ABSTRACT
Objective:
To investigate the diagnostic accuracy of a chest X-ray (CXR) score and of clinical and laboratory data in predicting the clinical course of patients with SARS coronavirus 2 (SARS-CoV-2) infection.
Methods:
This is a pilot multicenter retrospective study including patients with SARS-CoV-2 infection admitted to the ERs in three hospitals in Italy between February and March of 2020. Two radiologists independently evaluated the baseline CXR of the patients using a semi-quantitative score to determine the severity of lung involvement: a score of 0 represented no lung involvement, whereas scores of 1 to 4 represented the first (less severe) to the fourth (more severe) quartiles regarding the severity of lung involvement. Relevant clinical and laboratory data were collected. The outcome of patients was defined as severe if noninvasive ventilation (NIV) or intubation was necessary, or if the patient died.
Results:
Our sample comprised 140 patients. Most of the patients were symptomatic (132/138; 95.7%), and 133/140 patients (95.0%) presented with opacities on CXR at admission. Of the 140 patients, 7 (5.0%) showed no lung involvement, whereas 58 (41.4%), 31 (22.1%), 26 (18.6%), and 18 (12.9%), respectively, scored 1, 2, 3, and 4. In our sample, 66 patients underwent NIV or intubation, 37 of whom scored 1 or 2 on baseline CXR, and 28 patients died.
Conclusions:
The severity score based on CXR seems to be able to predict the clinical progression in cases that scored 0, 3, or 4. However, the score alone cannot predict the clinical progression in patients with mild-to-moderate parenchymal involvement (scores 1 and 2).
Keywords: Coronavirus infections; Radiography, thoracic; Pneumonia; Respiratory insufficiency; Severe acute respiratory syndrome
RESUMO
Objetivo:
Investigar a acurácia diagnóstica de um escore de radiografia de tórax (RxT) e também de dados clínicos e laboratoriais na previsão da evolução clínica de pacientes com infecção por SARS coronavirus 2 (SARS-CoV-2).
Métodos:
Estudo piloto multicêntrico retrospectivo incluindo pacientes com infecção por SARS-CoV-2 internados nos PSs de três hospitais na Itália entre fevereiro e março de 2020. Dois radiologistas avaliaram as RxT iniciais dos pacientes de forma independente utilizando um escore semiquantitativo para determinar a gravidade do comprometimento pulmonar: escore 0 representava ausência de comprometimento pulmonar, enquanto escores de 1 a 4 representavam o primeiro (menos grave) ao quarto (mais grave) quartil de gravidade do comprometimento pulmonar. Coletaram-se dados clínicos e laboratoriais relevantes. O desfecho dos pacientes foi definido como grave se foi necessária ventilação não invasiva (VNI) ou intubação ou se o paciente faleceu.
Resultados:
Nossa amostra foi composta por 140 pacientes. A maioria era sintomática (132/138; 95,7%), e 133/140 (95,0%) apresentavam opacidades na RxT da admissão. Dos 140 pacientes, 7 (5,0%) não apresentavam comprometimento pulmonar, enquanto 58 (41,4%), 31 (22,1%), 26 (18,6%) e 18 (12,9%), respectivamente, receberam escore 1, 2, 3 e 4. Em nossa amostra, 66 pacientes foram submetidos a VNI ou intubação, 37 dos quais receberam escore 1 ou 2 na RxT inicial, e 28 pacientes faleceram.
Conclusões:
O escore de gravidade baseado em RxT parece ser capaz de prever a evolução clínica em casos com escore 0, 3 ou 4. No entanto, o escore isoladamente não consegue prever a evolução clínica de pacientes com comprometimento leve a moderado do parênquima (escores 1 e 2).
Descritores: Infecções por coronavírus, Radiografia torácica, Pneumonia, Insuficiência respiratória, Síndrome respiratória aguda grave
INTRODUCTION
In December 2019, an epidemic caused by the SARS coronavirus 2 (SARS-CoV-2) occurred in China. The most incident symptoms and clinical signs are related to impairment of the respiratory system. 1 , 2 Forms of interstitial pneumonia can be diagnosed and can sometimes require invasive ventilatory support. A rapid accurate assessment of pulmonary parenchymal damage is needed in order to design a tailored therapeutic plan. 1 , 3 - 6 CT is currently deemed the most sensitive imaging tool when coronavirus disease 2019 (COVID-19) is suspected, based on the detection of specific and highly suggestive signs (e.g., ground-glass opacities with or without consolidation in the lung periphery), 2 , 5 - 11 and recently published studies have investigated the potential role of artificial intelligence based on CT images to assess the severity of the disease and to predict the final clinical outcome. 12 - 14 However, chest X-rays are frequently requested in patients with acute pulmonary symptoms admitted to the ER, as well as in critical patients in the ICU, being a technique that is inexpensive, is largely available at the bedside, and has low radiation exposure. The relatively low sensitivity of chest X-rays in patients with a SARS-CoV-2-related interstitial pneumonia 15 - 17 could be overcome by combining chest X-rays with clinical and laboratory data, including arterial blood gas (ABG) analysis.
