Background:
Prosthetic reconstruction in previously irradiated breasts has been associated with a higher risk of complications. Here we describe the surgical and cosmetic outcome of our breast reconstruction process based on primary fat grafting combined with prosthetic placement.
Methods:
In this multicenter retrospective study, 136 patients who underwent mastectomy and external chest wall radiotherapy between 2014 and 2018 were benefited from chest wall lipofilling and silicone implant placement were chosen. Patients were assessed for skin trophicity, thickness, and mobility and were allowed to undergo several lipofilling sessions before implant placement, if required. No patient had >3 lipofilling sessions. Cosmetic outcome was evaluated by the patient, surgeon, and nurse, using a Likert-type ordinal scale.
Results:
We included 136 patients: 79 patients (58%) received only 1 session of lipofilling before implant placement, 33 (24.6%) had 2 sessions, and 24 (17.4%) had 3 sessions. The volume of the third lipofilling was significantly higher and the volume of the prosthesis of these patients was significantly lower than those of patients undergoing 1 or 2 lipofillings. Reconstruction failure rate was 2.2% (3 patients had explantation); however, all benefited from prosthesis reconstruction a year after the initial procedures. The average satisfaction score was 4.7 out of 5 as evaluated by patients, 4.8 out of 5 by surgeons, and 4.8 out of 5 by nurses.
Conclusions:
Primary lipofilling combined with prosthesis placement after radiotherapy is a reconstructive method that yields a satisfactory cosmetic outcome with a low complication rate. Such minimally invasive breast reconstruction approach can be an alternative to flap-based reconstruction.
INTRODUCTION
Delayed breast reconstruction after total mastectomy and radiotherapy is essentially based on a free or pedicled autologous flap.
When autologous flap-based reconstruction is contraindicated, or declined by patients refusing additional scars or invasive surgeries, a prosthetic-based reconstruction could represent an alternative. Unfortunately, prosthetic breast reconstruction after radiation is associated with a high rate of complications and usually results in poor cosmetic outcomes compared with patients without radiation (relative risk, 2.58).1–3
Since the work by Coleman4 in the early 1990s, indications of autologous fat transfer (AFT) have considerably grown in the management of cosmetic sequelae after breast cancer surgery and particularly in the context of secondary breast reconstructions.5–8 Prepectoral AFT before prosthesis placement has been associated to increased skin trophicity and vascularization and improved cosmetic results. AFT allows to fill defect areas, improves outlines and overall shape, and leads to increased skin flexibility.8 The first evaluations of such approach show a significant improvement in the outcome of prosthetic reconstruction with reduced complications.9,10
In a recent meta-analysis of 21 studies analyzing 1,011 breast reconstructions in 834 patients, 2.84–4.66 sessions were required to complete reconstruction.11
The number of fat grafting sessions to complete breast reconstruction was significantly higher for irradiated compared with nonirradiated patients (4.27 versus 2.84; P < 0.05). The complication rate was mainly related to radiation therapy with 5.4% in the irradiated group compared with 1.1% in the nonirradiated group (considering only necrosis and ulceration). This was a significant shift from the high rate of complications associated to prosthetic reconstruction without lipofilling in irradiated patients. Such an approach associating fat grafting to prosthetic reconstruction could, thus, be an alternative to flap-based reconstruction if outcomes were confirmed.
Here we report our experience of 136 irradiated patients who underwent primary lipofilling associated to prosthetic reconstruction. We evaluated the morbidity of the surgical procedure and the cosmetic results.
