiated.[28,29]
It has been reported that renal function does not recover to the baseline in a substantial proportion of patients with PPI-induced AIN despite discontinuation of the causative drug and treatment with steroids.[7] Furthermore, long-term administration of PPIs may increase the risk of chronic renal injury. For example, when compared with H2 receptor antagonists, long-term PPI use is associated with a higher risk of renal disease that progresses to CKD and ESRD.[24,30,31] We suggest that renal biopsy may represent a useful technique for characterizing the pathological changes underlying the progression of AIN to chronic renal injury.[30,32] Furthermore, the use of renal biopsy could help in increasing our understanding of the factors influencing the prognosis of renal disease caused by long-term PPI use and the ability of renal function to recover after discontinuation of the drug.
There is also cross-reactivity in PPI-induced AIN, necessitating a skin prick test before the use of an alternative PPI and systemic desensitization for those who must use it.[3] A recent case report described acute renal injury caused by the administration of two different PPIs (omeprazole first, pantoprazole later) to the same individual.[33] The present case reported the prior use of three different PPIs (omeprazole, pantoprazole, and esomeprazole), suggesting that cross-reactivity may have contributed to the development of renal injury. Only two previous case reports have suggested that omeprazole[34] and pantoprazole[35] can induce ANCA-related vasculitis, and immunofluorescence microscopy of renal biopsy specimens demonstrated deposition of oligoimmune complex in a patient treated with pantoprazole.[35] The renal biopsy specimens from the patient reported here were positive for IgM antibody but negative for other antibodies, such as IgG and IgA, which is consistent with the deposition of oligoimmune complex. However, the underlying mechanism is still unclear. In this case, the patient also tested positive for the anti-MPO antibody, which was deposited in the renal interstitium (see Fig. 4 for details). To the best of our knowledge, this is a novel finding that has not been described in previous studies.
Figure 4.
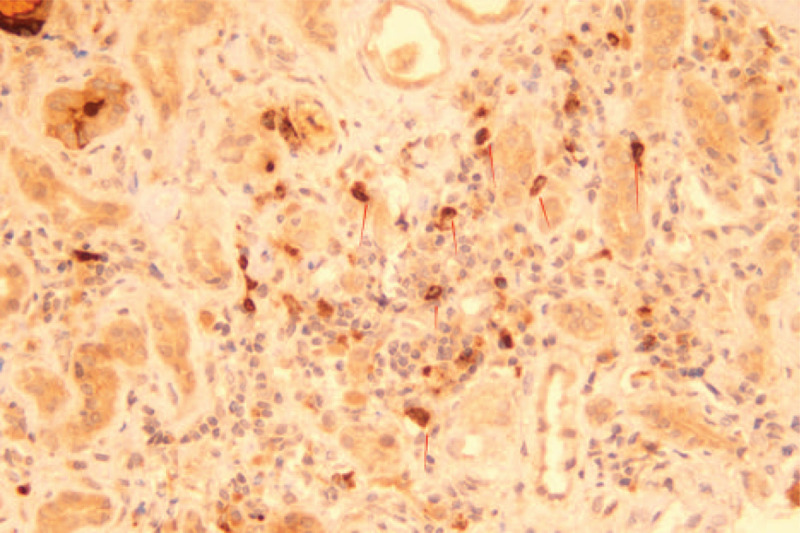
Myeloperoxidase staining of the renal interstitium. The arrow indicates the positively stained cells (200×).
The established treatment of drug-induced AIN involves discontinuation of the drug and administration of a glucocorticoid as an immunosuppressant.[36,37] Early intervention with a glucocorticoid agent upon confirmation of renal pathology is thought to reduce the risk of renal interstitial fibrosis and facilitate the recovery of impaired renal function[38] However, early diagnosis and intervention to prevent renal failure require close cooperation between physicians from different departments, in particular nephrology and pathology. Pathologic markers to guide therapy (such as the ratio of glomerular sclerosis to renal tubular atrophy, the degree of renal arteriolar occlusion, or the extent of interstitial fibrosis) are yet to be established. Nonetheless, it is envisaged that the development of standardized methods to evaluate the severity of interstitial nephritis and disease chronicity will facilitate decision-making by clinicians in the future.
