Abstract
The distribution, characteristics of extracellular polymeric substances (EPS) of Phanerochaete chrysosporium under Pb2+ stress and the influence on Pb removal were investigated. Polysaccharides was found to be the main composition in both soluble EPS (SEPS) and bounded EPS (BEPS). More polysaccharides and protein in BEPS were detected with the increased Pb2+ concentration. The ratio of Pb amount distributed in BEPS to the total Pb removed by the fungal biomass gradually decreased from 91.66 to 61.27% in group with 50 mg/L of initial Pb2+, but kept at about 35% or 25% in groups with higher Pb2+. It implies that BEPS played a certain role in the lead removal process, and the role of BEPS was relatively more important in the removal of lower concentration of Pb2+ and in the initial period of Pb removal. With FTIR analysis and Pb2+ adsorption experiment, more effective functional groups and better Pb2+ adsorption capacity was demonstrated in BEPS than in SEPS. SEM–EDS analysis demonstrated that part of Pb immobilized in BEPS was in the form of Pb precipitation. The increased molecular weight in SEPS and more polysaccharides in BEPS were probably beneficial for the adhesion of Pb precipitation.
Subject terms: Biotechnology, Environmental sciences
Introduction
With the rapid development of industry, heavy metal pollution in water has become more and more serious. Different from organic pollutants, heavy metals could not be degraded, but could be accumulated in organisms and even cause biological toxicity. Lead (Pb) is one common heavy metal pollutant in wastewater, especially in battery industrial wastewater. Severe Pb poisoning in human can cause damage to the nerves, kidneys and blood systems, so it is of great significance to remove Pb from wastewater1. Among the various methods currently used to remove Pb in wastewater, adsorption is a commonly used treatment method2. There are various adsorption materials, including microporous materials with large specific surface areas, such as activated carbon3, zeolite4 or metal–organic frameworks5, and biomaterials, for example bacteria6, fungi7,8, algae9, etc. Among those materials, the low-cost and non-toxicity biosorbents can be prepared with abundant raw sources, and have attracted more and more attention.
In the past few decades, many studies on the application of white rot fungi in the treatment of heavy metal wastewater have been reported8,10. White rot fungi were found to display high heavy metal adsorption capacity. In those studied, live or dead fungal biomass in the form of mycelium pellets or fungal fruit bodies were used8,11,12, as well as the immobilized biomass13. Researches on the removal mechanisms of heavy metals by white rot fungi found that most heavy metals were distributed around cell walls14,15. Extracellular polymeric substances (EPS), a complex mixture of biomacromolecules, are secreted to the outside of cells during the growth and metabolism of microorganisms16,17. The stress of heavy metals usually caused changes in the metabolic activities of microorganisms18. Joshi et al. demonstrated that the protein/polysaccharide ratio in EPS was greatly affected when Azotobacter was exposed to heavy metals19. The composition and molecular weight of EPS produced by Cordyceps cicadae also can be affected by environment condition20. Since the contribution of each component in EPS to the adsorption of heavy metals was different16, the changes of EPS components were likely to lead to the changes of the contribution of EPS to heavy metal removal in some way. But there was little information available on the role of EPS in heavy metal removal by white rot fungi until now, as well as the characteristics of EPS when the fungi were exposed to heavy metal.
The functions of EPS have been widely discussed, such as protecting microbial cells from external aggression, and serving as energy and carbon sources21. EPS contain many functional groups, such as hydroxyl, amine, carboxyl, thiol, phosphoric and etc., which are important for biological adsorbents to adsorb heavy metals22–24. Recently, biosorbents based on EPS have attracted much concern. EPS can be divided into two types: one is soluble extracellular polymeric substances (SEPS), which is distributed in the medium and sometimes called “soluble microbial products” (SMP), and the other is bounded extracellular polymeric substances (BEPS), which is distributed on the surface of microorganisms25. The two types of EPS are homologous, and SEPS also can be formed by the hydrolysis of BEPS26, which means that both types of EPS probably have similar functional groups to chelate heavy metals. However, most of the investigations on EPS’ role on heavy metal removal were limited to BEPS and focused on bacterial EPS. The role of BEPS produced by Phanerochaete chrysosporium (P. chrysosporium) in Pb2+ adsorption has been proved in our previous study14. It is still unknown whether the removal of heavy metals would be affected by SEPS. Considering the easier access to SEPS than BEPS and EPS’ dominant role in heavy metal adsorption, it is meaningful to investigate the SEPS’ adsorption capacity to heavy metals.
Hence, in this study, the characteristics of SEPS and BEPS produced by white rot fungi P. chrysosporium under Pb2+ stress and their role on Pb removal were investigated. The adsorb capability of Pb2+ in SEPS and that in BEPS were also compared. This study can further explain the removal mechanisms of heavy metals by white rot fungi, and explore the feasibility of direct use of white-rot fungal SEPS in the treatment of heavy metal pollution.
