Abstract
Introduction
Diabetes mellitus (DM) is a metabolic disease that can cause many complications. The occurrence of urinary tract infection is also considered to be the cause of complications in patients with DM. This study aimed to evaluate the prevalence of urinary tract infection (UTI) and antibiotic-resistant bacteria found in urine culture from patients with DM in Surabaya, Indonesia.
Methods
This study was conducted with a retrospective cross-sectional study design, using a database of 1551 patients with DM admitted to Haji Hospital, Surabaya, Indonesia, from January 2017 to December 2018. Data regarding the bacteria isolated from urine and antimicrobial sensitivity were analyzed.
Results
The prevalence of UTI was 3.93% - 61 patients were confirmed with urine culture for UTI diagnosis. The predominant isolates found were Escherichia coli (24.5%) and Enterococcus faecalis (8%). This study also showed multidrug-resistant organisms (MDRO) found in UTI such as Escherichia coli MDRO (3.3 %), Klebsiella pneumoniae MDRO (3.3%), Acinetobacter baumannii MDRO (1.6%), extended-spectrum beta-lactamase (ESBL) Escherichia coli (3.3%). The E. coli were sensitive to fosfomycin (93%), meropenem (93%) and nitrofurantoin (67%). No significant difference in the prevalence of UTI was found among ages, sex, and duration of disease in all patients with DM.
Conclusions
The cases of UTI seen in patients with DM show the importance of monitoring UTI occurrence in this patient category to ensure better treatment for these patients.
Keywords: Diabetes mellitus, UTI, Escherichia coli, Enterococcus faecalis
Introduction
Diabetes mellitus (DM) is considered a chronic disorder and its complications can lead to morbidity. Type 2 diabetes mellitus is commonly found in adult patients, being characterized by resistance to insulin, less production of insulin or overproduction of glucose. Interaction between environmental, genetic and other behavioral risk factors can cause type 2 diabetes mellitus.1,2 Updated data show a prevalence of 6.7% DM in patients in Indonesia (around more than 10 million people). These numbers will be increasing and, in the year 2045, the estimates will reach around 16.7 million becoming the sixth highest in the world.3
Diabetes has been shown to have secondary effects in the urogenital system that causes the increased probability of urinary tract infection.4 This type of infection frequently occurs due to high blood glucose levels, poor circulation of white blood cells in the body,5 and improper bladder emptying process due to the autonomic neuropathy which makes the urine stay too long in the bladder thus becoming a breeding field for bacteria.1,6 Patients with hyperglycemia have a high concentration of glucose (glycosuria) in urine that is suitable for microorganism’s growth. These hyperglycemic conditions will prevent the antimicrobial function by inactivating glucose-6-phosphate dehydrogenase (G6PD), limiting polymorphonuclear leukocyte mobility through the endothelium, and also escalating apoptosis of polymorphonuclear leukocytes.7,8
Urinary tract infection comprises several clinical syndromes including asymptomatic bacteriuria, acute cystitis, acute pyelonephritis, and severe urosepsis. UTI prevalence is determined by age and gender. The probability of females acquiring UTI is 50% to 60% in their lifetime. In a diabetic patient, several factors were counted to increase the risk of UTI, including advancing age, an extended period of complications, metabolic control, predominantly diabetic nephropathy, and cystopathy.9
The current trend shows an escalated threat of UTI among patients with DM. An increase in cases of type 2 DM also increases the risk of infection with multidrug-resistant pathogens, such as extended-spectrum beta-lactamase (ESBL) Enterobacteriaceae, vancomycin-resistant enterococci (VRE), fluoroquinolone-resistant uropathogens, or carbapenem-resistant Enterobacteriaceae (CRE).10-13 Thus, monitoring the prevalence of antimicrobial-resistant pathogens of UTI will assist practitioners to select a right choice of antibiotic to treat the infections. However, the information for these antimicrobial-resistant pathogens of the UTI is very scarce; thus, it limits the proper treatment for patients with DM and UTI. Therefore, this study was conducted to provide data regarding the identification of microorganisms involved in UTI and their antimicrobial patterns found in patients with DM from Surabaya.
Methods
This study was a retrospective cross-sectional study of 161 hospitalized patients with DM and UTI. This study was approved by the ethics committee in the public hospital were this study was conducted. These patients were identified from a total of 1551 patients with DM from Haji Hospital, a public hospital in Surabaya from January 2017 to December 2018. This public hospital is a government hospital with a minimum of two hundred beds. This hospital also serves as teaching hospital, and it is classified into type B hospital in Indonesia (there are four hospital classifications in Indonesia). It means that this hospital is used as a reference for other district hospitals in Indonesia for patients with National Health Insurance coverage. This hospital has seventeen specializations including pediatric, anesthesiologist, dental specialist, internist, radiologist, etc. The selection criteria for this study were: 1) positive DM; 2) diagnosed with UTI with urine culture method14 as a confirmation method for detecting bacteriuria. Patients were screened based on billing code (or medical record number) from all patients that conducted a glucose test.
