Abstract
Background
Coronavirus disease 2019 (COVID-19) is a growing pandemic that confers augmented risk for right ventricular (RV) dysfunction and dilation; the prognostic utility of adverse RV remodeling in COVID-19 patients is uncertain.
Objectives
The purpose of this study was to test whether adverse RV remodeling (dysfunction/dilation) predicts COVID-19 prognosis independent of clinical and biomarker risk stratification.
Methods
Consecutive COVID-19 inpatients undergoing clinical transthoracic echocardiography at 3 New York City hospitals were studied; images were analyzed by a central core laboratory blinded to clinical and biomarker data.
Results
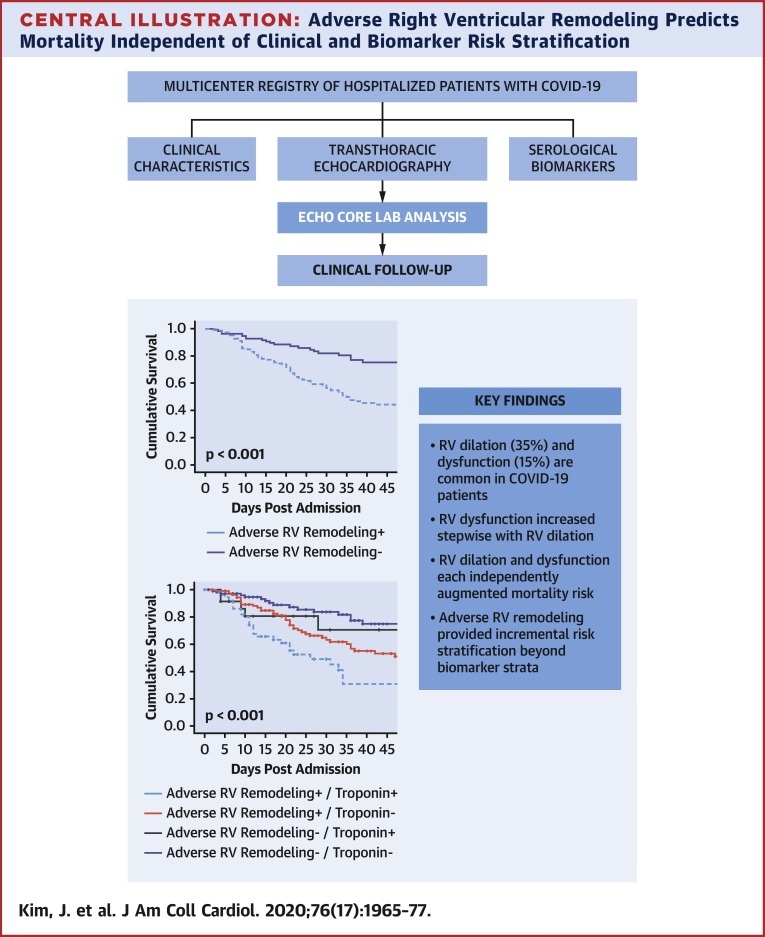
In total, 510 patients (age 64 ± 14 years, 66% men) were studied; RV dilation and dysfunction were present in 35% and 15%, respectively. RV dysfunction increased stepwise in relation to RV chamber size (p = 0.007). During inpatient follow-up (median 20 days), 77% of patients had a study-related endpoint (death 32%, discharge 45%). RV dysfunction (hazard ratio [HR]: 2.57; 95% confidence interval [CI]: 1.49 to 4.43; p = 0.001) and dilation (HR: 1.43; 95% CI: 1.05 to 1.96; p = 0.02) each independently conferred mortality risk. Patients without adverse RV remodeling were more likely to survive to hospital discharge (HR: 1.39; 95% CI: 1.01 to 1.90; p = 0.041). RV indices provided additional risk stratification beyond biomarker strata; risk for death was greatest among patients with adverse RV remodeling and positive biomarkers and was lesser among patients with isolated biomarker elevations (p ≤ 0.001). In multivariate analysis, adverse RV remodeling conferred a >2-fold increase in mortality risk, which remained significant (p < 0.01) when controlling for age and biomarker elevations; the predictive value of adverse RV remodeling was similar irrespective of whether analyses were performed using troponin, D-dimer, or ferritin.
Conclusions
Adverse RV remodeling predicts mortality in COVID-19 independent of standard clinical and biomarker-based assessment.
Key Words: COVID-19 (coronavirus), echocardiography, right ventricle
Abbreviations and Acronyms: CI, confidence interval; COVID-19, coronavirus disease 2019; echo, echocardiography; IQR, interquartile range; LV, left ventricle/ventricular; RV, right ventricle/ventricular; TAPSE, tricuspid annular plane excursion
Central Illustration
Coronavirus disease 2019 (COVID-19) is an evolving global pandemic. More than 15 million people have been diagnosed with COVID-19 worldwide (1). In the United States, >142,000 people have died with COVID-19, and >23,000 deaths have occurred in the New York City area (2). Given high rates of infection and the substantial morbidity and mortality risks conferred by this condition, evidence-based data regarding risk stratification of patients with COVID-19 is critical.
Emerging data suggests that cardiovascular injury occurs in the setting of COVID-19 infection. In an initial study of hospitalized patients with COVID-19, suspected cardiac injury was present in 7.2%, including nearly one-quarter (22%) of COVID-19–infected patients who required intensive care unit–level care (3). In another study, almost 12% of COVID-19–infected patients without known cardiovascular disease had elevated troponin levels or cardiac arrest during index hospitalization (4). Cardiac injury has also been reported to predict prognosis among COVID-19–infected patients, as evidenced by outcomes data that troponin elevation was present in 46% of nonsurvivors as opposed to 1% of survivors (5) and was associated with a >10-fold increased risk of mortality among hospitalized COVID-19 patients (6). Adverse cardiac chamber remodeling has been reported in patients with COVID-19. Given that this condition confers high risk for lung involvement, a key area of focus has been adverse right ventricular remodeling. Single-center studies have shown both RV dilation and dysfunction to commonly occur with COVID-19 infection, and to confer adverse prognosis (7,8). However, mechanistic determinants and incremental prognostic utility of RV remodeling to that of clinical or biomarker assessment are uncertain.
This study encompassed a multicenter cohort of hospitalized patients with COVID-19 infection undergoing transthoracic echocardiography (echo) at 3 hospitals throughout New York City. In all patients, echoes were transferred to a centralized core laboratory for blinded quantitative analyses. The goal was to test relative prognostic utility of adverse chamber remodeling in relation to conventional clinical and biomarker risk stratification of COVID-19.
Methods
Study population
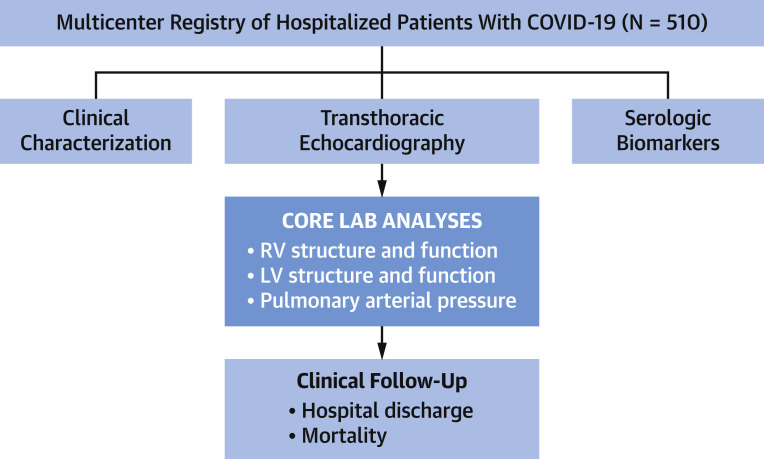
The population comprised consecutive inpatients (age ≥18 years) with COVID-19 (established via reverse transcriptase polymerase chain reaction) who underwent transthoracic echo between March 12, 2020, and May 17, 2020, at 3 hospitals within the New York Presbyterian Hospital network (Weill Cornell, Lower Manhattan, and Queens) (Figure 1 ). Pre-existing echoes (among patients who had undergone imaging ≥1 month [<5 years] prior to COVID-19 diagnosis) were retrieved from institutional databases and used to assess temporal changes in cardiac chamber geometry and function. No patients were excluded from the study based on clinical characteristics or echo results.