Results
Composition of SEPS
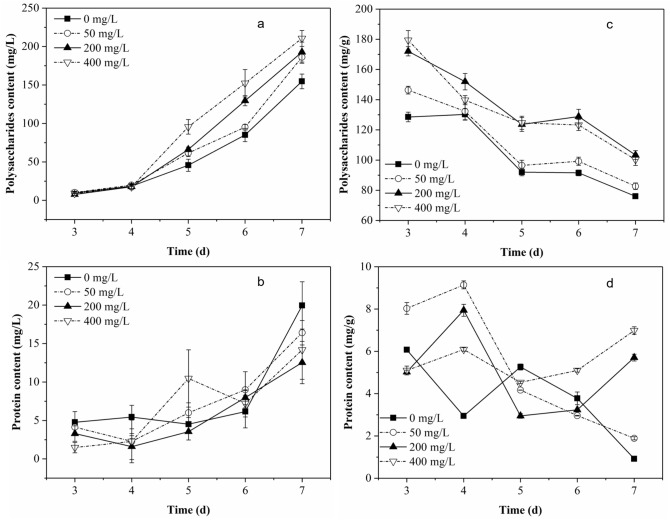
As shown in Fig. 1a and b, the main component in SEPS produced by P. chrysosporium was polysaccharides, and there was only a small amount of protein. As the culture progressed, the two components in the culture solution accumulated more. When the fungi were exposed to Pb2+, the production of the two components in SEPS were affected. The higher the initial concentration of Pb2+, the more polysaccharides accumulated in SEPS. This difference mainly appeared after 4-day culture. However, the effect of Pb2+ on the protein content in SEPS was different from that on polysaccharides content in SEPS. Higher initial concentration of Pb2+ resulted in a lower protein concentration in SEPS. After 7-day culture, the maximum polysaccharide content in SEPS in the group with 400 mg/L of Pb2+ reached 210.6 mg/L, while the maximum protein content in the group without Pb2+ addition was only about 20.0 mg/L.
Figure 1.
Contents of polysaccharides and protein in SEPS (a,b) and in BEPS (c,d) produced by P. chrysosporium as a function of time.
Composition of BEPS
It can be seen from Fig. 1c and d that the BEPS composition of P. chrysosporium was similar to that of SEPS (Fig. 1a,b), with polysaccharide as the main component in BEPS. The highest content of polysaccharides reached 179.4 mg/g, while the highest protein content was only 9.14 mg/g. Figure 1c displayed the changes of polysaccharides content in the BEPS. The polysaccharides content in BEPS in the groups with Pb2+ increased at first 3 days, and then decreased continuously. With more Pb2+ in the medium, the polysaccharide content in BEPS was higher. As shown in Fig. 1d, when the initial concentration of Pb2+ was 50 mg/L, the protein content in BEPS increased fastest in the previous 4 days. The higher the initial Pb2+ concentration, the slower the increase rate of protein content. In the later culture period (after 5 days), protein content began to increase again in the groups with 200 mg/L and 400 mg/L of Pb2+. In the groups containing Pb, both of the two compositions of BEPS increased first and then gradually decreased. In the Pb-free group, both the two compositions continued to decrease.
Proportion of Pb in different distributions and the role of BEPS during the Pb removal process
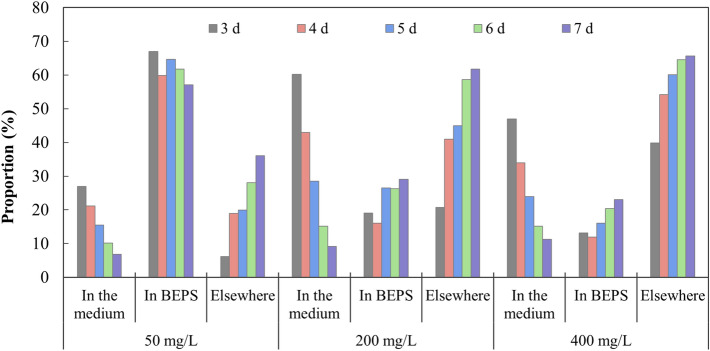
Figure 2 shows the proportion of Pb in different distributions in groups with different initial Pb2+ concentrations within 3 to 7 days. It can be seen that the proportion of Pb in the culture solution continued to decrease, while the proportion in elsewhere increased continuously. When the initial Pb2+ concentration varied, the changes of Pb proportion in BEPS were slightly different. With 50 mg/L of initial Pb2+, Pb proportion in BEPS accounted for more than 50% of the total Pb content, and it displayed a stable but slowly decreasing trend within 3 to 7 days. With initial Pb2+ concentrations of 200 mg/L and 400 mg/L, the proportion of Pb distributed in BEPS showed an increase at 4 d and a continuously increase trend from 4 to 7 days, but only accounting for less than 30% of the total Pb content.
Figure 2.
Proportion of Pb distributed in the medium, in BEPS and elsewhere during the removal process of Pb by P. chrysosporium.
The ratio of Pb content distributed in BEPS to the total amount of Pb removed by the fungal biomass was further analyzed, and the results are shown in Fig. 3. In the group with an initial Pb2+ concentration of 50 mg/L, the ratio decreased continuously from 91.66 to 61.27%. When the initial Pb2+ concentration was higher, the ratio decreased from 3 to 4 days, and then slowly increased, while the ratio maintained at a relatively stable level, around 35% or 25%, in groups with higher initial Pb2+ concentration during the whole removal process.
Figure 3.
