Supplemental Digital Content is available in the text.
Background:
Although patients with obesity-induced lymphedema can be treated by weight loss therapy, they find it difficult to lose the required amount of weight. The aims of this study were to clarify the characteristics of the lymphatic vessels in patients with obesity-induced lymphedema and to determine the feasibility and efficacy of lymphovenous anastomosis (LVA) in these patients.
Methods:
Twenty-two patients (44 edematous lower limbs) with a body mass index (BMI) >35 kg/m2 (obese group) and 91 patients with lymphedema (141 edematous lower limbs) and BMI <25 kg/m2 were enrolled as a control group (nonobese group) and underwent LVA. The diameter and depth of lymphatics and the effect of LVA were compared.
Results:
Lymphatics were detectable within 10-mm depth in the nonobese group and the obese group (3.0 ± 1.4 mm versus 3.5 ± 2.1 mm; P < 0.01). The lymphatic diameter was significantly greater in the obese group than in the nonobese group (0.79 ± 0.30 mm versus 0.54 ± 0.22 mm; P < 0.01). There was no significant difference in the rate of improvement in lymphedema after LVA between the nonobese group (9.1% ± 9.2%) and the obese group (8.9% ± 7.3%; P = 0.84). There was no correlation between the improvement rate of lymphedema and that of BMI in the obese group (P = 0.57).
Conclusions:
LVA is a feasible procedure even in morbidly obese patients. Considering that substantial weight loss is a difficult and time-consuming task for patients, LVA combined with not gaining weight is a good option for these patients.
INTRODUCTION
Obesity has recently been identified as a risk factor for lymphedema.1,2 Obese individuals with lymphedema are at risk of lymphorrhea and cellulitis, which significantly affects their quality of life and further increases health care costs.3–6 Obesity-induced lymphedema is defined as lymphedema occurred in obese patients [body mass index (BMI), >30] accompanied by bilateral lower extremity enlargement without any other potential cause of lymphedema (eg, filariasis, primary lymphedema, inguinal radiation/lymphadenectomy).1,2
It is important to recognize obesity-induced lymphedema so that weight loss interventions can be instituted before the onset of potentially irreversible lymphatic dysfunction. Although patients with obesity-induced lymphedema can be treated by weight loss therapy, they find it difficult to lose the required amount of weight. Furthermore, it takes a long time to lose the excess weight and obesity-induced lymphedema increases the surgical risk in patients undergoing laparoscopic sleeve gastrectomy.7 Microsurgical intervention, such as lymphaticovenous anastomosis (LVA), has the potential to decrease the complications of obesity-induced lymphedema.3 However, to perform LVA successfully in these patients, it is important to have a detailed knowledge of their lymphatics, including the diameter of the vessels, the depth of these vessels in relation to the skin surface, and their characteristics on indocyanine green (ICG) lymphography. The aims of this study were to clarify the characteristics of the lymphatic vessels in patients with obesity-induced lymphedema and to determine the feasibility and efficacy of LVA in these patients.
PATIENTS AND METHODS
The study protocol was approved by the Ethics Committee of Hiroshima University Hospital. Patients with a BMI >35 kg/m2 who visited or were referred to our institution with possible lower extremity lymphedema (LEL) between May 2017 and December 2018 were enrolled in the study as the obese group. All patients provided written informed consent to participate in this study.
The patients were initially referred for weight loss therapy, dietary instructions, and conventional compression therapy with elastic stockings exercise therapy. All patients underwent lymphoscintigraphy to assess lymphatic function in the lower extremities. An abnormal lymphoscintigram was defined as delayed transit of radiolabeled colloid (>50 minutes), dermal backflow (DB), and/or tortuous collateral lymphatic channels8–12 (Fig. 1A). The inclusion criteria were as follows: patients with a BMI >35 kg/m2 even after 6-month therapy (Fig. 1B); no history of heart failure, renal failure, cirrhosis of the liver, hypoproteinemia, deep vein thrombosis, chronic venous obstruction or venous reflux, thyroid dermopathy, other endocrine cause of edema, drug-induced edema; edema not present earlier in life; edema limited to the lower limbs; and edema refractory to conventional compression therapy with elastic stockings despite continuous use for at least 6 months. The pressure of compression garment was adjusted preoperatively between 18 and 32 mm Hg at the highest pressure the patients were able to continue compression therapy. Patients with arterial and/or venous malformation, generalized lymphedema, or iodine allergy (a contraindication to injection of ICG) were excluded. None of the patients had a family history of lymphedema. Twenty-two of these patients (44 edematous lower limbs) underwent LVA for lymphedema after confirmation of lymphedema on ICG lymphography (obese group). The diagnosis of lymphedema was established based on ICG lymphography and lymphoscintigraphy. Twelve of the 22 patients had experienced repeated episodes of cellulitis, and 7 had persistent lymphorrhea despite using compression therapy for at least 6 months. Three of the 22 patients had diabetes mellitus: 2 patients were controlled by insulin injection, and 1 patient was controlled by oral medication.
