Highlights
-
•
Timely access to RT remains a challenge in low- and middle-income countries.
-
•
COVID-19 pandemic has disrupted healthcare resources and capacity globally.
-
•
Radiobiological modelling indicates a somewhat lower α/β ratio for HNSCC.
-
•
Hypofractionated accelerated RT has significant resource-sparing potential.
-
•
Such regimens represent a suitable alternative to standard fractionation in HNSCC.
Keywords: Accelerated, Hypofractionation, Outcomes, Pandemic, Radiobiology
Abstract
The incidence of head and neck squamous cell carcinoma (HNSCC) is increasing worldwide, with over three quarters of cases now diagnosed in low and middle-income countries (LMICs) with resource-constraints. Loco-regional recurrence remains the predominant pattern of failure mandating adequate local therapy for acceptable loco-regional control and survival. There is high-quality evidence that intensification of treatment by either by adding concurrent chemotherapy or by altering radiotherapy (RT) fractionation improves outcomes in the curative-intent management of loco-regionally advanced HNSCC. Even conservative estimates indicate that >50% of patients in LMIC are unlikely to get access to timely RT, which will only get compounded with the coronavirus disease (COVID)-19 pandemic. The radiation oncology community has been systematically testing altered fractionation schedules in several solid cancers (breast, lung, and head-neck), given the cost-effectiveness, convenience, and compliance to short-course RT regimens. Radiobiological modelling suggests that standard fractionation of 6–7 weeks in HNSCC can be compressed safely into a 4-week schedule to counter accelerated repopulation by increasing the dose per fraction and delivering 5 fractions per week which is currently being tested in the ongoing multicentric trial of hypo- vs normo-fractionated accelerated RT (HYPNO study). Herein, we discuss the radiobiological basis of curative-intent hypofractionated-accelerated RT schedule delivering 55 Gy in 20 fractions over 4 weeks in HNSCC followed by critical appraisal of the published literature on such regimens with concurrent systemic therapy and its inherent resource-sparing potential applicable across large parts of the world particularly in the context of the ongoing COVID-19 pandemic.
Introduction
Head and neck squamous cell carcinoma (HNSCC) constitutes nearly 7% of the global cancer burden with an estimated worldwide incidence of over 600,000 new cases annually [1], [2]. Over three quarters of such cases are now seen in low and middle-income countries (LMICs), where they commonly present with loco-regionally advanced stage disease [2]. The rising incidence of HNSCC is largely driven by lifestyle related factors such as tobacco and alcohol consumption [2], [3], although human papilloma virus (HPV)-associated oropharyngeal cancer has emerged as a distinct entity and is being increasingly reported from high-income countries [2], [4]. Loco-regional recurrence remains the predominant pattern of failure mandating adequate local therapy in the form of surgery and/or radiotherapy (RT) for acceptable loco-regional control and survival. Recent emphasis on organ-preservation has spurred more widespread the use of definitive non-surgical approaches [5], [6], particularly for cancers of the larynx and pharynx. Traditionally, this was accomplished by radical RT using conventional fractionation typically defined as delivery of 1.8–2 Gy per fraction, one fraction per day, 5 fractions per week to a total dose of 66–70 Gy in 33–35 fractions over 6–7 weeks [7], [8].
