Abstract
Investigation of auditory verbal hallucinations (AVHs) in schizophrenics is complicated by psychiatric symptoms. Investigating healthy individuals with AVHs (H-AVHs) can obviate such confounding factors. The objective of this study was to explore the effects of antipsychotic treatment on AVHs and gray matter volumes (GMVs) in H-AVH subjects and whether such are effects are influenced by COMT-Val158Met genotype. Magnetic resonance imaging (MRI) and genotyping studies were completed for 42 H-AVH subjects and 42 well-matched healthy controls (HCs). COMT-Met/Met homozygotes (158th codon) were identified as COMT-Met genotype; COMT-Met/Val heterozygotes and COMT-Val/Val homozygotes were identified as COMT-Val genotype. Data were compared across groups (H-AVH vs. HC, and between genotypes) with two-sample t-tests. The H-AVH COMT-Met group showed a stronger response to antipsychotic treatment than the H-AVH COMT-Val group (p < 0.001). Both H-AVH genotype groups exhibited temporal lobe GMV reductions after treatment, and relative to their respective genotype-matched HC groups. Antipsychotic treatment effects in H-AVH subjects were influenced by COMT-Val158Met genotype and associated with widespread GMV reductions. These findings provide clues for further exploration of treatment targets for AVHs. Treatment associated GMV reductions, however, raise concerns about use of antipsychotics in H-AVH subjects.
Keywords: Auditory verbal hallucination, Anti-psychotic, Hi-AVHs, COMT, MRI
Introduction
Emprirical studies have indicated that some 0.7% of the general population has experienced auditory verbal hallucinations (AVHs) defined based on Jhons’ strict criteria (Johns et al. 2004; Sommer et al. 2010; Upthegrove et al. 2016). Various hypotheses have been proposed to explain the AVHs in patients with schizophrenia from different perspectives (Alderson-Day and Fernyhough 2016; Alderson-Day et al. 2017; Alderson-Day et al. 2015; Baumeister et al. 2017; Cho and Wu 2014; Conde et al. 2016; Curcic-Blake et al. 2017; Hugdahl 2015; Jones 2010; Liemburg et al. 2012; McCarthy-Jones et al. 2014; Northoff 2014; Wilkinson 2014; Wilkinson and Fernyhough 2017); however, none of the proposed hypotheses has achieved general acceptance (Wilkinson and Fernyhough 2017). To the best of our knowledge, no published research has focused on investigating the effects of antipsychotic medication on AVHs and accompanied brain alterations in H-AVH subjects.
The efficacy of antipsychotic drugs in patients with schizophrenia has been shown to be related to catechol-o-methyl transferase (COMT) genotype at the 158th codon, where a valine-to-methionine substitution (rs4680) is common (Huang et al. 2016; Olgiati et al. 2009; Sagud et al. 2010). COMT with Val in this location is much more efficient at removing dopamine than COMT with Met at this location. Hence, COMT-Val/Val homozygotes exhibit very efficient COMT activity, COMT-Met/Met homozygotes exhibit hypo-efficient COMT activity, and Val/Met heterozygotes carrying both variants of the enzyme exhibit an intermediate activity level, generally within normal range. COMT-Val158Met genotype (COMT genotype from here forward for simplicity) has also been shown to influence structural and functional aspects of auditory processing, including dopaminergic alterations in both healthy subjects and patients with schizophrenia (Edgar et al. 2012; Gothelf et al. 2011; Kang et al. 2010; Li et al. 2015; Lu et al. 2007; Steiner et al. 2018; Tian et al. 2013a; Tian et al. 2013b). Hence, a convergence of findings indicates that there may be reciprocal interactions between COMT genotype, dopamine levels, and structural/functional brain alterations in relation to neuropsychiatric symptoms, such as AVHs (Edgar et al. 2012; Gothelf et al. 2011; Huang et al. 2016; Kang et al. 2010; Li et al. 2015; Lu et al. 2007; Sagud et al. 2010; Steiner et al. 2018; Tian et al. 2013a; Tian et al. 2013b).
Schizophrenics with the COMT-Met/Met genotype respond more strongly to antipsychotics, in terms of positive symptom alleviation, than schizophrenics with the COMT-Val/Val genotype, and this response is associated with characteristic brain structural alterations (Edgar et al. 2012; Gong et al. 2016; Lei et al. 2015). Although AVHs are a classic positive symptom of schizophrenia (Reed et al. 2018; Tandon 2013), no study has reported the effects of antipsychotics on AVHs per se in schizophrenic patients. Typically, AVHs have been encompassed within a positive symptom cluster without explicit distinction (Huang et al. 2016; Olgiati et al. 2009; Sagud et al. 2010). To the best of our knowledge, there has been one study that has reported that adjuvant transcranial direct current stimulation alleviated AVHs more effectively in schizophrenia patients with a COMT-Met/Met genotype than in those with a COMT-Val/Val genotype (Chhabra et al. 2018). A recent systematic review reported that antipsychotics can improve AVHs in patients with borderline personality disorder (Slotema et al. 2018), which suggests that it may be feasible to explore antipsychotic effects on isolated AVHs. Moreover, several studies have recommended possible antipsychotic use to treat AVHs in otherwise healthy patients (Snitz et al. 2006; de Leede-Smith and Barkus 2013; Upthegrove et al. 2016; Vallath et al. 2018).
Exploratory studies of H-AVH subjects, including examining the effects of antipsychotic treatment, can provide fundamental information about the mechanisms underlying AVHs. Investigation of AVHs in H-AVH subjects can provide important information to help clarify the precise pathological features of AVHs and avoid many confounding factors, such as other psychiatric symptoms (Hugdahl 2015; Jones 2010; Wilkinson and Fernyhough 2017).