The patient population in this study was represented by inpatients that were diagnosed with positive DM (newly diagnosed or already in therapy for DM). Positive DM was defined using a random blood glucose test of more than 200 mg/dL (≥200 mg/dL). HbA1c test was also documented in the medical record. UTI positive cases were selected from 1551 patients positive for DM with a positive urine culture. Urinary tract infection diagnosis was conducted using urine culture, with a total plate count showing more than 105 CFU considered as UTI. Urinalysis was also performed to diagnose UTI, but this study focused only on UTI that was confirmed with a urine culture.
The history of the patients included age, gender, duration of diabetes, and the antimicrobial susceptibility pattern among several antibiotics. Data were collected from the medical records provided by the hospital. They were obtained from measured blood parameters such as fasting blood glucose and glycosylated hemoglobin (HbA1c; not all patients were tested for HbA1c).
Antimicrobial susceptibility testing
All identified bacteria were tested using Kirby Baue’s disc diffusion method against various antibiotics. Antimicrobial discs (Oxoid, Ltd, Hampshire, UK) including amoxicillin-clavulanic acid (30 µg), ceftriaxone (30 µg), gentamicin (10 µg), ampicillin (10 µg), ciprofloxacin (30 µg), nitrofurantoin (50 µg), norfloxacin (10 µg), tetracycline (25 µg) were placed onto inoculated Muller-Hinton agar plate. The zone of inhibition was measured using a caliper which was observed after overnight incubation at 37°C. The inhibition zone diameters were then compared to the Clinical and Laboratory Standards Institute (CLSI) guidelines for the resistance and sensitivity confirmation.15
Statistical analysis
Statistical analysis was carried out using Statistical Package for the Social Sciences (SPSS) software version 21 (IBM corporation, Armonk, NY, USA). Frequencies and percentages were chosen for categorical variables and continuous variables were presented as mean ± standard deviation. The t-test was used to compare means of age group while Mann-Whitney was used to compare means of random blood sugar values. The Chi-square test was used to find the association between categorical variables. Binary logistic analysis was also used to determine the risk factors for UTIs among patients with DM. The p-value of ≤0.05 was considered to be statistically significant.
Results
Characteristics of patients with DM
We have selected 1551 patients admitted to the H Hospital and diagnosed with DM from January 2017 to December 2018. The majority of patients were women with a ratio of 1:0.66 for females and males respectively. The average age of patients was 57±11.4 years within the range of 50-60 years of age (counted as 552 patients, 36.7%).
Prevalence of urinary tract infections
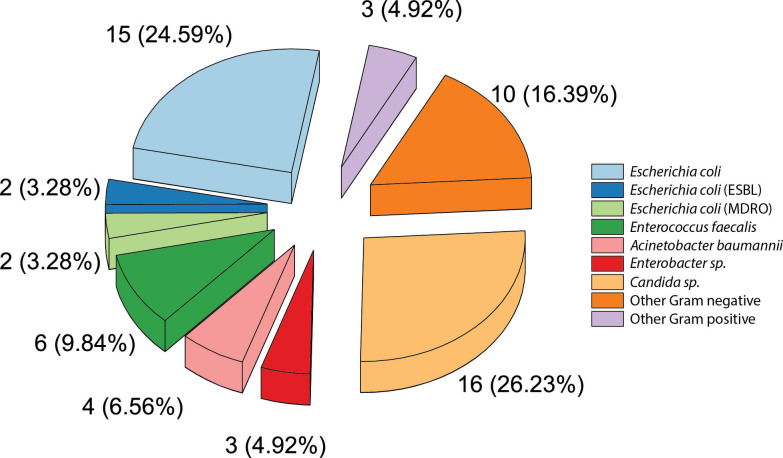
The patients with UTI detected through urine culture contributed to 3.93% of the total patients with DM (61 out of 1551 patients). One hundred sixty-one patients with DM were suspected for urinary tract infection and among those patients, 61 were confirmed to have UTI. The majority of patients with DM who were diagnosed with UTI were above 60 years of age (59%; 24.2% male and 34.8% female, Table 1). The predominant isolates found were Gram-negative bacteria (36 patients, 59%) such as Escherichia coli (15 patients, 24.59%), and Acinetobacter baumannii (4 patients, 6.56%). In addition, Escherichia coli ESBL and MDRO were detected in 2 patients. Other MDRO bacteria were also found in this study. Klebsiella pneumoniae was found in 2 patients and Acinetobacter baumannii MDRO was found in 1 patient (Figure 1). Meanwhile, the main Gram-positive bacteria found were Enterococcus faecalis (6 patients, 9.84%). Fungal infection by Candida spp. was also seen in urine culture from patients with DM-UTI (16 patients, 26.23%) – Figure 1. In three urine specimens, more than one species of microorganisms was isolated. Of those three specimens, one urine specimen contained Escherichia coli (ESBL), Escherichia coli, and Raoultella terrigena. From two other urine specimens, one specimen contained Raoultella terrigena and Enterococcus faecalis while other specimens contained Candida spp. and Escherichia coli. The distribution of these microorganisms found in UTI was not significantly different (p=0.137) in comparison to patients without DM. Escherichia coli was also seen in 9 patients (45%). Resistant pathogens were also observed in patients without DM (1 patient with Escherichia coli MDRO and 1 patient with Enterococcus faecalis MDRO).