Treatment intensification in HNSCC
There is now consistent and robust high-quality evidence that intensification of treatment either by combining with chemotherapy [9], [10] or altering the fractionation schedule [11], [12] improves outcomes in the curative-intent radiotherapeutic management of loco-regionally advanced HNSCC. Schedules of altered fractionation were generally designed to increase dose-intensity by delivering a higher total dose in the same overall treatment time (hyperfractionated RT), the same total dose in lesser (5–6 weeks) time (accelerated RT without total dose reduction), or a lesser total dose in even shorter (3–4 weeks) time (accelerated RT with total dose reduction). Accelerated RT where typically doses above the conventional 10 Gy per week are delivered has been shown to be associated with an improved benefit-risk ratio relative to standard normofractionated RT (2 Gy/fraction) in HNSCC, provided a careful balance between total dose, dose per fraction and overall treatment time is chosen [7], [13]. The Danish Head and Neck Cancer Group (DAHANCA) and the International Atomic Energy Agency (IAEA) reported significant improvements in loco-regional control using normofractionated-accelerated RT delivering 2 Gy/fraction, 6 fractions per week for total dose of 66–70 Gy in 33–35 fractions over 5.5 weeks compared to conventionally fractionated RT, delivering similar total dose (66–70 Gy) in 5 fractions per week over 6.5–7 weeks in large pivotal phase III randomized controlled trials [14], [15]. Radiobiological modelling suggests that this can be further compressed in 4-week schedule to counter accelerated repopulation by increasing the dose per fraction and delivering 5 fractions per week. Such a hypofractionated accelerated RT schedule delivering 55 Gy in 20 fractions over 4 weeks is currently being tested in the ongoing IAEA multicentric trial of hypo- vs normo-fractionated accelerated RT in non-nasopharyngeal HNSCC (HYPNO study), registered at clinical trials.gov (NCT0765503). Herein, we discuss the radiobiological basis of curative-intent hypofractionated-accelerated RT schedules in HNSCC followed by critical appraisal of the published literature on such regimens combined with systemic therapy and its inherent resource-sparing potential applicable across large parts of the world, particularly in the context of the ongoing coronavirus disease 19 (COVID-19) pandemic.
Radiobiological modelling
A systematic overview analysing 14 paired comparisons of altered fractionation RT (test arm) versus conventionally fractionated RT (control arm) from several phase III trials with a combined sample size of 6229 patients was conducted (unpublished data). Within each trial, a linear-quadratic (LQ) model with correction for overall treatment time was fitted to the observed tumor control data and the parameters were synthesized across trials using standard meta-analysis methodology. The best-fit estimates of the model parameters with 95% confidence interval (CI) provided an α/β ratio of 6.4 Gy (95%CI: 2.7–10.2 Gy) and Dproliferation = 0.65 Gy/day (95%CI: 0.55–0.76) to counter accelerated repopulation after 4-weeks. These estimates are roughly in agreement with previously data, but have narrower CI, particularly for the time factor, with added theoretical advantage of being derived only from randomized trials. Fractionation sensitivity of HNSCC is quantified by the parameter α/β (in Gy) and the estimate derived from this systematic overview is somewhat lesser than the often quoted ‘textbook’ value of α/β = 10 Gy [16]. This suggests that hypofractionated schedules could be associated with a slightly higher efficacy than estimated using conventional values. Radiobiological modelling and exploratory calculations suggest that a hypofractionated-accelerated schedule delivering 55 Gy in 20 fractions over 4 weeks (2.75 Gy per fraction, 5 fractions per week) could radiobiologically represent an attractive alternative to other altered fractionation regimens in HNSCC.
Comparison of fractionation schedules
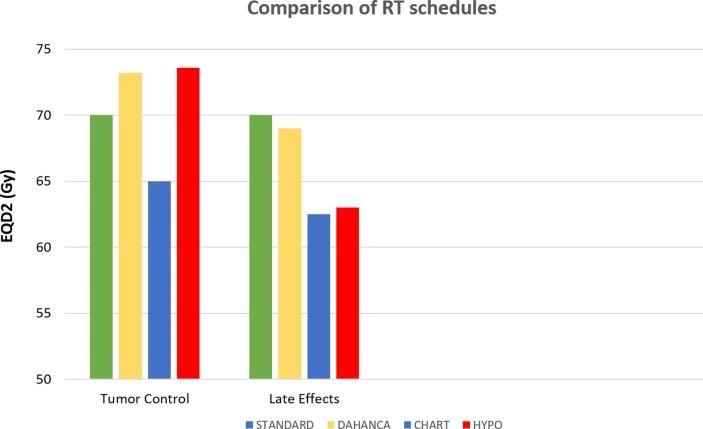