It is not yet clear how COMT genotype may be related to AVH severity in H-AVH subjects, particularly with respect to brain structural alterations and the effectiveness of antipsychotics for treating AVHs. We hypothesize that the effectiveness of antipsychotic treatment for AVH symptoms in H-AVH subjects may be influenced by COMT genotype and that this genotype variation will have accompanying structural brain alterations. In the present study, we employed genotyping and magnetic resonance imaging (MRI) with statistical parametric mapping (SPM) techniques to explore the influence of COMT genotype on AVH symptoms and the effectiveness of a 6-month antipsychotic drug treatment regimen on AVHs and gray matter volumes (GMVs) in H-AVH subjects.
Materials and methods
Sample
We used advertisements in 1000 local communities (total resident population > 200,000) to recruit H-AVH volunteers from January 1, 2016 to June 31, 2018. We enrolled 300 healthy people with diagnosed AVHs; among them, 115 subjects reported that they had suffered mental distress caused from the AVHs and volunteered to accept pharmacological treatment with risperidone (Johns and Johns, Xi’an Yang-Sen Pharmaceutical Co., Ltd.) at dosages in the range of 100–500 mg/d (chlorpromazine equivalent dosing). Exclusion criteria included diagnosis of any other mental disorder by psychiatrist, according to the Structured Clinical Interview for DSM-5, or diagnosis of a neurological disease by a neurologist according to standard neurological diagnostic criteria. From a cohort of subjects who participated in a prior pilot study, we recruited healthy control (HC) subjects matched to the H-AVH subjects with respect to COMT genotype, gender, age, and education level. The Tianjin Anding Hospital ethics review board approved this study and all patients provided written consent. The assessments were carried out in compliance with the Declaration of Helsinki guidelines and approved by the institutional ethics committee.
Self-report assessments
We used the Wisconsin Card Sorting Test (WCST, Westwood et al. 2016) and global assessment scale (GAS, Dauwan et al. 2016) to monitor benefit/risk ratios in H-AVH subjects. AVHs were assessed with the auditory verbal hallucinations rating scale (AHRS) (Haddock et al. 1999). The AHRS, which is one of two components of the Psychotic Symptom Rating Scales instrument set (the other assesses delusions), was shown to have excellent inter-rater reliability. The AHRS consists of 11 items addressing the following aspects of hallucinations: negative content amount; negative content degree; distress amount; distress intensity; frequency; duration; loudness; disruption; control; location; and (beliefs regarding) origin distress.
Genotyping
Blood collection and genotyping were performed as previously reported (Chhabra et al. 2018). Briefly, 5 ml of peripheral blood was collected in K2EDTA-treated vacutainers (Becton & Dickinson, Franklin Lakes, NJ), and genomic DNA was extracted using commercial spin columns (Qiagen, Inc., Limburg, the Netherlands). The quality of extracted DNA was determined by ultraviolet spectrophotometry (Thermo Scientific, Waltham, MA). We submitted genomic DNA subjected to COMT genotyping at rs4680 using the TaqMan 5′ nuclease allelic discrimination assay. The genotyping was performed by real-time polymerase chain reaction (PCR) in a 96-well plate (StepOne Plus™ Real-Time PCR Systems, Applied Biosystems) with predesigned, commercially available primers and allele-specific minor groove binding probes (FAM and VIC; Applied Biosystems, Foster City, CA) in a reaction volume of 10 μl (10 ng of genomic sample DNA, assay mix and PCR Universal Master Mix with AmpErase® uracil-DNA glycosylase) as follows: 60 °C for 30 s, and 95 °C for 10 min, followed by 50 cycles of 92 °C for 15 s and 60 °C for 90 s. PCR was performed in duplicate with both positive and negative controls. Genotypes were grouped by allele dominance (Chhabra et al. 2018). That is, COMT-Met/Met homozygotes were regarded as having the COMT-Met genotype and COMT-Met/Val heterozygotes and COMT-Val/Val homozygotes were regarded as having the COMT-Val genotype (Kang et al. 2010).
Imaging data acquisition
All MRI data were obtained on a 3.0-T MR system (Discovery MR750, General Electric, Milwaukee, WI). Tight but comfortable foam padding was used to stabilize head position, and earplugs were used to reduce scanner noise during image acquisition. A three-dimensional T1-weighted brain volume sequence with 188 sagittal slices was performed with the following parameters: repetition time = 8.2 ms; echo time = 3.2 ms; inversion time = 450 ms; flip angle = 12°; field of view = 256 mm2; matrix = 256 × 256; slice thickness = 1 mm, no gap.
GMV calculation
Voxel-wise GMVs were calculated by SPM in SPM8 software (http://www.fil.ion.ucl.ac.uk/spm/software/spm8/). Employing the standard unified segmentation model, we segmented images into gray matter, white matter, and cerebrospinal fluid. After affine registration of the gray matter concentration map into Montreal Neurological Institute space with diffeomorphic anatomical registration and exponentiated Lie algebra (DARTEL), gray matter concentration images were warped nonlinearly and converted to a 1.5-mm3 voxel size. The nonlinear determinants were derived from the spatial normalization step and multiplied by the gray matter concentration map to obtain the GMV of each voxel. GMV images were smoothed with a 6-mm3 full width at half maximum Gaussian kernel. The normalized, modulated, and smoothed GMV maps were used for statistical analyses after spatial preprocessing as described in detail previously (Zhuo et al. 2017).
Statistical analysis
Means are reported with standard deviations (SDs). A two-sample t-test was used to compare GMVs between groups (H-AVH vs. HC, and COMT-Met vs. COMT-Val) and time points (baseline vs. after 6 months of antipsychotic treatment) in a voxel-wise manner with adjustment for age and sex. The family-wise error method was used to correct for multiple comparisons (p < 0.05).