This pilot retrospective study aims at investigating the diagnostic accuracy of a chest X-ray score and of clinical and laboratory data in predicting the outcome of patients with SARS-CoV-2 pulmonary infection.
METHODS
Study population
A pilot retrospective multicenter study was carried out in three Italian institutions (Cattinara Hospital and Maggiore Hospital, both located in the city of Trieste; and Azienda Ospedaliera Universitaria, in the city of Sassari). Patients with SARS-CoV-2 infection, confirmed by a positive RT-PCR result from nasopharyngeal swabs performed at admission to the ER between February and March of 2020, were retrospectively identified. Patients were included if they were ≥ 18 years of age and had a chest X-ray performed at the onset of the respiratory symptoms. The most relevant clinical and epidemiological data, including smoking status, major comorbidities, and signs and symptoms at the onset of the disease were collected from the medical records of all patients (Table 1).
Table 1. Characteristics of the study patients (N = 140) at baseline and outcomes.a .
| Characteristic | Result | |
|---|---|---|
| Male, n (%) | 86/140 (61.4) | |
| Age, years | 71 [58.5-80.0] | |
| Age bracket, n (%) | < 50 years | 18/140 (12.9) |
| 50-75 years | 72/140 (51.4) | |
| > 75 years | 50/140 (35.7) | |
| Smoking status, n (%) | Never smoker | 100/139 (71.9) |
| Current smoker | 26/139 (18.7) | |
| Former smoker | 13/139 (9.4) | |
| RT-PCR, n (%) | 139/139 (100.0) | |
| Comorbidities | ||
| Presence of comorbidity, n (%) | 116/139 (83.5) | |
| BMI > 30 kg/m2, n (%) | 23/139 (16.6) | |
| COPD, n (%) | 15/139 (10.8) | |
| Diabetes, n (%) | 38/139 (27.3) | |
| Hypertension, n (%) | 79/139 (56.8) | |
| CHD, n (%) | 29/139 (20.9) | |
| Liver disease, n (%) | 6/139 (4.3) | |
| Cancer, n (%) | 24/139 (17.3) | |
| Kidney disease, n (%) | 23/138 (16.7) | |
| Immunodeficiency, n (%) | 0/139 (0.0) | |
| Symptoms | ||
| Presence of a symptom, n (%) | 132/138 (95.7) | |
| Fever, n (%) | 121/139 (87.1) | |
| Cough, n (%) | 69/138 (50.0) | |
| Sputum, n (%) | 17/138 (12.3) | |
| Dyspnea, n (%) | 79/139 (56.8) | |
| Baseline chest X-rays | ||
| Time from the onset of symptoms to performing X-ray, days | 4 [1-8] | |
| Chest X-ray score, n (%) | No lung involvement | 7/140 (5.0) |
| Lung involvement, 1-25% | 58/140 (41.4) | |
| Lung involvement, 26-50% | 31/140 (22.1) | |
| Lung involvement, 51-75% | 26/140 (18.6) | |
| Lung involvement, 76-100% | 18/140 (12.9) | |
| Baseline laboratory data | ||
| WBC/mL | 5,920 [4,145-8,850] | |
| CRP, mg/L | 29.6 [11.6-101.4] | |
| pH | 7.45 ± 0.04 | |
| PaO2, mmHg | 62.7 [53.4-76.6] | |
| PaCO2, mmHg | 34.5 ± 5.2 | |
| SaO2, % | 94.7 [91-96] | |
| HCO3, mmol/L | 24.5 ± 2.7 | |
| PaO2/FiO2 ratio | 279.0 [173.5-333.5] | |
| Mild hypoxia, n (%) | 25/97 (25.8) | |
| Moderate hypoxia, n (%) | 25/97 (25.8) | |
| Severe hypoxia, n (%) | 13/97 (13.4) | |
| Outcome | ||
| Oxygen therapy, n (%) | 96/140 (68.6) | |
| NIV, n (%) | 38/140 (27.1) | |
| Intubation, n (%) | 28/140 (20.0) | |
| Death, n (%) | 28/138 (20.9) | |
| Recovery, n (%) | 41/113 (36.3) | |
| Patient management, n (%) | Discharge | 9/115 (7.8) |
| Hospitalization | 66/115 (57.4) | |
| ICU | 40/115 (34.8) | |
BMI: body mass index; CHD: coronary heart disease; WBC: white blood cell; CRP: C-reactive protein; and NIV: noninvasive ventilation. aValues expressed as mean ± SD or median [interquartile range], except where otherwise indicated.