Table 1.
Patients with diabetes mellitus and urinary tract infection based on urine culture during 2017-2018
| Age | Male, n (%) | Female, n (%) | Total, n (%) |
|---|---|---|---|
| <20 years | 1 (0.6) | 1 (0.6) | 2 (1.2) |
| 20-30 years | 0 (0) | 2 (1.2) | 2 (1.2) |
| 31-40 years | 4 (2.5) | 3 (1.9) | 7 (4.4) |
| 41-50 years | 7 (4.4) | 6 (3.7) | 13 (8.1) |
| 51-60 years | 15 (9.3) | 27 (16.8) | 42 (26.1) |
| >60 years | 39 (24.2) | 56 (34.8) | 95 (59) |
| Total | 66 (41) | 95 (59) | 161 (100) |
Figure 1. Distribution of microorganisms isolated from 61 patients with DM-UTI patients (showed in number and percentage).
Other Gram-negative bacteria are included: Klebsiella pneumoniae, Klebsiella pneumoniae MDRO, Pseudomonas aeruginosa, Enterobacter spp., Raoultella terrigena, Acinetobacter baumannii MDRO, Pantoea spp., Kocuria rosea. Other Gram positive-bacteria are: Staphylococcus aureus, coagulase-negative Staphylococcus (CoNS).
Major factors observed in patients with DM contributing to development of UTI
Several elements are associated with the increase of urinary tract infection prevalence among patients with DM, such as age, gender, DM duration, and glycemic status. This study differentiates the prevalence of UTI based on productive age and non-productive age (over 60). There was no association among all those factors mentioned with UTI shown with Chi-Square test. Binary logistic analysis odds ratio and 95% CI value for age, gender, DM duration, and glycemic status are shown in Table 2.
Table 2.
Prevalence of urinary tract infection in patients with diabetes mellitus (DM) based on age, gender, DM duration and glycemic status between 2017-2018
| Characteristics | Urinary tract infection (UTI) | UTI vs. no UTI | ||||
| Positive, n (%) | Negative, n (%) | Significance | Odds ratio | 95% confidence interval | ||
| Age | ≥60 years | 36 (36.0) | 64 (64.0) | p=0.867 | 1.07 | 0.550-2.099 |
| <60 years | 21 (34.4) | 40 (65.6) | ||||
| Total | 57 (35.4) | 104 (64.6) | ||||
| Gender | Female | 38 (40.0) | 57 (60.0) | p=0.180 | 1.649 | 0.842-3.231 |
| Male | 19 (28.8) | 47 (71.2) | p=0.180 | 1.649 | 0.842-3.231 | |
| Total | 57 (35.4) | 104 (64.6) | ||||
| DM duration | > 1 years | 23 (43.4) | 30 (56.6) | p=0.162 | 1.669 | 0.847-3.288 |
| ≤ 1 years | 34 (61.82) | 74 (68.5) | p=0.162 | 1.669 | 0.847-3.288 | |
| Total | 57 (35.4) | 104 (64.6) | ||||
| Glycemic status | Controlled (< 200 mg/dL) | |||||
| Age ≥60 years | 13 (26.0) | 37 (74.0) | p=0.788 | 0.791 | 0.278-2.279 | |
| Age <60 years | 8 (30.8) | 18 (69.2) | p=0.788 | 0.791 | 0.278-2.279 | |
| Total | 21 (27.7) | 55 (72.4) | ||||
| Uncontrolled (≥200 mg/dL) | ||||||
| Age ≥60 years | 25 (49.1) | 26 (50.9) | p=0.179 | 2.010 | 0.814-4.966 | |
| Age <60 years | 11 (32.4) | 23 (67.6) | p=0.179 | 2.010 | 0.814-4.966 | |
| Total | 36 (42.4) | 49 (57.6) | ||||
Antimicrobial susceptibility pattern