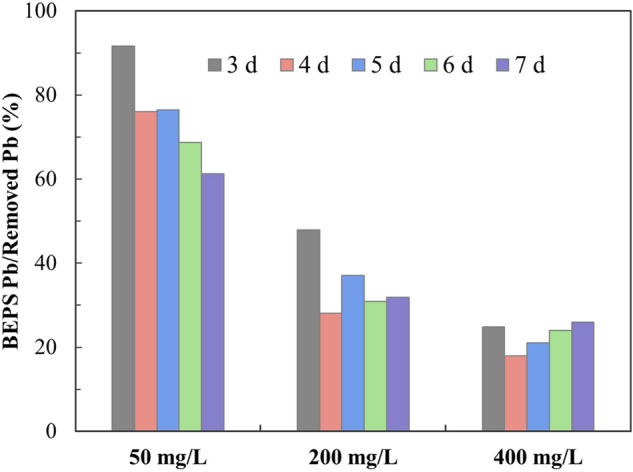
Ratio of Pb in BEPS to the total removed Pb during the removal process of Pb by P. chrysosporium.
Changes of EPS characterization during the Pb removal process
The molecular weight distribution of EPS was monitored, and the results are listed in Table 1. Because of the solid phase of BEPS and the expensive test costs of GPC-MALLS, we only tested the SEPS extracted from the groups with 0 mg/L and 200 mg/L of Pb2+. It was found that the molecular weight of SEPS in the group with 200 mg/L of Pb2+ was more widely distributed, and its highest molecular weight was higher than that in the group without Pb2+ addition.
Table 1.
The molecular weight distribution of SEPS in groups with different initial Pb2+ concentration.
| Pb2+ concentration (mg/L) | Mw (Da) | Mn (Da) | Mw/Mn |
|---|---|---|---|
| 0 | 1.499 × 106 | 1.238 × 106 | 1.211 |
| 200 | 2.860 × 106 | 2.586 × 106 | 1.106 |
| 4.994 × 104 | 3.994 × 104 | 1.250 |
Mn apparent number average molecular weight, Mw weight average molecular weight.
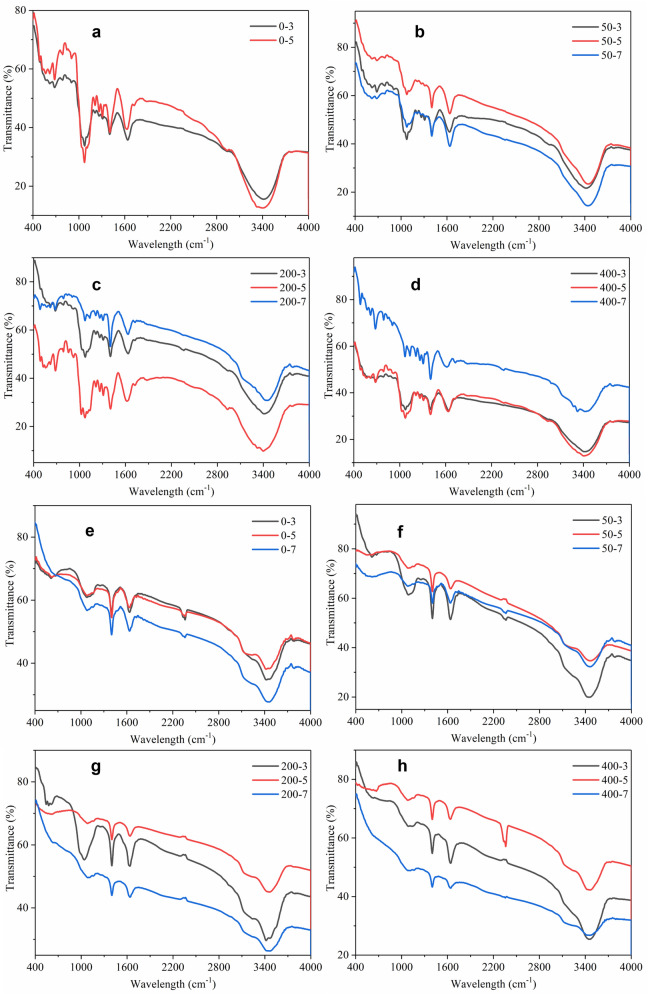
The FTIR spectra of BEPS and SEPS produced by P. chrysosporium at 3 days, 5 days and 7 days were analyzed, and the results are listed in Fig. 4. It can be seen that the functional groups in BEPS was more than that in SEPS, for example the signals at 1309–1213 cm−1 which was assigned to C–N stretch in amide III of protein, and the band at 2933 cm−1 which was assigned to C–H stretching vibration in methyl. The strong signal at 1637 cm−1 was assigned to C=O stretching, and the strong signal around 3400 cm−1 was assigned to O–H stretching vibrations, which both existed in the FTIR spectra of BEPS and SEPS. In the FTIR spectra of BEPS extracted from the groups with 200 mg/L and 400 mg/L of Pb2+, the bands at 1022 cm−1 and 1103 cm−1 which was assigned to C–O stretching vibration in alcohols or phenols disappeared at 7 days, and the band at 3320 cm−1 which was assigned to N–H stretch in amide II of protein was more obvious. The weak band at 1723 cm−1 which implied the trace evidence of uronic acid was absent in the FTIR spectra of SEPS in the groups with 200 and 400 mg/L of Pb2+, while the weak band at 1160 cm−1 only appeared in the FTIR spectra of SEPS, which was assigned to C–O and C–C stretching vibrations.
Figure 4.