Fig. 1.
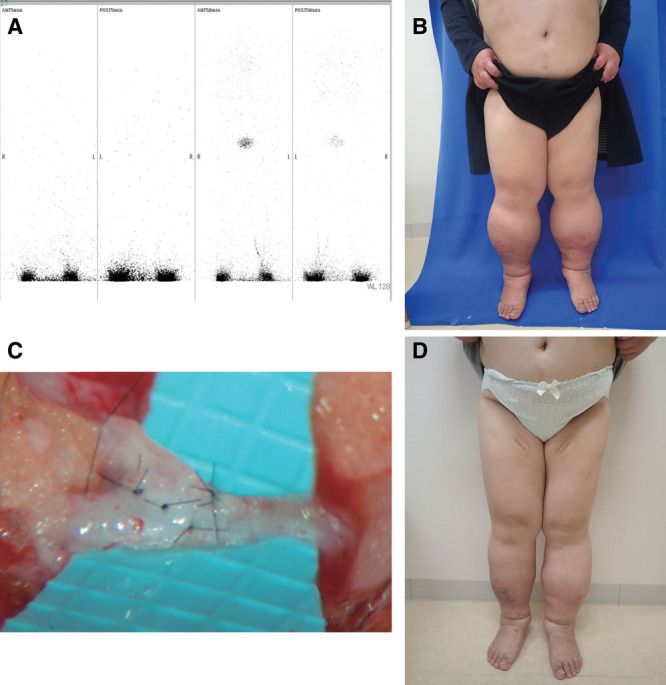
Patient details. A, An abnormal lymphoscintigram was defined as delayed transit of radiolabeled colloid (>50 minutes), DB, and/or tortuous collateral lymphatic channels. B, Patients with a BMI >35 kg/m2 with possible lower extremity lymphedema were enrolled in the study as the obese group. C, LVA procedures were performed for the patients with no improvement even after weight loss therapy, dietary instructions, and conventional compression therapy with elastic stockings exercise therapy. The veins and lymphatic vessels were anastomosed in an end-to-end or end-to-side fashion using 11-0 or 12-0 nylon microsutures. D, The effect of LVA was also investigated by comparing LEL index before and after LVA between the obese and nonobese groups.
Ninety-one patients with lymphedema (141 edematous lower limbs) and BMI <25 kg/m2 were also enrolled as a control group (nonobese group) using the same eligibility criteria. Nineteen of the 91 patients had experienced repeated episodes of cellulitis; no one had lymphorrhea. These patients also underwent LVA after establishing the diagnosis of lymphedema based on ICG lymphography.
Preoperative Investigations
ICG lymphography was performed in all cases as follows. First, 0.2 mL of ICG (Diagnogreen 0.25%; Daiichi Sankyo, Tokyo, Japan) was injected subcutaneously into each lower extremity at the first web space of the foot and at the lateral border of the Achilles tendon. Circumferential fluorescent images of the lymphatic drainage channels were then obtained using an infrared camera system (Photodynamic Eye; Hamamatsu Photonics K.K., Hamamatsu, Japan). ICG lymphography images were recorded in the plateau phase (12–18 hours after injection; ie, on the following day) when no further changes in the images obtained would be expected. All ICG lymphography images were reviewed by 2 plastic surgeons working independently. The characteristic lymphography patterns in the thigh and lower leg were then categorized according to anthropometric characteristics. The patients in the obese and nonobese groups were categorized according to whether the lymphedema was primary or secondary.