The antimicrobial susceptibility pattern of UTI isolates from patients with DM was analyzed. Escherichia coli was a predominant microorganism found in UTI patients. It showed resistance to almost all the tested antibiotics, except for fosfomycin and meropenem. Escherichia coli showed resistance to ampicillin (87%), ceftriaxone (87%), tetracycline (73%), cefixime (73%), norfloxacin (53%), trimethoprim-sulfamethoxazole (47%), cefpodoxime (47%), ciprofloxacin (40%), amoxicillin-clavulanic acid (33%), and nitrofurantoin (6%). Acinetobacter baumannii was resistant to almost all the tested antibiotics, such as tetracycline (100%), ceftriaxone (100%), ampicillin (100%), nitrofurantoin (100%), fosfomycin (75%), trimethoprim-sulfamethoxazole (75%), ciprofloxacin (50%), amoxicillin-clavulanic acid (25%), norfloxacin (25%), and meropenem (25%) – Table 3. A different pattern of antimicrobial susceptibility was observed in Gram-positive bacteria. Enterococcus faecalis was resistant to ciprofloxacin (67%), meropenem (50%), ceftriaxone (50%), norfloxacin (33%), levofloxacin (33%), ampicillin (33%) and tetracycline (33%), but not resistant to nitrofurantoin (100%), vancomycin (83%) and fosfomycin (67%) – Table 4. Those pathogens also showed resistance to several antibiotics such as fluoroquinolones group: ciprofloxacin, norfloxacin; beta-lactamase group: cefixime, cefpodoxime, amoxicillin-clavulanic acid; and trimethoprim. Fortunately, these resistant pathogens were still susceptible to nitrofurantoin and fosfomycin.
Table 3.
Antimicrobial susceptibility patterns for Gram-negative bacteria found in patients with urinary tract infection and diabetes mellitus
| Antimicrobial | Pattern | Escherichia coli (n=15) | Escherichia coli (ESBL) (n=2) | Escherichia coli (MDRO) (n=2) | Klebsiella pneumoniae (MDRO) (n=2) | Pseudomonas aeruginosa (n=2) | Enterobacter spp. (n=3) | Raoultella terrigena (n=2) | Acinetobacter baumannii (n=4) |
|---|---|---|---|---|---|---|---|---|---|
| Nitrofurantoin | S | 10 (67) | 2 (100) | 2 (100) | 0 (0) | 0 (0) | 1 (33) | 0 (0) | 0 (0) |
| I | 4 (27) | 0 (0) | 0 (0) | 1 (50) | 0 (0) | 1 (33) | 1 (50) | 0 (0) | |
| R | 1 (6) | 0 (0) | 0 (0) | 1 (50) | 2 (100) | 1 (33) | 1 (50) | 4 (100) | |
| Fosfomycin | S | 14 (93) | 2 (100) | 2 (100) | 1 (50) | 1 (100) | 3 (100) | 2 (100) | 0 (0) |
| I | 0 (0) | 0 (0) | 0 (0) | 1 (50) | 0 (0) | 0 (0) | 0 (0) | 1 (25) | |
| R | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 3 (75) | |
| Trimethoprim-sulfamethoxazole | S | 7 (47) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (33) | 1 (50) | 1 (25) |
| I | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| R | 7 (47) | 2 (100) | 2 (100) | 2 (100) | 0 (0) | 1 (33) | 1 (50) | 3 (75) | |
| Ciprofloxacin | S | 4 (27) | 0 (0) | 0 (0) | 0 (0) | 1 (50) | 0 (0) | 1 (50) | 2 (50) |
| I | 2 (13) | 1 (50) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| R | 6 (40) | 1 (50) | 2 (100) | 2 (100) | 1 (50) | 2 (67) | 1 (50) | 2 (50) | |
| Norfloxacin | S | 4 (27) | 1 (50) | 0 (0) | 0 (0) | 1 (50) | 0 (0) | 2 (100) | 1 (25) |
| I | 2 (13) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (33) | 0 (0) | 0 (0) | |
| R | 8 (53) | 1 (50) | 2 (100) | 1 (50) | 0 (0) | 1 (33) | 0 (0) | 1 (25) | |
| Amoxicillin-clavulanic acid | S | 4 (27) | 1 (50) | 1 (50) | 0 (0) | 0 (0) | 0 (0) | 1 (50) | 0 (0) |
| I | 6 (40) | 0 (0) | 0 (0) | 2 (100) | 0 (0) | 1 (33) | 0 (0) | 2 (50) | |
| R | 5 (33) | 1 (50) | 1 (50) | 0 (0) | 1 (50) | 2 (67) | 1 (50) | 2 (50) | |
| Cefixime | S | 2 (13.3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (67) | 0 (0) | 0 (0) |
| I | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| R | 11 (73.3) | 2 (100) | 0 (0) | 1 (50) | 1 (50) | 0 (0) | 2 (100) | 4 (100) | |
| Cefpodoxime | S | 2 (13.3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (33) | 0 (0) | 0 (0) |