Results
Group characteristics
Ultimately, 34 COMT-Met and 45 COMT-Val H-AVH subjects underwent dopamine antagonist treatment for 6 months. We obtained complete and fully analyzable MRI data from 25 COMT-Met subjects and 21 COM-Val subjects (at baseline and 6 months later). We factitiously discarded data from 3 COMT-Met subjects and 1 COMT-Val subject (see limitations paragraph in the Discussion), preserving 22 COMT-Met and 20 COMT-Val H-AVH subjects for further analysis. The two H-AVH genotype groups did not differ significantly with respect to gender ratio, age, educational level, AVH duration, and AVH symptom severity. The sociodemographic, genotype, and treatment response characteristics of the two H-AVH genotype groups are compared in Table 1. The HC group consisted of 22 COMT-Met and 20 COMT-Val subjects as well; the characteristics of the two HC genotype groups are summarized in Table 2.
Table 1.
Sociodemographic and clinical characteristics of the Hi-AVHs genotype groups
| Characteristic | COMT-Met | COMT-Val | t/x2 | p |
|---|---|---|---|---|
| Mean age, years | 24.91 ± 3.58 | 25.23 ± 3.61 | −0.293 | 0.771 |
| Gender, males:females | 7:15 | 10:10 | 1.437 | 0.231 |
| Mean education level | 11.91 ± 2.33 | 11.86 ± 1.81 | 0.072 | 0.943 |
| Mean AVH duration, months | 128.73 ± 40.84 | 129.86 ± 43.69 | −0.089 | 0.929 |
| Mean risperidone dosage, mg/d, chlorpromazine equivalent | 250.40 ± 50.10 | 300.15 ± 75.50 | −2.564 | 0.014 |
| Mean AHRS scores | ||||
| Baseline (Bl) | 28.41 ± 4.25 | 28.32 ± 5.40 | −0.279 | 0.781 |
| Posttreatment (Pt) | 12.18 ± 4.08 | 23.14 ± 5.89 | 6.781 | 0.000 |
| Baseline vs. posttreatment | t = 8.341 | t = 2.974 | – | – |
| p < 0.001 | p = 0.005 | |||
The auditory verbal hallucinations scale (AHRS, Gillian Haddock, University of Manchester, 1999) was used to assess AVH symptom severity
Table 2.
Sociodemographic characteristics of the HC genotype groups
| Characteristic | COMT-Met | COMT-Val | t/x2 | p |
|---|---|---|---|---|
| Mean age, years | 24.50 ± 2.50 | 25.00 ± 4.2 | 0.474 | 0.638 |
| Gender, males:females | 7:15 | 10:10 | 1.437 | 0.231 |
| Mean education level, years | 12.50 ± 3.68 | 13.50 ± 2.50 | 1.020 | 0.314 |
Antipsychotic dosage differed significantly between the two H-AVH genotype groups, with COMT-Val subjects receiving significantly higher risperidone dosages than COMT-Met subjects (Table 1). Despite their being treated with lower antipsychotic dosing, and otherwise comparable medication regimens, the treatment was markedly more effective at alleviating AVHs in COMT-Met H-AVH subjects than in COMT-Val H-AVH subjects (Table 1).
Baseline GMVs
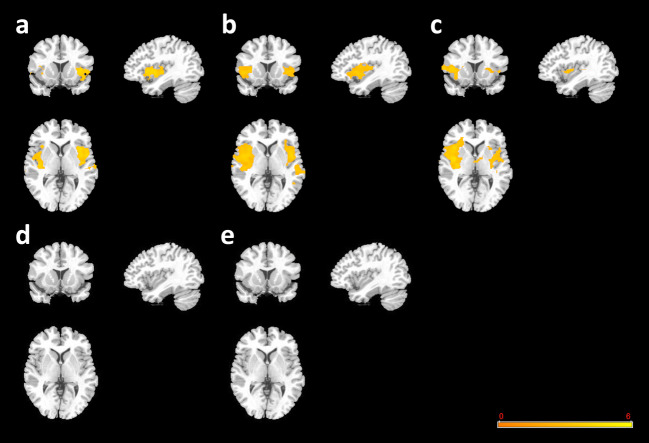
We observed enlarged GMVs, mainly in the temporal lobes, in all Hi-AVH subjects at baseline compared to GMVs observed in the HC reference group (Fig. 1a). Similarly, comparing GMVs in H-AVH subjects with the COMT-Met genotype versus GMVs in HCs with the same genotype, we found larger GMVs mainly in the temporal lobes (Fig. 1b). Interestingly, the scope of GMV differences between H-AVH subjects and HCs was more pronounced when only COMT-Met genotype groups were compared. H-AVH subjects with a COMT-Val genotype also had larger GMVs in their temporal lobes than HCs with a COMT-Val genotype (Fig. 1c). The GMV enlargements in the H-AVH COMT-Val group, however, were smaller, particularly in the left temporal lobe, than the enlargements seen for the whole H-AVH cohort and for the H-AVH COMT-Met genotype group. We did not observe significant GMV differences related to COMT genotype (Met vs. Val) within the HC (Fig. 1d) or H-AVH cohorts (Fig. 1e).
Fig. 1.