The current standard fractionation schedule in HNSCC comprising 70 Gy in 35 fractions over 7 weeks (2 Gy/fraction, 5 fractions per week) is compared for tumor control (α/β = 10 Gy) and late effects (α/β = 3 Gy) in terms of equivalent doses in 2 Gy-fractions (EQD2) with three altered fractionation RT schedules used for treating HNSCC (Figure 1 ). The DAHANCA schedule of 66 Gy in 33 fractions over 5.5 weeks (2 Gy per fractions, 6 fractions per week); the Continuous Hyperfractionated Accelerated Radiation Therapy (CHART) [17] schedule of 54 Gy in 36 fractions over 12 days (1.5 Gy per fraction, 3 fractions per day 4–6 h apart, 7 fractions per week); and the hypofractionated-accelerated schedule of 55 Gy in 20 fractions over 4 weeks (2.75 Gy per fraction, 5 fractions per week) are included for comparison. Both CHART and DAHANCA are corrected for incomplete recovery between fractions delivered in the same day, assuming 6-hour inter-fraction interval and half-life for repair (T1/2) of 4.4 h. Acceleration is assumed to kick in before 26 days after the first fraction of RT. As shown in the bar-chart (Fig. 1), both DAHANCA and hypofractionated-accelerated RT regimens are very nearly equivalent with respect to biological effect on tumor and more efficient than 70 Gy using standard fractionation. Details of bio-effect modelling for the control arm (normofractionated-accelerated RT) as well as the test arm (hypofractionated-accelerated RT) of HYPNO study for tumor control (α/β = 10 Gy) and late normal tissue effects (α/β = 3 Gy) are provided in an online supplementary file (S1). If the actual α/β for HNSCC is 6.4 Gy, i.e. the best estimate from the systematic overview and meta-analysis, then the hypofractionated-accelerated schedule of HYPNO trial would be marginally hotter with expectedly lesser late-effects than DAHANCA and be near as well tolerated as the CHART regimen.
Fig. 1.
Estimated equivalent dose in 2-Gy fractions (EQD2) of DAHANCA, CHART, and Hypofractionated-accelerated (HYPO) schedules of radiotherapy for tumor control (α/β = 10 Gy, Dproliferation = 0.65 Gy/day, and start of accelerated repopulation at 28 days) and late normal tissue toxicity (α/β = 3 Gy and no impact of overall treatment time) compared to STANDARD (70 Gy in 35 fractions over 7 weeks) fractionation in head and neck cancer.
Discussion
As per conservative estimates by IAEA [18], over 50% of patients in LMICs are unlikely to get access to timely RT, which will get further compounded by the COVID-19 pandemic. The radiation oncology community has been at the forefront of systematically testing altered fractionation schedules not only to improve the therapeutic index, but also to promote the safe and evidenced-based use of hypofractionation in several solid cancers (breast, lung, and head-neck), given the fact short-course RT is associated with cost-effectiveness, patient/care-giver convenience and better compliance. Short-course RT (40 Gy in 15 fractions over 3 weeks) is now well established as the contemporary standard of care in the post-operative radiotherapeutic management of early breast cancer [19]. However, the COVID-19 pandemic has prompted the breast oncology community to offer even shorter regimens (5-fraction RT) such as 28.5 Gy/5 fractions (5.7 Gy/fraction), once weekly over 5 weeks (FAST protocol) and 27 Gy/5 fractions (5.4 Gy/fraction) or 26 Gy/5 fractions (5.2 Gy/fraction), once daily in one week (FAST-Forward) for early stage node-negative breast cancer in clinical practice [20]. The European Society for Radiation Oncology (ESTRO)-American Society for Radiation Oncology (ASTRO) consensus statement provides pragmatic, graded, and balanced practice recommendations for thoracic RT in patients with lung cancer including judicious and appropriate use of hypofractionated regimens in order to address the challenges of the COVID-19 pandemic [21]. In head-neck cancer, hyperfractionated RT [11], [12] appears to be the best form of altered fractionation and is associated with an 8% improvement in overall survival compared to conventionally fractionated RT. However, hyperfractionation is more resource-intensive (delivering 2 fractions per day with no reduction in overall treatment time) that makes it impractical and undesirable, particularly in the current context of the COVID-19 pandemic, wherein the underlying principle is to reduce the number of fractions/visits to the hospital to reduce the risk-exposure to patients and staff, as well as to allow more efficient utilization of resources [22], [23], [24]. The recently published ASTRO-ESTRO consensus statement [25] on practice recommendations for risk-adapted head and neck cancer RT during COVID-19 pandemic turns out to be overly conservative towards altered fractionation schedules. There was strong agreement (oropharynx) and agreement (glottis and larynx) to stay with conventional dose-fractionation for definitive and even palliative head-neck RT in early pandemic scenario. Reassuringly, in the later pandemic stage, there was strong agreement to switch to more hypofractionated regimens for all sub-sites; unfortunately, without recommending any specific schedule. Panellists considered it unsafe to combine chemotherapy with higher (>2.5–2.8 Gy) dose per fraction, despite evidence for its safety. The resource-sparing potential of hypofractionated-accelerated schedule such as 55 Gy in 20 fractions over 4 weeks would make it the most suitable alternative for HNSCC in the present scenario.