| I | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| R | 7 (47) | 2 (100) | 0 (0) | 2 (100) | 0 (0) | 0 (0) | 1 (50) | 1 (25) | |
| Ampicillin | S | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| I | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| R | 13 (87) | 2 (100) | 2 (100) | 1 (50) | 1 (100) | 3 (100) | 1 (50) | 4 (100) | |
| Meropenem | S | 14 (93) | 2 (100) | 2 (100) | 1 (50) | 2 (100) | 1 (33) | 2 (100) | 1 (25) |
| I | 0 (0) | 0 (0) | 0 (0) | 1 (50) | 0 (0) | 1 (33) | 0 (0) | 2 (50) | |
| R | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (33) | 0 (0) | 1 (25) | |
| Ceftriaxone | S | 2 (13) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (33) | 0 (0) | 0 (0) |
| I | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (50) | 0 (0) | 0 (0) | 0 (0) | |
| R | 13 (87) | 1 (50) | 2 (100) | 2 (100) | 1 (50) | 2 (67) | 2 (100) | 4 (100) | |
| Tetracycline | S | 4 (27) | 1 (50) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (50) | 0 (0) |
| I | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (50) | 0 (0) | |
| R | 11 (73) | 1 (50) | 2 (100) | 2 (100) | 0 (0) | 3 (100) | 0 (0) | 4 (100) |
ESBL – extended spectrum beta-lactamase; I – intermediate; MDRO – multidrug-resistant organism; R – resistant; S – susceptible. Data are presented as n (%).
Table 4.
Antimicrobial susceptibility patterns for Gram-positive bacteria found in patients with urinary tract infection and diabetes mellitus
| Antimicrobial | Pattern | Enterococcus faecalis (n=6) | Staphylococcus aureus (n=1) | Coagulase-negative Staphylococcus (CoNS) (n=2) | Total (n=9) |
|---|---|---|---|---|---|
| Nitrofurantoin | S | 6 (100) | 1 (100) | 1 (50) | 8 (89) |
| I | 0 (0) | 0 (0) | 1 (50) | 1 (11) | |
| R | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Fosfomycin | S | 4 (67) | 1 (100) | 1 (50) | 6 (67) |
| I | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| R | 0 (0) | 0 (0) | 1 (50) | 1 (11) | |
| Trimethoprim-sulfamethoxazole | S | 0 (0) | 1 (100) | 0 (0) | 1 (11) |
| I | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| R | 0 (0) | 0 (0) | 1 (50) | 1 (11) | |
| Ciprofloxacin | S | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| I | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| R | 4 (67) | 0 (0) | 2 (100) | 6 (67) | |
| Norfloxacin | S | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| I | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| R | 2 (33) | 1 (100) | 2 (100) | 5 (56) | |
| Levofloxacin | S | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| I | 0 (0) | 0 (0) | 2 (100) | 2 (22) | |
| R | 2 (33) | 0 (0) | 0 (0) | 2 (22) | |
| Ampicilin | S | 4 (67) | 0 (0) | 0 (0) | 4 (44) |
| I | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| R | 2 (33) | 0 (0) | 1 (50) | 3 (33) | |
| Meropenem | S | 1 (17) | 0 (0) | 0 (0) | 1 (11) |
| I | 1 (17) | 0 (0) | 0 (0) | 1 (11) | |
| R | 3 (50) | 0 (0) | 1 (50) | 4 (44) | |
| Ceftriaxone | S | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| I | 0 (0) | 1 (100) | 0 (0) | 1 (11) | |
| R | 3 (50) | 0 (0) | 2 (100) | 5 (56) | |
| Tetracyline | S | 1 (17) | 0 (0) | 2 (100) | 3 (33) |
| I | 1 (17) | 0 (0) | 0 (0) | 1 (11) | |
| R | 2 (33) | 1 (100) | 0 (0) | 3 (33) | |
| Vancomycin | S | 5 (83) | 1 (100) | 2 (100) | 8 (89) |
| I | 1 (17) | 0 (0) | 0 (0) | 1 (11) | |
| R | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
I – intermediate; R – resistant; S – susceptible. Data are presented as n (%).
