Abstract
Artemisinin-based combination therapies (ACTs) have demonstrated in vitro inhibition of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Artemisinins have also shown anti-inflammatory effects, including inhibition of interleukin-6 (IL-6) that plays a key role in the development of severe coronavirus disease 2019 (COVID-19). There is now sufficient evidence for the effectiveness of ACTs, and in particular artesunate/pyronaridine, to support clinical studies for COVID-19 infections.
Keywords: SARS-CoV-2, drug repurposing, antiviral, antimalarial, cytokine storm, artesunate/pyronaridine
Drug Repurposing Accelerates the Discovery of New Cures
Using a drug that works for one disease to treat an unrelated condition can reduce suffering and save lives. Antimalarials provide abundant examples of such successes. When quinidine was purified from cinchona alkaloids, earlier empirical observations on its antiarrhythmic properties led Walter Frey to conclude that it was the most effective of the four cinchona alkaloids in 1918. A merchant first proposed examining cinchona alkaloids to treat arrhythmias because he noticed that when he took quinine to prevent malaria his heart irregularities resolved. Quinine was being used to treat discoid lupus erythematosus with indifferent results until Francis Page (a Registrar at the Middlesex Hospital in London) described the beneficial effects of mepacrine (an antimalarial agent that is structurally related to chloroquine and was used during World War II) in most of 18 patients. A few years later, building on chloroquine’s success as an antimalarial in the late 1940s, hydroxychloroquine was also used in a case series of seven patients, many of whom had not responded to other antimalarials but were successfully treated.
These descriptions illustrate how successful repurposing of drugs can provide lasting medical benefits despite having been developed in less rigorous regulatory environments. The COVID-19 pandemic now necessitates urgent attempts at repurposing antimalarials. Hydroxychloroquine seized the global attention of politicians, the public, and investigators. The basis for wanting to repurpose hydroxychloroquine derived from its in vitro activity against SARS-CoV-2 (the causative agent of COVID-19), its affordability, its well understood safety profile in other conditions, and the results of small uncontrolled studies suggesting antiviral and clinical benefits in patients. As illustrated previously, uncontrolled studies and case series can highlight interventions that may be useful, especially when it comes to the design of larger controlled trials. However, current standards demand that any new intervention (even using an old drug) should be tested rigorously which can produce results that do not always agree with the preliminary studies. This is highlighted by the results of a large, randomized trial [1] for hydroxychloroquine that did not demonstrate a benefit in mortality prevention for COVID-19
Antimalarials as Potential Therapeutic Agents for COVID-19
Are there any other promising antimalarials that might be worth investigating in the management of COVID-19? Pyronaridine (a mepacrine nucleus with an amodiaquine-like addition) was first made in 1970 at the Institute of Chinese Parasitic Disease and used as an antimalarial monotherapy given orally and parenterally to treat chloroquine-resistant Plasmodium falciparum infections. It has since been combined with artesunate (in a 3:1 ratio) to form an ACT that is safe and which cures otherwise multidrug-resistant infections [2]. In vitro studies comparing pyronaridine, artesunate, and hydroxychloroquine effectiveness against SARS-COV-2 show that pyronaridine and artesunate are more potent than hydroxychloroquine [3] in the human lung epithelial cell line Calu-3 (Table 1 ). Another ACT, mefloquine–artesunate has also shown potent antiviral activity against SARS-CoV-2 [4] with increased drug concentration in lung tissue, a potential clinical advantage in COVID-19 (Table 1).
Table 1.
In Vitro Antiviral Effect of Selected Antimalarials against SARS-CoV-2
| Drug | Cell line | IC50 (μM) | Refs |
|---|---|---|---|
| Pyronaridine | Vero | 1.1 | [3] |
| Calu-3 | 6.4 | [3] | |
| Artesunate | Vero | 53.0 | [3] |
| Calu-3 | 1.8 | [3] | |
| Hydroxychloroquine | Vero | 1.1 | [3] |
| Calu-3 | 103.0 | [3] | |
| Mefloquine-dihydroartemisinin⁎ | Vero | Between 4.1-2.5 & 2.0-1.3 | [4] |
| Desethylamodiaquine-dihydroartemisinin⁎ | Vero | Between 4.0-5.0 & 2.0-2.5 | [4] |
| Pyronaridine-dihydroartemisinin⁎ | Vero | > 0.5-1.0 | [4] |
| Lumefantrine-dihydroartemisinin⁎ | Vero | > 33.0-2.0 | [4] |
| Piperaquine-dihydroartemisinin⁎ | Vero | > 1.0-3.1 | [4] |
IC50 values are estimated based on the 3-point dose-response data (2x/1x/0.5x expected Cmax concentrations) in [4]. The concentrations of each drug used are shown.
