Abstract
Eight advanced durum-breeding lines were treated with 5-methyl-azacytidine to test the feasibility of generating sources of Fusarium head blight (FHB) resistance. Of the 800 treated seeds, 415 germinated and were advanced up to four (M4) generations by selfing. Thirty-two of the resulting 415 M4 lines were selected following preliminary screening and were further tested for FHB resistance for three years at two field locations, and in the greenhouse. Five of the 32 M4 lines showed less than 30% disease severity, as compared to the parental lines and susceptible checks. Fusarium-damaged kernels and deoxynivalenol analyses supported the findings of the field and greenhouse disease assessments. Two of the most resistant M4 lines were crossed to a susceptible parent, advanced to third generation (BC1:F3) and were tested for stability and inheritance of the resistance. About, one third of the BC1:F3 lines showed FHB resistance similar to their M4 parents. The overall methylation levels (%) were compared using FASTmC method, which did not show a significant difference between M4 and parental lines. However, transcriptome analysis of one M4 line revealed significant number of differentially expressed genes related to biosynthesis of secondary metabolites, MAPK signaling, photosynthesis, starch and sucrose metabolism, plant hormone signal transduction and plant-pathogen interaction pathways, which may have helped in improved FHB resistance.
Subject terms: Plant sciences, Plant biotechnology, Plant breeding, Plant cell biology, Plant development, Plant genetics, Plant immunity, Plant molecular biology, Plant stress responses
Introduction
Cereals, such as maize (Zea mays), rice (Oryza sativa) and wheat (Triticum aestivum L.), supply approximately 45% of the dietary energy worldwide (https://www.fao.org/3/CA1796EN/ca1796en.pdf). Among them, wheat is the most important food grain source (https://www.fao.org/assets/infographics/FAO-Infographic-wheat-en.pdf). Fusarium head blight (FHB) primarily caused by Fusarium graminearum, is a devastating disease of both durum (T. turgidum L.) and common wheat (T. aestivum L.)1–4. FHB has been a significant threat to wheat production due to frequent outbreaks in many wheat-growing regions, including the United States3,4. The estimated losses to FHB were reported to be in excess of 100 million bushels annually for 1991 and the six subsequent years in the United States5. In a recent study, FHB induced yield losses in the United States were estimated around $1.176 billion during 2015 and 20166. In addition to yield losses, food safety is compromised by contamination with mycotoxins, including deoxynivalenol (DON), that present a serious threat to human and animal health7.
The most prevalent strategies to control FHB include the application of fungicides, crop rotation and breeding for resistance8. Breeding for host resistance is considered as the most effective method to generate FHB resistance; however the FHB resistance is a complex trait and is controlled by multiple genes and also influenced by environmental factors9. Multiple sources of resistance have been identified and successfully utilized in developing FHB-resistance in bread wheat10. However, the plant breeding strategies have only been partly successful due to the lack of resistance sources in durum wheat11–14. Efforts have been made to introduce new sources of resistance from wild tetraploid wheat, hexaploid bread wheat and other alien species; however, these attempts have had only limited success15,16.
DNA methylation is important for plant growth and development17. Inadequate DNA methylation levels may result in abnormal growth and phenotype18,19. Plants can quickly adapt to changing environments by adjusting DNA methylation patterns20. For example, salt and low temperature treatments induced demethylation in the promoter region of NtGPDL (glycerophosphodiesterase-like protein) gene leading to stress tolerance in tobacco21. Transposable elements (TEs) were demethylated and transcriptionally reactivated to regulate neighboring genes as a defense response in Arabidopsis22. Further, demethylation of metl gene led to specific expression of stress response genes23, confirming that alteration in DNA methylation play crucial roles in plant response to environmental stresses. In another study, hypo-methylated mutants of Arabidopsis displayed enhanced resistance to the biotrophic pathogen Hyaloperonospora arabidopsidis, whereas two hyper-methylated mutants were susceptible24. Le et al.25 showed involvement of the DNA demethylases, ROS1, DML2, and DML3, in resistance to Fusarium oxysporum in Arabidopsis.
Cytosine methylation is known to be involved in many important biological processes, including defense response26. Cytosine methylations in the plant genome include CG, CHG, and CHH (where H denotes either A, T, or C) sequences27. In a study, treatment of susceptible rice plants with 5-azadeoxycytidine, which is an effective inhibitor of methyltransferase28,29, resulted in high levels of resistance to Xanthomonas oryzae and analyses indicated a complete lack of methylation in the promoter region of the Xa21G gene of the resistant plants30. The present study aimed to remove cytosine DNA methylation in advanced durum lines to test the feasibility of generating a novel source of FHB resistance. The treated lines showed promising results as compared with the parental lines and FHB-susceptible checks. A number of FHB responsive genes have been uncovered and their possible roles have been discussed in the present study.