The clinical course of the patients was considered nonsevere when there was no hospitalization or when only oxygen therapy was necessary during hospital stay. The clinical course was considered severe when there was a need for noninvasive ventilation (NIV) or intubation or if the patient died, considered as a composite outcome and as single outcomes.
Chest X-ray imaging
Chest X-rays were obtained using the following equipment: Definium 8000 (GE Healthcare, Chalfont St Giles, United Kingdom) and Visitor T30R (Villa Sistemi Medicali, Buccinasco, Italy) in the hospitals in Trieste; and Mobilett XP Hybrid (Siemens Healthineers, Erlangen, Germany) in the Azienda Ospedaliera Universitaria in Sassari. All chest X-rays were performed in a single frontal projection in a posteroanterior view if the patient was able to maintain the standing position; in the remaining cases, an anteroposterior view in the sitting or supine position was acquired.
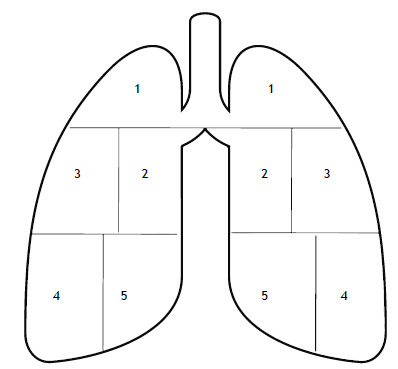
All chest X-rays were independently evaluated by two radiologists with experience in thoracic imaging (15 and 6 years’ experience, respectively); discrepant interpretations of the images were resolved by consensus. The radiologists used a semi-quantitative score in order to quantify the extent of pulmonary involvement (by less or more dense consolidations) on the chest X-rays (Figure 1). This severity score was adapted from the one proposed by Feng et al. 18 for patients with pneumonia secondary to avian influenza virus infection and was calculated as follows: each lung was divided craniocaudally into three main zones. The upper zone included the parenchymal region above the carina, the middle zone included the parenchyma below the carina and above the inferior pulmonary vein, and the lower zone involved the parenchyma below the inferior pulmonary vein; given their anatomical extent, the middle and lower zones were further divided into a lateral and a medial area (i.e. five regions per lung for a total of ten regions). A maximum of 10% of parenchymal involvement was assigned for each area. If an area was partly spared, a score of 5% was considered. The scores of each lung were summed up to provide the final severity score. A score of 0 was defined as a total lung involvement of 0%, whereas a score of 1, 2, 3, and 4 indicated a total lung involvement in the range of 1-25%, 26-50%, 51-75%, and 76-100%, respectively.
Figure 1. Chest X-ray score. Each lung is divided into three main zones (upper, middle, and lower) comprising five regions (for a total of ten regions), with a maximum of 10% of parenchymal involvement for each region.
Patients underwent a follow-up chest X-ray if there was worsening of the clinical symptoms, if a chest device was placed, or if the response to therapy needed to be assessed. These follow-up exams were scored as well.
Laboratory data
Baseline laboratory data obtained within 24 h from admission were recorded, including white blood cell (WBC) count, C-reactive protein (CRP), and ABG analysis (pH, PaO2, PaCO2, SaO2, and HCO3). In the present study, the major parameter obtained from the ABG analysis was the PaO2/FiO2 ratio, classified as follows: a PaO2/FiO2 ratio > 300 (normal); between 300 and 200 (mild hypoxia); between 200 and 100 (moderate hypoxia), and < 100 (severe hypoxia). 19
Statistical analysis
An ad hoc electronic database was created to compile all of the variables in our study. Qualitative variables were described as absolute and relative frequencies. Quantitative variables were expressed as means and standard deviations or as medians and interquartile ranges (IQR) in case of parametric or nonparametric distribution, respectively. Qualitative variables were compared with the chi-square test or the Fisher’s exact test when appropriate, whereas the Mann-Whitney test was used in order to detect any statistical differences in the comparison of nonparametric quantitative variables.
For correlations between the chest X-ray scores and the clinical outcomes of patients only the baseline chest X-rays were considered. Intraclass correlation coefficients were computed to assess interobserver reproducibility. Logistic regression analyses were carried out to assess the relationship of independent clinical, epidemiological, and demographic variables with individual and composite severe outcomes (i.e., NIV, intubation, or death). A two-tailed p-value < 0.05 was considered statistically significant. The Stata statistical software package, version 16 (StataCorp LP, College Station, TX, USA) was used for data processing and statistical analysis.