GMV baseline comparisons. a H-AVHs at baseline vs. HCs (genotype groups combined). b H-AVH COMP-Met group at baseline vs. HC COMT-Met group. c H-AVH COMP-Val group at baseline vs. HC COMT-Val group. d HC COMT-Met group vs. HC COMT-Val group. e H-AVH COMT-Met group at baseline vs. H-AVH COMT-Val group at baseline. Warm pseudocolor represents increased GMV in the former group relative to the second (reference) group
Treatment effects on GMVs in H-AVH subjects
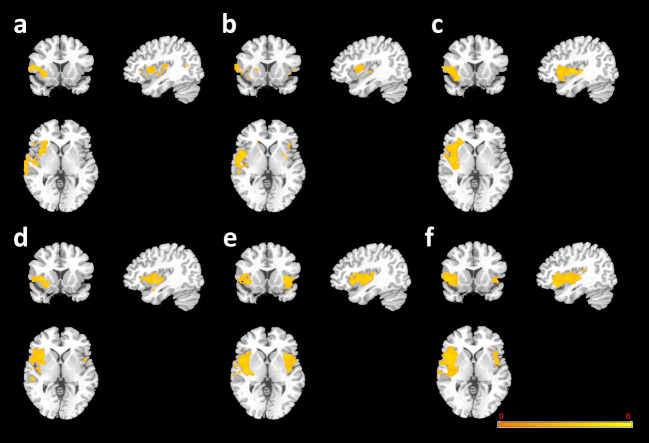
After 6 months of risperidone treatment, we observed obvious GMV reductions in the H-AVH participants compared to HCs (Fig. 2a) and compared to their own pretreatment baseline scans (Fig. 2b), with the latter difference appearing to be more widespread. Looking only at H-AVH subjects with a COMT-Met genotype after versus before treatment, the GMV reduction pattern was more prominent than in the combined genotypes comparison, particularly in the right temporal lobe (Fig. 2c). Conversely, looking only at H-AVH subjects with a COMT-Val genotype after versus before treatment, the GMV reductions were less pronounced than in the H-AVH COMT-Met group (Fig. 2d). After 6 months of risperidone treatment, both H-AVH subjects with a COMT-Met genotype (Fig. 2e) and H-AVH subjects with a COMT-Val genotype (Fig. 2f) showed reduced GMVs in their temporal lobes relative to their genotype-matched HC groups.
Fig. 2.
Antipsychotic treatment effects on GMVs in H-AVH subjects. a Posttreatment H-AVHs vs. HCs (both genotype groups combined). b Posttreatment H-AVH vs. baseline H-AVH (both genotype groups combined). c H-AVH COMT-Met group only at 6 months posttreatment vs. baseline. d H-AVH COMT-Val group only at 6 months posttreatment vs. baseline. e H-AVH COMT-Met group posttreatment vs. HC COMT-Met group. f H-AVH COMT-Val group posttreatment vs. HC COMT-Val group. Cool pseudocolor represents decreased GMV in the former group relative to the second (reference) group
Cognitive status
WCST and GAS scores obtained for all H-AVH subjects were within normal range at baseline (before treatment) and remained within normal range after 6 months of risperidone treatment.
Discussion
In the present study, we demonstrated for the first time that antipsychotic drug effects in H-AVH subjects are influenced by COMT genotype and that this distinction is accompanied by corresponding structural changes in the brain, particularly in the temporal lobes. Importantly, we compared GMVs after 6 months of risperidone treatment to pretreatment baseline GMVs within each H-AVH genotype group, providing supplementary information regarding the pathological features of H-AVH participants with specific COMT genotypes. Notably, alleviation of AVH symptoms was more pronounced in H-AVH subjects with a COMT-Met genotype than in H-AVH subjects with a COMT-Val genotype. Despite the limitations of this study (delineated below), our results provide clues to guide future studies.
Our findings of enlarged temporal lobe GMVs in H-AVH subjects, regardless of COMT genotype, compared to HCs, indicate that these alterations may reflect pathological features of AVHs, consistent with the hypotheses that AVHs may be related to structural abnormalities of the temporal lobe and that temporal lobe hyperactivity may be an intrinsic feature of AVH symptomology (Curcic-Blake et al. 2017; Hugdahl 2015; Kompus et al. 2011; Morch-Johnsen et al. 2017; Steinmann et al. 2014; Upthegrove et al. 2016; van Lutterveld et al. 2014; Wigand et al. 2015). Our findings of antipsychotic-induced reductions in temporal lobe GMVs provide additional indirect support for the hypothesis that an enlarged temporal lobe is an intrinsic feature of AVH symptomology (Hugdahl 2015; Kompus et al. 2011; Morch-Johnsen et al. 2017; Steinmann et al. 2014; van Lutterveld et al. 2014; Wigand et al. 2015). Our negative findings of no GMV differences between COMT-Met and COMT-Val genotype H-AVH groups at baseline suggest that, developmentally, COMT genotype does not influence temporal lobe GMV enlargement in H-AVH subjects, despite a genotype effect on AVH symptom severity and antipsychotic drug effectiveness for AVH symptom alleviation.
Partial correlation analysis and Pearson correlation analysis (according to variable properties) did not reveal any correlations among GMV alterations, AVH alterations, risperidone dosage, and duration of AVH symptoms at any examined time point (baseline, 6 months after treatment). These negative findings support the notion that AVH symptoms and antipsychotic-induced GMV reductions in H-AVH subjects may be related substantially to COMT genotype.
More importantly, we found that antipsychotic medication induced worrisome GMV reductions. Although many studies reported that antipsychotics can induce frontal-temporal GMV reduction in patients with schizophrenia (Ho et al. 2011; Andreasen et al. 2013; Lawrie 2018), the GMV reductions observed in our H-AVH participants in this study exceeded our expectations with respect to both scope and rapidity. These findings raise the concern that long-term antipsychotic use has the potential risk for widespread, detrimental GMV reductions. Therefore, we would not recommend antipsychotics as a first-line treatment for AVHs in otherwise healthy patients. Such patients may be better served by other approaches, such as psychotherapy, transcranial direct current stimulation, or avatar therapy (Dollfus et al. 2018; du Sert et al. 2018; Plewnia et al. 2018; Stephanie et al. 2018; Thomas et al. 2018; Craig et al. 2018).