Figure 1.
Study Design
Overall schematic of multicenter image/data acquisition and centralized core laboratory analysis. Note that all participatory sites used a similar echocardiography (echo) protocol (inclusive of left ventricular [LV] and right ventricular [RV] functional assessment). Ancillary clinical and biomarker data were collected using a standardized electronic medical record query. Echo analyses were performed by dedicated core laboratory investigators who were blinded to clinical and biomarker indices. Follow-up was performed for clinical events related to coronavirus disease 2019 (COVID-19) infection, including death or hospital discharge.
The research protocol was approved by the Weill Cornell and New York Presbyterian Hospital Queens Institutional Review Boards, which provided approval for use of pre-existing data for research purposes.
Echocardiography
Image acquisition
Echo was performed if requested in the context of clinical care. Leading clinical indications for echo during treatment for COVID-19 were dyspnea/respiratory decompensation (88%), hemodynamic instability (62%), known/suspected myocardial infarction (12%), and/or arrhythmia (7%).
Echoes were performed using commercial (full size [non-handheld]) equipment, on which examinations were performed using a tailored protocol so as to minimize acquisition time and thus diminish technologist viral exposure. Images were acquired in standard parasternal, as well as apical 2-, 3-, and 4-chamber orientations: RV systolic function assessment was performed using M-mode (for tricuspid annular plane excursion [TAPSE]) and tissue Doppler (for Sʹ) imaging, both of which were acquired in apical 4-chamber orientation. Echo contrast was administered infrequently (15% [75 of 510]) and was utilized at the discretion of sonographers.
Image analysis
Echoes were transferred to a centralized core laboratory (Weill Cornell), at which dedicated analyses were performed by pre-designated study investigators (J.K., R.B.D.) for whom experience and high reproducibility for quantitative left ventricular (LV) and RV remodeling indices has been documented (9,10). For patients with multiple examinations, analyses were performed using the initial echo performed during hospital admission.
RV systolic function was quantified via TAPSE and Sʹ; TAPSE was measured (on M-mode) as the distance of systolic excursion of the lateral tricuspid annulus along its longitudinal plane. Sʹ was measured on tissue Doppler as the peak tricuspid annular longitudinal velocity of excursion. Established cutoffs (TAPSE <1.6 cm, Sʹ <10 mm/s) were used for each parameter (11). Concordant with prior methods by our group (12), RV dysfunction was defined by impairment of both TAPSE and Sʹ so as to reduce false positive classification. RV size was quantified based on end-diastolic diameter, which was measured at the RV base (septum – free wall) in apical 4-chamber orientation. RV dilation was defined using a binary cutoff (>4.1 cm) in accordance with consensus guidelines (13).
LV systolic function, chamber size, and myocardial mass were quantified based on linear dimensions measured in parasternal long-axis, consistent with established quantitative methods validated in necropsy comparisons and epidemiological outcomes studies (14, 15, 16, 17, 18). A semiquantitative regional wall motion score was calculated in accordance with established criteria (13). Sex-specific binary cutoffs for LV chamber dilation and hypertrophy were derived from consensus guidelines and normative data samples (19,20).
Additional analyses were performed to assess sequelae of ventricular remodeling that could potentially affect prognosis. Left atrial size was measured based on diameter, as well as planimetered chamber volume using a modified biplane area-length method. Mitral and tricuspid regurgitation were graded in accordance with consensus guidelines (21). Pulmonary artery systolic pressure was calculated based on tricuspid regurgitant velocity and inferior vena cava caliber. Central venous pressure was calculated based on size and collapsibility of the inferior vena cava.
Clinical characterization
Clinical and laboratory indices were acquired via query of an established institutional registry, for which initial results have been previously reported (22).
Demographics included cardiovascular indices and baseline medication regimen at time of hospital admission, as was subsequent inpatient initiation of COVID-19–related therapies. Biomarker data encompassed pre-specified indices generally associated with adverse prognosis (troponin, ferritin, C reactive protein, D-dimer, white blood count, and hepatic transaminases). For patients with biomarkers obtained at multiple time points, peak values were used for study-related data analyses. Elevated thresholds for elevated biomarkers were defined based on site-specific laboratory thresholds at participatory hospitals.
Prognostic assessment
Clinical endpoints included in-hospital mortality and hospital discharge. All events were collected by reviewers blinded to echo analyses and confirmed by review of electronic medical records. Time to death was calculated in relation to hospital admission date.
Statistical methods
Continuous variables are reported as mean ± SD when normally distributed, and otherwise as median (interquartile range [IQR]). Normally distributed continuous indices were compared via Student’s t-tests (for 2-group comparisons) or analysis of variances (for multiple group comparisons); non-normally distributed indices were compared via the Mann-Whitney U test. Categorical variables are reported as frequencies/percent and compared using the chi-square test or, if <5 expected outcomes per cell, the Fisher exact test. Paired categorical testing (i.e., pre- and post-COVID echo) was performed using McNemar’s test. Kaplan-Meier analysis was used to calculate survival; patients were considered to be at risk for death between hospital admission and discharge. Cox proportional hazards analysis was used to evaluate univariable and multivariable associations of clinical, biomarker, and imaging parameters with mortality. Model overfitting was avoided by limiting the number of variables to 1 for every 10 outcomes. A 2-sided p < 0.05 was considered statistically significant. Analyses were performed using SPSS version 24.0 (IBM, Armonk, New York).
Results
Population characteristics
The population comprised 510 consecutive adult COVID-19 patients in whom echo was performed during hospitalization at a median interval of 6 days (IQR: 1 to 15 days) post-admission. RV chamber size and systolic function were respectively quantifiable in 97% and 53% of patients. Patients with and without quantifiable RV function on echo were similar with respect to age, sex, and cardiovascular risk factors (all p = NS).
RV dilation was present in more than one-third of patients (35%), a prevalence >2-fold higher than that of RV dysfunction (15%). Clinical and hemodynamic indices in relation to adverse RV remodeling (dysfunction, dilation) for each respective parameter are detailed in Table 1 . As shown, patients with RV dilation were more likely to be men (p = 0.003), paralleling a similar trend for RV dysfunction (p = 0.11). Regarding therapeutic intervention, mechanical ventilation was more common among patients with RV dilation (p = 0.01), accompanied by slight, albeit statistically significant, decrements in blood pressure (p < 0.01). Among ventilated patients who underwent echo, 85% of examinations were performed at the time of mechanical ventilation.
Table 1.
