Abstract
Aim
Patients with type 2 diabetes mellitus (T2DM) represent a high-risk population for both cardiovascular diseases and severe coronavirus disease 2019 (COVID-19). Recent studies have reported interactions between statin treatment and COVID-19-related outcomes. The study reported here specifically assessed the association between routine statin use and COVID-19-related outcomes in inpatients with T2DM.
Methods
The Coronavirus–SARS-CoV-2 and Diabetes Outcomes (CORONADO) study was a nationwide observational study aiming to describe the phenotypic characteristics and prognosis of T2DM patients with COVID-19 admitted to 68 French hospitals between 10 March and 10 April 2020. The composite primary outcome comprised tracheal intubation and/or death within 7 and 28 days of admission. The association between statin use and outcomes was estimated by logistic regression analysis after applying inverse probability of treatment weighting (IPTW) using a propensity score-weighting approach.
Results
Of the 2449 patients with T2DM (881 women, 1568 men; aged 70.9 ± 12.5 years) suitable for analysis, 1192 (49%) were using statin treatment before admission. In unadjusted analyses, patients using statins had rates of the primary outcome similar to those of non-users within both 7 (29.8% vs 27.0%, respectively; P = 0.1338) and 28 days (36.2% vs 33.8%, respectively; P = 0.2191) of admission. However, mortality rates were significantly higher in statin users within 7 (12.8% vs 9.8%, respectively; P = 0.02) and 28 days (23.9% vs 18.2%, respectively; P < 0.001). After applying IPTW, significant associations were observed with statin use and the primary outcome within 7 days (OR [95% CI]: 1.38 [1.04–1.83]) and with death within both 7 (OR [95% CI]: 1.74 [1.13–2.65]) and 28 days (OR [95% CI]: 1.46 [1.08–1.95]).
Conclusion
Routine statin treatment is significantly associated with increased mortality in T2DM patients hospitalized for COVID-19.
Keywords: COVID-19, Mortality, Outcomes, Statins, Type 2 diabetes mellitus
Introduction
Cardiovascular risk factors such as arterial hypertension, dyslipidaemia, diabetes mellitus and obesity are highly prevalent among patients with coronavirus disease 2019 (COVID-19) [1], [2]. Since the beginning of the COVID-19 pandemic, one important issue has been the impact of certain cardiovascular therapies, such as renin–angiotensin–aldosterone system (RAAS) blockers, on the prognosis for COVID-19 [3], [4]. Statins are the most widely used lipid-lowering drugs and are often recommended for patients at high cardiovascular risk, but recent studies have highlighted an interaction between the use of statins and COVID-19-related outcomes [5], [6]. While in-hospital use of statins has been reported to be associated with a lower risk of mortality in patients with COVID-19 [5], no specific data are as yet available for patients with type 2 diabetes mellitus (T2DM), a high-risk population for both cardiovascular disease and COVID-19-related outcomes.
For this reason, the present study aimed to assess the association between routine statin use and COVID-19-related outcomes in patients with T2DM participating in the Coronavirus–Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) and Diabetes Outcomes (CORONADO) study [7].
Methods
The CORONADO (ClinicalTrials.gov: NCT04324736) nationwide observational study aimed to describe the phenotypic characteristics and prognosis of patients with diabetes admitted with COVID-19 between 10 March and 10 April 2020 at 68 French hospitals. The protocol received all the necessary regulatory approvals, and the study design has been previously reported elsewhere [7]. In brief, inclusion criteria were: (i) hospitalization for biologically and/or clinically/radiologically confirmed COVID-19; and (ii) a personal history of diabetes or newly diagnosed diabetes on admission. The composite primary outcome combined tracheal intubation for mechanical ventilation and/or death within 7 days of admission, with an additional follow-up for up to 28 days after admission. Given the specific purposes of the present study, our analysis was restricted to only CORONADO participants with T2DM and with available information on routine statin use. Use of routine medications was identified by noting prescription drugs on admission and upon examination, with possible further questioning by general practitioners (GPs) or pharmacists when deemed necessary.
To control for confounding factors that might influence both statin use and outcomes, a propensity score (PS) was computed. This PS was the result of a logistic regression model combining variables selected on the basis of their relevance in clinical practice and/or statistical relevance (P < 0.10 in association with the primary outcome). Stabilized weighting was computed for patients based on the PS and were used in logistic regression models as the inverse probability of treatment weighting (IPTW) [8]. Covariate balance before and after weighting was assessed by the standardized mean difference approach. The threshold for statistical significance was set at 0.05. All statistical tests were two-sided and performed using R software, version 3.6.2 (R Core Team, R Foundation for Statistical Computing, Vienna, Austria; https://cran.r-project.org/bin/windows/base/old/3.6.2/), with the PSW package for propensity score analyses [9].