The FTIR spectra of BEPS (a–d) and SEPS (e–h) produced by P. chrysosporium at 3 days, 5 days and 7 days in groups with initial Pb2+ concentration of 0 mg/L, 50 mg/L, 200 mg/L and 400 mg/L.
SEM–EDS analysis of the mycelium before and after BEPS extraction
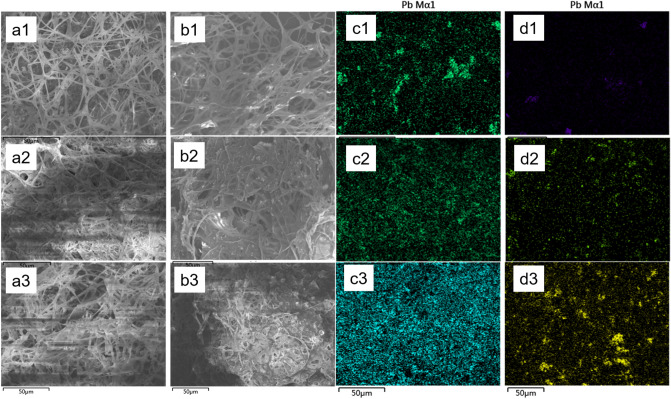
Figure 5 displays the SEM and their corresponding Pb mapping analysis result of the mycelium before and after BEPS extraction. It can be seen that the fungal mycelium was filamentous and intertwined to form a spatial network (Fig. 5a,b). Before BEPS extraction, there were many particles containing Pb immobilized around the mycelium or in the mycelium network (Fig. 5a,c). After BEPS extraction, most of those small particles disappeared (Fig. 5b,d). It indicates that those particles were probably immobilized by the BEPS. When the initial Pb2+ concentration was higher, more particles containing Pb especially the larger particles were left on the mycelium. Because of the high-speed centrifuge force during the extraction process, the mycelium network was broken to a certain extent and some mycelium even stick together after BEPS extraction (Fig. 5b). The mass fractions of Pb in the microregion of the fungal mycelium before and after BEPS extraction were obtained by EDS analysis, and the results are listed in Table 2. After BEPS extraction, the mass fraction of Pb in the group with 50 mg/L of initial Pb2+ changed from 6.28 to 0.48%, the mass faction of Pb in the group with 200 mg/L of initial Pb2+ from 16.22 to 2.16%, and that in the group with 400 mg/L of initial Pb2+ from 20.13 to 4.40%.
Figure 5.
SEM images of mycelium before (a) and after (b) BEPS extraction in the group with initial Pb2+ concentration of 50 mg/L (1), 200 mg/L (2), or 400 mg/L (3) and their corresponding Pb mapping images (c,d).
Table 2.
Changes in the mass fraction of Pb distribution on the surface of the white rot fungal mycelium before and after BEPS extraction.
| Initial Pb2+ concentration (mg/L) | Mass fraction of Pb (%) | |
|---|---|---|
| Before BEPS extraction | After BEPS extraction | |
| 50 | 6.28 | 0.48 |
| 200 | 16.22 | 2.16 |
| 400 | 20.13 | 4.4 |
Pb2+ adsorption by SEPS and BEPS
The adsorption efficiency and adsorption capacity of Pb2+ by SEPS and BEPS were listed in Table 3. The adsorption capacity of of Pb2+ by BEPS was 51.2 mg/g, higher than that of SEPS, i.e. 16.4 mg/g.
Table 3.
Adsorption efficiency and adsorption capacity of Pb2+ by BEPS and SEPS.
| Group | Adsorption efficiency of Pb2+ (%) | Adsorption capacity of Pb2+ (mg/g) |
|---|---|---|
| BEPS | 12.8 | 51.2 |
| SEPS | 4.1 | 16.4 |
Discussion
SEPS was also called as SMP25. It can be produced from substrate metabolism and microbial cell lysis25. In the first 4-day culture, the rate of total sugar consumption was quite fast, and it was slower in groups with higher initial concentration of Pb2+. This should have caused a higher SMP content in the blank, but there was not a significant difference in the SEPS content in all groups (Fig. 1a,b). On the one hand, it may be due to the fact that the metabolites produced from glucose metabolism were mainly small molecules, such as organic acids10, with a few macromolecules. Those small molecules were removed during the dialysis process, thereby reducing the part of SEPS due to the medium metabolism. On the other hand, environment stress such as salinity usually lead to the release of more extracellular substances by microbe18 and even caused cell lysis27. In this study, when the fungi were stressed by Pb2+, cell lysis was more likely to occur, which would increase the substances produced from cell lysis, which are usually macromolecules. After 4-day culture, the total sugar content in the medium significantly reduced. At this time, the fungus probably started to use the intracellular material to obtain carbon sources and energy, and the intracellular macromolecules released from cell lysis increased. The difference in SEPS amount after 4 d indicates that the higher the initial Pb2+, the more active the cell lysis activity in the later period.