PATIENTS AND METHODS
Patients and Procedures
We conducted a multicenter retrospective study evaluating our routine clinical practice between 2014 and 2018. The participating centers were the Nord Artois - Haut de France Breast Institute, the Nice Breast Institute, and the Centre du Sein Paris. The study was reviewed by Institutional Review Board at the Weill Cornell Medicine in Qatar (IRB19-00155). We only included patients who underwent modified radical mastectomy and external chest wall radiotherapy. All reconstructive procedures were discussed and validated in a multidisciplinary tumor board before surgery, and our procedures were performed as routine clinical care. The type of reconstruction was chosen in accordance with patient’s choice and surgeon’s assessment of skin condition (flexibility, prethoracic thickness, trophicity, range of motion of the upper limb). A common surgical approach was elaborated by the different surgeons of this study. During the initial period, all surgeons worked for few cases together to establish a robust process, including: (1) patient preparation, (2) fat harvesting, (3) lipofilling, and (4) prosthesis placement. The surgeons spent also several other surgical sessions together during the course of the study to ensure consistent uniformity. Finally, the surgeons also participated together to follow up evaluations to ensure also uniformity in patients’ evaluation. All procedures were carried as described in the following sections:
First Step: Chest Wall Lipofilling
The first step of the reconstruction consisted of a chest wall lipofilling, carried out in an outpatient setting and at least 3 months after the end of radiotherapy. The fat was retrieved using a 3-mm liposuction cannula, connected to a 600-ml Redon vial (FMM, Nice, France), connected to a liposuction device (Liposurg, Nouvag, France). The fat was then centrifuged 30 seconds at 3,000 revolutions per minute and injected through multiple passages in different planes. Injections were performed in a radially and retrograde way using a 10-mm syringe and a 1.6-mm cannula. Percutaneous rigotomy maneuvers were also performed when required to remove adhesions between the skin and the deeper plane. The volume of fat injected was distributed as close as possible to the natural shape of the patients’ breast; particular attention was given to the definition of the inframammary fold, as we never used abdominal flap or stitches (Fig. 1). Patients received cefazolin antibioprophylaxis at the beginning of the surgery.
Fig. 1.
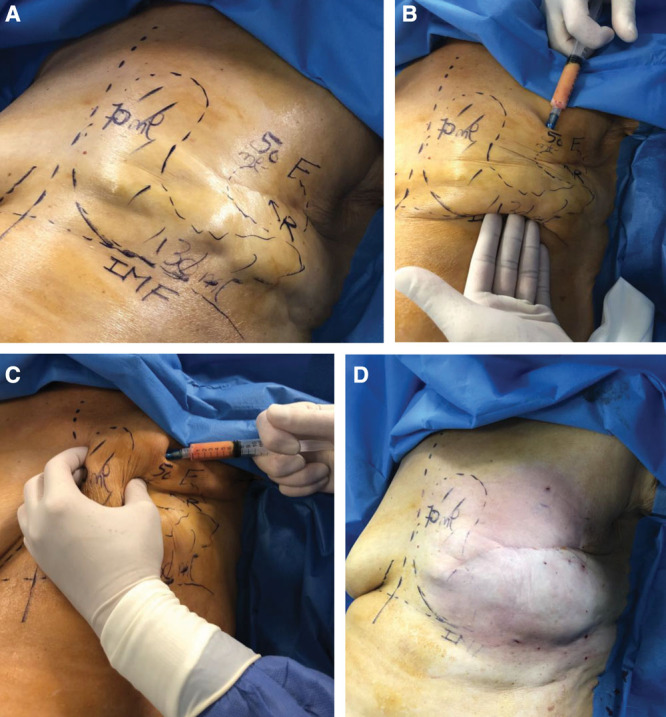
Lipofilling strategy for the first session in a patient 6 months after the end of radiotherapy. A, Different zones and planned injected volumes. B and C, Definition of the breast contour and IMF. D, Final immediate postoperative results. F indicates fibrosis; IMF, inframammary fold; numbers, volume to be injected; R, retraction.
All patients were reviewed 4 weeks and 3 months after lipofilling to evaluate the skin condition (thickness, flexibility, and laxity), and the surgeon decided on 1 of the following 2 options:
Further lipofilling procedure if the chest wall was not thick and mobile enough for a breast implant.
Implantation of a silicone prosthesis if the lipofilling resulted in optimal skin trophicity, thickness, and mobility (Fig. 2).
Fig. 2.