Studies using hypofractionated-accelerated RT (55 Gy in 20 fractions over 4 weeks) with concurrent systemic therapy are summarized in Table 1 [26], [27], [28], [29], [30], [31], [32]. Safety and efficacy outcomes of these short-course regimens are largely similar to the usually more protracted schedules. Given the clinical equipoise, there should not be much hesitation in offering such short-course hypofractionated-accelerated regimens in clinical practice in the context of the ongoing pandemic. COVID-context regimens need not necessarily be based on high-quality (level I) evidence from randomized trials, but could be supported by prospective phase II data, retrospective studies, or even personal/institutional experience. A few years ago, the Royal College of Radiology (RCR) in the UK, had omitted hypofractionated-accelerated RT (55 Gy in 20 fractions over 4 weeks) as an option for definitive curative-intent RT of HNSCC in their updated dose-fractionation guidelines [33]. Notably, the recent RCR advisory [34] enlists the same hypofractionated-accelerated regimen as one of the evidence-based and preferred therapeutic options in the definitive curative-intent management of HNSCC during the COVID-19 pandemic. There may be some scope to further tweak this schedule to derive the most optimal regimen by striking the right balance between tumor control probability (TCP) and late normal tissue complication probability (NTCP). Based on mathematical and optimized radiobiological modelling [35], a regimen delivering 54 Gy in 18 fractions over 3.5 weeks (3 Gy per fraction, 5 fractions per week) was recently predicted to substantially increase the TCP, particularly for late-stage disease (from 35% to 49% for advanced stages) while decreasing severe late NTCP (from 13% to <2%) compared to standard-fractionation (70 Gy in 35 fractions over 7 weeks). The authors further reported that any regimen delivering >3 Gy per fraction though associated with marginally increased TCP was predicted to be suboptimal due to the unacceptably high late NTCP. Several attempts have been made to model the contribution of chemotherapy given concomitantly with RT in terms of BED for squamous cancers of various sites including HNSCC [36], [37], [38] that would lead to resultant improvement in TCP. Although this can vary somewhat based on the model used, it is widely accepted that concurrent chemotherapy adds between 4.5 and 6.8 Gy (EQD2) for squamous cell carcinoma [37], [38]. Such chemo-potentiation has not been included in the radiobiological modelling of equivalent doses in the HYPNO study protocol as concurrent weekly cisplatin is optional in the study, which is attempting to answer a pure fractionation question. Identifying the optimal hypo-fractionated-accelerated RT regimen in loco-regionally advanced HNSCC continues to remain an area of active research. One of the four experimental arms in an ongoing phase III randomized controlled trial ‘Comparing Alternative Regimens for Escalating treatment of intermediate and high-risk oropharyngeal cancer’ (ComPARE) uses 64 Gy in 25 fractions over 5 weeks with concurrent cisplatin (either 100 mg/m2 three-weekly or 40 mg/m2 weekly) against standard-fractionation RT (EudraCT No: 2014-003389-26).
Table 1.
Studies using hypofractionated-accelerated radiotherapy (55 Gy/20 fx/4wk) with concurrent systemic therapy in head and neck squamous cell carcinoma.