The results in Figs. 1c and d indicates that the increase of BEPS around the fungal mycelium was promoted with the presence of Pb2+ at earlier growth stage, and it was faster than the biomass growth. While the fungal biomass growth became faster at later stages. Bi et al.28 found similar results that the extracellular polysaccharides production by Microcystis aeruginosa was stimulated by Pb2+. Ma et al.29 demonstrated that polysaccharide had an important role in Pseudomonas aeruginosa adhesion and was critical for maintenance of the biofilm structure. The increase of polysaccharide content in BEPS may enhanced the adhesion of fungal mycelium. Protein moieties in the BEPS has been demonstrated to be the main macromolecules involved in metal ion binding19,30. However, in this study, protein only accounted for a small part of BEPS, and its potential for Pb2+ binding was limited. The above results indicated that polysaccharides in both SEPS and BEPS was more sensitive to Pb2+ variation, and can be considered as a response to Pb2+ toxicity to protect mycelium safe.
Figures 2 and 3 together demonstrate that BEPS played a certain role in the lead removal process, and the role was relatively more important in the removal of lower concentration of Pb2+ and in the initial period of lead removal. Although the SEPS content increased under Pb2+ stress (Fig. 1), it did not inhibit the removal of lead by mycelium (Fig. 2), which also shows that the binding between SEPS and Pb2+ may be not enough to slow down the lead removal process.
FTIR results show that the main groups in BEPS and SEPS produced during the growth of P. chrysosporium are hydroxyl and carboxyl groups (Fig. 4). There was also amino group in BEPS, which was absent in SEPS. The more obvious characteristic peak of amino group in FTIR spectra of BEPS in groups with higher initial Pb2+ concentration seemed to be consistent with the changes of protein content in BEPS. In addition, there was also uronic acid in BEPS, which indicates the existence of free carboxyl groups. Free carboxyl and amino groups were both effective functional groups in chelating heavy metal ions31. The FTIR spectra of SEPS was similar with the typical FTIR of polysaccharides (Fig. 4). With only a small part of protein was detected in composition analysis of SEPS and less proportion of protein amount to the total amount in SEPS than that in BEPS, no obvious characteristic band of amino group was found in FTIR spectra of SEPS in Fig. 4. Joshi and Juwarkar19 found that the cell wall of Azotobacter composed mainly of polysaccharides lead to lack of binding of metal ions by whole cells. The above results confirms that BEPS probably had a stronger adsorption capacity for heavy metal ions than SEPS, and the main component in BEPS that had the ability to adsorb heavy metals probably was the protein moiety. Increased protein content in BEPS and decreased protein content in SEPS were detected in the groups with higher initial Pb2+ concentration, which was beneficial to adsorb more Pb2+ by BEPS. The Pb2+ adsorption experiment with EPS also demonstrated the better Pb2+ adsorption capacity by BEPS than SEPS (Table 3). Increase of molecular weight of SEPS in Pb2+-containing groups was found in this study (Table 2). SEPS have been used as bioflocculants to adsorb and remove particulates from wastewater32. SEPS with higher molecular weight usually have longer molecular chains and display stronger ability to capture particulates33. The increase in molecular weight of SEPS could help to capture the particles containing Pb and form larger particles, which could be removed from water by adhering to the surface of the mycelium.
The change of EPS characteristics would affect the role of EPS in heavy metal ion adsorption and heavy metal removal. However, the increase or decrease of BEPS content (Fig. 1) also did not slow down the removal of lead (Fig. 2). It probably indicates that BEPS’s role in the removal of lead by P. chrysosporium might not be accomplished mainly by its functional groups, but some other forces. In previous studies, it was found that white rot fungi secreted oxalic acid under Pb2+ stress, forming lead oxalate precipitation, and the change of oxalic acid content had a great impact on the removal of lead34. The BEPS of P. chrysosporium contained viscous polysaccharides as its main component, so BEPS was likely to remove Pb mainly by adhering lead oxalate precipitation. Therefore, the effect of BEPS had a significant relationship with the precipitation of lead oxalate. Previous studies have confirmed that oxalic acid was secreted more by white rot fungi in the early stages of exposure to Pb2+, and would be degraded by oxalate decarboxylase in the later stages to reduce the content10. This is consistent with that BEPS played a more important role in the early stage of lead removal. Those results indicate that BEPS may achieved the removal of Pb mainly due to its adhesion to heavy metal precipitation. Compared with soluble polysaccharide in SEPS, polysaccharides in BEPS was in the solid phase25 which was more suitable for the immobilization of metal-containing particles.
The EDS analysis results demonstrate that BEPS immobilized most lead-containing substances on the fungal mycelium surface (Fig. 5). SEM–EDS together prove that Pb in BEPS was probably removed in the form of Pb precipitation. Those findings are consistent with the speculation from Fig. 3, and further confirmed the role that BEPS played in the process of Pb removal. After BEPS extraction, part of the remaining Pb on the mycelial surface was likely to be distributed on the cell wall of the mycelium. The fungal cell wall was composed of chitin, cellulose, cellulose derivatives and melanin35. Many researches have reported the adsorption of heavy metals by fungal cell walls36. As for the Pb-containing particles on the mycelial surface EPS after BEPS extraction, it was probably because that the mycelia rapidly and tightly adhered each other during the BEPS extraction process, so that the Pb-containing particles trapped in that place were firmly fixed, making it difficult to be removed by high-speed centrifugation. Besides, more polysaccharides in BEPS was detected in the groups with more Pb2+, so that the corresponding mycelium probably was stickier, resulting in more larger Pb-containing particles left on the mycelial surface after BEPS extraction.