Limitations
This study has at least nine notable limitations, and we hope sincerely that constructive dialogue with international scholars will provide guidance for our subsequent studies. First, despite our multiple retention efforts, approximately half of the subjects did not complete the full 6-month study. Given the importance of longitudinal monitoring to clarify the dynamic trajectory of AVH characteristics, even greater efforts are needed to retain a large study sample. Second, to explore potentially objective evaluation indices, we discarded some subjects’ data due to excessive deviation from the bulk of the cohort (outlier exclusion). Moving forward from the present pilot study, it will be important to strengthen our methods to enable heterogeneous subject samples to be analyzed. Third, we adopted a relatively simple GMV metric for exploring brain alterations. We intend to apply more precise image data analysis methods in future studies. Fourth, we focused on COMT genotype; other genes (e.g. FOX2 and NRG1) would be of interest to examine in a similar context. Furthermore, genomic, transcriptomic, and even proteomic methods may provide complementary information in the future. Fifth, we used a 3.0-T scanner though there are higher-resolution scanners (e.g. 7.0-T) in use in China. We hope that a strategic collaboration may enable us to conduct future studies with a higher resolution MRI scanner. Sixth, in this pilot study, we did not analyze different treatment periods or reciprocal gene interaction effects. Seventh, we did not include a schizophrenia patient comparison group. Eighth, we did not compare data between the participants who fully completed the study and those who did not. Thus, it is possible that those who did not complete the study did experience the same magnitude of symptom benefit and/or GMV reduction as the analyzed participants. Finally, we administered only the WSCT and GAS to monitor cognitive ability. We intend to administer more precise cognitive tests in future studies.
Conclusion
COMT genotype was found to influence antipsychotic drug effects on AVH symptoms in H-AVH subjects. Compared to COMT-Val subjects, COMT-Met subjects responded more strongly to antipsychotic treatment with respect to both AVH symptoms and the magnitude of GMV reductions observed. Although the H-AVH subjects retained normal-range cognitive ability, as evidenced by the WSCT and GAS, throughout the study, the marked GMV reductions observed raise concerns that antipsychotic pharmacotherapy may not be well suited for H-AVH subjects. We would suggest that other approaches, such as psychological therapy, transcranial direct current stimulation, or avatar therapy (Dollfus et al. 2018; du Sert et al. 2018; Plewnia et al. 2018), be administered first to this otherwise healthy population. Despite the aforementioned limitations, these findings provide primary information toward explaining the mechanisms of AVHs and highlighting potential targets for AVH treatment in H-AVH subjects as well as, perhaps, schizophrenic patients.
Funding
This work was supported by grants from the Tianjin Health Bureau Foundation (2014KR02 to C.Z.), the National Natural Science Foundation of China (81871052 to C.Z., 81801679 and 81571319 to Y.X.), and Key Projects of the Natural Science Foundation of Tianjin, China (17JCZDJC35700 to C.Z.)
Compliance with ethical standards
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
The Tianjin Anding Hospital ethics review board approved this study and all patients provided written consent. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Chuanjun Zhuo, Langlang Cheng and Gongying Li contributed equally to this work.
Contributor Information
Chuanjun Zhuo, Email: chuanjunzhuotjmh@163.com, Email: chuanjunzhuotjmh@ieee.com.
Xiaodong Lin, Email: 44716171@qq.com.
Chunhua Zhou, Email: zhouchunhua80@126.com.
References
- Alderson-Day B, Fernyhough C. Auditory verbal hallucinations: social, but how? Journal of Consciousness Studies: Controversies in Science & the Humanities. 2016;23:163–194. [PMC free article] [PubMed] [Google Scholar]
- Alderson-Day B, McCarthy-Jones S, Fernyhough C. Hearing voices in the resting brain: a review of intrinsic functional connectivity research on auditory verbal hallucinations. Neuroscience and Biobehavioral Reviews. 2015;55:78–87. doi: 10.1016/j.neubiorev.2015.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderson-Day B, Lima CF, Evans S, Krishnan S, Shanmugalingam P, Fernyhough C, Scott SK. Distinct processing of ambiguous speech in people with non-clinical auditory verbal hallucinations. Brain: A Journal of Neurology. 2017;140:2475–2489. doi: 10.1093/brain/awx206. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Liu D, Ziebell S, Vora A, Ho BC. Relapse duration, treatment intensity, and brain tissue loss in schizophrenia: a prospective longitudinal MRI study. The American Journal of Psychiatry. 2013;170:609–615. doi: 10.1176/appi.ajp.2013.12050674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumeister D, Sedgwick O, Howes O, Peters E. Auditory verbal hallucinations and continuum models of psychosis: a systematic review of the healthy voice-hearer literature. Clinical Psychology Review. 2017;51:125–141. doi: 10.1016/j.cpr.2016.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhabra H, Shivakumar V, Subbanna M, Kalmady SV, Bose A, Agarwal SM, Sreeraj VS, Dinakaran D, Narayanaswamy JC, Debnath M, Venkatasubramanian G. Gene polymorphisms and response to transcranial direct current stimulation for auditory verbal hallucinations in schizophrenia. Acta Neuropsychiatrica. 2018;30:218–225. doi: 10.1017/neu.2018.4. [DOI] [PubMed] [Google Scholar]