Discussion
Several major factors were associated with the prevalence of urinary tract infections observed in patients with DM, listed as age, gender, DM duration, glycemic status. The productive ages in Indonesia are in the range of 18 to 55 years of age16 or below 60 years. Thus, this study differentiates the prevalence of UTI based on productive and non-productive age (over 60). The database showed a high number of patients with DM (41%) who were over 60 years of age. The prevalence of UTI in patients with DM was 3.93%, but there was no association between the risk of UTI and DM based on factors such as age, gender, and duration of DM. This present study was similar to other studies that showed no association between age and gender with the incidence of UTI.17,18 In contrast, Mama et al.19 reported the difference based on sex (female and male) which showed a significant result in the prevalence of UTI. The apparent gender preference was affected by several factors, including body mass index (BMI), waist size, clustering of metabolic syndrome, and no-leisure time physical activity.20 Prediabetes factors that contributed to different prevalence between genders were impaired fasting glucose (more often seen in men) and impaired glucose tolerance (more often found in women). Imbalance of sex hormones also affected gender preference, such as higher testosterone, low sex hormone-binding globulin (SHBG; and women usually have higher levels compared to men), polycystic ovary syndrome which can be found in females only with hyperinsulinemia and androgen excess related to obesity.20 Meanwhile, another biological factor was past gestational diabetes mellitus (with 71% higher incidence of diabetes mellitus among prediabetic women). In addition, psychosocial behaviors and unhealthy lifestyles also contributed to the progress of UTI.20
The predominant microorganism found in UTI was Escherichia coli (24.6%). This result was consistent with other studies that showed Escherichia coli as dominant uropathogen.17,18 The possible reason Escherichia coli was a causative agent of UTI is that this bacterium can occupy and reproduce within uroepithelial cells that supply a survival advantage to escape recognition and apoptosis by both innate and adaptive immune defense mechanisms.20 Meanwhile, Enterococcus faecalis (9.8%) as another dominant microorganism found in this study is usually part of the endogenous flora of the body that can cause community-acquired infection. Microorganisms from patien’s sources can cause infection with some alterations in host defenses.21 The third common bacterium found as the cause of UTI in this study was Acinetobacter baumannii (6.6%). In recent years, Acinetobacter baumannii is widely known as an important nosocomial pathogen, especially for hospitalized patients in intensive care units (ICU).22 Since this bacterium survives for a long time in the hospital, it can enhance the opportunity for cross infection.23
This study reported the occurrence of ESBL Enterobacteriaceae (Escherichia coli ESBL) and MDRO Enterobacteriaceae (Escherichia coli MDRO, Klebsiella pneumoniae MDRO, Acinetobacter baumannii MDRO). The result was consistent with the previous studies showing that patients with type 2 DM are susceptible to multidrug resistant pathogens.10,13 Based on our data, there is no association between the duration of DM with the prevalence of UTI in DM patients. It also has no contribution to the prevalence of resistant pathogen found in UTI. It was found that all patients with antibiotic resistant pathogens had been hospitalized, suggesting that all infections would have been nosocomial in origin. However, this conclusion needs further confirmation by genetic studies. Antibiotic resistant pathogens can decrease the effectiveness of therapy. Infection with antibiotic resistant pathogens can increase the mortality rate due to an inappropriate empiric antimicrobial therapy.24
In addition, fungal infection was caused by Candida spp. (26.2%). Patients with DM have an increased susceptibility to fungal infection. This fungal infection was caused by several factors. Most notably, the immunosuppression state of the patient will become dangerous in the presence of a high concentration of glucose, favoring the transition of fungi from commensal to pathogen.25 Since candiduria can complicate DM, patients should be carefully evaluated for systemic candidiasis and should be treated aggressively.26
The antimicrobial pattern showed that several microorganisms including antibiotic resistant pathogens still showed sensitivity to first-line regimen antibiotics such as fosfomycin (81% in Gram-negative bacteria and 67% in Gram-positive bacteria), nitrofurantoin (47% in Gram-negative bacteria and 89% in Gram-positive bacteria). Several microorganisms showed sensitivity towards beta-lactam antibiotics class, especially the carbapenem group such as meropenem (75% in Gram-negative bacteria but only 11% in Gram-positive bacteria). Gram-positive bacteria causing UTI in patients with DM showed a sensitivity towards vancomycin (89%), a glycopeptide antibiotic. From our study, we can give information that bacteria are still susceptible to several antibiotics to treat UTI such as fosfomycin, nitrofurantoin, and carbapenem. Meanwhile, the selection of glycopeptide antibiotics such as vancomycin should be confirmed for its efficacy, especially for treating pyelonephritis.27 Fosfomycin and nitrofurantoin can be used to treat uncomplicated cystitis patients. Carbapenem as beta-lactam antibiotics can be chosen for treating complicated urinary tract infections.
Another first line regimen usually used in treating UTI is trimethoprim-sulfamethoxazole, against which we saw high resistance in Gram-negative bacteria (58%). Almost all microorganisms in this study exhibited resistance to other beta-lactam antibiotics (amoxicillin-clavulanic acid, cefixime, cefpodoxime) with their resistance degree ranging from 39% to 67%. Fluoroquinolone antibiotics (ciprofloxacin, norfloxacin, levofloxacin) showed 22-67% resistance. Cephalosporin antibiotics, such as ceftriaxone, showed resistance in Gram-positive bacteria and Gram-negative bacteria (56% and 86%, respectively). With broad-spectrum penicillin, ampicillin also presented high resistance in Gram-negative bacteria (86%) and showed lower resistance in Gram-positive bacteria (33%). High resistance was observed against tetracycline antibiotics with 75% for Gram-negative bacteria and 33% for Gram positive. Since high resistance was observed, it was important to check the resistance level of several antibiotics such as trimethoprim-sulfamethoxazole, beta-lactam (such as cephalosporin, ampicillin), fluoroquinolone, and tetracycline before treating UTI in patients with DM.