PPIs are over-the-counter drugs that are widely used without prescription.[28] Many clinicians have expressed concerns about the safety implications of the misuse of PPIs due to widespread availability of these drugs and a lack of regulation.[20,39,40] PPIs are more frequently used by the elderly population than by the young people, which may contribute to a higher prevalence of AIN and CKD as well as a higher proportion of patients in need of dialysis.[29]
The following factors may significantly affect the clinical outcomes—lack of the awareness of PPI-induced AKI or other types of allergic manifestations, individual allergic idiosyncrasy, time of identification after the onset of PPI hypersensitivity reaction, therapeutic drug reaction to this syndrome.[41]
Most patients with PPI-induced DRESS can achieve a good prognosis if confirmation of the diagnosis, discontinuation of the PPI, and initiation of glucocorticoid-based therapy are performed in a timely manner.[11] However, the early diagnosis and treatment of PPI-induced DRESS requires a multidisciplinary approach. The present case highlights the need for clinicians to remain on high alert for potential life-threatening complications when prescribing PPIs. Furthermore, renal biopsy may help in identifying the pathogenesis and facilitate timely intervention in patients who develop unexplained renal injury after starting a course of PPI therapy.
Author contributions
Conception and design: Min Xia.
Administrative support: Min Xia, Xuelin He.
Provision of study materials or patients: Guanghui Ying, Xiapei Fei, Chenqin Zha, haogui Chen, Yishu Bao.
Collection and assembly of data: Qien He, Zhujun Wang,Min Xia.
Data analysis and interpretation: Qien He.
Manuscript writing: All authors.
Final approval of manuscript: All authors.
Footnotes
Abbreviations: AIN = acute interstitial nephritis, c-ANCA = cytoplasmic anti-neutrophil cytoplasmic antibodies, CRRT = continuous renal replacement therapy, DRESS = drug rash with eosinophilia and systemic symptoms, IgE = immunoglobulin E, IPA = invasive pulmonary aspergillosis, MPO = anti-myeloperoxidase, p-ANCA = perinuclear cytoplasmic anti-neutrophil cytoplasmic antibodies, PPIs = proton pump inhibitors.
How to cite this article: He Q, Ying G, Fei X, Zha C, Chen Z, Bao Y, Long J, Wang Z, He X, Xia M. Drug rash with eosinophilia and systemic symptoms and severe renal injury induced by proton pump inhibitor therapy: A case report. Medicine. 2020;99:42(e22509).
This study was supported by Ningbo City Department Level project [2019 ky654].
Written informed consent was obtained from the patient/her relative for publication of this report.
The authors have no conflicts of interest to disclose.
This is a case report, and the relevant case information has been fully provided in the article.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
All data generated or analyzed during this study are included in this published article [and its supplementary information files]
The data that support the findings of this study are available from a third party, but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are available from the authors upon reasonable request and with permission of the third party.