- Cho R, Wu W. Is inner speech the basis of auditory verbal hallucination in schizophrenia? Frontiers in Psychiatry. 2014;5:75. doi: 10.3389/fpsyt.2014.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conde T, Goncalves OF, Pinheiro AP. A cognitive neuroscience view of voice-processing abnormalities in schizophrenia: a window into auditory verbal hallucinations? Harvard Review of Psychiatry. 2016;24:148–163. doi: 10.1097/hrp.0000000000000082. [DOI] [PubMed] [Google Scholar]
- Craig TK, Rus-Calafell M, Ward T, Leff JP, Huckvale M, Howarth E, Emsley R, Garety PA. AVATAR therapy for auditory verbal hallucinations in people with psychosis: a single-blind, randomised controlled trial. Lancet Psychiatry. 2018;5:31–40. doi: 10.1016/S2215-0366(17)30427-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curcic-Blake B, Ford JM, Hubl D, Orlov ND, Sommer IE, Waters F, et al. Interaction of language, auditory and memory brain networks in auditory verbal hallucinations. Progress in Neurobiology. 2017;148:1–20. doi: 10.1016/j.pneurobio.2016.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauwan M, Begemann MJ, Heringa SM, Sommer IE. Exercise improves clinical symptoms, quality of life, global functioning, and depression in schizophrenia: a systematic review and meta-analysis. Schizophrenia Bulletin. 2016;42:588–599. doi: 10.1093/schbul/sbv164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Leede-Smith S, Barkus E. A comprehensive review of auditory verbal hallucinations: lifetime prevalence, correlates and mechanisms in healthy and clinical individuals. Frontiers in Human Neuroscience. 2013;7:367. doi: 10.3389/fnhum.2013.00367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dollfus S, Jaafari N, Guillin O, Trojak B, Plaze M, Saba G, Nauczyciel C, Montagne Larmurier A, Chastan N, Meille V, Krebs MO, Ayache SS, Lefaucheur JP, Razafimandimby A, Leroux E, Morello R, Marie Batail J, Brazo P, Lafay N, Wassouf I, Harika-Germaneau G, Guillevin R, Guillevin C, Gerardin E, Rotharmel M, Crépon B, Gaillard R, Delmas C, Fouldrin G, Laurent G, Nathou C, Etard O. High-frequency neuronavigated rTMS in auditory verbal hallucinations: a pilot double-blind controlled study in patients with schizophrenia. Schizophrenia Bulletin. 2018;44:505–514. doi: 10.1093/schbul/sbx127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- du Sert OP, Potvin S, Lipp O, Dellazizzo L, Laurelli M, Breton R, Lalonde P, Phraxayavong K, O'Connor K, Pelletier JF, Boukhalfi T, Renaud P, Dumais A. Virtual reality therapy for refractory auditory verbal hallucinations in schizophrenia: a pilot clinical trial. Schizophrenia Research. 2018;197:176–181. doi: 10.1016/j.schres.2018.02.031. [DOI] [PubMed] [Google Scholar]
- Edgar JC, Hunter MA, Huang M, Smith AK, Chen Y, Sadek J, Lu BY, Miller GA, Cañive JM. Temporal and frontal cortical thickness associations with M100 auditory activity and attention in healthy controls and individuals with schizophrenia. Schizophrenia Research. 2012;140:250–257. doi: 10.1016/j.schres.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Q, Lui S, Sweeney JA. A selective review of cerebral abnormalities in patients with first-episode schizophrenia before and after treatment. The American Journal of Psychiatry. 2016;173:232–243. doi: 10.1176/appi.ajp.2015.15050641. [DOI] [PubMed] [Google Scholar]
- Gothelf D, Hoeft F, Ueno T, Sugiura L, Lee AD, Thompson P, Reiss AL. Developmental changes in multivariate neuroanatomical patterns that predict risk for psychosis in 22q11.2 deletion syndrome. Journal of Psychiatric Research. 2011;45:322–331. doi: 10.1016/j.jpsychires.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddock G, McCarron J, Tarrier N, Faragher EB. Scales to measure dimensions of hallucinations and delusions: the psychotic symptom rating scales (PSYRATS) Psychological Medicine. 1999;29:879–889. doi: 10.1017/S0033291799008661. [DOI] [PubMed] [Google Scholar]
- Ho BC, Andreasen NC, Ziebell S, Pierson R, Magnotta V. Long-term antipsychotic treatment and brain volumes: a longitudinal study of first-episode schizophrenia. Archives of General Psychiatry. 2011;68:128–137. doi: 10.1001/archgenpsychiatry.2010.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang E, Zai CC, Lisoway A, Maciukiewicz M, Felsky D, Tiwari AK, Bishop JR, Ikeda M, Molero P, Ortuno F, Porcelli S, Samochowiec J, Mierzejewski P, Gao S, Crespo-Facorro B, Pelayo-Terán JM, Kaur H, Kukreti R, Meltzer HY, Lieberman JA, Potkin SG, Müller DJ, Kennedy JL. Catechol-O-methyltransferase Val158Met polymorphism and clinical response to antipsychotic treatment in schizophrenia and schizo-affective disorder patients: a meta-analysis. The International Journal of Neuropsychopharmacology. 2016;19:pyv132. doi: 10.1093/ijnp/pyv132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugdahl K. Auditory hallucinations: A review of the ERC "VOICE" project. World Journal of Psychiatry. 2015;5:193–209. doi: 10.5498/wjp.v5.i2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns LC, Cannon M, Singleton N, Murray RM, Farrell M, Brugha T, Bebbington P, Jenkins R, Meltzer H. Prevalence and correlates of self-reported psychotic symptoms in the British population. The British Journal of Psychiatry: the Journal of Mental Science. 2004;185:298–305. doi: 10.1192/bjp.185.4.298. [DOI] [PubMed] [Google Scholar]