The focus of this study is to emphasize that there are UTI cases in patients with DM that are caused by antimicrobial-resistant pathogens. While this remarkable information in Indonesia is very limited, we can encourage other hospitals or clinics to monitor the prevalence of antimicrobial-resistant pathogens and, finally, these data can be used to assist practitioners in prescribing the right choice of antibiotics for patients with UTI in Indonesia, especially in Surabaya.
To address the limitations of this study, as the patients were only collected from one public hospital in Surabaya, this restricts the conclusion of antimicrobial-resistant pathogens finding in patients with UTI-DM. Thus, it is very important that for the next study, the patient data collections can be added from several hospitals in Surabaya to give a better understanding of antimicrobial-resistant pathogens cases in patients with UTI-DM.
Conclusions
The prevalence of UTI was 3.93% in patients with DM and this number is relatively comparable with other studies. The most common etiology for UTI was Escherichia coli in Haji Hospital Surabaya, Indonesia. Antibiotic resistant pathogens found in this study were Escherichia coli ESBL and MDRO, Klebsiella pneumoniae MDRO, and Acinetobacter baumannii MDRO. The pathogens responsible for UTI in patients with DM in this study displayed susceptibility to fosfomycin, nitrofurantoin, meropenem, and vancomycin. In contrast, for antibiotics such as ceftriaxone, ampicillin, tetracycline, cefixime, ciprofloxacin or norfloxacin, resistance should be tested before using them as UTI treatment.
Acknowledgements
The authors are grateful to all the staff from Microbiology Laboratory, Haji Hospital Surabaya for their effort to support all the data used in this study. The authors also want to thank Prof Loh Teck Chwen from Universiti Putra Malaysia for his fruitful advice and suggestion to this manuscript.
Footnotes
Authors’ contributions statement: N and NA performed the experiment and data analysis. DW Indriati designed the experiment. N, DW Indriani and DW Indriati wrote the draft paper. DW Indriani and SP helped the statistical analysis and figure. All authors read and approved the final version of the study.
Conflicts of interest: All authors – none to declare.
Funding: This study was supported by research grant from Universitas Airlangga.
References
- 1.Chen L, Magliano DJ, Zimmet PZ. The worldwide epidemiology of type 2 diabetes mellitus--present and future perspectives. Nat Rev Endocrinol. 2011;8:228–36. doi: 10.1038/nrendo.2011.183. [DOI] [PubMed] [Google Scholar]
- 2.Olokoba AB, Obateru OA, Olokoba LB. Type 2 diabetes mellitus: a review of current trends. Oman Med J. 2012;27:269–73. doi: 10.5001/omj.2012.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.International DiabetesFederation. IDF Diabetes Atlas 8th Edition. International Diabetes Federation; Brussels, Belgium: 2017. [Google Scholar]
- 4.Geerlings SE, Stolk RP, Camps MJ, et al. Asymptomatic bacteriuria can be considered a diabetic complication in women with diabetes mellitus. Adv Exp Med Biol. 2000;485:309–14. doi: 10.1007/0-306-46840-9_41. [DOI] [PubMed] [Google Scholar]
- 5.Delamaire M, Maugendre D, Moreno M, Le Goff MC, Allannic H, Genetet B. Impaired leucocyte functions in diabetic patients. Diabet Med. 1997;14:29–34. doi: 10.1002/(SICI)1096-9136(199701)14:1<29::AID-DIA300>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 6.Hosking DJ, Bennett T, Hampton JR. Diabetic autonomic neuropathy. Diabetes. 1978;27:1043–55. doi: 10.2337/diab.27.10.1043. [DOI] [PubMed] [Google Scholar]
- 7.Peleg AY, Weerarathna T, McCarthy JS, Davis TM. Common infections in diabetes: pathogenesis, management and relationship to glycaemic control. Diabetes Metab Res Rev. 2007;23:3–13. doi: 10.1002/dmrr.682. [DOI] [PubMed] [Google Scholar]
- 8.Casqueiro J, Casqueiro J, Alves C. Infections in patients with diabetes mellitus: A review of pathogenesis. Indian J Endocrinol Metab. 2012;16(Suppl 1):S27–S36. doi: 10.4103/2230-8210.94253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown JS, Wessells H, Chancellor MB, et al. Urologic complications of diabetes. Diabetes Care. 2005;28:177–85. doi: 10.2337/diacare.28.1.177. [DOI] [PubMed] [Google Scholar]