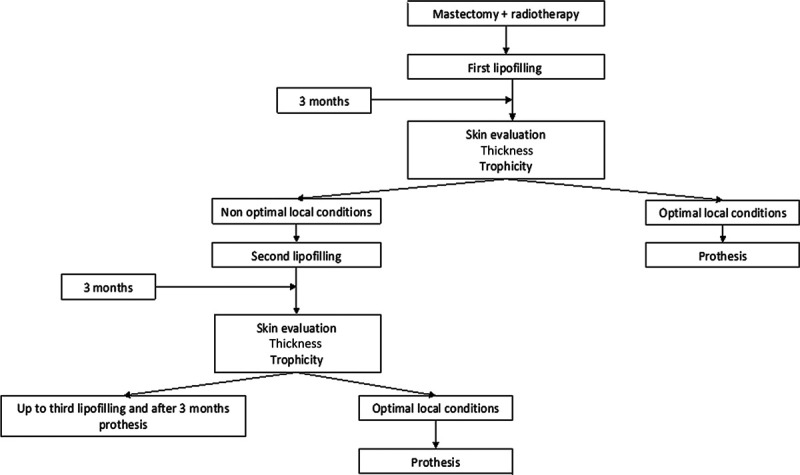
Surgical strategy.
Second Step: Implant Placement
Patients were assessed for skin trophicity, thickness, and mobility and were allowed to undergo up to 3 lipofilling sessions before implant placement, if required.
Round or anatomical implants were chosen based on the width, height, and projection of the breast to be reconstructed. The incision was systemically performed in the mastectomy scar at its external part, thus avoiding new scars. Prepectoral dissection was carried out 1 cm under the horizontal tangent passing through the inframammary fold of the contralateral breast.
For a few of the first patients in our study, a tissue expander was used. In these cases, the expander was only filled in up to 150 ml during the surgery. The patients underwent subsequent filling (50 ml per sessions) every 2 weeks up to the appropriate volume. They subsequently underwent a replacement of the expander by a permanent prosthesis concomitantly to another lipofilling session. There were no objective criteria to choose. We used permanent prosthesis when skin elasticity was optimal and allowed direct placement of the prosthesis under the grafted fat layer. Many patients in this study underwent skin therapy mobilization manually or using an endermology machine (LPG, Sophia Antipolis, France) that further improved skin elasticity, allowing us to avoid expander or tissue stretching.
No drain was placed during the surgery. Postoperative care consisted of classical dressings of the scars. Patients were assessed by a nurse every day for 1 week. Patients wore a compression garment at liposuction areas for 15 days. The symmetrization mammoplasty was performed when required.
The cosmetic results were evaluated by patients, surgeons, and nurses, using a Likert-type ordinal scale, ranging from 1 (very disappointed) to 5 (very satisfied).
Statistical Analysis
All quantitative data were expressed as mean ± standard error of the mean. Statistical analysis was performed by using SigmaPlot 11 (Systat Software Inc., Chicago, IL). A Shapiro–Wilk normality test, with a P = 0.05 rejection value, was used to test normal distribution of data before further analysis. All pairwise multiple comparisons were performed by 1-way analysis of variance followed by Holm–Sidak post hoc tests for data with normal distribution or by Kruskal–Wallis analysis of variance on ranks followed by Tukey post hoc tests, in case of failed normality test. Paired comparisons were performed by Student’s t tests or by Mann–Whitney rank sum tests in case of unequal variance or failed normality test. Statistical significance was accepted for P value <0.05.
RESULTS
Between January 2014 and December 2018, 136 patients underwent combined lipofilling and prosthesis-based reconstruction. Demographic characteristics of the patients were concordant with previously described cohorts in the literature (Table 1). The average age of the patients was 52.5 years (33–75 years). The mean body mass index was 23.6 (22–30; ±9.6). The average time between the end of radiation therapy and the first lipofilling session was 19.6 months (3–60 months). Computed tomography scan performed after lipofilling sessions demonstrated vascularized viable fat tissue (Fig. 3).
Table 1.
Patients Characteristics and Their Influence on Outcome
| Parameters | Values |
|---|---|
| Age, y | 52.5 (33–75) |
| Mastectomy indication | |
| Multifocality | 89 |
| Recurrence | 26 |
| Tumor size | 21 |
| Sessions of lipofilling before prosthesis | |
| 1 lipofilling | 79 |
| 2 lipofillings | 33 |
| 3 lipofillings | 24 |
| BMI | 23.6 (22–30) |
| 1 lipofilling | 23.2 (22–28) |
| 2 lipofillings | 23.7 (23–29) |
| 3 lipofillings | 24.1 (23–30) |
| Smoking | n = 30 |
| 1 lipofilling | 19 |
| 2 lipofillings | 8 |
| 3 lipofillings | 3 |
| Timing (mo) | |
| Follow-up | 32.4 (6–72) |
| Radiotherapy–lipofillings | 20.8 (3–60) |
| Lipofilling 1–lipofilling 2 | 3 (2–6) |
| Lipofilling 2–prosthesis | 3.4 (1–8) |
| Lipofilling 3–prosthesis | 3.4 (1–8) |
| Prosthesis used | |
| Anatomical shape | 18 |
| Round shape | 118 |
BMI, body mass index.