Lymphaticovenous Anastomosis
After obtaining the results of ICG lymphography, LVA was performed along the greater saphenous vein based on our experience of performing LVA when ICG lymphography showed a DB pattern or no enhancement; when ICG lymphography showed a linear pattern, LVA was performed exactly where this pattern was demonstrated on ICG lymphography after marking of the lymphatic vessels. All LVA procedures were performed under local anesthesia. Under a surgical microscopic view, several 1- to 5-cm skin incisions were made in the lower leg and thigh regions, and the subcutaneous veins and lymphatic vessels suitable for anastomosis were identified. The veins and lymphatic vessels were anastomosed in an end-to-end or end-to-side fashion using 11-0 or 12-0 nylon microsutures (Fig. 1C).
The diameter of each lymphatic vessel was identified during the procedure and was measured using a Crownjun Microscale submillimeter scale (Kono Seisakusyo Co., Ltd., Ichikawa, Japan). We also recorded the depth of each lymphatic vessel from the skin surface using the same scale as lymphatic vessels. We calculated the mean vessel diameter and the mean depth of the lymphatic vessels in the lower leg and thigh regions in each lower limb.
We allowed the patients to walk immediately after surgery, however, prohibited for 1 week the patient from keeping a state in which lower her foot for >1 hour, concerning about wound healing from the LVAs. Light compression had been performed immediately after surgery by elastic bandage made of cotton combined with wound management. All patients resumed compression therapy at the end of the second postoperative week using the same compression garment as the preoperative ones.
Postoperative Evaluation
Limb circumference was measured before and after the LVA procedure. Circumferential measurements of the affected lower limb were obtained at 5 anatomic locations (10 cm above the knee, knee, 10 cm below the knee, ankle, and foot) before and 1 year after LVA in the supine position after confirming that there was no cellulitis. The LEL index was calculated by dividing the sum of the squares of the circumference in the 5 areas of the affected lower extremity by the BMI.13
The rate of improvement in lymphedema (Fig. 1D) was calculated by dividing the difference in the LEL index between before and after surgery by the preoperative value for each case as follows:
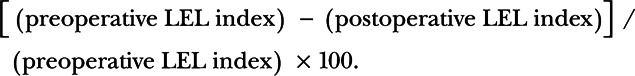 |
One set of tape measurements was obtained by a nurse or physiotherapist not otherwise involved in the research and randomly assigned to each patient in the outpatient clinic to reduce the possibility of measurement bias.
The rate of improvement in BMI was calculated by dividing the difference in BMI between before and after treatment by the pretreatment value for each case. The effect of LVA was also investigated by comparing LEL index before and after LVA between the obese and the nonobese groups (Fig. 1D). The effect of weight loss instructions in the obese group was assessed by comparing BMI values recorded before and after treatment. Patient age and sex, International Society of Lymphology (ISL) classification,14 and duration of illness were also recorded.
Statistical Analysis
The data are shown as the mean ± SD (range). Between-group differences in patient age, duration of illness, LEL index, the number of LVA for each lower limb, depth of lymphatic vessels, and rate of improvement in the LEL index were compared using Student t test. Differences in the distributions according to patient sex and whether lymphedema was primary or secondary were compared using the χ2 test for independence. Between-group differences in the distribution of ISL classification were compared using the Mann-Whitney U test. Differences in the various lymphography patterns were compared between the groups using the Kruskal-Wallis test.
Between-group differences in lymphatic vessel diameter and depth and in the improvement rate were compared using Student t test. Differences in LEL index between before and after LVA and those of BMI between before and after receiving weight loss instructions were compared using the paired t test. The correlation between the rate of improvement in the LEL index and the rate of improvement in BMI was investigated using Spearman coefficient by rank test. All statistical analyses were performed using Statcel 4 software (OMS Publishing, Inc., Tokyo, Japan). A P value of <0.05 was considered statistically significant.
RESULTS
All surgical procedures were performed uneventfully. There were no complications, such as lymphorrhea or delayed wound healing. There has been no recurrence of cellulitis or lymphorrhea following LVA as of this writing. Compression therapy and diet and exercise therapy were continued after LVA.