Results
Out of the entire CORONADO study population, 2449 patients with both T2DM and COVID-19 [94.6% with positive SARS-CoV-2 polymerase chain reaction (PCR) tests] were included in our present study. Of these patients, 1192 (48.7%) had been treated with statins before hospitalization, and 1257 (51.3%) were not. Their baseline characteristics prior to admission according to routine use of statins are presented in Table S1 (see supplementary materials associated with this article online). Compared with statin non-users, patients taking statins were slightly older (aged 71.7 vs 70.2 years, respectively; P = 0.0014), more frequently male (67.8% vs 60.5%, respectively; P = 0.0002) and more often also affected by comorbidities, respectively including hypertension (86.6% vs 74.1%, P < 0.0001), macrovascular (53.8% vs 26.6%, P < 0.0001) and microvascular (49.5% vs 41.1%, P = 0.0005) diabetes complications, heart failure (14.2% vs 9.9%, P = 0.0014) and treated obstructive sleep apnoea (OSA) (14.5% vs 8.1%, P < 0.0001). Clinical and biological characteristics of these participants on admission are presented in Tables S1 and S2 (see supplementary materials associated with this article online). In brief, estimated glomerular filtration rate (eGFR) was slightly lower in statin users vs non-users (66.2 vs 70.0 mL/min, respectively; P = 0.02). On the other hand, long-term glycaemic control (HbA1c: 8.0% vs 8.2%, P = 0.16), admission plasma glucose concentrations (165 vs 173 mg/dL, P = 0.09) and inflammatory biological parameters on admission (C-reactive protein: 80 vs 89 mg/L, P = 0.29; lymphocyte count: 995 vs 980 103/mm3, P = 0.62) did not differ between statin users and non-users, respectively.
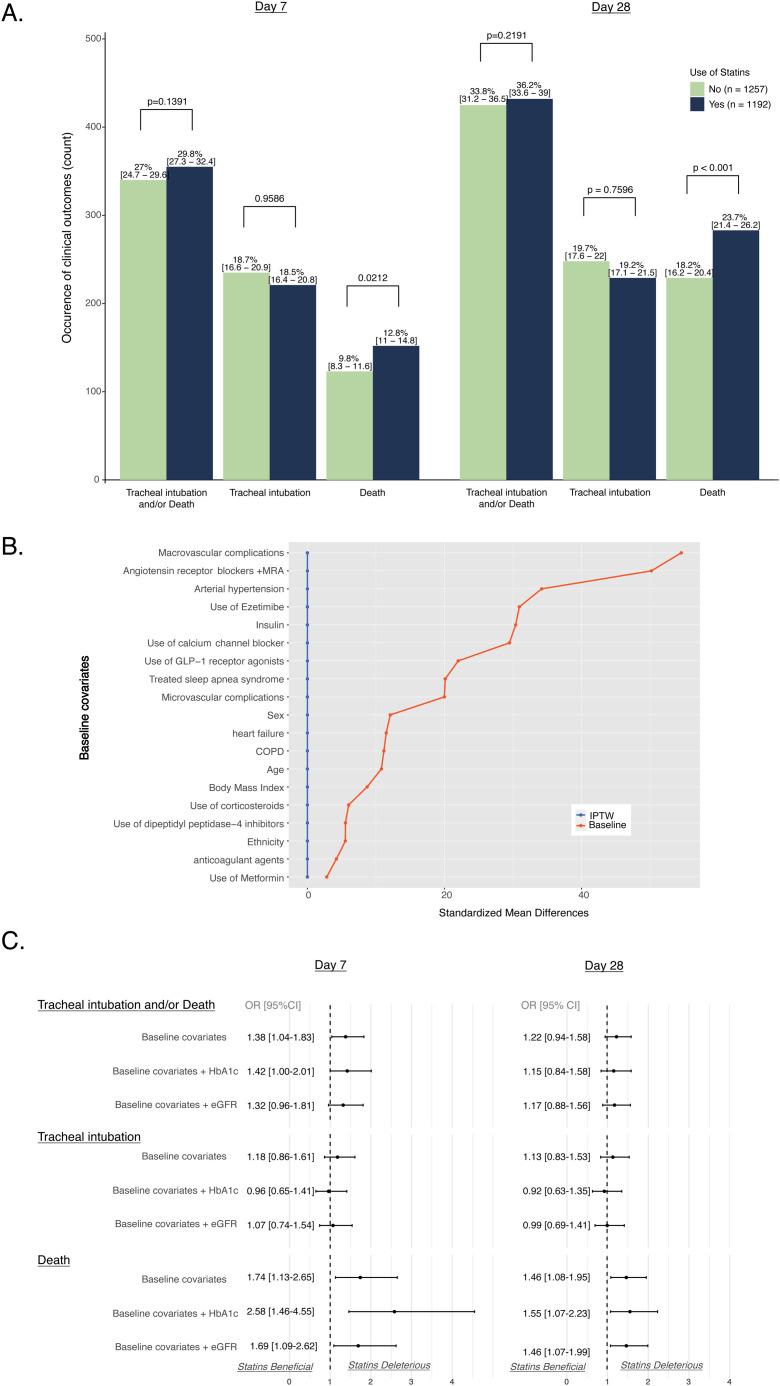
As shown in Fig. 1 A, the composite primary outcome was observed within 7 days of admission at similar rates in patients whether treated or not with statins (29.8% vs 27.0%, respectively; P = 0.1338). However, mortality rates were significantly higher in statin users within 7 (12.8% vs 9.8%; P = 0.02) and 28 days (23.9% vs 18.2%; P < 0.001), respectively. After applying IPTW (Fig. 1B), a significant association was observed for routine statin use and the primary outcome within 7 days [odds ratio (OR) [95% confidence interval (CI)]: 1.38 [1.04–1.83], but also for death within 7 (OR: 1.74 [1.13–2.65]) and 28 days (OR: 1.46 [1.08–1.95]; Fig. 1C). In contrast, routine statin use was not significantly associated with any increased risk of tracheal intubation for mechanical ventilation. Similar results were obtained in sensitivity analyses performed after additional adjustments for HbA1c and eGFR values.
Fig. 1.