COVID-19 and the Cytokine Storm
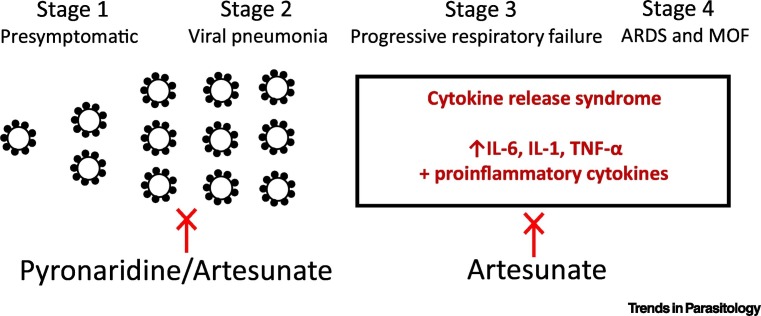
Are there other reasons, beyond antiviral properties, that would make artemisinins, and in particular artesunate, useful in managing patients with COVID-19? To answer this question, we need to understand the pathophysiology of COVID-19 progression (Figure 1 ). In most patients, the natural history of COVID-19 infection is self-limiting with a presymptomatic or asymptomatic phase lasting a few days (Stage 1) followed by symptoms of fever, cough, and systemic malaise. After a median time of 5 days, the disease progresses from the upper to the lower respiratory tract as a viral pneumonia (Stage 2). Around day 7–10, the detectable upper respiratory viral load decreases, antibody responses are generated, and patients start to recover. However, at 7–14 days a small proportion of patients develop a cytokine release syndrome (CRS)/cytokine storm (CS), the hallmarks of which appear to be elevated host markers of inflammation [e.g., increased C-reactive protein (CRP), ferritin, D-dimers] and lymphopenia. Patients experiencing CRS often develop progressive respiratory failure (Stage 3) that can then lead to acute respiratory distress syndrome (ARDS) and multiorgan failure (MOF) (Stage 4).
Figure 1.
COVID-19 Disease Progression and Stages with the Potential to Be Targeted by Antimalarial Drugs.
During Stages 1 and 2 of infection there is viral replication, which may be targeted by antimalarials, including pyronaridine and artesunate. During Stages 3 and 4 of infection, a cytokine storm syndrome occurs that is characterized by increased proinflammatory markers. These latter stages may be targeted by the anti-inflammatory effects of artemisinins, including artesunate. Abbreviations: ARDS, acute respiratory distress syndrome; COVID-19, coronavirus disease 2019; IL-6, interleukin-6; IL-1, interleukin-1; MOF, multiorgan failure; TNF, tumor necrosis factor.
Interleukin-6 (IL-6) is promptly and transiently produced in response to tissue injury and infection, stimulating acute-phase responses, hematopoiesis, and immune reactions, which contribute to host defense [5]. In chronic inflammation and autoimmune diseases, dysregulated continual synthesis of IL-6 leads to significant pathophysiological effects. Apart from IL-6, other proinflammatory cytokines, such as IL-1, interferon-gamma (IFN-γ) and tumor necrosis factor (TNF) are produced during CRS/CS and contribute to pathophysiological processes that result in MOF. Although rare, symptoms similar to Kawasaki disease are another delayed immunological manifestation of SARS-CoV-2 infection in children [6].
In patients admitted to intensive care units, around 60–70% develop ARDS, followed by shock (30%), myocardial dysfunction (20–30%), and acute kidney injury (10–30%). In these critically ill patients, between 42 and 100% will require mechanical ventilation. Risk factors for developing severe COVID-19 disease include older age, male sex, comorbidities (including chronic lung disease, cardiovascular disease, diabetes, obesity, cancer, and organ transplantation). Genetic factors and ethnicity may also play a role [7]. Several therapeutic agents have been proposed for the treatment of CRS, including corticosteroids, intravenous immunoglobulin, selective cytokine blockade, and Janus kinase (JAK) inhibition [8].