Clinical Characteristics
| Overall (N = 510) | RV Dysfunction∗ + (n = 41) | RV Dysfunction∗ − (n = 227) | p Value | RV Dilation∗ + (n = 172) | RV Dilation∗ − (n = 314) | p Value | |
|---|---|---|---|---|---|---|---|
| Demographic indices | |||||||
| Age, yrs | 64 ± 14 | 66 ± 15 | 65 ± 14 | 0.82 | 65 ± 14 | 64 ± 14 | 0.49 |
| Male | 66 (335) | 73 (30) | 60 (136) | 0.11 | 74 (127) | 61 (190) | 0.003 |
| Body surface area, m2 | 1.87 ± 0.24 | 1.88 ± 0.27 | 1.86 ± 0.23 | 0.74 | 1.91 ± 0.24 | 1.85 ± 0.22 | 0.007 |
| Heart rate, beats/min | 93 ± 21 | 93 ± 21 | 91 ± 21 | 0.45 | 93 ± 23 | 93 ± 20 | 0.85 |
| Systolic blood pressure, mm Hg | 121 ± 21 | 122 ± 21 | 124 ± 20 | 0.53 | 118 ± 21 | 123 ± 20 | 0.008 |
| Diastolic blood pressure, mm Hg | 67 ± 14 | 67 ± 14 | 68 ± 14 | 0.69 | 64 ± 14 | 68 ± 14 | 0.006 |
| Cardiovascular risk factors | |||||||
| Hypertension | 63 (323) | 71 (29) | 64 (145) | 0.40 | 61 (104) | 65 (203) | 0.36 |
| Diabetes mellitus | 41 (207) | 39 (16) | 39 (89) | 0.98 | 36 (61) | 44 (137) | 0.08 |
| Obesity† | 34 (175) | 24 (10) | 34 (76) | 0.25 | 34 (58) | 34 (106) | 0.99 |
| Coronary artery disease‡ | 20 (100) | 22 (9) | 20 (45) | 0.76 | 17 (30) | 21 (66) | 0.34 |
| Tobacco use§ | 24 (124) | 24 (10) | 22 (49) | 0.69 | 26 (44) | 23 (72) | 0.51 |
| Pulmonary disease | |||||||
| Asthma | 7 (37) | 10 (4) | 8 (19) | 0.76 | 7 (12) | 7 (21) | 0.90 |
| Chronic obstructive pulmonary disease | 6 (29) | 5 (2) | 8 (17) | 0.75 | 8 (14) | 4 (12) | 0.043 |
| Baseline CV medications | |||||||
| ACE inhibitor/ARB | 32 (162) | 39 (16) | 30 (67) | 0.23 | 27 (47) | 35 (109) | 0.10 |
| Statin | 38 (192) | 46 (19) | 39 (88) | 0.36 | 39 (67) | 37 (116) | 0.66 |
| Beta-blocker | 30 (154) | 39 (16) | 35 (79) | 0.60 | 37 (63) | 26 (83) | 0.02 |
| Aspirin | 24 (120) | 22 (9) | 24 (55) | 0.75 | 27 (47) | 21 (66) | 0.12 |
| Diuretic agent | 14 (69) | 22 (9) | 14 (32) | 0.20 | 16 (28) | 12 (37) | 0.16 |
| In-hospital clinical course | |||||||
| Intensive care unit admission‖ | 68 (345) | 66 (27) | 61 (139) | 0.58 | 74 (128) | 64 (201) | 0.02 |
| Vasopressor use¶ | 61 (310) | 63 (26) | 55 (125) | 0.34 | 68 (117) | 57 (180) | 0.02 |
| Hypoxia# | 85 (431) | 88 (36) | 82 (187) | 0.39 | 89 (153) | 83 (259) | 0.06 |
| Supplemental oxygenation | |||||||
| Nasal cannula | 49 (181) | 35 (10) | 52 (83) | 0.08 | 49 (63) | 51 (112) | 0.76 |
| Face-mask ventilation | 43 (156) | 55 (16) | 39 (62) | 0.10 | 43 (55) | 41 (91) | 0.77 |
| Mechanical ventilation | 60 (308) | 56 (23) | 54 (123) | 0.82 | 68 (117) | 56 (177) | 0.01 |
| Acute respiratory distress syndrome | 58 (298) | 54 (22) | 52 (118) | 0.84 | 69 (118) | 53 (167) | 0.001 |
| Chest x-ray findings | |||||||
| Infiltrates | 84 (429) | 90 (36) | 83 (189) | 0.28 | 89 (153) | 82 (257) | 0.046 |
| Pleural effusion | 5 (27) | 5 (2) | 6 (13) | 1.00 | 6 (11) | 5 (16) | 0.55 |
| End-organ injury | |||||||
| Myocardial infarction∗∗ | 11 (58) | 12 (5) | 13 (30) | 0.86 | 10 (17) | 12 (38) | 0.46 |
| Heart failure or cardiogenic shock | 12 (63) | 17 (7) | 11 (24) | 0.29 | 16 (28) | 10 (32) | 0.051 |
| Acute renal injury†† | 54 (275) | 61 (25) | 49 (110) | 0.14 | 61 (104) | 48 (152) | 0.01 |
| COVID-19−directed medications | |||||||
| Hydroxychloroquine | 75 (381) | 59 (24) | 73 (165) | 0.07 | 78 (134) | 73 (229) | 0.23 |
| Steroids | 45 (229) | 34 (14) | 43 (97) | 0.30 | 48 (82) | 44 (137) | 0.39 |
| Tocilizumab | 12 (62) | 10 (4) | 13 (29) | 0.59 | 12 (20) | 12 (39) | 0.80 |
| Remdesivir | 12 (62) | 15 (6) | 11 (24) | 0.43 | 16 (27) | 11 (35) | 0.15 |
Values are mean ± SD or % (count). Bold p values are statistically significant.
Right ventricular (RV) dilation was defined as RV basal dimension >4.1 cm and dysfunction as tricuspid annular plane excursion <1.6 cm and RV Sʹ 10 cm/s.
Obesity was defined as body mass index ≥30 kg/m2.
Coronary artery disease was defined as history of prior myocardial infarction and/or coronary revascularization.
Tobacco use indicated current and past smoking.
Intensive care unit admission included any intensive care unit stay during hospitalization.
Vasopressor use was defined as need for any vasopressor support during hospitalization.
Hypoxia was defined as any need for supplemental oxygenation.
Myocardial infarction was defined in accordance with American College of Cardiology/American Heart Association/European Society of Cardiology universal criteria (36).
Acute renal injury was defined as an increase in serum creatinine by 0.3 mg/dl (26.5 mol/l) within 48 h or an increase in serum creatinine to 1.5× baseline within the prior 7 days.
Institutional database queries identified 73 patients in the study population (14%) who had also undergone echo (≥1 month) prior to COVID-19 diagnosis (median 14.5 months [IQR: 5.8 to 28.8 months]). Among this group, RV dilation was ∼1.5-fold more common on echo performed following COVID-19 diagnosis, compared with antecedent echo (55.2% vs. 38.8%; p = 0.06), paralleling a similar pattern for RV dysfunction (28.2% vs. 12.8%; p = 0.21), as well as the aggregate parameter of adverse RV remodeling (74.5% vs. 45.5%; p = 0.002).
RV remodeling in relation to serological biomarkers
Serological biomarkers conventionally associated with adverse prognosis in systemic infections such as COVID-19 were tested in relation to RV remodeling indices. As shown in Table 2 , biomarker profiles varied in relation to adverse RV remodeling pattern. Troponin elevation was >2-fold more common among patients with compared to those without RV dysfunction (50% vs. 21%; p < 0.001) whereas stratification based on presence or absence of RV dilation yielded similarly high prevalence in both groups (28% vs. 26%; p = 0.81). Conversely, despite near-uniform elevations across the study population, D-dimer levels were nearly 1.5-fold higher among patients with RV dilation (5,166 ng/ml [IQR: 2,465 to 13,716 ng/ml]) compared with those without RV dilation (3,528 ng/ml [IQR: 1,488 to 11,440 ng/ml]; p = 0.009); a lesser magnitude of difference was present when D-dimer levels were compared between patients with and without RV dysfunction (p = 0.39). Regarding inflammatory biomarkers, ferritin levels were higher among patients with compared to those without RV dysfunction (p < 0.001), paralleling a similar trend when patients were partitioned based on presence or absence of RV dilation (p = 0.08).
Table 2.