(A) Baseline associations between statin use and study outcomes: bar graphs represent the number of patients with related outcomes; percentages (95% confidence intervals) represent frequencies; P values represent univariable associations between statin use and clinical outcomes assessed by Fisher’s exact test. (B) Baseline distribution balance after propensity score (PS) analyses: PS was computed using a logistic regression model with statin treatment as the dependent variable and the following as explanatory (independent) variables: gender; age; ethnicity; body mass index; arterial hypertension; history of micro- or macrovascular diabetes complications; heart failure; treated obstructive sleep apnoea or chronic obstructive pulmonary disease (COPD); and use of any of the following drugs/drug classes on admission [metformin, dipeptidyl peptidase-4 inhibitors, glucagon-like peptide (GLP)-1 receptor agonists; insulin; ezetimibe; and renin–angiotensin–aldosterone system blockers, including angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, mineralocorticoid receptor antagonists (MRA), calcium-channel blockers, anticoagulant agents and corticosteroids]. IPTW, inverse probability of treatment weighting. (C) PS-weighted associations between statin use and clinical outcomes: baseline covariates were used to compute PS in all multivariable models (see B); sensitivity analyses used these baseline variables plus routine HbA1c and routine estimated glomerular filtration rates (eGFR).
Discussion
CORONADO is the first registered cohort study focused on the phenotypic characteristics of T2DM patients hospitalized for COVID-19. In the present post-hoc analysis, a PS approach with IPTW demonstrated that previous statin treatment was significantly associated with increased mortality in patients with T2DM in hospital because of COVID-19 for up to 28 days after admission.
These results are somewhat surprising as previous observational studies of the general population had highlighted the potentially beneficial effects of statins on COVID-19 prognosis, including its in-hospital use [5], [6]. Most notably, in a large population-based cohort study performed in England, a recent prescription for statins was associated with a decrease in COVID-19-related mortality in those with T2DM [hazard ratio (HR)] [95% CI]: 0.72 [0.69–0.75] [10]. One potential explanation for this observed discrepancy with our present study is that CORONADO was focused only on patients with T2DM hospitalized for COVID-19 and was therefore associated with more severe prognosis. Indeed, the 28-day mortality rate in the non-statin group was 18.2% in CORONADO vs 6.8% in the study of Zhang et al. [5]. Thus, the possibility that the effect of statins on COVID-19 prognosis varies according to the stage and severity of disease cannot be excluded. In accordance with this speculation, the mortality HR was also lower at 0.4% in non-statin users in the study by Holman et al. [10], with cardiorenal morbidity affecting only 21.7% of the studied population, a considerably lower rate than in CORONADO, where 42% of participants exhibited cardiovascular complications.