There were 91 cases/141 limb, unilateral; 41 cases, bilateral; 50 cases in nonobese group, 22 cases/44 limbs, all cases bilateral in obese group. Mean BMI (kg/m2) was 21.4 ± 2.0 (range, 13–95) in the nonobese group and 40.3 ± 5.6 (35–59) in the obese group. There was a significant between-group difference in sex distribution. There was no significant between-group difference in patient age; LEL index; distribution of I, II, II later, and III ISL grades; duration of illness; or length of follow-up. Etiologies of lymphedema in nonobese group were primary, 34; uterine cancer, 44; ovarian cancer, 3; other cancer, 9; and other surgery, 1 (cases). Etiologies of lymphedema in obese groups were obesity, 21 and ovarian cancer, 1. There was no significant between-group difference in the number of LVA for each lower limb (Table 1).
Table 1.
Patient Profile Comparison between the Nonobese Group and Obese Groups
| Group | Nonobese | Obese | P | ||
|---|---|---|---|---|---|
| Cases/limbs, n | 91/141 | 22/44 | |||
| Unilateral/bilateral (cases) | 41/50 | 0/22 | |||
| BMI, kg/m2 | 21.4 ± 2.0 (13–95) | 40.3 ± 5.6 (35–59) | |||
| Sex, male/female | 10/81 | 11/11 | *0.00002 | χ2 test | |
| Age, y | 62.5 ± 16.6 (13–95) | 55.5 ± 12.9 (38–78) | 0.07 | Student t test | |
| LEL index | 239 ± 45 (147–428) | 239 ± 35 (144–307) | 0.99 | Student t test | |
| ISL classification I/II/II later III | 0/59/74/8 | 0/20/16/8 | 0.7 | Mann-Whitney U test | |
| Illness duration, y | 6.6 ± 8.8 (1–50) | 5.5 ± 6.0 (1–18) | 0.56 | Student t test | |
| length of follow-up, mo | 20.7 ± 7.6 (6–36) | 20.4 ± 9.6 (8–48) | 0.89 | Student t test | |
| No. LVA for each lower limbs | 4.3 ± 2.0 (1–8) | 4.5 ± 1.6 (1–9) | 0.93 | Mann-Whitney U test | |
| Primary | 34 | 0 | |||
| Obese | 21 | ||||
| Uterine cancer | 44 | 0 | |||
| Ovarian cancer | 3 | 1 | |||
| Other cancer | 9 | 0 | |||
| Other surgery | 1 | 0 | |||
There was significant between-group difference in sex distribution. There was no significant between-group difference in patient age; LEL index; distribution of I, II, II later, and III ISL grades; duration of illness; or length of follow-up. Etiologies of lymphedema in nonobese group was primary: 34, uterine cancer: 44, ovarian cancer: 3, other cancer: 9, and other surgery: 1 (cases). In obese groups, obesity: 21, and ovarian cancer: 1. There was no significant between-group difference in the number of LVA for each lower limb.
The pattern seen on ICG lymphography was visually interpreted as linear, DB, or low enhancement (LE). The pattern was deemed to be linear when the superficial lymphatic vessels were seen to be arranged in a linear manner. The LE pattern was observed only in the distal portion of the lower extremity around the foot with no enhancement in the proximal portion. The pattern was deemed to be DB when deterioration was visible in the enhanced lymphatics (Table 2). There was no difference in the categorization of ICG lymphography patterns between the 2 independent observers. The interclass correlation coefficient between 2 observers was 0.96 (P < 0.001). A significant between-group difference in ICG pattern was seen in the thigh and lower leg area (Table 2).
Table 2.
Comparison of ICG Patterns between Nonobese Group and Obese Group
| ICG Patterns | Differences in the Distribution of ICG Lymphography Patterns between the Nonobese and the Obese | ||||||
|---|---|---|---|---|---|---|---|
| Linear | LE | DB | |||||
| Nonobese | Obese | Nonobese | Obese | Nonobese | Obese | ||
| Thigh, n (%) | 80 (57.0) | 9 (20.5) | 3 (2.2) | 33 (75) | 58 (40.8) | 2 (4.5) | P = <0.01 |
| Lower leg, n (%) | 81 (57.4) | 7 (15.9) | 3 (2.1) | 22 (50 | 57 (40.4) | 15 (34.1) | P = <0.01 |
The pattern seen on ICG lymphography was visually interpreted as linear, DB, or LE. The pattern was deemed to be linear when the superficial lymphatic vessels were seen to be arranged in a linear manner. The LE pattern was observed only in the distal portion of the lower extremity around the foot with no enhancement in the proximal portion. The pattern was deemed to be DB when deterioration was visible in the enhanced lymphatics. There was no difference in the categorization of ICG lymphography patterns between the 2 independent observers. The interclass correlation coefficient between 2 observers was 0.96, P < 0.001. A significant between-group difference in ICG pattern was seen in the thigh and lower leg area between the nonobese group and the obese group.