- Jones SR. Do we need multiple models of auditory verbal hallucinations? Examining the phenomenological fit of cognitive and neurological models. Schizophrenia Bulletin. 2010;36:566–575. doi: 10.1093/schbul/sbn129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang C, Xu X, Liu H, Yang J. Association study of catechol-O-methyltransferase (COMT) gene Val158Met polymorphism with auditory P300 in Chinese Han patients with schizophrenia. Psychiatry Research. 2010;180:153–155. doi: 10.1016/j.psychres.2008.07.008. [DOI] [PubMed] [Google Scholar]
- Kompus K, Westerhausen R, Hugdahl K. The "paradoxical" engagement of the primary auditory cortex in patients with auditory verbal hallucinations: a meta-analysis of functional neuroimaging studies. Neuropsychologia. 2011;49:3361–3369. doi: 10.1016/j.neuropsychologia.2011.08.010. [DOI] [PubMed] [Google Scholar]
- Lawrie SM. Are structural brain changes in schizophrenia related to antipsychotic medication? A narrative review of the evidence from a clinical perspective. Therapeutic Advances in Psychopharmacology. 2018;8:319–326. doi: 10.1177/2045125318782306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei W, Li N, Deng W, Li M, Huang C, Ma X, Wang Q, Guo W, Li Y, Jiang L, Zhou Y, Hu X, Mary McAlonan G, Li T. White matter alterations in first episode treatment-naive patients with deficit schizophrenia: a combined VBM and DTI study. Scientific Reports. 2015;5:12994. doi: 10.1038/srep12994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li ML, Xiang B, Li YF, Hu X, Wang Q, Guo WJ, Lei W, Huang CH, Zhao LS, Li N, Ren HY, Wang HY, Ma XH, Deng W, Li T. Morphological changes in gray matter volume correlate with catechol-O-methyl transferase gene Val158Met polymorphism in first-episode treatment-naive patients with schizophrenia. Neuroscience Bulletin. 2015;31:31–42. doi: 10.1007/s12264-014-1491-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liemburg EJ, Vercammen A, Ter Horst GJ, Curcic-Blake B, Knegtering H, Aleman A. Abnormal connectivity between attentional, language and auditory networks in schizophrenia. Schizophrenia Research. 2012;135:15–22. doi: 10.1016/j.schres.2011.12.003. [DOI] [PubMed] [Google Scholar]
- Lu BY, Martin KE, Edgar JC, Smith AK, Lewis SF, Escamilla MA, Miller GA, Cañive JM. Effect of catechol O-methyltransferase val(158)met polymorphism on the p50 gating endophenotype in schizophrenia. Biological Psychiatry. 2007;62:822–825. doi: 10.1016/j.biopsych.2006.11.030. [DOI] [PubMed] [Google Scholar]
- McCarthy-Jones S, Green MJ, Scott RJ, Tooney PA, Cairns MJ, Wu JQ, Oldmeadow C, Carr V, Australian Schizophrenia Research Bank Preliminary evidence of an interaction between the FOXP2 gene and childhood emotional abuse predicting likelihood of auditory verbal hallucinations in schizophrenia. Journal of Psychiatric Research. 2014;50:66–72. doi: 10.1016/j.jpsychires.2013.11.012. [DOI] [PubMed] [Google Scholar]
- Morch-Johnsen L, Nesvag R, Jorgensen KN, Lange EH, Hartberg CB, Haukvik UK, et al. Auditory cortex characteristics in schizophrenia: associations with auditory hallucinations. Schizophrenia Bulletin. 2017;43:75–83. doi: 10.1093/schbul/sbw130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northoff G. Are auditory hallucinations related to the brain's resting state activity? 'A neurophenomenal resting state hypothesis. Clinical Psychopharmacology Neuroscience: the Official Scientific Journal Korean College of Neuropsychopharmacology. 2014;12:189–195. doi: 10.9758/cpn.2014.12.3.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olgiati P, Mandelli L, Lorenzi C, Marino E, Adele P, Ferrari B, de Ronchi D, Serretti A. Schizophrenia: genetics, prevention, and rehabilitation. Acta Neuropsychiatrica. 2009;21:109–120. doi: 10.1111/j.1601-5215.2009.00360.x. [DOI] [PubMed] [Google Scholar]
- Plewnia C, Brendel B, Schwippel T, Martus P, Cordes J, Hasan A, Fallgatter AJ. Treatment of auditory hallucinations with bilateral theta burst stimulation (cTBS): protocol of a randomized, double-blind, placebo-controlled, multicenter trial. European Archives of Psychiatry and Clinical Neuroscience. 2018;268:663–673. doi: 10.1007/s00406-017-0861-3. [DOI] [PubMed] [Google Scholar]
- Reed GM, Keeley JW, Rebello TJ, First MB, Gureje O, Ayuso-Mateos JL, Kanba S, Khoury B, Kogan CS, Krasnov VN, Maj M, de Jesus Mari J, Sharan P, Stein DJ, Zhao M, Akiyama T, Andrews HF, Asevedo E, Cheour M, Domínguez-Martínez T, el-Khoury J, Fiorillo A, Grenier J, Gupta N, Kola L, Kulygina M, Leal-Leturia I, Luciano M, Lusu B, Martínez-López JNI, Matsumoto C, Odunleye M, Onofa LU, Paterniti S, Purnima S, Robles R, Sahu MK, Sibeko G, Zhong N, Gaebel W, Lovell AM, Maruta T, Pike KM, Roberts MC, Medina-Mora ME. Clinical utility of ICD-11 diagnostic guidelines for high-burden mental disorders: results from mental health settings in 13 countries. World Psychiatry: Official Journal of the World Psychiatry Association (WPA) 2018;17:306–315. doi: 10.1002/wps.20581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagud M, Muck-Seler D, Mihaljevic-Peles A, Vuksan-Cusa B, Zivkovic M, Jakovljevic M, et al. Catechol-O-methyl transferase and schizophrenia. Psychiatria Danubina. 2010;22:270–274. [PubMed] [Google Scholar]