Fig. 3.
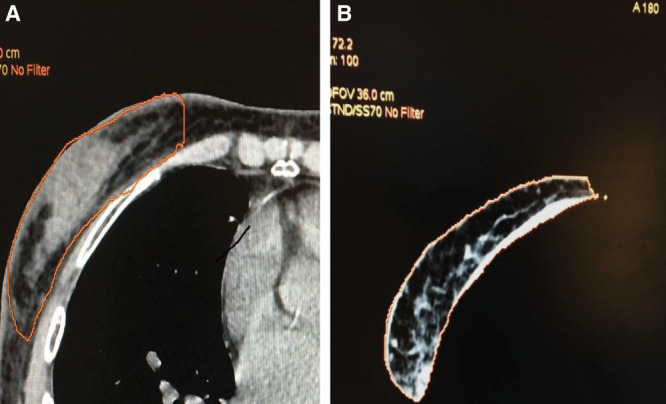
CT scan evaluation of the first lipofilling 3 months after the procedures demonstrating fat tissue (A) displaying functional vascularization (B). CT indicates computed tomography.
Twenty-three patients had an early reconstruction with the procedure initiated between 3 and 6 months. There was no correlation between the delay before the first lipofilling and the number of lipofilling sessions, volume of prosthesis, or complication rate.
The average delay between the lipofilling sessions was 3 months (2–6 months). The average delay between the final lipofilling and prosthesis placement was 3.4 months (1–8 months).
The average volume of injected fat during the first lipofilling was 210.7 ml (50–310 ml; ±78.6) (Fig. 4A). Seventy-nine patients (58%) underwent prosthesis placement after the first session of lipofilling. Thirty-three patients (24.6%) had a second lipofilling procedure before prosthesis implantation. The average volume of injected fat during the second lipofilling session was 207 ml (100–270 ml; ±66.5). Twenty-four patients (17.4%) had 3 lipofillings before breast implant placement. The average volume in the third lipofilling was 270 ml (150–350; ±48.9). The volume of the third lipofilling was significantly higher than the first and second lipofilling (P < 0.05). Patients requiring a second or a third lipofilling usually had a higher volume of fat injected in the first or second session, respectively; however, this did not reach statistical significance.
Fig. 4.
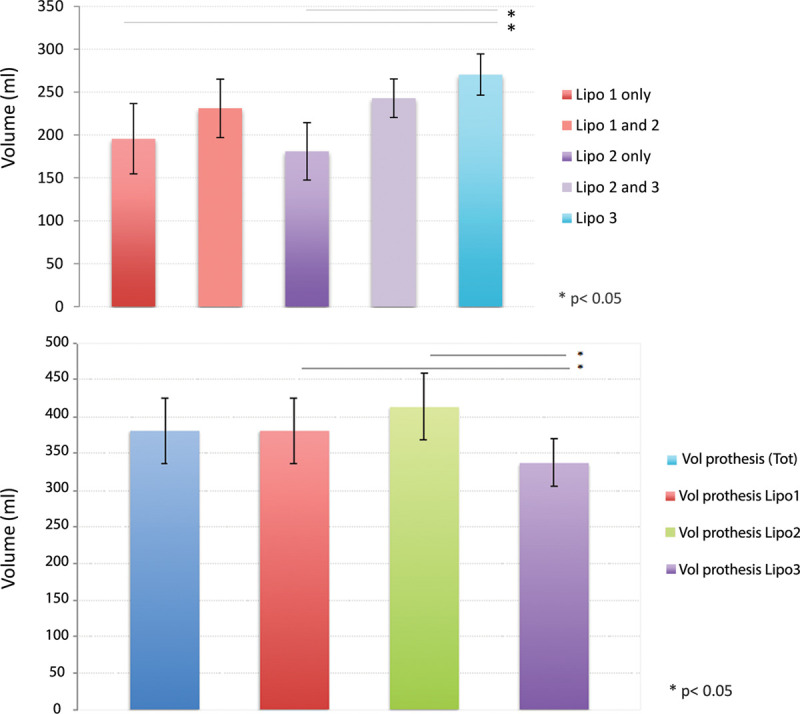
Volume of fat injected at different sessions and volume of prosthesis implanted. A, Volume of fat injected during the different lipofilling sessions. We separated the volume of fat injected for patients undergoing only 1 or 2 lipofillings from the one injected in patients undergoing subsequent sessions of lipofilling. B, Volume of prosthesis for the different lipofilling groups. Error bars are SD. Lipo indicates number of lipofilling session.