There was a significant between-group difference in lymphatic depth between the nonobese group and the obese group [3.0 ± 1.4 mm (range, 1–10 mm) mm versus 3.5 ± 2.1 mm (2–10 mm); P = 0.006; left in Supplemental Digital Content 1]. [See figure, Supplemental Digital Content 1, which displays that there was significant between-group difference in the depth of lymphatics (mm) between the nonobese group and the obese group (left). Significant between-group difference in the depth of lymphatics (mm) was also seen in each area, thigh (middle above) or lower leg (middle below). There was no significant between-group difference within the obese group when the group was divided according to the type of ICG image: LE pattern versus linear pattern (P = 0.54), LE versus linear + DB (P = 0.49) in thigh area (right above), LE pattern versus linear pattern (P = 0.65), LE versus DB (P = 0.42), and LE versus linear + DB (P = 0.56) in lower leg area (right below), http://links.lww.com/PRSGO/B374.]
A significant between-group difference was also found in lymphatic depth in both the thigh (SDC1, upper middle) and the lower leg (SDC1, lower middle). However, in the obese group, there was no significant difference in lymphatic depth according to the type of ICG pattern in either the thigh area (SDC1, right upper) or the lower leg area (SDC1, right lower). There was a significant between-group difference in lymphatic diameter between the nonobese group and the obese group [0.54 ± 0.22 mm (range, 0.2–1.25 mm) versus 0.79 ± 0.30 mm (0.3–1.75 mm); P < 0.01; left in Supplemnetal Digital Content 2]. [See figure, Supplemental Digital Content 2, which displays that there was a significant between-group difference in the diameter of lymphatics between the nonobese group and the obese group (left). Significant between-group difference in the diameter of lymphatics was also seen in each area, thigh and lower leg (right), http://links.lww.com/PRSGO/B375.] There was also a significant between-group difference in lymphatic diameter between the nonobese group and the obese group in the thigh area (SDC2, right upper) and in the lower leg area (SDC2, right lower).
There was no significant difference in the rate of improvement in lymphedema after LVA between the nonobese group and the obese group (upper left in Supplemental Digital Content 3). [See figure, Supplemental Digital Content 3, which displays that there was no significant difference in the lymphedema improvement rate LEL between the nonobese group and the obese group. Significant difference of LEL index was seen between before and after LVA in the nonobese group and the obese group over 35 (above right 2). Significant difference of BMI was also seen between before and after therapy in the obese group (below left). There was no correlation between improvement rate of LEL index and improvement rate of BMI in the over 35 group (P = 0.57; below right), http://links.lww.com/PRSGO/B376.]
However, there was a significant difference in the mean LEL index value between before and after LVA in the nonobese group (241 ± 44 [147–364] versus 218 ± 41 [150–372]; P < 0.01; SDC3, middle upper) and in the obese group (238 ± 35 [144–307] versus 217 ± 28 [152–274]; SDC3, right upper). There was also a significant decrease in BMI between before and after treatment in the obese group (SDC3, left lower). There was no correlation between the rate of improvement in the LEL index and that in BMI in the obese group (SDC3, right lower).
DISCUSSION
A previous study suggested that patients with BMI >50 kg/m2 are at increased risk of lymphedema.15 However, the results of this study in Japanese patients indicate that obesity-induced lymphedema can occur even when BMI is >35. There may be regional or racial differences in the BMI value at which the risk of obesity-induced lymphedema is increased.
The 2 groups in this study were enrolled using the same inclusion criteria, except for BMI. There were no significant between-group differences in the severity of lymphedema at baseline, as indicated by LEL index, ISL classification, and duration of illness, which suggests that the severity of lymphedema was similar between the obese and the nonobese groups. Therefore, the nonobese group was considered suitable as a control group.