RESULTS
The study involved 140 patients, 86 (61.1%) being male. The median age was 71 (IQR: 58.8-80.0) years. Only 26 patients (18.7%) were current smokers. The most common comorbidity was hypertension, in 79 patients (56.8%), followed by diabetes, in 38 (27.3%). Almost all of the patients were symptomatic at admission to the ER (n = 134; 95.7%). Common symptoms were fever (in 87.1%), dyspnea (in 56.8%), and cough (in 50.0%). During follow-up, 73 of the 140 patients met the composite severe outcome criteria, and 28 (20.9%) died. The mean time between hospital admission and death was 7.0 ± 3.8 days (Table 1).
Of the 140 patients, 7 (5%) had no lung involvement (were scored 0) on the baseline chest X-ray, 58 (41.4%) were scored 1, 31 (22.1%) were scored 2, 26 (18.6%) were scored 3, and 18 (12.9%) were scored 4. Follow-up X-rays of the chest were performed in 74 patients, all of which showing scores ≥ 1-mean follow-up period = 6 days (range: 1-17 days). Scores of 1, 2, 3, and 4 were found, respectively, in 14, 11, 23, and 26 patients. None of the patients with a baseline score of 3 or 4 showed a decrease in their follow-up scores (Table 2). The intraclass correlation coefficient to assess interobserver reproducibility was 0.95 (95% CI: 0.93-0.96).
Table 2. Chest X-ray findings during the follow-up period (n = 78).
| Chest X-ray score | Baseline | Follow-up | p |
|---|---|---|---|
| No lung involvement, n (%) | 1 (1.3) | - | - |
| Lung involvement, 1-25%, n (%) | 35 (44.9) | 15 (19.2) | 0.0006 |
| Lung involvement, 26-50%, n (%) | 17 (21.8) | 11 (14.1) | 0.21 |
| Lung involvement, 51-75%, n (%) | 14 (18.0) | 25 (32.1) | 0.04 |
| Lung involvement, 76-100%, n (%) | 11 (14.1) | 27 (34.6) | 0.003 |
| Chest x-ray score, median (IQR) | 2 (1-3) | 3 (2-4) | < 0.0001 |
IQR: interquartile range.
Routine blood tests were performed in all patients at admission to the ER, and ABG analyses were performed in 97 patients (69.0%).
A nonsevere clinical course (no NIV, intubation, or death) was associated with a statistically significant smaller proportion of patients with a score of 3 or 4 on the baseline chest X-ray (10.5% and 4.5%, respectively; Table 3). None of the patients who were scored 0 on the baseline chest X-ray score had a severe clinical course. Patients with a severe clinical course had significantly higher median absolute WBC (p = 0.02) and CRP (p = 0.0006). In addition, the median PaO2/FiO2 ratio was lower in patients with severe disease-207.5 (IQR: 127.5-285.0)-in comparison with that of those with nonsevere disease-326.0 (IQR: 279.0-387.5; p < 0.0001; Figure 2).
Table 3. Demographic, radiological, and laboratory characteristics of the study patients, by outcome-composite outcome (noninvasive ventilation/intubation/death) and each of the outcomes separately.a .
| Characteristic | NIV/intubation/death | |||
|---|---|---|---|---|
| No (n = 67) | Yes (n = 73) | p | ||
| Male, n (%) | 37 (55.2) | 49 (67.1) | 0.15 | |
| Age, years | 71 [54-80] | 71 [60-80] | 0.42 | |
| Chest X-ray score, n (%) | No lung involvement | 7 (10.5) | 0 (0.0) | 0.005 |
| Lung involvement, 1-25% | 33 (49.3) | 25 (34.3) | 0.07 | |
| Lung involvement, 26-50% | 17 (24.4) | 14 (19.2) | 0.38 | |
| Lung involvement, 51-75% | 7 (10.5) | 19 (26.0) | 0.02 | |