References
- [1].Lin CY, Wang CW, Hui CR, et al. Delayed-type hypersensitivity reactions induced by proton pump inhibitors: a clinical and in vitro T-cell reactivity study. Allergy 2018;73:221–9. [DOI] [PubMed] [Google Scholar]
- [2].Kepil Özdemir S, Öner Erkekol F, Ünal D, et al. Management of hypersensitivity reactions to proton pump inhibitors: a retrospective experience. Int Arch Allergy Immunol 2016;171:54–60. [DOI] [PubMed] [Google Scholar]
- [3].Otani IM, Banerji A. Immediate and delayed hypersensitivity reactions to proton pump inhibitors: evaluation and management. Curr Allergy Asthma Rep 2016;16:17. [DOI] [PubMed] [Google Scholar]
- [4].Lobera T, Navarro B, Del Pozo MD, et al. Nine cases of omeprazole allergy: cross-reactivity between proton pump inhibitors. J Investig Allergol Clin Immunol 2009;19:57–60. [PubMed] [Google Scholar]
- [5].Bose S, Guyer A, Long A, et al. Evaluation and management of hypersensitivity to proton pump inhibitors. Ann Allergy Asthma Immunol 2013;111:452–7. [DOI] [PubMed] [Google Scholar]
- [6].Lombardo C, Bonadonna P. Hypersensitivity reactions to proton pump inhibitors. Curr Treat Options Allergy 2015;2:110–23. [Google Scholar]
- [7].Simpson IJ, Marshall MR, Pilmore H, et al. Proton pump inhibitors and acute interstitial nephritis: report and analysis of 15 cases. Nephrology (Carlton) 2006;11:381–5. [DOI] [PubMed] [Google Scholar]
- [8].Roujeau JC, Stern RS. Severe adverse cutaneous reactions to drugs. N Engl J Med 1994;331:1272–85. [DOI] [PubMed] [Google Scholar]
- [9].Kepil Özdemir S, Yilmaz I, Aydin Ö, et al. Immediate-type hypersensitivity reactions to proton pump inhibitors: usefulness of skin tests in the diagnosis and assessment of cross-reactivity. Allergy 2013;68:1008–14. [DOI] [PubMed] [Google Scholar]
- [10].Caboni S, Gunera-Saad N, Ktiouet-Abassi S, et al. Esomeprazole-induced DRESS syndrome. Studies of cross-reactivity among proton-pump inhibitor drugs. Allergy 2007;62:1342–3. [DOI] [PubMed] [Google Scholar]
- [11].Bourneau-Martin D, Leclech C, Jamet A, et al. Omeprazole-induced drug reaction with eosinophilia and systemic symptoms (DRESS). Eur J Dermatol 2014;24:413–5. [DOI] [PubMed] [Google Scholar]
- [12].Barbaud A, Collet E, Milpied B, et al. A multicentre study to determine the value and safety of drug patch tests for the three main classes of severe cutaneous adverse drug reactions. Br J Dermatol 2013;168:555–62. [DOI] [PubMed] [Google Scholar]
- [13].Kardaun SH, Sidoroff A, Valeyrie-Allanore L, et al. Variability in the clinical pattern of cutaneous side-effects of drugs with systemic symptoms: does a DRESS syndrome really exist? Br J Dermatol 2007;156:609–11. [DOI] [PubMed] [Google Scholar]
- [14].Ruffenach SJ, Siskind MS, Lien YH. Acute interstitial nephritis due to omeprazole. Am J Med 1992;93:472–3. [DOI] [PubMed] [Google Scholar]
- [15].Ra A, Tobe SW. Acute interstitial nephritis due to pantoprazole. Ann Pharmacother 2004;38:41–5. [DOI] [PubMed] [Google Scholar]
- [16].Post AT, Voorhorst G, Zanen AL. Reversible renal failure after treatment with omeprazole. Neth J Med 2000;57:58–61. [DOI] [PubMed] [Google Scholar]
- [17].Geevasinga N, Coleman PL, Roger SD. Rabeprazole-induced acute interstitial nephritis. Nephrology (Carlton) 2005;10:7–9. [DOI] [PubMed] [Google Scholar]
- [18].Geevasinga N, Kairaitis L, Rangan GK, et al. Acute interstitial nephritis secondary to esomeprazole. Med J Aust 2005;182:235–6. [DOI] [PubMed] [Google Scholar]
- [19].Jose J, Saravu K, Khera K, et al. Acute interstitial nephritis related to lansoprazole administration. J Pak Med Assoc 2008;58:206–7. [PubMed] [Google Scholar]
- [20].Nast CC. Medication-induced interstitial nephritis in the 21st century. Adv Chronic Kidney Dis 2017;24:72–9. [DOI] [PubMed] [Google Scholar]
- [21].Berney-Meyer L, Hung N, Slatter T, et al. Omeprazole-induced acute interstitial nephritis: a possible Th1-Th17-mediated injury? Nephrology (Carlton) 2014;19:359–65. [DOI] [PubMed] [Google Scholar]