Serological Biomarkers Stratified by RV Remodeling
| Laboratory Indices∗ | Overall (N = 510) | RV Dysfunction + (n = 41) | RV Dysfunction − (n = 227) | p Value | RV Dilation + (n = 171) | RV Dilation − (n = 315) | p Value |
|---|---|---|---|---|---|---|---|
| Troponin, ng/ml | 0.07 (0.00–0.29) | 0.16 (0.08–0.75) | 0.05 (0.00–0.26) | 0.001 | 0.09 (0.01–0.33) | 0.06 (0.00–0.26) | 0.06 |
| >ULN | 27 (120) | 50 (17) | 21 (43) | <0.001 | 28 (42) | 26 (73) | 0.81 |
| >5× ULN | 11 (49) | 21 (7) | 9 (19) | 0.07 | 11 (17) | 10 (28) | 0.75 |
| Ferritin, ng/ml | 1,511 (740–2,586) | 2,389 (1,574–3,511) | 1,409 (617–2,126) | <0.001 | 1,599 (832–3,053) | 1,465 (656–2,414) | 0.08 |
| >ULN | 91 (431) | 97 (35) | 89 (192) | 0.22 | 93 (147) | 90 (262) | 0.24 |
| >5× ULN | 56 (264) | 83 (30) | 52 (112) | <0.001 | 61 (96) | 53 (156) | 0.14 |
| D-dimer, ng/ml | 3,762 (1,786–11,545) | 4,380 (1,959–16,022) | 3,504 (1,700–10,581) | 0.39 | 5,166 (2,465–13,716) | 3,528 (1,488–11,440) | 0.009 |
| >ULN | 99 (469) | 97 (32) | 99 (217) | 0.43 | 100 (161) | 98 (287) | 0.09 |
| >5× ULN | 81 (387) | 88 (29) | 80 (175) | 0.26 | 88 (141) | 79 (230) | 0.02 |
| CRP, mg/dl | 24.4 (14.0–32.0) | 25.4 (15.5–31.5) | 23.6 (13.4–32.2) | 0.77 | 26.0 (14.4–32.3) | 23.6 (13.2–32.0) | 0.31 |
| >ULN | 98 (467) | 100 (36) | 97 (210) | 0.60 | 99 (160) | 97 (285) | 0.11 |
| >5× ULN | 93 (442) | 97 (35) | 90 (194) | 0.22 | 94 (151) | 91 (269) | 0.33 |
| AST, U/l | 110 (57–234) | 161 (50–688) | 108 (54–204) | 0.10 | 118 (64–341) | 104 (54–205) | 0.06 |
| >ULN | 88 (434) | 85 (33) | 86 (189) | 0.78 | 89 (147) | 87 (266) | 0.55 |
| >5× ULN | 34 (165) | 49 (19) | 29 (63) | 0.01 | 39 (64) | 31 (93) | 0.07 |
| ALT, U/l | 93 (48–211) | 113 (41–409) | 86 (41–189) | 0.39 | 96 (51–247) | 92 (43–178) | 0.16 |
| >ULN | 73 (346) | 65 (26) | 70 (142) | 0.57 | 77 (120) | 71 (208) | 0.16 |
| >5× ULN | 23 (106) | 30 (12) | 20 (40) | 0.14 | 27 (42) | 19 (56) | 0.054 |
| WBC, 109/l | 19.4 (13.3–28.8) | 18.7 (12.4–27.1) | 18.7 (12.4–27.5) | 0.85 | 20.6 (13.7–29.1) | 19.1 (12.7–28.6) | 0.36 |
Values are median (interquartile range) or % (count). Bold p values are statistically significant.
ALT = alanine aminotransferase; AST = aspartate aminotransferase; ULN = upper limit of normal; WBC = white blood cells.
Abnormal biomarker cutoffs defined in accordance with bioassays at participatory study sites (troponin-I >0.5 ng/ml, troponin-T >0.1 ng/ml, ferritin >274 ng/ml, D-dimer >229 mg/ml, C-reactive protein (CRP) >0.9 mg/dl, AST >34 U/l, ALT >49 U/l).
Cardiac structure and function
Table 3 details left- and right-sided imaging parameters in relation to adverse RV remodeling (dysfunction, dilation). As shown, both adverse RV remodeling indices were associated with LV dysfunction, as evidenced by lower LV ejection fraction among patients with RV dysfunction (p < 0.001), paralleling a similar trend when patients were stratified based on RV dilation (p = 0.08). Regarding left-sided chamber remodeling, patients with RV dilation more commonly had increased LA volume (p < 0.001) as well as a trend toward increased LV chamber size (p = 0.12). Of note, whereas pulmonary artery systolic pressure was higher among patients with RV dilation (p < 0.001), stratification based on RV performance demonstrated nonsignificant differences between groups stemming from equivalent elevations in patients with (44.8 ± 12.2 mm Hg) compared to those without RV dysfunction (42.3 ± 12.4 mm Hg; p = 0.31).
Table 3.
Imaging Characteristics
| Overall (N = 510) | RV Dysfunction + (n = 41) | RV Dysfunction − (n = 227) | p Value | RV Dilation + (n = 172) | RV Dilation − (n = 314) | p Value | |
|---|---|---|---|---|---|---|---|
| LV function/morphology∗ | |||||||
| LVEF, % | 54.1 ± 14.4 | 45.2 ± 17.1 | 55.9 ± 13.5 | <0.001 | 52.5 ± 15.3 | 54.9 ± 13.7 | 0.08 |
| LVEF <55% | 41 (206) | 68 (27) | 35 (80) | <0.001 | 44 (76) | 40 (124) | 0.30 |
| LV stroke volume, ml | 62.1 ± 24.0 | 51.1 ± 26.3 | 65.5 ± 21.9 | 0.001 | 63.0 ± 24.8 | 61.5 ± 23.4 | 0.58 |
| LV cardiac output, l/min | 5.7 ± 2.4 | 4.7 ± 2.4 | 5.8 ± 2.2 | 0.005 | 5.7 ± 2.4 | 5.7 ± 2.3 | 0.91 |
| LV wall motion scores | 23 ± 10 | 27 ± 12 | 22 ± 9 | 0.006 | 24 ± 11 | 22 ± 9 | 0.14 |
| LV wall motion score index | 1.41 ± 0.62 | 1.69 ± 0.73 | 1.35 ± 0.57 | 0.006 | 1.48 ± 0.67 | 1.39 ± 0.58 | 0.14 |
| Regional wall motion abnormality | 13 (63) | 23 (9) | 12 (26) | 0.06 | 13 (21) | 13 (40) | 0.81 |
| LV end-diastolic volume, ml/m2 | 67.6 ± 23.5 | 74.9 ± 36.2 | 66.6 ± 21.9 | 0.19 | 70.7 ± 28.2 | 66.4 ± 20.2 | 0.12 |
| LV end-diastolic dilation∗ | 14 (54) | 22 (8) | 14 (25) | 0.21 | 13 (18) | 14 (34) | 0.79 |
| LV end-systolic volume, ml/m2 | 34.6 ± 22.6 | 47.7 ± 36.3 | 31.9 ± 19.0 | 0.02 | 37.9 ± 27.9 | 33.1 ± 19.2 | 0.08 |
| LV end-systolic dilation† | 29 (111) | 36 (13) | 26 (47) | 0.22 | 30 (41) | 29 (69) | 0.79 |
| LV myocardial mass, g/m2 | 84.9 ± 28.8 | 96.8 ± 43.8 | 83.4 ± 25.5 | 0.08 | 87.6 ± 30.1 | 83.4 ± 26.5 | 0.16 |
| LV hypertrophy‡ | 17 (67) | 22 (8) | 18 (33) | 0.59 | 18 (25) | 17 (40) | 0.70 |
| Relative wall thickness | 0.33 ± 0.07 | 0.33 ± 0.07 | 0.33 ± 0.07 | 0.74 | 0.33 ± 0.07 | 0.33 ± 0.06 | 0.77 |
| LA morphology | |||||||
| LA volume, ml/m2 | 32.0 ± 16.0 | 38.0 ± 15.9 | 31.5 ± 15.6 | 0.08 | 38.5 ± 18.2 | 29.2 ± 14.1 | <0.001 |
| LA diameter, cm | 3.3 ± 0.9 | 3.7 ± 1.4 | 3.3 ± 0.8 | 0.09 | 3.4 ± 0.9 | 3.2 ± 0.8 | 0.09 |
| RV function/morphology | |||||||
| RV diameter, cm | 4.0 ± 0.7 | 4.3 ± 1.0 | 3.9 ± 0.7 | 0.02 | 4.8 ± 0.5 | 3.5 ± 0.4 | <0.001 |
| cm/m2 | 2.1 ± 0.4 | 2.3 ± 0.4 | 2.1 ± 0.4 | 0.043 | 2.5 ± 0.4 | 1.9 ± 0.3 | <0.001 |
| Tricuspid annular plane excursion, cm | 1.9 ± 0.5 | 1.3 ± 0.2 | 2.0 ± 0.5 | <0.001 | 1.8 ± 0.6 | 1.9 ± 0.5 | 0.36 |
| RV Sʹ, cm/s | 13.4 ± 4.5 | 8.4 ± 1.3 | 14.6 ± 4.2 | <0.001 | 12.3 ± 4.6 | 13.9 ± 4.0 | 0.01 |
| Hemodynamic and valvular indices | |||||||
| Pulmonary artery systolic pressure, mm Hg | 42.5 ± 12.0 | 44.8 ± 12.2 | 42.3 ± 12.4 | 0.31 | 46.7 ± 12.9 | 39.6 ± 10.6 | <0.001 |
| Mitral regurgitation (≥2+) | 8 (33) | 20 (8) | 4 (9) | 0.002 | 10 (16) | 6 (15) | 0.08 |
| Tricuspid regurgitation (≥2+) | 13 (57) | 17 (7) | 14 (29) | 0.55 | 23 (36) | 8 (21) | <0.001 |
| Central venous pressure, mm Hg | 8.2 ± 3.7 | 9.6 ± 4.1 | 7.7 ± 3.8 | 0.005 | 9.5 ± 3.7 | 7.5 ± 3.6 | <0.001 |
Values are mean ± SD or % (count). Cardiac remodeling indices acquirable as follows (data reported as % [count]): left ventricular ejection fraction (LVEF): 99% (n = 506), left ventricular (LV) wall motion score: 95% (n = 486), LV internal diameter end-diastole: 79% (n = 404), left atrial (LA) diameter: 77% (n = 392), LA volume: 45% (n = 230), pulmonary artery systolic pressure: 55% (n = 283). Upper-limit normative cutoffs for LV quantitative indices defined in accordance with established literature. Bold p values are statistically significant.