| Lung involvement, 76-100% | 3 (4.5) | 15 (20.6) | 0.005 | |
| PaO2/FiO2 ratio | 326.0 [279.0-387.5] | 207.5 [127.5-285.0] | < 0.0001 | |
| WBC/mL | 5,600 [4,000-7,220] | 6,980 [4,230-9,920] | 0.02 | |
| CRP, mg/L | 20.6 [4.4-71.8] | 59.1 [18.8-134.6] | 0.0006 | |
| Characteristic | NIV | |||
| No (n = 102) | Yes (n = 38) | p | ||
| Male, n (%) | 60 (58.8) | 26 (68.4) | 0.30 | |
| Age, years | 72.0 [60.0-81.0] | 66.5 [58.0-72.0] | 0.03 | |
| Chest X-ray score, n (%) | No lung involvement | 7 (6.9) | 0 (0.0) | 0.19 |
| Lung involvement, 1-25% | 42 (41.2) | 16 (42.1) | 0.92 | |
| Lung involvement, 26-50% | 23 (22.6) | 8 (21.1) | 0.85 | |
| Lung involvement, 51-75% | 17 (16.7) | 9 (23.7) | 0.34 | |
| Lung involvement, 76-100% | 13 (12.8) | 5 (13.2) | 0.95 | |
| PaO2/FiO2 ratio | 285.5 [185.5-354.5] | 254 [127.5-292.0] | 0.04 | |
| WBC/mL | 6,140 [4,445-8,930] | 4,935 [4.020-8,520] | 0.33 | |
| CRP, mg/L | 22.1 [9.0-82.9] | 90.8 [19.5-155.5] | 0.001 | |
| Characteristic | Intubation | |||
| No (n = 112) | Yes (n = 28) | p | ||
| Male, n (%) | 62 (55.4) | 24 (85.7) | 0.004 | |
| Age, years | 71.5 [57.0-81.0] | 67.5 [62.5-72.0] | 0.30 | |
| Chest X-ray score, n (%) | No lung involvement | 7 (6.3) | 0 (0.0) | 0.35 |
| Lung involvement, 1-25% | 48 (42.9) | 10 (35.7) | 0.49 | |
| Lung involvement, 26-50% | 28 (25.0) | 3 (10.7) | 0.13 | |
| Lung involvement, 51-75% | 18 (16.1) | 8 (28.6) | 0.13 | |
| Lung involvement, 76-100% | 11 (9.8) | 7 (25.0) | 0.03 | |
| PaO2/FiO2 ratio | 287.5 [223.0-352.0] | 181.0 [138.0-240.0] | 0.005 | |
| WBC/mL | 5,765 [4,070-8,835] | 6,860 [4,450-9,140] | 0.34 | |
| CRP, mg/L | 39.6 [11.8-102.7] | 20.8 [10.7-68.4] | 0.49 | |
| Characteristic | Death | |||
| No (n = 110) | Yes (n = 28) | p | ||
| Male, n (%) | 68 (61.8) | 16 (57.1) | 0.65 | |
| Age, years | 67.0 [56.0-76.0] | 81.5 [75.0-86.5] | < 0.0001 | |
| Chest X-ray score, n (%) | No lung involvement | 7 (6.4) | 0 (0.0) | 0.34 |
| Lung involvement, 1-25% | 51 (46.4) | 6 (21.4) | 0.02 | |
| Lung involvement, 26-50% | 25 (22.7) | 6 (21.4) | 0.88 | |
| Lung involvement, 51-75% | 19 (17.3) | 6 (21.4) | 0.91 | |
| Lung involvement, 76-100% | 8 (7.3) | 10 (35.7) | < 0.0001 | |
| PaO2/FiO2 ratio | 285 [222-348] | 167 [77-209] | 0.002 | |
| WBC/mL | 5,610 [4,010-8,000] | 9,330 [5,115-11,500] | 0.001 | |
| CRP, mg/L | 25.7 [10.5-92.1] | 71.1 [19.8-147.8] | 0.02 | |
WBC: white blood cell; CRP: C-reactive protein; and NIV: noninvasive ventilation. aValues expressed as median [interquartile range], except where otherwise indicated.
Figure 2. In A and B, chest X-rays of a 72-year-old male patient. In A, the chest X-ray was scored 2 due to right perihilar opacities (arrow). The patient presented with a PaO2/FiO2 ratio = 86 and CRP = 58 mg/L at admission to the ER; NIV was required. In B, a follow-up chest X-ray seven days later was scored 2 again (bilateral opacities). In C and D, chest X-rays of a 62-year-old male patient. In C, the chest X-ray was scored 1 due to unilateral opacity (arrow). The patient presented with a PaO2/FiO2 ratio = 103 and CRP = 165 mg/L at the onset of symptoms. In D, a chest X-ray performed two days later was scored 4 due to the presence of bilateral and diffuse opacities. The patient was referred to the ICU and intubated.