The frequency of the LE pattern was significantly higher in both the thigh and the lower leg areas in our obese group. Given that the lymphatics in the obese group were significantly deeper than those in the nonobese group, thick adipose tissue is conceivably the reason why this pattern was significantly more common in the obese patients. However, ICG fluorescence is usually visible to a depth of 1 cm even though the injected ICG dye diffuses into the subcutaneous fatty tissue,16 which is not consistent with our findings. The actual depth of penetration of ICG through living tissue may be less than that previously reported. However, we found no between-group difference in lymphatic depth in the obese group according to whether the ICG pattern was LE or linear. At present, we have no explanation for this finding. Although the lymphatics in the obese group were found to be located significantly deeper than those in the nonobese group, they were detectable within 10 mm of the skin surface during the LVA procedure even in the thigh area in the obese group. Based on our findings, it is conceivable that the lymphatics were not located deeper in morbidly obese patients. Therefore, LVA can be considered feasible even in these patients.
The lymphatic diameter in both the thigh and the lower leg areas was significantly greater in the obese group than in the nonobese group. The findings of our study are consistent with those of an earlier study in hypercholesterolemic mice in which lymphatic dysfunction was shown to result in part from profound structural abnormalities in the lymphatic vasculature, namely, the initial lymphatic vessels were greatly enlarged and the collecting vessels showed a marked decrease in coverage by smooth muscle cells.17 Our findings were also supported by another animal study that found the lymphatic vessels to be significantly more dilated in mice with diet-induced obesity than in controls.18 The mechanism by which obesity impairs the lymphatic function in the lower extremities was unknown. However, it has been suggested that the amount of lymph produced by the leg increases as BMI increases and the amount of ambulation/muscle contraction required to transport the fluid decreases.2 LVA is easier to perform in dilated lymphatics, which could increase the success rate of anastomosis. Therefore, LVA is a feasible procedure even in morbidly obese patients.
In our study, there was a significant improvement in the LEL index after LVA in both groups with no significant between-group difference, which indicates that LVA for lymphedema is as successful in obese patients as it is in patients with normal weight. Although we cannot exclude the impact of weight loss, there was no correlation between the rate of improvement in the LEL index and that of BMI, suggesting that the improvement in the LEL index in the obese group was the result of both weight loss and LVA.
We consider that weight loss could be the most effective treatment for obesity-induced lymphedema, despite a case report in which obesity-related lymphedema is not reversible following massive weight loss.19 However, LVA is a promising microsurgical treatment for obesity-induced lymphedema; however, considering that substantial weight loss is a difficult and time-consuming task for patients, LVA combined with weight loss or at least not gaining weight is a good option for these patients.
LVA also has the potential to reduce lymphedema-induced cellulitis or lymphorrhea, which often accompanies lymphedema in obese patients.20,21 Our study showed that the lymphatics in obese patients are dilated and located at depths that are amenable to LVA, which is effective when combined with even mild weight loss.
This study has some limitations. One of them is nonheterogenous group of patients. Two groups have different disease etiology. The other is BMI in our patients was typically around 40 kg/m2. Thus, further investigations are needed in patients with more severe obesity-induced lymphedema. Additional limitations include the small number of cases and short follow-up period. Therefore, future studies should include a larger number of cases with longer follow-up duration.
CONCLUSION
LVA is feasible in patients with obesity-induced lymphedema and is a potentially effective treatment for lymphedema in morbidly obese patients.
Supplementary Material
Footnotes
Published online 27 May 2020.
Disclosure: The authors have no financial interest to declare in relation to the content of this article.
Related Digital Media are available in the full-text version of the article on www.PRSGlobalOpen.com.