LV end-diastolic dilation: women >81.4 ml/m2, men >88.5 ml/m2;
LV end-systolic dilation: women >34.9 ml/m2, men >40.3 ml/m2;
LV myocardial mass: women >95 g/m2, men >115 g/m2.
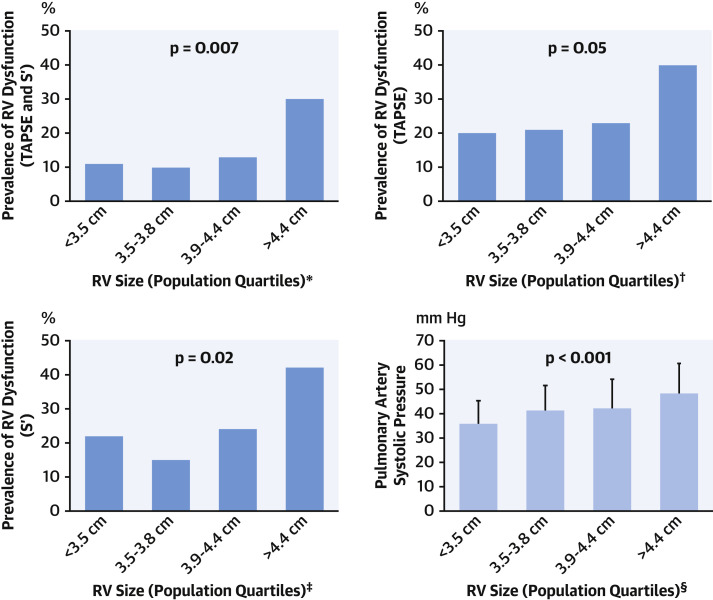
Figure 2 reports prevalence of RV dysfunction in relation to strata (population-based quartiles) of RV size. As shown, whereas RV dysfunction generally increased in relation to RV size, marked increments in prevalence of dysfunction occurred primarily among patients with advanced RV geometric remodeling—as evidenced by a >2-fold increment among patients in the highest quartile of RV size as compared with those in the preceding strata (30% vs. 13%; p = 0.018). Consistent with the notion that impaired RV contractile performance occurred subsequent to development of geometric remodeling, cross-sectional data demonstrated RV dysfunction to be present in less than one-quarter (23%) of patients with RV dilation.
Figure 2.
RV Size in Relation to Contractile Dysfunction and PA Pressure
(Dark blue) Prevalence of right ventricular (RV) dysfunction as defined using the composite of abnormal tricuspid annular plane excursion (TAPSE) and Sʹ (upper left) as well as individual linear indices (upper right, lower left) in relation to population-based quartiles of RV size. Note stepwise increments in prevalence of RV dysfunction with increasing RV size (all p ≤ 0.05), including a >2-fold increase in dysfunction (≥30%) among patients in the highest quartile compared with other strata. (Light blue) Pulmonary artery systolic pressure (mean ± SD) among groups stratified by RV size (bottom right). Consistent with above noted impairments in function, RV afterload as quantified by pulmonary artery (PA) pressure increased in relation to strata of RV size (p < 0.001). ∗Number affected per quartile: <3.5 cm: 7 of 65; 3.5 to 3.8 cm: 6 of 60; 3.9 to 4.4 cm: 9 of 72; >4.4 cm: 18 of 61. †Number affected per quartile: <3.5 cm: 11 of 56; 3.5 to 3.8 cm: 12 of 57; 3.9 to 4.4 cm: 15 of 65; >4.4 cm: 21 of 52. ‡Number affected per quartile: <3.5 cm: 11 of 50; 3.5 to 3.8 cm: 6 of 40; 3.9 to 4.4 cm: 13 of 54; >4.4 cm: 22 of 53. §Number affected per quartile: <3.5 cm: 28 of 52; 3.5 to 3.8 cm: 50 of 68; 3.9 to 4.4 cm: 52 of 77; >4.4 cm: 73 of 86.
Clinical outcomes
Mortality was assessed following echo to test the additive prognostic utility of adverse RV remodeling’s impact on hospitalization-related clinical prognosis. More than three-fourths (77%) of the population had follow-up to a time point sufficient for a study-related clinical endpoint comprised of death (32%) or hospital discharge (45%). Median duration of follow-up after hospital admission was 20 days (IQR: 9 to 39 days) in the overall population, encompassing a median follow-up of 23 days (IQR: 10 to 46 days) among survivors and 17 days (IQR: 9 to 26 days) among patients with subsequent death.
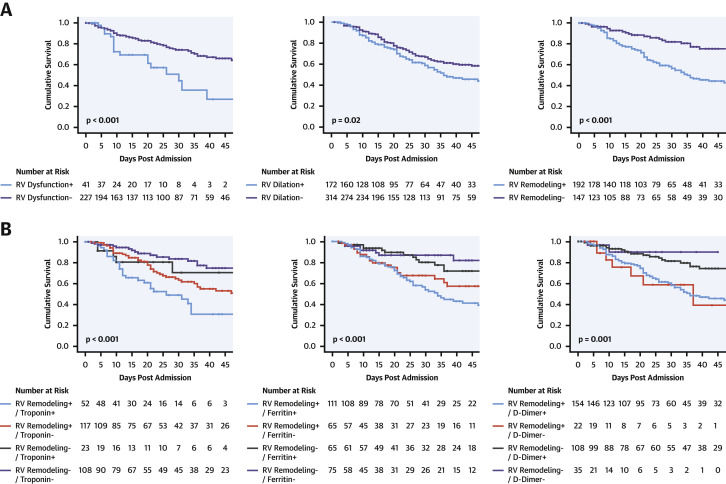
Table 4 provides univariable Cox modeling results for clinical, biomarker, and echo-quantified imaging indices in relation to mortality. As shown, age conferred increased risk for death (hazard ratio [HR]: 1.15 per decade; 95% confidence interval [CI]: 1.02 to 1.30 per decade; p = 0.02), despite nonsignificant associations for conventional clinical risk factors for atherosclerotic heart disease (all p = NS). Biomarker data demonstrated a strong association between elevated troponin and risk for death (HR: 1.30; 95% CI: 1.04 to 1.63; p = 0.02), paralleled by similar magnitude of association for D-dimer (HR: 1.62; 95% CI: 1.20 to 2.19; p = 0.002) and ferritin (HR: 1.82; 95% CI: 1.25 to 2.63; p = 0.002). Regarding imaging data, RV dysfunction and dilation were each associated with augmented mortality risk (p < 0.05) in univariate analysis. Multivariate regression including both indices demonstrated RV dysfunction (HR: 2.57; 95% CI: 1.49 to 4.43; p = 0.001) and dilation (HR: 1.43; 95% CI: 1.05 to 1.96; p = 0.02) to each be independently associated with risk for death. Consistent with this, Table 4 provides multivariate analyses inclusive of age, biomarkers, and adverse RV remodeling: as shown, echo-quantified adverse RV remodeling (dilation or dysfunction) conferred a >2-fold increase in risk for death, which was significant (p < 0.01) even after controlling for age and biomarker elevations; multivariate regression yielded similar results regarding predictive value of adverse RV remodeling irrespective of whether analyses were performed using troponin, D-dimer, or ferritin. Additionally, multivariate analysis demonstrated adverse RV remodeling to predict mortality (HR: 2.73; 95% CI: 1.72 to 4.35; p < 0.001) independent of need for mechanical ventilation (HR: 1.35; 95% CI: 0.78 to 2.33; p = 0.29). Conversely, patients without adverse RV remodeling (no RV dysfunction or dilation) were more likely to survive to hospital discharge (HR: 1.39; 95% CI: 1.01 to 1.90; p = 0.041).