| Author [ref] (year) | Number of pts (N) | Disease stage | Radiotherapy regimen | Concurrent systemic therapy | Median FU | Loco-regional control & survival | Acute toxicity | Late toxicity |
|---|---|---|---|---|---|---|---|---|
| Sanghera [26] (2007) | 81 | Stage II-IV | 55 Gy/20 fx/4wk | Methotrexate (100 mg/m2) D1 & 14 | 24 mth | 2-yr LRC = 75.4% | Grade 3/4 mucositis = 65 (80%) | 1-year feeding tube dependency = 11% |
| Or Carboplatin (AUC = 4.5) on D1 & 21 | 2-yr DFS = 68.6% | Prolonged grade 3 mucositis = 7 (9%) | ||||||
| 2-yr OS = 71.6% | Grade 3 dysphagia = 44 (54%) | |||||||
| Jegannathen [27] (2010) | 43 | Stage II-IV | 55 Gy/20 fx/4wk | Cisplatin (80–100 mg/m2) wk 1 & 4 | 3.9 yr | 3-yr LRC = 70% | Grade 3 mucositis = 39 (90%) | 1-year feeding tube dependency = 14% |
| Or Carboplatin (AUC = 5) week 1 & 4 | 3-yr DFS = 60% | Prolonged grade 3 mucositis = 24 (56%) | ||||||
| Or Methotrexate (100 mg/m2) wk 1 & 3 | 3-yr OS = 60% | Prophylactic tube feeding = 11 (26%) | ||||||
| Or Capecitabine (500 mg/m2) twice daily | Reactive tube feeding = 25 (58%) | |||||||
| $Tobias [28] (2010) | 212 | Stage III-IV | 55 Gy/20 fx/4wk | Vincristine, Bleomycin, Methotrexate and Fluorouracil | 10 yr | RT + Sim CT | Hospitalization for supportive care during RT + Sim CT = 28% | Significant late (>6-month) toxicity = 6% |
| 5-yr DFS = 42% | ||||||||
| 5-yr OS = 50% | ||||||||
| Chan [29] (2011) | 150 | Stage II-IV | 55 Gy/20 fx/4wk | Carboplatin (at median dose AUC = 4) | 25 mth | 2-yr LRC = 78.3% | Grade 3/4 mucositis = 121 (81%) | 1-year feeding tube dependency = 9% |
| 2-yr DFS = 67.2% | Prolonged grade 3 mucositis = 9% | |||||||
| 2-yr OS = 74.9% | Grade 3 dermatitis = 58 (39%) | |||||||
| Jegannathen [30] (2011) | 50 | Stage III-IV | 55 Gy/20 fx/4wk | Capecitabine (450–550 mg/m2) twice daily | 6 yr | 3-yr LRC = 78% | Grade 3/4 mucositis = 47 (96%) | 1-year feeding tube dependency = 6% |
| 3-yr DFS = 62% | Feeding tube = 22 (44%) | |||||||
| 3-yr OS = 72% | ||||||||
| Beniaghat [31] (2014) | 85 | Stage II-IV | 55 Gy/20 fx/4wk | Carboplatin at AUC of 4 on D1 & 21 (n = 69) Or | 26 mth | 2-yr LRC = 68% | Grade 3 mucositis = 85 (100%) | 1-year feeding tube dependency seen only in one patient |
| Cetuximab 400 mg/m2 (loading dose 1wk before RT), 250 mg/m2 once weekly (n = 16) | 2-yr OS = 80% | Prolonged grade 3 mucositis = 9 (11%) | ||||||
| Grade 3 dermatitis = 36 (43%) | ||||||||
| Prophylactic tube feeding = 36 (43%) | ||||||||
| Reactive tube feeding = 8 (17%) | ||||||||
| Jacinto [32] (2018) | 20 | Stage III-IV | 55 Gy/20 fx/4wk | Cisplatin (35 mg/m2) once weekly | NR | ORR = 95% at 2 mth | Grade 3 mucositis = 6 (30%) | 1-year feeding tube dependency = 1 (5%) |
| CR at primary = 85% | Grade 3 dermatitis = 8 (40%) | |||||||
| CR at nodes = 40% | Median weight loss = 7.8% | |||||||
| Reactive tube feeding = 15 (75%) | ||||||||
Pts = patients, RT = radiotherapy; fx = fractions; FU = follow-up; wk = weeks; mth = months; yr = years; AUC = area under curve; D = day; LRC = loco-regional control; DFS = disease-free survival; OS = overall survival; Sim = simultaneous; CT = chemotherapy; NR = not reported; ORR = overall response rate; and CR = complete response.