The RECOVERY trial has shown a mortality benefit of dexamethasone in patients with moderate and severe COVID-19, supporting the importance of anti-inflammatory interventions to manage the complications of CRS. Tocilizumab is an anti-IL-6 antibody that binds membrane-bound and soluble IL-6 receptors, blocking IL-6 from exerting its proinflammatory effects. It is currently licensed for the treatment of CRS related to chimeric antigen receptor (CAR)-T cell therapy and rheumatoid arthritis. Tocilizumab has also been trialed in different studies to treat severe COVID-19, including the specific features of CRS [9], and may be beneficial in reducing mortality and morbidity, although more studies are needed.
Artemisinins as Potential Therapeutic Agents for COVID-19
In addition to their in vitro SARS-CoV-2 effects, as noted earlier, artemisinins, including artesunate, also have anti-inflammatory properties. These include those directed at IL-6-mediated pathways. The anti-inflammatory effects of artesunate in a range of disease states are detailed later and suggest that artemisinins may be beneficial in managing COVID-19 patients.
Artesunate in Sepsis and Hemorrhagic Shock
In a sepsis model, artesunate inhibited lipopolysaccharide (LPS)/endotoxin-induced IL-6 and TNF-α release [10] from bone-marrow-derived monocytes, peritoneal macrophages, and the RAW264.7 mouse cell line. Toll-like receptor 4 (TLR4) is utilized by monocytes/macrophages of the innate immune system to recognize LPS. This then triggers activation of TNF receptor-associated factor 6 (TRAF6) which, in turn, activates nuclear factor (NF)-κB. NF-κB is known to promote the release of downstream proinflammatory cytokines such as IL-6 and TNF-α.
In a rat model of hemorrhagic shock, artesunate attenuated the expression of proinflammatory proteins IL6, TNF-α, NF-κB, and nitric oxide synthase (NOS). This protected against MOF [11]. Pathway analysis by RNAseq supported an effect of artesunate on the protein kinase B (PKB or Akt)-survival pathway, resulting in IL-1 receptor-associated kinase 1 (IRAK1) downregulation. Treating rats with artesunate enhanced the phosphorylation (activation) of endothelial (e)NOS and Akt as well as the phosphorylation (inhibition) of glycogen synthase kinase-3β (GSK-3β). Akt activation is linked to the prevention of a range of organ injuries and phosphorylates eNOS at Ser117, enhancing production of nitric oxide (NO). This is pivotal to preserve microvascular perfusion and prevent MOF.
Artesunate in Models of Acute Lung Injury and Nephritis
In a rat model of LPS-induced lung injury, artesunate reduced levels of IL-6, IL-1β, and TNF-α. TLR4 expression and NF-κB activation were also attenuated by artesunate, which upregulated expression of nuclear factor erythroid 2-related factor 2 (Nrf2) and heme oxygenase-1 (HO-1) [12]. In another rat model [13], artesunate inhibited renal reperfusion-stimulated lung inflammation by attenuating serum and pulmonary IL-6, macrophage-inflammatory protein 2 (MIP-2), prostaglandin E2 (PGE2), NO and malondialdehyde (MDA) levels, and activated the HO-1 pathway.
In a rat model of nephritis, artesunate attenuated IL-6 levels, TNF-α, transforming growth factor (TGF)-β1, TLR4, and NF-κB expression [14]. Artesunate also ameliorated high glucose-induced injury in rat glomerular mesangial cells via suppression of the TLR4/NF-κB/nod-like receptor protein 3 (NLRP3) inflammasome pathway [15].
Concluding Remarks
There is sufficient evidence for the antiviral and anti-inflammatory effects of antimalarials to support further clinical therapeutic studies for COVID-19 infections. In particular, pyronaridine has demonstrated in vitro antiviral effects on SARS-CoV-2 in a human lung epithelial cell line, while artesunate, in addition to similar antiviral effects, has anti-inflammatory effects via IL-6 mediated pathways in other disease states that suggest it may be beneficial in the treatment of COVID-19 (Table 1, Figure 1). Thus, the ACT artesunate/pyronaridine deserves further investigation as a COVID-19 treatment option. The safety of this antimalarial combination is established in malaria in children and adults, providing some reassurance for studies in COVID-19. Several Phase II studies are being implemented, and their design may benefit from the varied mechanisms of action that have been outlined, including assessment of the broad-spectrum anti-inflammatory properties of artesunate. In addition, care should be taken to test this combination with rigor and not over promise its potential so as to avoid the issues that surrounded the use of hydroxychloroquine.