Seven patients developed a cystic seroma; however, prosthesis placement could easily be performed after excision of the cyst. One patient developed a minor pneumothorax and required 24 hours hospital stay under intravenous analgesia and was subsequently discharged. She had a follow-up to resolution of chest x-ray.
Twenty-eight patients required an expander before final prosthesis placement mostly during the first year of our experience. We used expanders in 19 patients undergoing a single lipofilling (34.5%), in 9 patients with 2 lipofilling (15.7%), and in none of patients undergoing 3 lipofillings.
The average volume of the prosthesis was 380 ml (150–620; ±91.2) (Fig. 4B).
The mean volume of prosthesis used was 381 ml (195–525; ±92.7) for 1 lipofilling, 413 ml (290–620; ±92.5) for 2 lipofillings, and 336 ml (150–490; ±65.2) for 3 lipofillings.
Patient undergoing 3 lipofillings had a significantly lower volume of prosthesis compared to 1 or 2 lipofillings (Fig. 3B). There were no significant differences in the final cup size between the 3 groups.
After prosthesis placement, we encountered 1 case of immediate postoperative infection and 2 cases of cutaneous necrosis with scar disunity. In these 3 cases, the implant had to be explanted, resulting in an exposure rate of 2.2%. All underwent final prosthesis insertion a year after the initial procedures without further lipofilling.
The average duration of surgery for the lipofilling sessions was 48 minutes (34–56 minutes; ±12.7). The average duration of surgery for the prosthesis placement was 53 minutes (45–70 minutes; ±21.3).
The cosmetic result of the surgical procedures was evaluated in all patients who had a final prosthetic reconstruction (Table 2). The average satisfaction score was 4.7 out of 5 as evaluated by patients, 4.8 out of 5 by surgeons, and 4.8 out of 5 by nurses (Likert scale). Unsatisfactory results were due to capsular contraction (or lack of symmetry as displayed in Fig. 5). The initial cosmetic results appeared to be stable over time in our study with a follow-up of 22.7 months (8–45). Eleven percentage of patients developed a capsular contracture with no difference in distribution in the different groups (number, volume of lipofillings, and the use of expander). Twelve patients (9%) required subsequent surgeries during the follow-up period. Three underwent another session of lipofilling to improve overall breast shape after prosthesis placement. Nine patients required prosthesis replacement to achieve a higher cup size.
Table 2.
Evaluation of Cosmetic Results
| Cosmetic Results | Very Disappointed | Disappointed | Middle Satisfied | Satisfied | Very Satisfied |
|---|---|---|---|---|---|
| N = 136 | |||||
| Patient | 5 | 0 | 5 | 13 | 113 |
| Nurse | 2 | 2 | 4 | 13 | 115 |
| Surgeon | 3 | 2 | 5 | 16 | 110 |
Fig. 5.