Outcome data includes all patients treated with definitive RT with simultaneous CT in the UKHAN1 trial. Hazard ratios (HR) with 95% confidence intervals (CI) of survival for simultaneous chemoradiotherapy vs RT alone (in patients without surgery) was 0.77 (95%CI = 0.56–1.07) for conventional fractionation regimen; 0.83 (95%CI = 0.51–1.33) for Christie’s regimen; and 0.55 (95%CI = 0.35–0.87) for Manchester/Birmingham regimen (same as HYPNO schedule). In all regimens, the CI included the overall HR of 0.72 indicating no evidence of a differential treatment effect according to the RT fractionation.
Challenges of hypofractionated-accelerated schedules
The use of short-course regimens using a higher dose per fraction comes with its unique set of challenges. While it is easier to compensate for missed treatments in the standard schedule by either delivering it on the weekend or by giving 6 fractions in the week following the missed fraction, the same cannot be done easily for hypofractionated-accelerated schedules, to keep the delivered dose per week within safe and acceptable limits. Rapid shrinkage of a tumor, particularly a large nodal mass, due to the higher dose per fraction may increase the perceived need for adaptive re-planning with resultant unfavourable impact on workflow and resources. There exist due concerns of an increased risk of acute and possibly even late toxicity of such short-course regimens, particularly when combined with concurrent chemotherapy; however, many of these concerns are mostly theoretical and largely unfounded. Nonetheless, it is important to remember that such schedules are just about at the limits of acute normal tissue tolerance, precluding the use of concurrent high-dose three-weekly cisplatin, otherwise considered as standard of care in HNSCC. In any case, most head and neck oncologists would presently prefer avoiding high-dose three-weekly cisplatin concurrently with RT during the ongoing pandemic. However, the addition of concurrent weekly low-dose cisplatin is much safer, less resource-intensive, and can be left to the discretion of the treating physician. Nonetheless, addition of any such concurrent chemotherapy should not compromise the delivery of definitive RT.
Conclusion
Short-course hypofractionated-accelerated RT represents an attractive and suitable alternative to the more protracted regimens in non-nasopharyngeal HNSCC and can be offered in clinical practice during the ongoing pandemic which threatens to disrupt the healthcare resources and capacity globally.
Source of funding
No funding support was involved in the preparation of this manuscript.
Declaration of Competing Interest
All the authors are involved as Investigators from Tata Memorial Centre, Mumbai, India on an international multicentric randomized controlled trial comparing accelerated hypofractionated vs normofractionated RT (HYPNO study) in loco-regionally advanced head and neck cancer which has received financial support from the International Atomic Energy Agency (IAEA), Vienna, Austria.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.oraloncology.2020.105045.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Bray F., Ferlay J., Soerjomataram I. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Gupta B., Johnson N.W., Kumar N. Global epidemiology of head and neck cancers: a continuing challenge. Oncology. 2016;91:13–23. doi: 10.1159/000446117. [DOI] [PubMed] [Google Scholar]
- 3.Lee Y.C.A., Hashibe M. Tobacco, alcohol, and cancer in low and high income countries. Ann Glob Health. 2014;80(5):378–383. doi: 10.1016/j.aogh.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 4.Gillison M.L., Chaturvedi A.K., Andersen W.F. Epidemiology of human papillomavirus-positive head and neck squamous cell carcinoma. J Clin Oncol. 2015;33(29):3233–3242. doi: 10.1200/JCO.2015.61.6995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bernier J., Vermorken J.B., Debruyne C. From chemoprevention and organ preservation programmes to postoperative management: major achievements and strategies of the EORTC Head and Neck Cancer Group. Eur J Cancer. 2002;38(Suppl 4):S75–S81. doi: 10.1016/s0959-8049(01)00462-2. [DOI] [PubMed] [Google Scholar]
- 6.Forastierre A.A., Ismalia N., Lewin J.S. Use of larynx-preservation strategies in the treatment of laryngeal cancer: America Society of Clinial Oncoloy clinical practive guidelines update. J Clin Oncol. 2018;36(11):1143–1169. doi: 10.1200/JCO.2017.75.7385. [DOI] [PubMed] [Google Scholar]
- 7.Nguyen L.N., Ang K.K. Radiotherapy for cancer of the head and neck: altered fractionation regimens. Lancet Oncol. 2002;3:693–701. doi: 10.1016/s1470-2045(02)00906-3. [DOI] [PubMed] [Google Scholar]
- 8.Board of the Faculty of Clinical Oncology. Radiotherapy Dose-Fractionation, The Royal College of Radiologists, London; 2006.