Acknowledgements
H.M.S. is supported by the Wellcome Trust Institutional Strategic Support Fund (204809/Z/16/Z) awarded to St George’s University of London.
References
- 1.Horby P. Effect of hydroxychloroquine in hospitalized patients with COVID-19: preliminary results from a multi-centre, randomized, controlled trial. medRxiv. 2020 Published online July 15, 220. https://www.medrxiv.org/content/10.1101/2020.07.15.20151852v1. [Google Scholar]
- 2.Quang Bui P. Pyronaridine-artesunate efficacy and safety in uncomplicated Plasmodium falciparum malaria in areas of artemisinin-resistant falciparum in Viet Nam (2017–2018) Clin. Infect. Dis. 2020;70:2187–2195. doi: 10.1093/cid/ciz580. [DOI] [PubMed] [Google Scholar]
- 3.Bae J.-Y. Pyronaridine and artesunate are potential antiviral drugs against COVID-19 and influenza. bioRxiv. 2020 doi: 10.1101/2020.07.28.225102. Published online July 28, 2020. [DOI] [Google Scholar]
- 4.Gendrot M. Antimalarial artemisinin-based combination therapies (ACT) and COVID-19 in Africa: In vitro inhibition of SARS-CoV-2 replication by mefloquine-artesunate. Int. J. Infect. Dis. 2020;99:437–440. doi: 10.1016/j.ijid.2020.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Conti P. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): anti-inflammatory strategies. J. Biol. Regul. Homeost. Agents. 2020;34:327–331. doi: 10.23812/CONTI-E. [DOI] [PubMed] [Google Scholar]
- 6.Ronconi G. SARS-CoV-2, which induces COVID-19, causes Kawasaki-like disease in children: role of pro-inflammatory and anti-inflammatory cytokines. J. Biol. Regul. Homeost. Agents. 2020;34:767–773. doi: 10.23812/EDITORIAL-RONCONI-E-59. [DOI] [PubMed] [Google Scholar]
- 7.Pareek M. Ethnicity and COVID-19: an urgent public health research priority. Lancet. 2020;395:1421–1422. doi: 10.1016/S0140-6736(20)30922-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mehta P. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baker E.H. Insights from compassionate use of tocilizumab for COVID-19 to inform appropriate design of randomised controlled trials. Br. J. Clin. Pharmacol. 2020 doi: 10.1111/bcp.14466. Published online July 12, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuang M. Artesunate attenuates pro-inflammatory cytokine release from macrophages by inhibiting TLR4-mediated autophagic activation via the TRAF6-Beclin1-PI3KC3 pathway. Cell. Physiol. Biochem. 2018;47:475–488. doi: 10.1159/000489982. [DOI] [PubMed] [Google Scholar]
- 11.Sordi R. Artesunate protects against the organ injury and dysfunction induced by severe hemorrhage and resuscitation. Ann. Surg. 2017;265:408–417. doi: 10.1097/SLA.0000000000001664. [DOI] [PubMed] [Google Scholar]
- 12.Zhao D. Artesunate protects LPS-induced acute lung injury by inhibiting TLR4 expression and inducing Nrf2 activation. Inflammation. 2017;40:798–805. doi: 10.1007/s10753-017-0524-6. [DOI] [PubMed] [Google Scholar]
- 13.Liu Z. Artesunate inhibits renal ischemia reperfusion-stimulated lung inflammation in rats by activating HO-1 pathway. Inflammation. 2018;41:114–121. doi: 10.1007/s10753-017-0669-3. [DOI] [PubMed] [Google Scholar]
- 14.Wan R.J., Li Y.H. Effects of artesunate prevent nephritis via the Toll-like receptor 4/nuclear factor kappaB signaling pathway in rats. Mol. Med. Rep. 2017;16:6389–6395. doi: 10.3892/mmr.2017.7362. [DOI] [PubMed] [Google Scholar]
- 15.Sun Z. Artesunate ameliorates high glucose-induced rat glomerular mesangial cell injury by suppressing the TLR4/NF-kappaB/NLRP3 inflammasome pathway. Chem. Biol. Interact. 2018;293:11–19. doi: 10.1016/j.cbi.2018.07.011. [DOI] [PubMed] [Google Scholar]