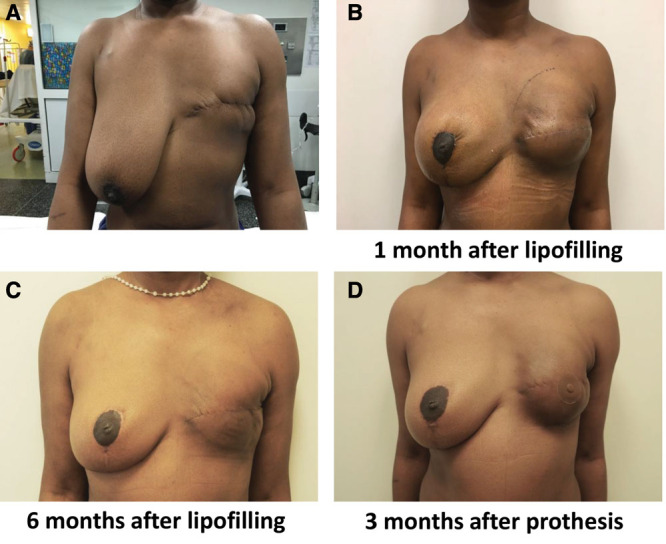
Patient with unsatisfactory results. A, T0 before procedure. B, One month after lipofilling. C, Six months after the first lipofilling. D, Three months after prosthesis placement.
DISCUSSION
Here we report the results of 136 combined lipofilling and prosthetic reconstruction in patients with mastectomy and radiation therapy. Our study demonstrates a low complication rate. Our reconstruction failure rate was 2.2%; however, all the patients have finally benefited from prosthetic reconstruction. The procedures in all cases except one were performed in an outpatient setting and were associated with a high rate of patients’ satisfaction.
The association of lipofilling and prosthesis placement could be considered as a minimally invasive breast reconstruction. The lipofilling sessions are scarless and can be carried out in an outpatient setting; the prosthetic placement is performed through the mastectomy incision and is also performed in an outpatient setting. Such minimally invasive breast reconstruction protocol allowed reconstruction of small and large breast volume as displayed in Figure 6.
Fig. 6.
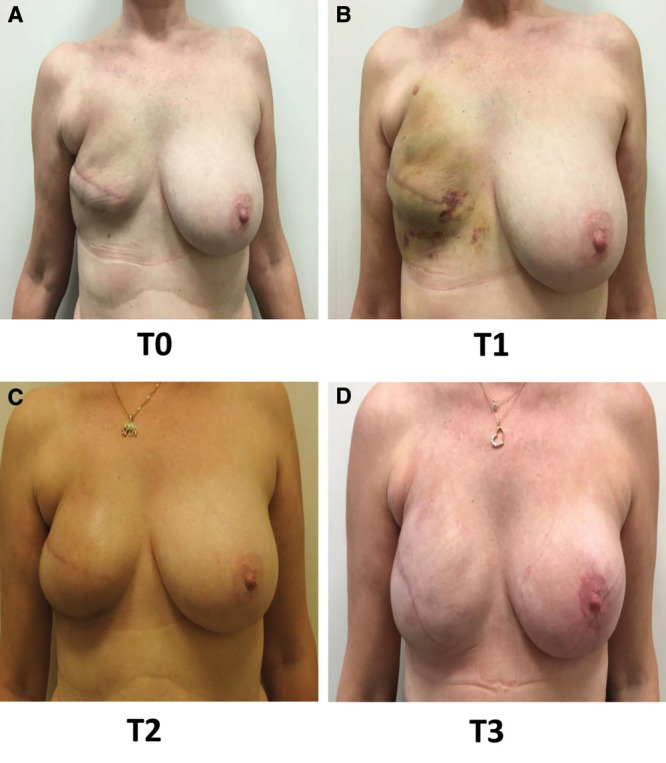
Typical lipofilling sequences. A, T0: 3 weeks after first lipofilling. B, T1: 1 month after first lipofilling. C, T2: 3 months after second lipofilling. D, T3: 3 months after prosthesis placement and lipofilling.
The main concerns of secondary breast implant reconstruction are high early and long-term failure rate due to previous radiation therapy inducing either prosthesis rejection and lack of scarring (acute reaction) or capsular contraction (late reaction). However, the complication rate seems to be significantly reduced by skin preparation using fat injection. Several studies have now reported the use and outcome of autologous fat transplantation; patients who had radiation therapy require more lipofilling sessions.9,12–14 The complication rate of lipofilling-based protocol seems to be quite low across the different studies, in particular regarding reconstruction failure. Recently, a study by Bennett et al15 showed a significantly higher rate of complications at 2 years for any flap-based reconstruction compared to prosthesis or expander-based reconstruction. Prosthesis-based reconstruction alone were associated to higher rate of failure, hence the benefit of preparing the prosthesis placement by AFT.2,16
Concordantly with previous studies, AFT significantly improved the reconstructive outcome. AFT enables the prosthesis-based reconstruction of patients with major skin trophicity issues such as fibrosis, retractions, and adherences as displayed in Figure 7. The trophic effect of AFT is clinically obvious, and several studies have demonstrated improved skin status after AFT with a reduction of the skin damage grade.