REFERENCES
- 1.Greene AK, Grant FD, Slavin SA.Lower-extremity lymphedema and elevated body-mass index. N Engl J Med. 2012;366:2136–2137. [DOI] [PubMed] [Google Scholar]
- 2.Greene AK, Grant FD, Slavin SA, et al. Obesity-induced lymphedema: clinical and lymphoscintigraphic features. Plast Reconstr Surg. 2015;135:1715–1719. [DOI] [PubMed] [Google Scholar]
- 3.Mihara M, Hara H, Todokoro T, et al. The effect of lymphatico-venous anastomosis for an intractable ulcer at the lower leg in a marked obese patient. Microsurgery. 2014;34:64–67. [DOI] [PubMed] [Google Scholar]
- 4.Todd M, Lay-Flurrie K, Drake J.Managing ulceration and lymphorrhea in chronic oedema. Br J Community Nurs. 2017;22suppl 5S34–S41. [DOI] [PubMed] [Google Scholar]
- 5.Olszewski WL.Cellulitis and bacteria in peripheral lymphedema. Lymphology. 2018;51:54–56. [PubMed] [Google Scholar]
- 6.Sharkey AR, King SW, Ramsden AJ, et al. Do surgical interventions for limb lymphoedema reduce cellulitis attack frequency? Microsurgery. 2017;37:348–353. [DOI] [PubMed] [Google Scholar]
- 7.Li J, Lai D, Wu D.Laparoscopic Roux-en-Y gastric bypass versus laparoscopic sleeve gastrectomy to treat morbid obesity-related comorbidities: a systematic review and meta-analysis. Obes Surg. 2016;26:429–442. [DOI] [PubMed] [Google Scholar]
- 8.Gloviczki P, Calcagno D, Schirger A, et al. Noninvasive evaluation of the swollen extremity: experiences with 190 lymphoscintigraphic examinations. J Vasc Surg. 1989;9:683–689; discussion 690. [DOI] [PubMed] [Google Scholar]
- 9.Szuba A, Shin WS, Strauss HW, et al. The third circulation: radionuclide lymphoscintigraphy in the evaluation of lymphedema. J Nucl Med. 2003;44:43–57. [PubMed] [Google Scholar]
- 10.Witte CL, Witte MH, Unger EC, et al. Advances in imaging of lymph flow disorders. Radiographics. 2000;20:1697–1719. [DOI] [PubMed] [Google Scholar]
- 11.Moshiri M, Katz DS, Boris M, et al. Using lymphoscintigraphy to evaluate suspected lymphedema of the extremities. AJR Am J Roentgenol. 2002;178:405–412. [DOI] [PubMed] [Google Scholar]
- 12.Scarsbrook AF, Ganeshan A, Bradley KM.Pearls and pitfalls of radionuclide imaging of the lymphatic system. Part 2: evaluation of extremity lymphoedema. Br J Radiol. 2007;80:219–226. [DOI] [PubMed] [Google Scholar]
- 13.Yamamoto T, Matsuda N, Todokoro T, et al. Lower extremity lymphedema index: a simple method for severity evaluation of lower extremity lymphedema. Ann Plast Surg. 2011;67:637–640. [DOI] [PubMed] [Google Scholar]
- 14.Executive Committee The diagnosis and treatment of peripheral lymphedema: 2016 consensus document of the International Society of Lymphology. Lymphology. 2016;49:170–184. [PubMed] [Google Scholar]
- 15.Greene AK.Diagnosis and management of obesity-induced lymphedema. Plast Reconstr Surg. 2016;138:111e–118e. [DOI] [PubMed] [Google Scholar]
- 16.Kitai T, Inomoto T, Miwa M, et al. Fluorescence navigation with indocyanine green for detecting sentinel lymph nodes in breast cancer. Breast Cancer. 2005;12:211–215. [DOI] [PubMed] [Google Scholar]
- 17.Lim HY, Rutkowski JM, Helft J, et al. Hypercholesterolemic mice exhibit lymphatic vessel dysfunction and degeneration. Am J Pathol. 2009;175:1328–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weitman ES, Aschen SZ, Farias-Eisner G, et al. Obesity impairs lymphatic fluid transport and dendritic cell migration to lymph nodes. PLoS One. 2013;8:e70703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greene AK, Grant FD, Maclellan RA.Obesity-induced lymphedema nonreversible following massive weight loss. Plast Reconstr Surg Glob Open. 2015;3:e426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mihara M, Hara H, Tsubaki H, et al. Combined conservative treatment and lymphatic venous anastomosis for severe lower limb lymphedema with recurrent cellulitis. Ann Vasc Surg. 2015;29:1318.e11–1318.e15. [DOI] [PubMed] [Google Scholar]
- 21.Scaglioni MF, Fontein DBY, Arvanitakis M, et al. Systematic review of lymphovenous anastomosis (LVA) for the treatment of lymphedema. Microsurgery. 2017;37:947–953. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