- [22].Corsonello A, Lattanzio F, Bustacchini S, et al. Adverse events of proton pump inhibitors: potential mechanisms. Curr Drug Metabol 2018;19:142–54. [DOI] [PubMed] [Google Scholar]
- [23].Torpey N, Barker T, Ross C. Drug-induced tubulo-interstitial nephritis secondary to proton pump inhibitors: experience from a single UK renal unit. Nephrol Dial Transplant 2004;19:1441–6. [DOI] [PubMed] [Google Scholar]
- [24].Lazarus B, Chen Y, Wilson FP, et al. Proton pump inhibitor use and the risk of chronic kidney disease. JAMA Int Med 2016;176:238–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Toth-Manikowski S, Grams ME. Proton pump inhibitors and kidney disease—GI upset for the nephrologist? Kidney Int Rep 2017;2:297–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Klatte DCF, Gasparini A, Xu H, et al. Association between proton pump inhibitor use and risk of progression of chronic kidney disease. Gastroenterology 2017;153:702–10. [DOI] [PubMed] [Google Scholar]
- [27].Joyce E, Glasner P, Ranganathan S, et al. Tubulointerstitial nephritis: diagnosis, treatment, and monitoring. Pediatr Nephrol 2017;32:577–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Geevasinga N, Coleman PL, Webster AC, et al. Proton pump inhibitors and acute interstitial nephritis. Clin Gastroenterol Hepatol 2006;4:597–604. [DOI] [PubMed] [Google Scholar]
- [29].Muriithi AK, Leung N, Valeri AM, et al. Clinical characteristics, causes and outcomes of acute interstitial nephritis in the elderly. Kidney Int 2015;87:458–64. [DOI] [PubMed] [Google Scholar]
- [30].Xie Y, Bowe B, Li T, et al. Proton pump inhibitors and risk of incident CKD and progression to ESRD. J Am Soc Nephrol 2016;27:3153–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Muriithi AK, Leung N, Valeri AM, et al. Biopsy-proven acute interstitial nephritis, 1993-2011: a case series. Am J Kidney Dis 2014;64:558–66. [DOI] [PubMed] [Google Scholar]
- [32].Morschel CF, Mafra D, Eduardo JCC. The relationship between proton pump inhibitors and renal disease. J Bras Nefrol 2018;40:301–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Torlot FJ, Whitehead DJ. Acute interstitial nephritis caused by two different proton pump inhibitors. Br J Hosp Med (Lond) 2016;77:50–1. [DOI] [PubMed] [Google Scholar]
- [34].Singer S, Parry RG, Deodhar HA, et al. Acute interstitial nephritis, omeprazole and antineutrophil cytoplasmic antibodies. Clin Nephrol 1994;42:280. [PubMed] [Google Scholar]
- [35].Jacobs-Kosmin D, Derk CT, Sandorfi N. Pantoprazole and perinuclear antineutrophil cytoplasmic antibody-associated vasculitis. J Rheumatol 2006;33:629–32. [PubMed] [Google Scholar]
- [36].Clarkson MR, Giblin L, O’Connell FP, et al. Acute interstitial nephritis: clinical features and response to corticosteroid therapy. Nephrol Dial Transplant 2004;19:2778–83. [DOI] [PubMed] [Google Scholar]
- [37].Prendecki M, Tanna A, Salama AD, et al. Long-term outcome in biopsy-proven acute interstitial nephritis treated with steroids. Clin Kidney J 2017;10:233–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].González E, Gutiérrez E, Galeano C, et al. Early steroid treatment improves the recovery of renal function in patients with drug-induced acute interstitial nephritis. Kidney Int 2008;73:940–6. [DOI] [PubMed] [Google Scholar]
- [39].Gross M, Labenz J. Prescription of PPI in Germany: too often, too long, too much? MMW Fortschr Med 2018;160:37–40. [DOI] [PubMed] [Google Scholar]
- [40].Leven C, Hudier L, Picard S, et al. Prospective study of drug-induced interstitial nephritis in eleven French nephrology units. Presse Med 2014;43:e369–76. [DOI] [PubMed] [Google Scholar]
- [41].Arora P, Gupta A, Golzy M, et al. Proton pump inhibitors are associated with increased risk of development of chronic kidney disease. BMC Nephrol 2016;17:112.Published 2016 Aug 3. [DOI] [PMC free article] [PubMed] [Google Scholar]