Fig. 7.
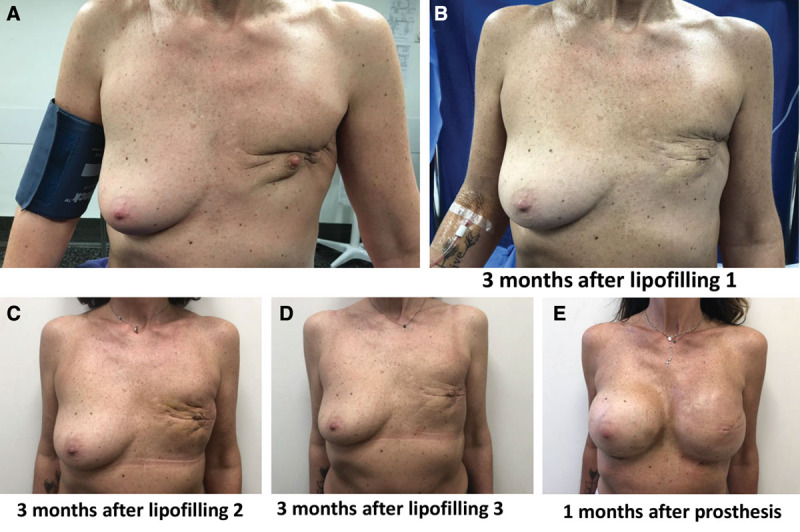
Lipofilling process in a difficult case with skin adherence and retraction. A, T0: Before reconstructive surgery. B, T1: 3 months after first lipofilling. C, T2: 3 months after second lipofilling. D, T3: 3 months after third lipofilling. E, T4: 1 month after prosthesis placement.
Patients (95.5%) rated the reconstruction as very satisfactory or satisfactory. This is higher than the 80% rate reported by Sarfati et al,9 most probably due to technical improvement during the last few years. Because this was a pilot study, we unfortunately used a Likert scale that might lack granularity to precisely characterize cosmetic outcome, and our results should be confirmed in a subsequent study using more appropriate scales such as F-36, EORTC-Br23, or Breast Q v2.
The optimal results of prosthesis-based reconstruction associated to AFT can be explained by the described improvement of skin vascularization and trophicity17 due to a complex interplay between cell graft and irradiated skin. The fat grafted is composed of different cell types beside adipocytes such as endothelial progenitor cells, mesenchymal stem cells, adipocytes, and adipocytes-derived stem cells. Together, they all secrete multiple growth factors and increase vessel density, resulting in an increase of the subcutaneous tissue thickness and an improvement in cutaneous trophicity.18
CONCLUSIONS
The low complication rate and the good cosmetic results of lipofilling-based breast reconstruction concordant with the literature advocate for AFT when a prosthetic reconstruction is considered in secondary reconstruction on irradiated skin. Our study concordant with others suggests that such minimally invasive breast reconstruction approach could be a feasible alternative to flap-based reconstruction.
Footnotes
Published online 26 May 2020.
Disclosure: The authors have no financial interest to declare in relation to the content of this article.