Table 4.
Predictors of All-Cause Mortality
| Hazard Ratio (95% CI) | p Value | |
|---|---|---|
| Univariable Cox models for all-cause mortality | ||
| Clinical history | ||
| Age (per 10 yrs) | 1.15 (1.02–1.30) | 0.02 |
| Male | 1.21 (0.86–1.69) | 0.28 |
| Hypertension | 0.87 (0.63–1.19) | 0.37 |
| Diabetes mellitus | 0.99 (0.73–1.36) | 0.96 |
| Coronary artery disease | 0.94 (0.64–1.40) | 0.77 |
| Tobacco use | 0.86 (0.59–1.24) | 0.41 |
| Asthma | 0.93 (0.53–1.64) | 0.80 |
| Chronic obstructive pulmonary disease | 0.74 (0.35–1.57) | 0.43 |
| Laboratory markers∗ | ||
| Troponin | 1.30 (1.04–1.63) | 0.02 |
| Ferritin | 1.82 (1.25–2.63) | 0.002 |
| D-dimer | 1.62 (1.20–2.19) | 0.002 |
| C-reactive protein | 1.20 (0.66–2.17) | 0.56 |
| AST | 1.73 (1.30–2.28) | <0.001 |
| ALT | 1.31 (0.97–1.78) | 0.08 |
| WBC | 1.20 (0.66–2.18) | 0.55 |
| Imaging markers | ||
| RV dilation | 1.43 (1.05–1.96) | 0.02 |
| RV dysfunction | 2.57 (1.49–4.43) | 0.001 |
| Adverse RV remodeling† | 2.76 (1.73–4.39) | <0.001 |
| LVEF (per 10%) | 0.95 (0.85–1.06) | 0.33 |
| LVEF <55% | 1.26 (0.92–1.72) | 0.16 |
| LV end-diastolic volume (per 10 ml/m2) | 0.96 (0.89–1.05) | 0.40 |
| LV end-diastolic dilation | 0.80 (0.43–1.48) | 0.47 |
| LV end-systolic volume (per 10 ml/m2) | 0.95 (0.86–1.05) | 0.31 |
| LV end-systolic dilation | 0.93 (0.61–1.40) | 0.71 |
| LV myocardial mass (per 10 g/m2) | 0.96 (0.89–1.04) | 0.29 |
| LV hypertrophy | 0.59 (0.32–1.09) | 0.09 |
| LA volume (per 10 ml/m2) | 0.91 (0.76–1.09) | 0.29 |
| PASP (per 10 mm Hg) | 1.04 (0.88–1.23) | 0.66 |
| Multivariable Cox models for all-cause mortality | ||
| Model 1, chi-square = 40.58 | <0.001 | |
| Age (per 10 yrs) | 1.31 (1.10–1.55) | 0.002 |
| AST | 1.88 (1.35–2.63) | <0.001 |
| Adverse RV remodeling | 2.70 (1.68–4.36) | <0.001 |
| Model 2, chi-square = 28.99 | <0.001 | |
| Age (per 10 yrs) | 1.27 (1.08–1.50) | 0.004 |
| D-dimer | 1.48 (1.01–2.17) | 0.043 |
| Adverse RV remodeling | 2.55 (1.59 – 4.08) | <0.001 |
| Model 3, chi-square = 25.71 | <0.001 | |
| Age (per 10 yrs) | 1.27 (1.07–1.51) | 0.006 |
| Ferritin | 1.49 (0.93–2.41) | 0.10 |
| Adverse RV remodeling | 2.63 (1.63–4.25) | <0.001 |
| Model 4, chi-square = 15.64 | 0.001 | |
| Age (per 10 yrs) | 1.25 (1.04–1.50) | 0.02 |
| Troponin | 1.37 (1.02 -1.84) | 0.03 |
| Adverse RV remodeling | 2.16 (1.25 -3.71) | 0.006 |
Bold p values are statistically significant.
PASP = pulmonary artery systolic pressure; other abbreviations as in Table 3.
Analyses based on log-transformed data.
RV dilation or dysfunction.
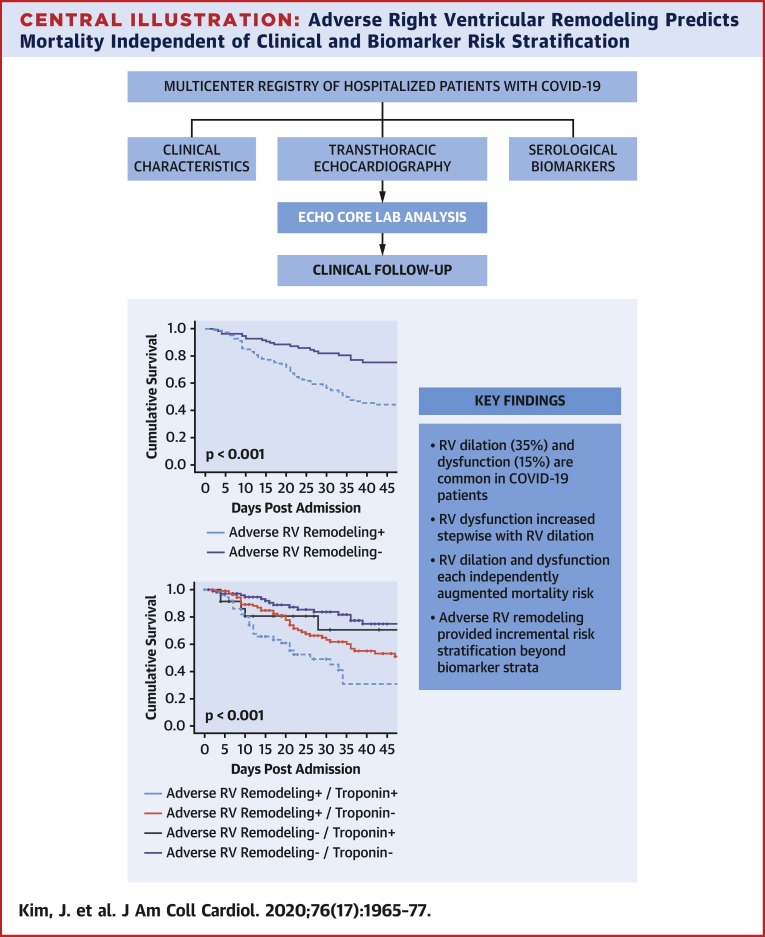
Figure 3A provides Kaplan-Meier survival curves for patients stratified based on RV dysfunction, RV dilation, or adverse RV remodeling (dilation or dysfunction). Results demonstrate prognostic utility for each echo-quantified RV parameter (p < 0.05) in relation to all-cause mortality. Figure 3B demonstrates that RV indices provided additional risk stratification beyond biomarker strata, as evidenced by greatest risk for death among patients with both adverse RV remodeling and positive biomarkers, and lesser risk among patients with isolated biomarker elevations (p ≤ 0.001).
Figure 3.