- 9.Pignon J.P., Bourhis J., Domenge C., Designe L. Chemotherapy added to locoregional treatment for head and neck squamous-cell carcinoma: three meta-analyses of updated individual data. MACH-NC Collaborative Group. Meta-Analysis of Chemotherapy on Head and Neck Cancer. Lancet. 2000;355:949–955. [PubMed] [Google Scholar]
- 10.Pignon J.P., le Maitre A., Maillard E., Bourhis J. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol. 2009;92:4–14. doi: 10.1016/j.radonc.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 11.Bourhis J., Overgaard J., Audry H. Hyperfractionated or accelerated radiotherapy in head and neck cancer: a meta-analysis. Lancet. 2006;368:843–854. doi: 10.1016/S0140-6736(06)69121-6. [DOI] [PubMed] [Google Scholar]
- 12.Lacas B., Bourhis J., Overgaard Role of radiotherapy fractionation in head and neck cancers (MARCH): an updated analysis. Lancet Oncol. 2017;18(9):1221–1237. doi: 10.1016/S1470-2045(17)30458-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bernier J., Bentzen S.M. Altered fractionation and combined radio-chemotherapy approaches: pioneering new opportunities in head and neck oncology. Eur J Cancer. 2003;39:560–571. doi: 10.1016/s0959-8049(02)00838-9. [DOI] [PubMed] [Google Scholar]
- 14.Overgaard J., Hansen H.S., Specht L. Five compared with six fractions per week of conventional radiotherapy of squamous-cell carcinoma of head and neck: DAHANCA 6 and 7 randomised controlled trial. Lancet. 2003;362:933–940. doi: 10.1016/s0140-6736(03)14361-9. [DOI] [PubMed] [Google Scholar]
- 15.Overgaard J., Mohanti B.K., Begum N. Five versus six fractions of radiotherapy per week for squamous-cell carcinoma of the head and neck (IAEA-ACC study): a randomised, multicentre trial. Lancet Oncol. 2010;11:553–560. doi: 10.1016/S1470-2045(10)70072-3. [DOI] [PubMed] [Google Scholar]
- 16.Stuschke M., Thames H.D. Fractionation sensitivities and dose-control relations of head and neck carcinomas: analysis of the randomized hyperfractionation trials. Radiother Oncol. 1999;51(2):113–121. doi: 10.1016/s0167-8140(99)00042-0. [DOI] [PubMed] [Google Scholar]
- 17.Dische S., Saunders M.I., Barrett A. A randomized multicentre trial of CHART versus conventional radiotherapy in head and neck cancer. Radiother Oncol. 1997;44:123–136. doi: 10.1016/s0167-8140(97)00094-7. [DOI] [PubMed] [Google Scholar]
- 18.Zubizarreta E.H., Fidarova E., Healy B. Need for radiotherapy in low and middle-income countries - the silent crisis continues. Clin Oncol. 2015;27(2):107–114. doi: 10.1016/j.clon.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 19.Haviland J.S., Owen J.R., Dewar J.A. The UK Standardization of Breast Radiotherapy (START) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-year follow-up results of two randomized controlled trials. Lancet Oncol. 2013;14(11):1086–1094. doi: 10.1016/S1470-2045(13)70386-3. [DOI] [PubMed] [Google Scholar]
- 20.Coles C.E., Aristei C., Bliss J. International guidelines on radiation therapy for breast cancer during the COVID-19 pandemic. Clin Oncol. 2020;32(5):279–281. doi: 10.1016/j.clon.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guckenberger M., Belka C., Bezjak A. Practice recommendations for lung cancer radiotherapy during the COVID-19 pandemic: An ESTRO-ASTRO consensus statement. Radiother Oncol. 2020;151:314–321. doi: 10.1016/j.radonc.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pino L., Perez C., Cardona A. Cancer centre recommendations to mitigate COVID-19 impact in patients with cancer: low-resource settings version. JCO Glob Oncol. 2020;6:559–560. doi: 10.1200/GO.20.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simcock R., Thomas T.V., Mercy C.E. COVID-19: Global radiation oncology’s targeted response for pandemic preparedness. Clin Transl Radiat Oncol. 2020;22:55–68. doi: 10.1016/j.ctro.2020.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Felice F., Polimeni A., Valentini V. The impact of coronavirus (COVID-19) on head and neck cancer patients’ care. Radiother Oncol. 