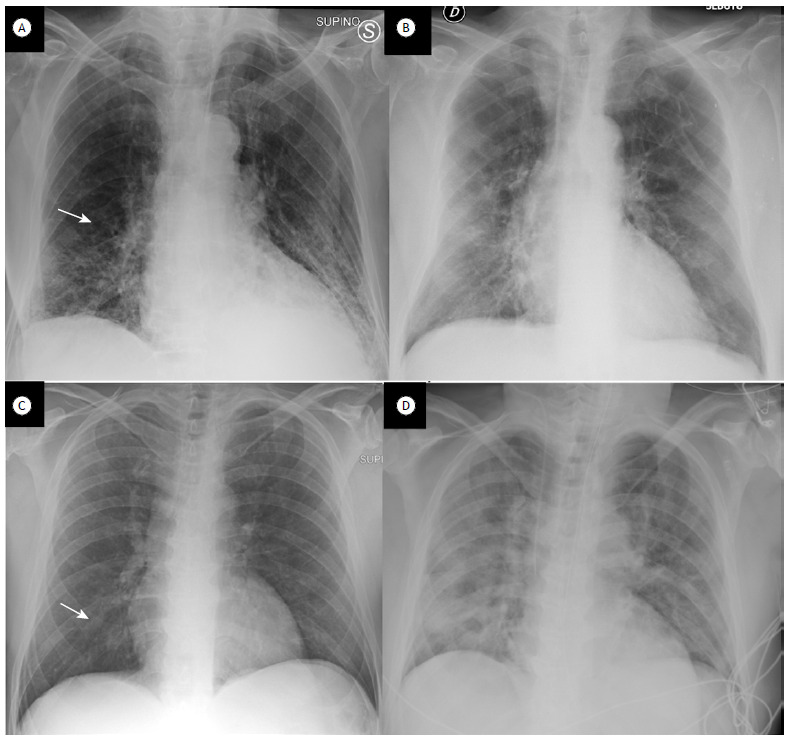
Table 3 shows the demographic, radiological, and laboratory characteristics of the study patients, by outcome-composite outcome (NIV/intubation/death) and each of the outcomes separately. No radiological differences were found between those who underwent NIV and those who did not, whereas the proportion of patients with a score of 4 was higher among those who were intubated (25%; p = 0.03) or died (35.7%; p < 0.0001). Median PaO2/FiO2 ratio was statistically lower in patients who underwent NIV (n = 254; p = 0.04), were intubated (n = 181; p = 0.005), or died (n = 167; p = 0.002). Median absolute WBC was significantly higher only in patients who died (9,330 cells/mL; p = 0.001), whereas median CRP was statistically more elevated in patients under NIV (90.8 mg/L; p = 0.001) and in those who died (71.1 mg/L; p = 0.02).
Logistic regression analyses showed that severe outcomes (NIV, intubation, or death) were associated with diabetes (OR: 4.1; p = 0.049) and moderate hypoxia (OR: 19.0; p = 0.02). The same risk factors were found for the individual outcome “intubation” (Tables S1-S4 in the supplementary material (246.6KB, pdf) ).
DISCUSSION
In our study, we performed a semi-quantitative analysis of lung involvement based on chest X-rays in patients with SARS-CoV-2 infection. Of the 140 patients, 133 (95.0%) showed lung opacities at admission, and 89 (63,5%) presented with mild-to-moderate lung involvement (scores 1 and 2).
HRCT has greater sensitivity and specificity in identifying viral pneumonia when compared with chest X-rays, especially in an early phase of the disease. 13 , 15 Even though the sensitivity of CT is greater than is that of chest X-ray, 14 - 16 the latter remains the first imaging technique of choice in patients with respiratory illnesses due to its wide availability, rapidity, low radiation exposure, and low costs. 17 Furthermore, the use of portable X-ray machines can minimize the risk of transmission and diffusion of the disease, because the infection is contained inside the isolation room of the patient. 20 Wong et al. 16 showed that chest X-rays are useful for demonstrating the presence of pulmonary abnormalities in patients with SARS-CoV-2 infection and for providing a baseline for both future examinations and the monitoring of response to therapy. Our study demonstrated the probable predictive role of X-rays in patients with no lung involvement (score 0) and in those with extensive lung disease (scores 3 and 4). In patients with mild lung parenchymal involvement (scores 1 and 2), the chest X-ray score alone was unable to predict the clinical outcome.
Follow-up X-rays of the chest are generally required to evaluate possible complications, the radiological progression of the disease, and the response to therapy, as well as to assess the placement of chest devices (e.g., central venous catheter, endotracheal cannula, pleural tube, etc.). Although the correlation of a follow-up chest X-ray score with the outcome of patients was not analyzed in the present study, this should be investigated in future studies in order to determine whether there is a correlation between radiological and clinical progression of the disease, as well as to further evaluate the predictive value of a chest X-ray score obtained at baseline.
In our study, baseline CRP levels were significantly higher in patients who needed NIV, died, or met the composite outcome criteria (NIV/intubation/death). A recent study suggested that CRP levels correlate with a CT scan severity score and may predict SARS-CoV-2 lung infection or unfavorable outcomes in patients with viral pneumonia. 21 Our data confirm the relationship between CRP and poor prognosis even in patients with mild-to-moderate lung impairment.