- Slotema CW, Blom JD, Niemantsverdriet MBA, Sommer IEC. Auditory verbal hallucinations in borderline personality disorder and the efficacy of antipsychotics: a systematic review. Frontiers in Psychiatry. 2018;9:347. doi: 10.3389/fpsyt.2018.00347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snitz BE, Macdonald AW, Carter CS. Cognitive deficits in unaffected first-degree relatives of schizophrenia patients: a meta-analytic review of putative endophenotypes. Schizophrenia Bulletin. 2006;32:179–194. doi: 10.1093/schbul/sbi048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer IE, Daalman K, Rietkerk T, Diederen KM, Bakker S, Wijkstra J, Boks MPM. Healthy individuals with auditory verbal hallucinations; who are they? Psychiatric assessments of a selected sample of 103 subjects. Schizophrenia Bulletin. 2010;36:633–641. doi: 10.1093/schbul/sbn130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner, G.Z., Fernandez, F.M., Coles, M., Karamacoska, D., Barkus, E., Broyd, S.J., et al. (2018). Interrogating the relationship between Schizotypy, the catechol-O-methyltransferase (COMT) Val158Met polymorphism, and neuronal oscillatory activity. Cerebral Cortex (New York, N.Y.: 1991). 10.1093/cercor/bhy171. [DOI] [PubMed]
- Steinmann S, Leicht G, Mulert C. Interhemispheric auditory connectivity: structure and function related to auditory verbal hallucinations. Frontiers in Human Neuroscience. 2014;8:55. doi: 10.3389/fnhum.2014.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephanie L, Susan LR, Wei Lin T, Monique S, Neil T. Does mindfulness help people adapt to the experience of hearing voices? Psychiatry Research. 2018;270:329–334. doi: 10.1016/j.psychres.2018.09.013. [DOI] [PubMed] [Google Scholar]
- Tandon R. Schizophrenia and other psychotic disorders in DSM-5. Clinical Schizophrenia & Related Psychoses. 2013;7:16–19. doi: 10.3371/csrp.ta.032513. [DOI] [PubMed] [Google Scholar]
- Thomas ML, Bismark AW, Joshi YB, Tarasenko M, Treichler EBH, Hochberger WC, Zhang W, Nungaray J, Sprock J, Cardoso L, Tiernan K, Attarha M, Braff DL, Vinogradov S, Swerdlow N, Light GA. Targeted cognitive training improves auditory and verbal outcomes among treatment refractory schizophrenia patients mandated to residential care. Schizophrenia Research. 2018;S0920-9964:30470–304705. doi: 10.1016/j.schres.2018.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian T, Qin W, Liu B, Jiang T, Yu C. Functional connectivity in healthy subjects is nonlinearly modulated by the COMT and DRD2 polymorphisms in a functional system-dependent manner. The Journal of Neuroscience. 2013;33:17519–17526. doi: 10.1523/jneurosci.2163-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian T, Qin W, Liu B, Wang D, Wang J, Jiang T, Yu C. Catechol-O-methyltransferase Val158Met polymorphism modulates gray matter volume and functional connectivity of the default mode network. PLoS One. 2013;8:e78697. doi: 10.1371/journal.pone.0078697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upthegrove R, Broome MR, Caldwell K, Ives J, Oyebode F, Wood SJ. Understanding auditory verbal hallucinations: a systematic review of current evidence. Acta Psychiatrica Scandinavica. 2016;133:352–367. doi: 10.1111/acps.12531. [DOI] [PubMed] [Google Scholar]
- Vallath S, Luhrmann T, Bunders J, Ravikant L, Gopikumar V. Reliving, replaying lived experiences through auditory verbal hallucinations: implications on theories and management. Frontiers in Psychiatry. 2018;9:528. doi: 10.3389/fpsyt.2018.00528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Lutterveld R, Diederen KM, Otte WM, Sommer IE. Network analysis of auditory hallucinations in nonpsychotic individuals. Human Brain Mapping. 2014;35:1436–1445. doi: 10.1002/hbm.22264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westwood H, Stahl D, Mandy W, Tchanturia K. The set-shifting profiles of anorexia nervosa and autism spectrum disorder using the Wisconsin card sorting test: a systematic review and meta-analysis. Psychological Medicine. 2016;46:1809–1827. doi: 10.1017/S0033291716000581. [DOI] [PubMed] [Google Scholar]
- Wigand M, Kubicki M, Clemm von Hohenberg C, Leicht G, Karch S, Eckbo R, Pelavin PE, Hawley K, Rujescu D, Bouix S, Shenton ME, Mulert C. Auditory verbal hallucinations and the interhemispheric auditory pathway in chronic schizophrenia. The World Journal of Biological Psychiatry. 2015;16:31–44. doi: 10.3109/15622975.2014.948063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson S. Accounting for the phenomenology and varieties of auditory verbal hallucination within a predictive processing framework. Consciousness and Cognition. 2014;30:142–155. doi: 10.1016/j.concog.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson, S. & Fernyhough, C. (2017). Wellcome trust-funded monographs and book chapters auditory verbal hallucinations and inner speech: A predictive processing perspective In Z. Radman (Ed.), Before consciousness: In search of the fundamentals of mind. Imprint Academic, Ltd. Copyright (c) Imprint Academic, 2017. Individual contributions (c) the respective authors 2017., Exeter (UK). [PubMed]
- Zhuo C, Zhu J, Wang C, Qu H, Ma X, Tian H, Liu M, Qin W. Brain structural and functional dissociated patterns in schizophrenia. BMC Psychiatry. 2017;17:45. doi: 10.1186/s12888-017-1194-5. [DOI] [PMC free article] [PubMed] [Google Scholar]