The above results demonstrated that polysaccharides was the main composition in both SEPS and BEPS produced by P. chrysosporium, with a small amount of protein. More polysaccharides in EPS, more protein in BEPS, and less protein in SEPS were detected in the group with higher initial Pb2+. BEPS played a certain role in Pb removal process, and the role was relatively more important in the removal of lower concentration of Pb2+ and in the initial period of Pb removal. FTIR analysis and Pb2+ adsorption experiment with EPS both proved the better Pb2+ adsorption capacity in BEPS than that in SEPS. SEM–EDS analysis demonstrated that part of Pb immobilized in BEPS was removed in the form of Pb precipitation. The increase of molecular weight in SEPS and more polysaccharides in BEPS in the group with higher initial Pb2+ both could help to immobilize more Pb-containing particles. This study illustrated the role of EPS in Pb removal and the changes of EPS characteristics when P. chrysosporium was exposed to the stress of Pb2+. Those findings were helpful to further understand the removal mechanisms of heavy metals by white rot fungi.
Methods
Microorganism and culture condition
The basidiomycete P. chrysosporium BKMF-1767 provided by China Center for Type Culture Collection (Wuhan, China) was maintained on potato dextrose agar slants at 4 ℃. The strain was cultured at 37 ℃ for 7 days. Spore suspension was used as inoculum which was prepared by scraping spores on the agar surface and diluting in ultrapure water. Spore concentration was measured and adjusted to 2.5 × 106 spores/mL.
1.0 mL of spore suspensions was inoculated and cultured in 200 mL of sterile trace element solution in 500 mL flasks at 150 rpm, 30 ℃. 20 mmol/L of sodium tartrate buffer was used to as the solvent of the culture medium (pH 4.5). Per liter of medium contained 2 g KH2PO4, 0.1 g CaCl2, 0.5 g MgSO4, 0.115 g FeSO4·7H2O, 0.112 g MnSO4·H2O, 0.089 g ZnSO4·7H2O, 0.05 g CuSO4·5H2O, 0.001 g Vitamin B1, 0.12 g NH4Cl, 10 g glucose. Pb2+ in the form of Pb(NO3)2 was dosed to the medium to desired concentrations before inoculation. The flasks without Pb2+ added were run as control. All experiments were performed in triplicates and mean values were used in the analysis.
Extraction of SEPS and BEPS
Fungal biomass was filtrated from culture medium, washed for 2 or 3 times using 200 mL of ultrapure water. Afterwards, the fungal mycelium was resuspended in 50 mL of ultrapure water, and centrifuged at 10,000 rpm for 15 min at 4 ℃. The suspension was recognized as the solution of BEPS. The culture medium at different culture period was collected, dialyzed (molecular weight cutoff: 3500 Da) against deionized water for 5 days. The obtained solution was designated as SEPS.
Dry weight of fungal mycelia was measured after the biomass was dried in a vacuum freezing dryer for 24 h to a constant weight. Field emission scanning electron microscopy (SEM, Zeiss Σigma, Germany) and energy dispersive X-ray spectrometer (EDS, Oxford Inca, UK) were used to observe the morphology and element composition of the fungal mycelium before and after BEPS extraction.
Characterization of EPS
The carbohydrates content in the extracted EPS was determined by anthranone-sulfuric acid method with glucose as standard. The total protein content in the extracted EPS solution was measured by Coomassie brilliant blue method with bovine serum albumin as standard. The SEPS solution in different groups were diluted to one uniform mass concentration according to the total content of polysaccharides and proteins, before the characterization of molecular weight distribution of SEPS with gel permeation chromatography coupled with a multi-angle laser-light scattering detector (GPC-MALLS, DAWN HELEOS-II Wyatt, USA). Freeze-dried EPS were used for Fourier transform infrared spectroscopy (FTIR) analysis, which was applied on a Thermo Nicolet Nexus 470 FTIR spectrometer. The samples were milled with desiccative potassium bromide (KBr) power and pressed into pellets using a tabulating machine. The spectral region of 400–4000 cm−1 was scanned at a spectral resolution of 2 cm−1.
Pb2+ adsorption experiment with SEPS and BEPS
SEPS and BEPS extracted at 7 days from the group without Pb2+ addition was concentrated to about 500 mg/L by evaporation. 30 mL of concentrated EPS solution was put into the dialysis bags (molecular weight cutoff: 3500 Da), which were placed into 30 mL of 200 mg/L Pb2+ solution. After 24-h adsorption, Pb2+ concentration in solution was used for Pb content analysis. One blank group was set with deionized water instead of EPS in the dialysis bag.
Analysis of Pb content
The samples were acidified with 3% (v/v) HNO3 and then filtered through 0.45 µm filter membrane. The filtrate was stored at 4 ℃ for Pb2+ estimation with an atomic absorption spectroscopy (AAS). The instrument was calibrated with Pb2+ standard solutions.
Acknowledgements
This study was jointly supported by the National Natural Science Foundation of China (51608142), and the Guangxi Science and Technology Planning Project (Guike-AD19110151, 2018GXNSFGA281001, 2016GXNSFBA380076).