RV Dysfunction in Relation to Survival
(A) Kaplan-Meier survival analysis for patient groups partitioned based on presence or absence of right ventricular (RV) dysfunction (left), RV dilation (center), and adverse RV remodeling as defined by the composite of dilation or dysfunction (right). As shown, mortality was increased among COVID-19 patients with echo-quantified dilation or dysfunction (all p < 0.05). (B) Kaplan-Meier survival curves inclusive of both biomarker and RV remodeling strata. Note that mortality risk increased stepwise in relation to both biomarker and adverse remodeling strata, as evidenced by highest rates of death among patients with both abnormal biomarkers and adverse RV remodeling (p < 0.001).
Discussion
Our findings yield new insights regarding cardiovascular sequelae of COVID-19, as well as prognostic risk factors for this growing epidemic. Key findings are as follows. First, among a multicenter cohort of 510 hospitalized adults for whom centralized echo analysis was performed to quantify cardiac remodeling, RV dilation and dysfunction were common (35% and 15% respective prevalence). Second, both adverse RV remodeling indices were associated with LV dysfunction, as evidenced by lower LV ejection fraction among patients with RV dysfunction (p < 0.001). Whereas RV dysfunction generally increased in relation to RV size, marked increments in prevalence of dysfunction occurred primarily among patients with advanced RV remodeling, as evidenced by a >2-fold increment among patients in the highest quartile of RV size compared with those in the preceding strata (30% vs. 13%; p = 0.018). Third, both RV dilation and dysfunction were independently associated with increased mortality risk. Conversely, patients without adverse RV remodeling (i.e., no RV dysfunction or dilation) were more likely to achieve hospital discharge (HR: 1.39; 95% CI: 1.01 to 1.90; p = 0.041). In multivariate analysis inclusive of age, biomarkers, and adverse RV remodeling, echo-quantified adverse RV remodeling (dilation or dysfunction) conferred a >2-fold increase in risk for death, which was significant (p < 0.01) even after controlling for age and biomarker elevations—multivariate regression yielded similar results regarding predictive value of adverse RV remodeling irrespective of whether analyses were performed using troponin, D-dimer, or ferritin (Central Illustration ).
Central Illustration.
Adverse Right Ventricular Remodeling Predicts Mortality Independent of Clinical and Biomarker Risk Stratification
(Top) Study design. (Bottom) Kaplan-Meier survival curves inclusive of troponin and right ventricular (RV) remodeling strata; mortality risk increased stepwise in relation to both biomarker and adverse remodeling strata with highest risk of death among patients with adverse RV remodeling and abnormal biomarkers.
Our results extend logically on recent single-center studies that have shown prognostic utility of RV dilation and systolic dysfunction in patients with COVID-19. In an initial study of 120 patients with COVID-19 undergoing echo in Wuhan, China, Li et al. (8) reported impaired RV function to predict mortality independent of sex and clinically diagnosed ARDS physiology. However, one-fifth of otherwise eligible patients were excluded from analysis—including 16% based on poor echo image quality—raising uncertainty as to whether findings from this study reflect data that can be routinely acquired in clinical practice or large-scale epidemiological research. Regarding generalizability, it should also be noted that mortality in this prior study (15%) was lower than that in our cohort (32%)—and that mortality in our study was within the range (24% to 32%) reported among patients of similar age and intensive care unit–level acuity in prior epidemiological studies in the United States (23, 24, 25) and Europe (26,27). In a subsequent U.S. study of 101 patients, Argulian et al. (7) assessed RV size using an equivalent approach to our study and reported RV dilation to predict mortality in patients with COVID-19. Whereas this study supports premise of our study, limited sample size and laboratory data prohibited assessment of whether the predictive utility of adverse RV remodeling dysfunction was additive to that of biomarker derived prognostic markers, which was a focus of the current research.
Regarding mechanism, our observed link between adverse RV remodeling and death may stem from hemodynamic stimuli in which RV dilation is an initially compensatory adaptation to increased RV afterload and/or augmented pulmonary circulatory requirements in context of COVID-mediated hypoxia, but ultimately leads to increased RV wall stress and subsequent dysfunction. Consistent with this notion, our findings demonstrated that RV dysfunction was 2-fold less common than dilation and typically occurred in patients in whom dilation was greatest. More specifically, hypercoagulability and high rates of thrombotic events are known to occur in COVID-19 patients among whom coagulopathy can involve the venous, arterial, and microcirculatory systems (28, 29, 30). Thromboembolism and microthrombi due to COVID-19 infection-related inflammation, hypoxia, and diffuse intravascular coagulation has the potential to augment RV afterload leading to RV dilation and ultimately resulting in RV dysfunction/failure.
It is also possible that our observed association between RV dysfunction and elevated troponin reflects RV myocardial injury stemming from hypoxia or inflammatory myocarditis. Consistent with the latter, myocarditis has been reported in patients with acute COVID-19 infection (31), and it is possible that such pathology could affect both LV and RV myocardium. It is also plausible that COVID-mediated systemic inflammation activates molecular pathways that depress myocardial contractility. For example, prior animal studies have shown inflammatory cytokines impair cardiac systolic function (32,33). Based on this, it is possible that RV dilation and dysfunction occur as sequelae of inflammation and produce deleterious alterations in RV systolic or diastolic performance that are insufficient to meet hemodynamic demands imposed by systemic infection, thus providing a nidus for heart failure, arrhythmia, and death.
Study limitations
First, it is important to recognize that echoes were acquired for clinical purposes, such that critically ill patients may have been less likely to undergo testing. Moreover, given risks of COVID-19 transmission, echoes were lacking quantitative indices in some cases including pulmonary artery systolic pressure and LA volumes in 45% and 55%, respectively, which could have affected the prognostic role of these variables as predictors of mortality. Regarding this, it should be noted that RV chamber size was quantifiable in nearly all (97%) patients in our study and that patients with and without quantifiable RV dysfunction were similar with respect to traditional clinical risk factors, supporting the generalizability of our findings. Second, our analyses were performed using 2-dimensional linear indices as opposed to broader assessments such as 3-dimensional volumetric echo or strain. However, our current data builds on prior validation work by our group showing that echo-derived linear indices of LV and RV chamber geometry correlate with cardiac magnetic resonance imaging–derived 3-dimensional volumetric data (9,34), and that both TAPSE and Sʹ predict clinical outcomes, including impaired effort tolerance (12) and mortality (35). More broadly, given that the echo indices tested in our study can be rapidly acquired and require no specialized software for data analysis, our finding that they strongly impacted clinical prognosis supports the notion that such RV-focused analyses should be incorporated into echo-based triage and risk stratification of patients with known or suspected COVID-19 infection. Finally, whereas our study demonstrated adverse RV remodeling to be linked to LV dysfunction as well as elevated troponin and systemic inflammatory biomarkers, further studies are warranted to test whether the physiological basis of these associations stems from alterations in RV loading conditions or direct cardiotoxic effects on the RV. Related to this, it is important to note that whereas our results demonstrate adverse RV remodeling to strongly predict mortality in critically ill patients with COVID-19, available study data was insufficient to establish the mechanism for RV dilation or dysfunction. For example, given that COVID-19 can induce a prothrombotic milieu, it is possible that RV dilation resulted from augmented afterload and/or hypoxia due to pulmonary emboli. Whereas critical illness and transmission risks prohibited referral for imaging assessment for thromboembolic events and/or pre-existing lung disease (e.g., chronic obstructive pulmonary disease) in a systematic manner, further research is warranted to test these issues as well as whether prognosis in patients with COVID-19 varies based on quantitative methods employed for RV assessment or mechanism of RV dilation or dysfunction.
Conclusions
Findings of this study demonstrate adverse RV remodeling—as assessed by echo-quantified RV dilation or dysfunction—to be a powerful prognostic indicator in patients with COVID-19, for which predictive utility is incremental to routine clinical and/or biomarker-based assessments. Future research is warranted to elucidate inflammatory pathways and myocardial tissue properties responsible for RV dysfunction in patients with acute COVID-19, as well as whether COVID-19 survivors with adverse RV remodeling are at residual risk for adverse clinical outcomes.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Adverse RV remodeling, reflected echocardiographically as RV dilation or dysfunction, is a powerful prognostic indicator in patients with COVID-19, incremental to clinical and biomarker assessments. Patients with both adverse RV remodeling and elevated levels of troponin, ferritin, or D-dimer face the highest risk of mortality.