- 10.Inns T, Millership S, Teare L, Rice W, Reacher M. Service evaluation of selected risk factors for extended-spectrum beta-lactamase Escherichia coli urinary tract infections: a case-control study. J Hosp Infect. 2014;88:116–9. doi: 10.1016/j.jhin.2014.07.009. [DOI] [PubMed] [Google Scholar]
- 11.Vlietinck AJ, De Bruyne T, Apers S, Pieters LA. Plant-derived leading compounds for chemotherapy of human immunodeficiency virus (HIV) infection. Planta Med. 1998;64:97–109. doi: 10.1055/s-2006-957384. [DOI] [PubMed] [Google Scholar]
- 12.Papadimitriou-Olivgeris M, Drougka E, Fligou F, et al. Risk factors for enterococcal infection and colonization by vancomycin-resistant enterococci in critically ill patients. Infection. 2014;42:1013–22. doi: 10.1007/s15010-014-0678-1. [DOI] [PubMed] [Google Scholar]
- 13.Nitzan O, Elias M, Chazan B, Saliba W. Urinary tract infections in patients with type 2 diabetes mellitus: review of prevalence, diagnosis, and management. Diabetes Metab Syndr Obes. 2015;8:129–36. doi: 10.2147/DMSO.S51792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmiemann G, Kniehl E, Gebhardt K, Matejczyk MM, Hummers-Pradier E. The diagnosis of urinary tract infection: a systematic review. Dtsch Arztebl Int. 2010;107:361–7. doi: 10.3238/arztebl.2010.0361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing, 26th ed.. CLSI supplement M100S. Wayne, PA, USA: Clinical and Laboratory Standards Institute; 2016. [Google Scholar]
- 16.Mihardja L, Soetrisno U, Soegondo S. Prevalence and clinical profile of diabetes mellitus in productive aged urban Indonesians. J Diabetes Investig. 2014;5:507–12. doi: 10.1111/jdi.12177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bonadio M, Costarelli S, Morelli G, Tartaglia T. The influence of diabetes mellitus on the spectrum of uropathogens and the antimicrobial resistance in elderly adult patients with urinary tract infection. BMC Infect Dis. 2006;6:54. doi: 10.1186/1471-2334-6-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aswani SM, Chandrashekar U, Shivashankara K, Pruthvi B. Clinical profile of urinary tract infections in diabetics and non-diabetics. Australas Med J. 2014;7:29–34. doi: 10.4066/AMJ.2014.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mama M, Manilal A, Gezmu T, Kidanewold A, Gosa F, Gebresilasie A. Prevalence and associated factors of urinary tract infections among diabetic patients in Arba Minch Hospital, Arba Minch province, South Ethiopia. Turk J Urol. 2018;45:56–62. doi: 10.5152/tud.2018.32855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mulvey MA. Adhesion and entry of uropathogenic Escherichia coli . Cell Microbiol. 2002;4:257–71. doi: 10.1046/j.1462-5822.2002.00193.x. [DOI] [PubMed] [Google Scholar]
- 21.Silverman J, Thal LA, Perri MB, Bostic G, Zervos MJ. Epidemiologic evaluation of antimicrobial resistance in community-acquired enterococci. J Clin Microbiol. 1998;36:830–2. doi: 10.1128/JCM.36.3.830-832.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mule P, Patil N, Gaikwad S. Urinary tract infections by multidrug resistant Acinetobacter species- a retrospective analysis. Acta Sci Microbiol. 2018;1:47–55. [Google Scholar]
- 23.Sinha M, Srinivasa H. Mechanisms of resistance to carbapenems in meropenem- resistant Acinetobacter isolates from clinical samples. Indian J Med Microbiol. 2007;25:121–5. doi: 10.4103/0255-0857.32717. [DOI] [PubMed] [Google Scholar]
- 24.Melzer M, Petersen I. Mortality following bacteraemic infection caused by extended spectrum beta-lactamase (ESBL) producing E. coli compared to non-ESBL producing E. coli . J Infect. 2007;55:254–9. doi: 10.1016/j.jinf.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 25.Rodrigues CF, Rodrigues ME, Henriques M. Candida sp. infections in patients with diabetes mellitus. J Clin Med. 2019;8:76. doi: 10.3390/jcm8010076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sambani E, Toulkeridis G, Stangou M, et al. Diabetic patient with fungal renal infection and fungus balls: case study. J Clin Nephrol Kidney Dis. 2017;2:1006. [Google Scholar]
- 27.Gupta K, Hooton TM, Naber KG, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: A 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011;52:e103–20. doi: 10.1093/cid/cir102. [DOI] [PubMed] [Google Scholar]