Our results showed that baseline WBC counts were significantly higher in patients requiring NIV, in those who met the composite outcome criteria, and in those who died. This is in line with one meta-analysis 22 that investigated biochemical and immune biomarker abnormalities associated with severe illness and mortality in patients with COVID-19. The results showed that patients who died had a significant increase in the WBC count, due to both an increase in the neutrophil count and a decrease in the lymphocyte count.
SARS-CoV-2 infects alveolar epithelial cells in the lungs and causes pneumonia or ARDS (in severe cases). 23 In our study, the degree of hypoxia of the patients was assessed using the PaO2/FiO2 ratio. Severely ill patients had a lower baseline PaO2/FiO2 ratio values than did nonseverely ill patients. In addition, the patients who died had a significantly lower PaO2/FiO2 ratio at baseline. These results confirm that a low PaO2/FiO2 ratio at baseline is predictive of poor outcomes in patients with COVID-19, similarly to what occurs in patients with ARDS caused by other conditions. Patients with such characteristics should be promptly evaluated by ICU specialists for early intubation and respiratory maneuvers with the patient in the prone position, which have been shown to reduce lung stress and strain in patients with ARDS. 24
These considerations might have clinical relevance in both low- and high-income countries and highlight the potential role of routine clinical and laboratory parameters integrated with chest X-rays, a low-cost and largely available technique, to stratify infected patients according to their risk of worsening.
Our study has limitations. Firstly, no control CT scans were available, which might have caused underdetection of opacities on chest X-rays. Only 2 patients underwent a CT scan within two days after the onset of symptoms because of a discrepancy between clinical symptoms and the radiological severity of the disease. Those patients presented with doubtful findings on their chest X-rays but had severe respiratory insufficiency. Another limitation was the small sample size, which is due to the nature of the study, and our results therefore require confirmation with a greater number of patients.
In conclusion, the severity score based on chest X-rays seems to be able to predict the clinical outcome in patients with COVID-19 when there is no lung involvement (score 0) and in severe cases (scores 3 and 4). However, a radiographic score alone is unable to predict the clinical outcome in patients with mild-to-moderate parenchymal involvement (scores 1 and 2). In these cases, the score should be associated with clinical and laboratory data in order to identify, at the onset of symptoms, those patients who may require ventilatory support during hospitalization.
Footnotes
Study carried out in the Dipartimento di Radiologia, Università degli Studi di Trieste, Trieste, Italia.
Financial support: None.
REFERENCES
- 1.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chung M, Bernheim A, Mei X, Zhang N, Huang M, Zeng X. CT Imaging Features of 2019 Novel Coronavirus (2019-nCoV) Radiology. 2020;295(1):202–207. doi: 10.1148/radiol.2020200230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernheim A, Mei X, Huang M, Yang Y, Fayad ZA, Zhang N. Chest CT Findings in Coronavirus Disease-19 (COVID-19) Relationship to Duration of Infection. Radiology. 2020;295(3):200463–200463. doi: 10.1148/radiol.2020200463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zu ZY, Jiang MD, Xu PP, Chen W, Ni QQ, Lu GM, et al. Coronavirus Disease 2019 (COVID-19): A Perspective from China [published online ahead of print, 2020 Feb 21] Radiology. 2020:200490–200490. doi: 10.1148/radiol.2020200490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li K, Wu J, Wu F, Guo D, Chen L, Fang Z. The Clinical and Chest CT Features Associated With Severe and Critical COVID-19 Pneumonia. Invest Radiol. 2020;55(6):327–331. doi: 10.1097/RLI.0000000000000672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Y, Xia L. Coronavirus Disease 2019 (COVID-19) Role of Chest CT in Diagnosis and Management. AJR Am J Roentgenol. 2020;214(6):1280–1286. doi: 10.2214/AJR.20.22954. [DOI] [PubMed] [Google Scholar]