2020;147 doi: 10.1016/j.radonc.2020.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thomson D.J., Palma D., Guckenberger M. Practice recommendations for risk-adapted head and neck cancer radiotherapy during the COVID-19 pandemic: an ASTRO-ESTRO consensus statement. Int J Radiat Oncol Biol Phys. 2020;107(4):618–627. doi: 10.1016/j.ijrobp.2020.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanghera P., McConkey C., Ho K.F. Hypofractionated-accelerated radiotherapy with concurrent chemotherapy for locally advanced squamous cell carcinoma of the head and neck. Int J Radiat Oncol Biol Phys. 2007;67(5):1342–1351. doi: 10.1016/j.ijrobp.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 27.Jegannathen A., Swindell R., Yap B. Can synchronous chemotherapy be added to accelerated hypofractionated radiotherapy in patients with base of tongue cancer? Clin Oncol. 2010;22(3):185–191. doi: 10.1016/j.clon.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 28.Tobias J.S., Monson K., Gupta N. Chemoradiotherapy for locally advanced head and neck cancer: 10-year follow-up of the UK Head and Neck (UKHAN1) trial. Lancet Oncol. 2010;11(1):66–74. doi: 10.1016/S1470-2045(09)70306-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chan A.K., Sanghera P., Choo B.A. Hypofractionated accelerated radiotherapy with concurrent carboplatin for locally advanced squamous cell carcinoma of the head and neck. Clin Oncol. 2011;23(1):34–39. doi: 10.1016/j.clon.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 30.Jegannathen A., Mais K., Sykes A. Synchronous chemoradiotherapy in patients with locally advanced squamous cell carcinoma of the head and neck using capecitabine: a single-centre, open-label, single-group phase II study. Clin Oncol. 2011;23(2):149–158. doi: 10.1016/j.clon.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 31.Benghiat H., Sanghera P., Cashmore J. Four-week hypofractionated accelerated intensity modulated radiotherapy and synchronous carboplatin or cetuximab in biologically staged oropharyngeal carcinoma. Cancer Clin Oncol. 2014;3(2):2. [Google Scholar]
- 32.Jacinto A.A., Batahla Filho E.S., Viana L.S. Feasibility of concomitant cisplatin with hypofractionated radiotherapy for locally advanced head and neck squamous cell carcinoma. BMC Cancer. 2018;18:1026. doi: 10.1186/s12885-018-4893-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Royal College of Radiologists. Head and neck cancer. Radiotherapy dose fractionation (2nd Edn). At https://www.rcr.uk/system/files/publication/filed_publication_files/bfco163_6_head_neck.pdf; 2016 (last accessed 6th April 2020).
- 34.Roques T, Prestwich R. Head and neck cancer and COVID-19. Available from https://www.rcr.ac.uk/default/files/head-and-neck-cancer-treatment-covid-19.pdf (last accessed on 6th April 2020).
- 35.Shuryak I., Hall E.J., Brenner D.J. Optimized hypofractionation can markedly improve tumor control and decrease late effects for head and neck cancer. Int J Radiat Oncol Biol Phys. 2019;104(2):272–278. doi: 10.1016/j.ijrobp.2019.02.025. [DOI] [PubMed] [Google Scholar]
- 36.Jones B., Dale R. The potential for mathematical modeling in the assessment of radiation dose equivalent of cytotoxic chemotherapy given concomitantly with radiotherapy. Br J Radiol. 2005;78:939–944. doi: 10.1259/bjr/40226390. [DOI] [PubMed] [Google Scholar]
- 37.Hartley A., Sanghera P., Glaholm J. Radiobiological modelling of the therapeutic ratio for the addition of synchronous chemotherapy to radiotherapy in locally advanced squamous cell carcinoma of the head and neck. Clin Oncol. 2010;22(2):125–130. doi: 10.1016/j.clon.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 38.Petit L., Meade S., Sanghera P. Panoramic radiobiological modelling of the contribution of concomitant chemotherapy to biological effective dose in squamous cell carcinoma. Cancer Clin Oncol. 2014;3(1):1–10. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.