REFERENCES
- 1.Kelley BP, Ahmed R, Kidwell KM, et al. A systematic review of morbidity associated with autologous breast reconstruction before and after exposure to radiotherapy: are current practices ideal? Ann Surg Oncol. 2014;21:1732–1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kronowitz SJ, Robb GL.Radiation therapy and breast reconstruction: a critical review of the literature. Plast Reconstr Surg. 2009;124:395–408. [DOI] [PubMed] [Google Scholar]
- 3.Lee KT, Mun GH.Prosthetic breast reconstruction in previously irradiated breasts: a meta-analysis. J Surg Oncol. 2015;112:468–475. [DOI] [PubMed] [Google Scholar]
- 4.Coleman SR.Long-term survival of fat transplants: controlled demonstrations. Aesthetic Plast Surg. 1995;19:421–425. [DOI] [PubMed] [Google Scholar]
- 5.Groen JW, Negenborn VL, Twisk DJWR, et al. Autologous fat grafting in onco-plastic breast reconstruction: a systematic review on oncological and radiological safety, complications, volume retention and patient/surgeon satisfaction. J Plast Reconstr Aesthetic Surg JPRAS. 2016;69:742–764. [DOI] [PubMed] [Google Scholar]
- 6.Petit JY, Lohsiriwat V, Clough KB, et al. The oncologic outcome and immediate surgical complications of lipofilling in breast cancer patients: a multicenter study–Milan-Paris-Lyon experience of 646 lipofilling procedures. Plast Reconstr Surg. 2011;128:341–346. [DOI] [PubMed] [Google Scholar]
- 7.Silva-Vergara C, Fontdevila J, Weshahy O, et al. Breast cancer recurrence is not increased with lipofilling reconstruction: a case-controlled study. Ann Plast Surg. 2017;79:243–248. [DOI] [PubMed] [Google Scholar]
- 8.de Blacam C, Momoh AO, Colakoglu S, et al. Evaluation of clinical outcomes and aesthetic results after autologous fat grafting for contour deformities of the reconstructed breast. Plast Reconstr Surg. 2011;128:411e–418e. [DOI] [PubMed] [Google Scholar]
- 9.Sarfati I, Ihrai T, Duvernay A, et al. Autologous fat grafting to the postmastectomy irradiated chest wall prior to breast implant reconstruction: a series of 68 patients. Ann Chir Plast Esthet. 2013;58:35–40. [DOI] [PubMed] [Google Scholar]
- 10.Ribuffo D, Atzeni M, Guerra M, et al. Treatment of irradiated expanders: protective lipofilling allows immediate prosthetic breast reconstruction in the setting of postoperative radiotherapy. Aesthetic Plast Surg. 2013;37:1146–1152. [DOI] [PubMed] [Google Scholar]
- 11.Herly M, Ørholt M, Larsen A, et al. Efficacy of breast reconstruction with fat grafting: a systematic review and meta-analysis. J Plast Reconstr Aesthet Surg. 2018;71:1740–1750. [DOI] [PubMed] [Google Scholar]
- 12.Salgarello M, Visconti G, Farallo E.Autologous fat graft in radiated tissue prior to alloplastic reconstruction of the breast: report of two cases. Aesthetic Plast Surg. 2010;34:5–10. [DOI] [PubMed] [Google Scholar]
- 13.Serra-Renom JM, Muñoz-Olmo JL, Serra-Mestre JM.Fat grafting in postmastectomy breast reconstruction with expanders and prostheses in patients who have received radiotherapy: formation of new subcutaneous tissue. Plast Reconstr Surg. 2010;125:12–18. [DOI] [PubMed] [Google Scholar]
- 14.Irani Y, Casanova D, Amar E.Autologous fat grafting in radiated tissue prior to breast prosthetic reconstruction: is the technique reliable?. Ann Chir Plast Esthet. 2012;57:59–66. [DOI] [PubMed] [Google Scholar]
- 15.Bennett KG, Qi J, Kim HM, et al. Comparison of 2-year complication rates among common techniques for postmastectomy breast reconstruction. JAMA Surg. 2018;153:901–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clough KB, O’Donoghue JM, Fitoussi AD, et al. Prospective evaluation of late cosmetic results following breast reconstruction: I. Implant reconstruction. Plast Reconstr Surg. 2001;107:1702–1709. [DOI] [PubMed] [Google Scholar]
- 17.Mojallal A, Lequeux C, Shipkov C, et al. Improvement of skin quality after fat grafting: clinical observation and an animal study. Plast Reconstr Surg. 2009;124:765–774. [DOI] [PubMed] [Google Scholar]
- 18.Rigotti G, Marchi A, Galiè M, et al. Clinical treatment of radiotherapy tissue damage by lipoaspirate transplant: a healing process mediated by adipose-derived adult stem cells. Plast Reconstr Surg. 2007;119:1409–1422; discussion 1423. [DOI] [PubMed] [Google Scholar]