TRANSLATIONAL OUTLOOK: More research is needed to elucidate the inflammatory pathways and myocardial pathology responsible for RV dysfunction in patients with COVID-19, and determine whether survivors with adverse RV remodeling remain at risk of adverse outcomes.
Acknowledgments
The authors thank the chart abstractors, which included a team of Weill Cornell Medicine medical students and New York-Presbyterian/Weill Cornell Medical Center house-staff, as well as Mangala Rajan for her assistance with data extraction.
Footnotes
This study is supported by the National Institutes of Health (K23HL140092 to Dr. Kim; R01HL128278 to Drs. Kim, Devereux, and Weinsaft), and the Bruce B. Lerman Clinical Scholar Award (to Dr. Kim). The authors have reported that they have no relationships relevant to the contents of this paper to disclose. Tasneem Z. Naqvi, MBBS, served as Guest Associate Editor for this paper. P.K. Shah, MD, served as Guest Editor-in-Chief for this paper.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACCauthor instructions page.
References
- 1.World Health Organization WHO coronavirus disease (COVID-19) dashboard. https://covid19.who.int/?gclid=Cj0KCQjwqfz6BRD8ARIsAIXQCf0wM4jt_ayhOyRj96X872a8w_eoVkX01nP0yoSIXXrPK16ikwmPAGMaAozpEALw_wcB Available at: Accessed September 14, 2020.
- 2.Centers for Disease Control and Prevention Cases in the U.S. https://covid.cdc.gov/covid-data-tracker/#cases_totaldeaths Available at: Accessed September 14, 2020.
- 3.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zheng Y.Y., Ma Y.T., Zhang J.Y., Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020;17:259–260. doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shi S., Qin M., Shen B. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5:802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Argulian E., Sud K., Vogel B. Right ventricular dilation in hospitalized patients with COVID-19 Infection. J Am Coll Cardiol Img. 2020 May 15 doi: 10.1016/j.jcmg.2020.05.010. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Y.L.H., Zhu S., Xie Y. Prognostic value of right ventricular longitudinal strain in patients with COVID-19. J Am Coll Cardiol Img. 2020 Apr 28 doi: 10.1016/j.jcmg.2020.04.014. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim J., Srinivasan A., Seoane T. Echocardiographic linear dimensions for assessment of right ventricular chamber volume as demonstrated by cardiac magnetic resonance. J Am Soc Echocardiogr. 2016;29:861–870. doi: 10.1016/j.echo.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palmieri V., Dahlof B., DeQuattro V. Reliability of echocardiographic assessment of left ventricular structure and function: the PRESERVE study. Prospective Randomized Study Evaluating Regression of Ventricular Enlargement. J Am Coll Cardiol. 1999;34:1625–1632. doi: 10.1016/s0735-1097(99)00396-4. [DOI] [PubMed] [Google Scholar]
- 11.Rudski L.G., Lai W.W., Afilalo J. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23:685–713. doi: 10.1016/j.echo.2010.05.010. quiz 786–8. [DOI] [PubMed] [Google Scholar]
- 12.Kim J., Di Franco A., Seoane T. Right ventricular dysfunction impairs effort tolerance independent of left ventricular function among patients undergoing exercise stress myocardial perfusion imaging. Circ Cardiovasc Imaging. 2016;9 doi: 10.1161/CIRCIMAGING.116.005115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lang R.M., Badano L.P., Mor-Avi V. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39.e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 14.Devereux R.B., Alonso D.R., Lutas E.M. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57:450–458. doi: 10.1016/0002-9149(86)90771-x. [DOI] [PubMed] [Google Scholar]
- 15.Devereux R.B., Reichek N. Echocardiographic determination of left ventricular mass in man. Anatomic validation of the method. Circulation. 1977;55:613–618. doi: 10.1161/01.cir.55.4.613. [DOI] [PubMed] [Google Scholar]
- 16.Devereux R.B., Roman M.J., Palmieri V. Prognostic implications of ejection fraction from linear echocardiographic dimensions: the Strong Heart Study. Am Heart J. 2003;146:527–534. doi: 10.1016/S0002-8703(03)00229-1. [DOI] [PubMed] [Google Scholar]
- 17.Palmieri V., Roman M.J., Bella J.N. Prognostic implications of relations of left ventricular systolic dysfunction with body composition and myocardial energy expenditure: the Strong Heart Study. J Am Soc Echocardiogr. 2008;21:66–71. doi: 10.1016/j.echo.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Devereux R.B., Wachtell K., Gerdts E. Prognostic significance of left ventricular mass change during treatment of hypertension. JAMA. 2004;292:2350–2356. doi: 10.1001/jama.292.19.2350. [DOI] [PubMed] [Google Scholar]
- 19.Lang R.M., Bierig M., Devereux R.B. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 20.Devereux R.B., de Simone G., Arnett D.K. Normal limits in relation to age, body size and gender of two-dimensional echocardiographic aortic root dimensions in persons >/=15 years of age. Am J Cardiol. 2012;110:1189–1194. doi: 10.1016/j.amjcard.2012.05.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zoghbi W.A., Adams D., Bonow R.O. Recommendations for noninvasive evaluation of native valvular regurgitation: a report from the American Society of Echocardiography Developed in Collaboration with the Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr. 2017;30:303–371. doi: 10.1016/j.echo.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 22.Goyal P., Choi J.J., Pinheiro L.C. Clinical characteristics of Covid-19 in New York City. N Engl J Med. 2020;382:2372–2374. doi: 10.1056/NEJMc2010419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cummings M.J., Baldwin M.R., Abrams D. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395:1763–1770. doi: 10.1016/S0140-6736(20)31189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Price-Haywood E.G., Burton J., Fort D., Seoane L. Hospitalization and mortality among black patients and white patients with Covid-19. N Engl J Med. 2020;382:2534–2543. doi: 10.1056/NEJMsa2011686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Richardson S., Hirsch J.S., Narasimhan M. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Docherty A.B., Harrison E.M., Green C.A. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369:m1985. doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grasselli G., Zangrillo A., Zanella A. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323:1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Helms J., Tacquard C., Severac High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46:1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoskins P.P. Supporting colleagues—but how? Okla Nurse. 1988;33:19. [PubMed] [Google Scholar]
- 30.Klok F.A., Kruip M., van der Meer N.J.M. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Doyen D., Moceri P., Ducreux D., Dellamonica J. Myocarditis in a patient with COVID-19: a cause of raised troponin and ECG changes. Lancet. 2020;395:1516. doi: 10.1016/S0140-6736(20)30912-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kubota T., McTiernan C.F., Frye C.S. Dilated cardiomyopathy in transgenic mice with cardiac-specific overexpression of tumor necrosis factor-alpha. Circ Res. 1997;81:627–635. doi: 10.1161/01.res.81.4.627. [DOI] [PubMed] [Google Scholar]
- 33.Van Tassell B.W., Seropian I.M., Toldo S., Mezzaroma E., Abbate A. Interleukin-1beta induces a reversible cardiomyopathy in the mouse. Inflamm Res. 2013;62:637–640. doi: 10.1007/s00011-013-0625-0. [DOI] [PubMed] [Google Scholar]
- 34.Dele-Michael A.O., Fujikura K., Devereux R.B. Left ventricular stroke volume quantification by contrast echocardiography - comparison of linear and flow-based methods to cardiac magnetic resonance. Echocardiography. 2013;30:880–888. doi: 10.1111/echo.12155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim J., Krichevsky S., Xie L. Incremental utility of right ventricular dysfunction in patients with myeloproliferative neoplasm-associated pulmonary hypertension. J Am Soc Echocardiogr. 2019;32:1574–1585. doi: 10.1016/j.echo.2019.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thygesen K., Alpert J.S., Jaffe A.S. Fourth universal definition of myocardial infarction (2018) J Am Coll Cardiol. 2018;72:2231–2264. doi: 10.1016/j.jacc.2018.08.1038. [DOI] [PubMed] [Google Scholar]