- 7.Ai T, Yang Z, Hou H, Zhan C, Chen C, Lv W, et al. Correlation of Chest CT and RT-PCR Testing in Coronavirus Disease 2019 (COVID-19) in China: A Report of 1014 Cases [published online ahead of print, 2020 Feb 26] Radiology. 2020:200642–200642. doi: 10.1148/radiol.2020200642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu J, Wu X, Zeng W, Guo D, Fang Z, Chen L. Chest CT Findings in Patients With Coronavirus Disease 2019 and Its Relationship With Clinical Features. Invest Radiol. 2020;55(5):257–261. doi: 10.1097/RLI.0000000000000670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu YH, Dong JH, An WM, Lv XY, Yin XP, Zhang JZ. Clinical and computed tomographic imaging features of novel coronavirus pneumonia caused by SARS-CoV-2. J Infect. 2020;80(4):394–400. doi: 10.1016/j.jinf.2020.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simpson S, Kay FU, Abbara S, Bhalla S, Chung JH, Chung M. Radiological Society of North America Expert Consensus Statement on Reporting Chest CT Findings Related to COVID-19 Endorsed by the Society of Thoracic Radiology, the American College of Radiology, and RSNA - Secondary Publication. J Thorac Imaging. 2020;35(4):219–227. doi: 10.1097/RTI.0000000000000524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Han R, Huang L, Jiang H, Dong J, Peng H, Zhang D. Early Clinical and CT Manifestations of Coronavirus Disease 2019 (COVID-19) Pneumonia [published online ahead of print, 2020 Mar 17] AJR Am J Roentgenol. 2020:1–6. doi: 10.2214/AJR.20.22961. [DOI] [PubMed] [Google Scholar]
- 12.Colombi D, Bodini FC, Petrini M, Maffi G, Morelli N, Milanese G, et al. Well-aerated Lung on Admitting Chest CT to Predict Adverse Outcome in COVID-19 Pneumonia [published online ahead of print, 2020 Apr 17] Radiology. 2020:201433–201433. doi: 10.1148/radiol.2020201433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi H, Qi X, Yoon SH, Park SJ, Lee KH, Kim JY, et al. Extension of Coronavirus Disease 2019 (COVID-19) on Chest CT and Implications for Chest Radiograph Interpretation. Radiol Cardiothorac Imag. 2019;2(2):e200107. doi: 10.1148/ryct.2020200107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang R, Li X, Liu H, Zhen Y, Zhang X, Xiong Q. Chest CT Severity Score An Imaging Tool for Assessing Severe COVID-19. Radiology: Cardiothoracic Imaging. 2020;2(2):e200047. doi: 10.1148/ryct.2020200047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoon SH, Lee KH, Kim JY, Lee YK, Ko H, Kim KH. Chest Radiographic and CT Findings of the 2019 Novel Coronavirus Disease (COVID-19) Analysis of Nine Patients Treated in Korea. Korean J Radiol. 2020;21(4):494–500. doi: 10.3348/kjr.2020.0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wong HYF, Lam HYS, Fong AH, Leung ST, Chin TW, Lo CSY, et al. Frequency and Distribution of Chest Radiographic Findings in COVID-19 Positive Patients [published online ahead of print, 2019 Mar 27] Radiology. 2019:201160–201160. doi: 10.1148/radiol.2020201160. [DOI] [Google Scholar]
- 17.Bandirali M, Sconfienza LM, Serra R, Brembilla R, Albano D, Pregliasco FE. Chest Radiograph Findings in Asymptomatic and Minimally Symptomatic Quarantined Patients in Codogno, Italy during COVID-19 Pandemic. Radiology. 2020;295(3):E7. doi: 10.1148/radiol.2020201102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feng F, Jiang Y, Yuan M, Shen J, Yin H, Geng D. Association of radiologic findings with mortality in patients with avian influenza H7N9 pneumonia. PLoS One. 2014;9(4):e93885. doi: 10.1371/journal.pone.0093885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.World Health Organization . Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected: interim guidance, 13 March 2020. Geneva: World Health Organization; c2020. https://apps.who.int/iris/handle/10665/331446 [Google Scholar]
- 20.Rubin GD, Ryerson CJ, Haramati LB, Sverzellati N, Kanne JP, Raoof S. The Role of Chest Imaging in Patient Management during the COVID-19 Pandemic A Multinational Consensus Statement from the Fleischner Society. Radiology. 2020;296(1):172–180. doi: 10.1148/radiol.2020201365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tan C, Huang Y, Shi F, Tan K, Ma Q, Chen Y. C-reactive protein correlates with computed tomographic findings and predicts severe COVID-19 early. J Med Virol. 2020;92(7):856–862. doi: 10.1002/jmv.25871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Henry BM, de Oliveira MHS, Benoit S, Plebani M, Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19) a meta-analysis. Clin Chem Lab Med. 2020;58(7):1021–1028. doi: 10.1515/cclm-2020-0369. [DOI] [PubMed] [Google Scholar]
- 23.Geng YJ, Wei ZY, Qian HY, Huang J, Lodato R, Castriotta RJ. Pathophysiological characteristics and therapeutic approaches for pulmonary injury and cardiovascular complications of coronavirus disease 2019. Cardiovasc Pathol. 2020;47:107228–107228. doi: 10.1016/j.carpath.2020.107228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mentzelopoulos SD, Roussos C, Zakynthinos SG. Prone position reduces lung stress and strain in severe acute respiratory distress syndrome. Eur Respir J. 2005;25(3):534–544. doi: 10.1183/09031936.05.00105804. [DOI] [PubMed] [Google Scholar]