Author contributions
N.L. conceived the experiment and prepared the manuscript, J.L. conducted the experiment, R.Y. and L.W. analyzed the results. All authors reviewed the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chen G, Jin Y, Wang W, Zhao Y. Colorimetric assay of lead using unmodified gold nanorods. Gold Bull. 2012;45:137–143. doi: 10.1007/s13404-012-0057-6. [DOI] [Google Scholar]
- 2.Sessarego S, Rodrigues SCG, Xiao Y, Lu Q, Hill JM. Phosphonium-enhanced chitosan for Cr(VI) adsorption in wastewater treatment. Carbohyd. Polym. 2019;211:249–256. doi: 10.1016/j.carbpol.2019.02.003. [DOI] [PubMed] [Google Scholar]
- 3.Kołodyńska D, Krukowska J, Thomas P. Comparison of sorption and desorption studies of heavy metal ions from biochar and commercial active carbon. Chem. Eng. J. 2016;307:353–363. doi: 10.1016/j.cej.2016.08.088. [DOI] [Google Scholar]
- 4.Dutta A, Patra PDA, Bhaumik A. Porous organic–inorganic hybrid nickel phosphonate: adsorption and catalytic applications. Microporous Mesoporous Mater. 2012;155:208–214. doi: 10.1016/j.micromeso.2012.01.017. [DOI] [Google Scholar]
- 5.Jamali A, Tehrani AA, Shemirani F, Morsali A. Lanthanide metal-organic frameworks as selective microporous materials for adsorption of heavy metal ions. Dalton Trans. 2016 doi: 10.1039/C6DT00782A. [DOI] [PubMed] [Google Scholar]
- 6.Wan W, et al. A manganese-oxidizing bacterial consortium and its biogenic Mn oxides for dye decolorization and heavy metal adsorption. Chemosphere. 2020;253:126627. doi: 10.1016/j.chemosphere.2020.126627. [DOI] [PubMed] [Google Scholar]
- 7.Zhu Z, Song Q, Dong F. Taxonomy characterization and plumbum bioremediation of novel fungi. J. Basic Microbiol. 2018;58:368–376. doi: 10.1002/jobm.201700469. [DOI] [PubMed] [Google Scholar]
- 8.Kahraman S, Erdemoglu S, Yesilada O. Biosorption of copper(II) by live and dried biomass of the white rot fungi Phanerochaete chrysosporium and Funalia trogii. Eng. Life Sci. 2005;5:72–77. doi: 10.1002/elsc.200420057. [DOI] [Google Scholar]
- 9.Li Y, et al. Enhanced Pb(II) removal by algal-based biosorbent cultivated in high-phosphorus cultures. Chem. Eng. J. 2019;361:167–179. doi: 10.1016/j.cej.2018.12.070. [DOI] [Google Scholar]
- 10.Li N-J, et al. Oxalate production at different initial Pb2+ concentrations and the influence of oxalate during solid-state fermentation of straw with Phanerochaete chrysosporium. Biores. Technol. 2011;102:8137–8142. doi: 10.1016/j.biortech.2011.05.092. [DOI] [PubMed] [Google Scholar]
- 11.Hanif MA, Bhatti HN, Ali MA. Heavy metal uptake potential of various types of white rot fungi: a preliminary study and investigation of metal toxicity. J. Biotechnol. 2008;136:S30. doi: 10.1016/j.jbiotec.2008.07.057. [DOI] [Google Scholar]
- 12.Baldrian P. Interactions of heavy metals with white-rot fungi. Enzyme Microb. Technol. 2003;32:78–91. doi: 10.1016/S0141-0229(02)00245-4. [DOI] [Google Scholar]
- 13.Mat Don M, Yahaya Y, Bhatia S. Biosorption of Pb(II) ions by immobilized cells of Pycnoporus sanguineus in a packed bed column. Pertanika J. Sci. Technol. 2009;17:191–199. [Google Scholar]
- 14.Li N, et al. Contribution characteristics of the in situ extracellular polymeric substances (EPS) in Phanerochaete chrysosporium to Pb immobilization. Bioprocess. Biosyst. Eng. 2017;40:1447–1452. doi: 10.1007/s00449-017-1802-2. [DOI] [PubMed] [Google Scholar]
- 15.Li N, et al. Response of extracellular carboxylic and thiol ligands (oxalate, thiol compounds) to Pb2+ stress in Phanerochaete chrysosporium. Environ. Sci. Pollut. Res. 2015 doi: 10.1007/s11356-015-4429-3. [DOI] [PubMed] [Google Scholar]
- 16.Sheng G-P, et al. Thermodynamic analysis on the binding of heavy metals onto extracellular polymeric substances (EPS) of activated sludge. Water Res. 2013;47:607–614. doi: 10.1016/j.watres.2012.10.037. [DOI] [PubMed] [Google Scholar]
- 17.Yue Z-B, Li Q, Li C-C, Chen T-H, Wang J. Component analysis and heavy metal adsorption ability of extracellular polymeric substances (EPS) from sulfate reducing bacteria. Biores. Technol. 2015;194:399–402. doi: 10.1016/j.biortech.2015.07.042. [DOI] [PubMed] [Google Scholar]
- 18.Wang Z, et al. Effect of salinity on extracellular polymeric substances of activated sludge from an anoxic–aerobic sequencing batch reactor. Chemosphere. 2013;93:2789–2795. doi: 10.1016/j.chemosphere.2013.09.038. [DOI] [PubMed] [Google Scholar]