In addition, as CORONADO patients using statins also had a greater number of comorbidities than non-users, the use of PS-weighting took these differences into account. As for the potential underlying molecular mechanisms that could support the deleterious effects of statins, it has been reported that these drugs increase cellular expression of angiotensin-converting enzyme 2 (ACE2), the primary receptor for SARS-CoV-2 [11].
Our present study has several limitations that must be acknowledged. Even though many covariates were captured in CORONADO, it remains nonetheless possible that some residual confounding factors were still persistent in the PS analysis. There was also a lack of information regarding the continuation (or not) of statin treatment after hospital admission, especially in comparison to a previous study assessing the benefits of in-hospital use of statins [5]. Unfortunately, information on plasma lipid values with statin therapy was also absent. Moreover, as it has been reported that lower low-density lipoprotein (LDL) cholesterol values are associated with severity of COVID-19 [12], it would have been of interest to verify the extent to which the LDL-lowering effect of statins mediated their influence on COVID-19 prognosis. Finally, as these results were obtained solely in patients with T2DM, they cannot be generalized to the general population.
Conclusion
Albeit observational, our present results do not support the hypothesis of a protective role of routine statin use against COVID-19, at least not in hospitalized patients with T2DM. Indeed, the potentially deleterious effects of routine statin treatment on COVID-19-related mortality demands further investigation and, as recently highlighted [13], only appropriately designed and powered randomized controlled trials will be able to properly address this important issue.
Acknowledgments and funding
We thank the sponsor of the study [Délégation à la Recherche Clinique et à l'Innovation (DRCI) CHU Nantes], the Clinical Project Manager M. Saignes and assistant J. Saunier, clinical research associates S. El Andaloussi, J. Martin-Gauthier and E. Rebouilleau, and data managers B. Guyomarc’h and T. Roman. We gratefully acknowledge all the medical and clinical research staff involved in the diagnosis and treatment of patients with COVID-19 at the participating centres, and also thank all of the general practitioners, specialists, pharmacists and biological laboratory staff responsible for hospitalized patients for providing their additional medical information to the investigators. Finally, we thank the Société Francophone du Diabète (SFD) and Société Française d’Endocrinologie (SFE) for disseminating the study design and organization, and the Fédération Française des Diabétiques (FFD) for participating in the organization of the study.
This study received the following funding: the Fondation Francophone de Recherche sur le Diabète (FFRD), supported by Novo Nordisk, MSD, Abbott, AstraZeneca and Eli Lilly; the Fédération Française des Diabétiques (FFD) CORONADO initiative emergency grant; Société Francophone du Diabète (SFD) CORONADO initiative emergency grant; Air Liquide Healthcare International; CORONADO initiative emergency grant, Allergan; CORONADO initiative emergency grant, AstraZeneca; CORONADO initiative emergency grant, LifeScan; CORONADO initiative emergency grant, NHC Pharmaceuticals; CORONADO initiative emergency grant, Novo Nordisk; CORONADO initiative emergency grant, Sanofi; and PHRC National COVID-19 Hospitalization and Care Organization Division (DHOS), part of the Hospital Clinical Research Program (PHRC COVID-19-20-0138). All research facilities are gratefully acknowledged for providing research associates and research technicians for clinical investigations pro bono. Funders of this study played no role in the study design, data collection, data analysis, data interpretation or writing of the report.