- 19.Joshi PM, Juwarkar AA. In vivo studies to elucidate the role of extracellular polymeric substances from Azotobacter in immobilization of heavy metals. Environ. Sci. Technol. 2009;43:5884–5889. doi: 10.1021/es900063b. [DOI] [PubMed] [Google Scholar]
- 20.Sharma S, Gautam N, Atri N. Optimization, composition, and antioxidant activities of exo- and intracellular polysaccharides in submerged culture of Cordyceps gracilis (Grev.) Durieu & Mont. Evid. Based Complement. Altern. Med. 2015;2015:462864. doi: 10.1155/2015/462864. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21.Sheng G-P, Yu H-Q, Li X-Y. Extracellular polymeric substances (EPS) of microbial aggregates in biological wastewater treatment systems: a review. Biotechnol. Adv. 2010;28:882–894. doi: 10.1016/j.biotechadv.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 22.Zhang W, et al. Influence of wastewater sludge treatment using combined peroxyacetic acid oxidation and inorganic coagulants re-flocculation on characteristics of extracellular polymeric substances (EPS) Water Res. 2016;88:728–739. doi: 10.1016/j.watres.2015.10.049. [DOI] [PubMed] [Google Scholar]
- 23.Chen Y-P, et al. Functional groups characteristics of EPS in biofilm growing on different carriers. Chemosphere. 2013;92:633–638. doi: 10.1016/j.chemosphere.2013.01.059. [DOI] [PubMed] [Google Scholar]
- 24.Yin Y, Hu Y, Xiong F. Sorption of Cu(II) and Cd(II) by extracellular polymeric substances (EPS) from Aspergillus fumigatus. Int. Biodeterior. Biodegrad. 2011;65:1012–1018. doi: 10.1016/j.ibiod.2011.08.001. [DOI] [Google Scholar]
- 25.Ni B-J, Rittmann BE, Yu H-Q. Soluble microbial products and their implications in mixed culture biotechnology. Trends Biotechnol. 2011;29:454–463. doi: 10.1016/j.tibtech.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 26.Laspidou CS, Rittmann BE. A unified theory for extracellular polymeric substances, soluble microbial products, and active and inert biomass. Water Res. 2002;36:2711–2720. doi: 10.1016/S0043-1354(01)00413-4. [DOI] [PubMed] [Google Scholar]
- 27.Lin Y-C, Leano E, Pang K-L. Effects of Cu(II) and Zn(II) on growth and cell morphology of thraustochytrids isolated from fallen mangrove leaves in Taiwan. Bot. Mar. 2010 doi: 10.1515/bot.2010.070. [DOI] [Google Scholar]
- 28.Bi X-D, Zhang S-L, Dai W, Xing K-Z, Yang F. Effects of lead(II) on the extracellular polysaccharide (EPS) production and colony formation of cultured Microcystis aeruginosa. Water Sci. Technol. 2013;67:803–809. doi: 10.2166/wst.2012.632. [DOI] [PubMed] [Google Scholar]
- 29.Ma L, Jackson K, Landry R, Parsek M, Wozniak D. Analysis of Pseudomonas aeruginosa conditional psl variants reveals roles for the psl polysaccharide in adhesion and maintaining biofilm structure postattachment. J. Bacteriol. 2007;188:8213–8221. doi: 10.1128/JB.01202-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sheng G-P, Yu H-Q, Yue Z-B. Production of extracellular polymeric substances from Rhodopseudomonas acidophila in the presence of toxic substances. Appl. Microbiol. Biotechnol. 2005;69:216–222. doi: 10.1007/s00253-005-1990-6. [DOI] [PubMed] [Google Scholar]
- 31.Adesulu-Dahunsi AT, et al. Extracellular polysaccharide from Weissella confusa OF126: production, optimization, and characterization. Int. J. Biol. Macromol. 2018;111:514–525. doi: 10.1016/j.ijbiomac.2018.01.060. [DOI] [PubMed] [Google Scholar]
- 32.Subudhi S, et al. Purification and characterization of exopolysaccharide bioflocculant produced by heavy metal resistant Achromobacter xylosoxidans. Carbohydr. Polym. 2016;137:441–451. doi: 10.1016/j.carbpol.2015.10.066. [DOI] [PubMed] [Google Scholar]
- 33.Zhao C, et al. Production of ultra-high molecular weight poly-γ-glutamic acid with Bacillus licheniformis P-104 and characterization of its flocculation properties. Appl. Biochem. Biotechnol. 2013;170:562–572. doi: 10.1007/s12010-013-0214-2. [DOI] [PubMed] [Google Scholar]
- 34.Zeng G, et al. The stability of Pb species during the Pb removal process by growing cells of Phanerochaete chrysosporium. Appl. Microbiol. Biotechnol. 2015;99:3685–3693. doi: 10.1007/s00253-014-6275-5. [DOI] [PubMed] [Google Scholar]
- 35.Hopke A, Brown AJP, Hall RA, Wheeler RT. Dynamic fungal cell wall architecture in stress adaptation and immune evasion. Trends Microbiol. 2018;26:284–295. doi: 10.1016/j.tim.2018.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dhankhar R, Hooda A. Fungal biosorption: an alternative to meet the challenges of heavy metal pollution in aqueous solutions. Environ. Technol. 2011;32:467–491. doi: 10.1080/09593330.2011.572922. [DOI] [PubMed] [Google Scholar]