Duality of interest
B.C. reports grants, non-financial support and/or personal fees from Abbott, Air Liquide Healthcare, Allergan, Amgen, Akcea Therapeutics, AstraZeneca, Eli Lilly, GENFIT, Gilead Sciences, LifeScan, Merck Sharp & Dohme (MSD), NHC Pharmaceuticals, Novo Nordisk, Pierre Fabre, Regeneron and Sanofi. P.M. has served on advisory boards for AstraZeneca and Boehringer Ingelheim, and received honoraria from Novo Nordisk, MSD and Sanofi for oral presentations. M.P. reports grants, non-financial support and/or personal fees from Amgen, Novo Nordisk and Sanofi. L.P. reports personal fees and/or non-financial support from Eli Lilly, MSD, Novo Nordisk and Sanofi. M.W. reports personal fees from Air Liquide, Allergan, AstraZeneca, LifeScan, NHC Pharmaceuticals, Novo Nordisk and Sanofi. P.G. reports grants and/or personal fees from Abbott, Air Liquide, Allergan, Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, LifeScan, Merck Sharp & Dohme, Mundipharma, NHC Pharmaceuticals, Novo Nordisk, Sanofi and Servier. S.H. reports grants, non-financial support and/or personal fees from Air Liquide, Allergan, AstraZeneca, Bayer, Boehringer Ingelheim, Dinno Santé, Eli Lilly, LifeScan, LVL, MSD, NHC Pharmaceuticals, Novartis, Novo Nordisk, Pierre Fabre, Sanofi, Servier and Valbiotis. The other authors have nothing to disclose.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.diabet.2020.10.001.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Apicella M., Campopiano M.C., Mantuano M., Mazoni L., Coppelli A., Del Prato S. Covid-19 in people with diabetes: understanding the reasons for worse outcomes. Lancet Diabetes Endocrinol. 2020;8:782–792. doi: 10.1016/S2213-8587(20)30238-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scheen A.J., Marre M., Thivolet C. Prognostic factors in patients with diabetes hospitalized for COVID-19: findings from the CORONADO study and other recent reports. Diabetes Metab. 2020;46:265–271. doi: 10.1016/j.diabet.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fosbol E.L., Butt J.H., Ostergaard L., Andersson C., Selmer C., Kragholm K. Association of angiotensin-converting enzyme inhibitor or angiotensin receptor blocker use with Covid-19 diagnosis and mortality. JAMA. 2020;324:168–177. doi: 10.1001/jama.2020.11301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Messerli F.H., Siontis G.C.M., Rexhaj E. Covid-19 and renin angiotensin blockers: current evidence and recommendations. Circulation. 2020;141:2042–2044. doi: 10.1161/CIRCULATIONAHA.120.047022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang X.J., Quin J.J., Cheng X., Shen L., Zhao Y.C., Yuan Y. In-Hospital use of statins is associated with a reduced risk of mortality among individuals with Covid-19. Cell Metab. 2020;32 doi: 10.1016/j.cmet.2020.06.015. 32: 176-87.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kow C.S., Hasan S.S. Meta-analysis of effect of statins in patients with Covid-19. Am J Cardiol. 2020;S0002–9149(August (20)):30823–30827. doi: 10.1016/j.amjcard.2020.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cariou B., Hadjadj S., Wargny M., Pichelin M., Al-Salameh A., Allix I. Phenotypic characteristics and prognosis of inpatients with Covid-19 and diabetes: the CORONADO study. Diabetologia. 2020;63:1500–1515. doi: 10.1007/s00125-020-05180-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Austin P.C. The performance of different propensity-score methods for estimating differences in proportions (risk differences or absolute risk reductions) in observational studies. Stat Med. 2010;29:2137–2148. doi: 10.1002/sim.3854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mao H., Liang Li L. 2018. PSW: propensity score weighting methods for dichotomous treatments. R package version 1.1-3.https://CRAN.R-project.org/package=PSW [Google Scholar]
- 10.Holman N., Knighton P., Kar P., O’Keefe J., Curley M., Weaver A. Risk factors for Covid-19-related mortality in people with type 1 and type 2 diabetes in England: a population-based cohort study. Lancet Diabetes Endocrinol. 2020;8:823–833. doi: 10.1016/S2213-8587(20)30271-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shin Y.H., Min J.J., Lee J.H., Kim E.H., Kim G.E., Kim M.H. The effect of fluvastatin on cardiac fibrosis and angiotensin-converting enzyme-2 expression in glucose-controlled diabetic rat hearts. Heart Vessels. 2017;32:618–627. doi: 10.1007/s00380-016-0936-5. [DOI] [PubMed] [Google Scholar]
- 12.Wei X., Zeng W., Su J., Wan H., Yu X., Cao X. Hypolipidemia is associated with the severity of Covid-19. J Clin Lipidol. 2020;14:297–304. doi: 10.1016/j.jacl.2020.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fajgenbaum D.C., Rader D.J. Teaching old drugs new tricks: statins for Covid-19? Cell Metab. 2020;32:145–147. doi: 10.1016/j.cmet.2020.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.