Materials and methods
Select germplasm and treatment with DNA methylation inhibitor
Eight advanced durum lines; D0-3028, D0-3708, D0-41708, D0-4581, D0-6710, D0-6855, E-25 and TRT-4 (D0-3028 and D0-4581 were later released as Carpio and Joppa;31,32) were selected for treatment with 5-azacytidine (Millipore Sigma), which is an effective inhibitor of methyltransferase28,29. A total of 100 seeds for each of the eight durum lines were placed in individual Petri dishes with 0.5 mM 5-azacytidine solutions for 3 days as described by Akimoto et al.30. Following treatment, seeds were washed with 10% detergent and sterilized double distilled water. The treated seeds were planted individually in plastic pots containing 30% soil and 70% Pro-Line C/B Growing Mix (https://jollygardener.com/soil_jg_mixes.htm). Upon germination, the pots containing plantlets were transferred to a greenhouse set at 25 °C under 16 h photoperiod and grown to full maturity. The plants were selfed and advanced for four generations (M4) without selection (Supplementary Fig. S1). The M4 seeds were bulked and used in all the experiments.
Experiment design and inoculations
A total of 415 M4 lines were planted in the greenhouse for elimination screening and lines that showed more than 50% susceptibility were eliminated. Of the 415 M4 lines tested, 32 were selected following elimination screening and used for multi-year multi-location field testing. The 32 M4 lines, eight parental lines and two checks, one susceptible (MN00269) and one resistant (Alsen), were planted in the mist-irrigated field nurseries with a randomized complete block design with three replicates each at two locations, Saint Paul, Minnesota (MN) and Fargo, North Dakota (ND) during the summer of 2015. An additional two years (2016 and 2017) of field evaluations were performed at Saint Paul. Lines at Saint Paul were grown in single row plots (ca. 5 feet long) and were spray inoculated and mist-irrigated to facilitate disease development as described by Guatam and Dill-Macky33. The inoculum in Saint Paul consisted of macroconidia from a mixture of 15–30 F. graminearum isolates each year. Each line in the experiment at Fargo was planted in a hill plot. The Fargo nursery was inoculated when the majority of the plants were at the boot stage by applying Fusarium-colonized corn seeds at a rate of 35.6 g m−2. The corn was soaked to imbibe water, autoclaved, and infected with spores produced from 20 F. graminearum strains. The strains used in each nursery collected from commercial wheat fields in Minnesota and North Dakota and used in that state’s screening nursery34.
Disease evaluations were also conducted in the greenhouse, using the concentration of 100,000 conidia/ml, with the goal of measuring Type II resistance (resistance to spread in the spike). In greenhouse experiments, the same 42 lines evaluated in the field were evaluated for four experiments conducted in Fall 2015, Spring 2016, Fall 2016 and Spring 2017. The 42 lines examined in the field experiments were included in each greenhouse experiment. Seeds of each line were planted in a plastic pot with five seeds planted for each replicate and each experiment included three replications. Twelve to 15 plants per lines were inoculated and assessed for disease development in each experiment. The greenhouse settings for photoperiod and temperature were 16 h light and 22 °C, respectively. A single virulent isolate of F. graminearum was used for inoculum in each of these experiments. Approximately, 100 μL of inoculum was pipetted into two adjacent spikelets (the fourth and fifth spikes from the bottom of the spike) at anthesis. Each inoculated spike was then misted with water and covered with a plastic bag for 48 h.
Disease and mycotoxin analysis
FHB severity was examined visually 21 DAI by counting the total and the number of symptomatic spikelets in nondestructively selected 20 heads in each plot. Disease severity for each line was calculated as the percentage of infected spikelets of all spikelets assessed. Spikes (ca. 30 per plot) were harvested at maturity and dried for 5 days at 95 °C. Dried spikes were threshed using a belt thresher and the grain cleaned manually. Fusarium damaged kernels (FDK) analysis was done by counting out 100 arbitrarily selected seeds per plot and visually categorizing each grain as healthy or symptomatic, with FDK being expressed as a percentage. Following the FDK analysis, the samples were submitted for deoxynivalenol (DON) analyses using gas chromatography—mass spectrometry (GC–MS, detection limit 0.05 ppm) as described elsewhere35.
In the greenhouse experiments spikes (ca. 15 per entry) were assessed visually by counting the total and the number of symptomatic spikelets in each inoculated spike.
Statistical analyses
The FHB severity data were analyzed using R, version 3.2.2 (https://www.r-project.org/). Data from the two locations, Saint Paul and Fargo, were analyzed separately by using 80% trimmed mean to reduce the effect of outliers. Separate ANOVA models were applied to determine which model resulted in more variation among the lines compared to within the lines.
Stability and inheritance of resistance
The stability and inheritance of resistances, which were generated by alterations in the methylation patterns, were examined in two of the most promising lines. These lines were crossed with a susceptible parental cultivar; Ben36 and the resulting backcross-derived lines were advanced for three generations (BC1:F3) without a selection pressure. The BC1:F3 families were then tested in the field in one year and in one greenhouse experiment. These lines were tested together with the resistant M4 (parent) lines and susceptible checks.
DNA methylation level sample preparation
A total of 10 lines, comprising of four best and three worst performing lines and their parental lines, were selected for genome wide DNA methylome level analysis using the FASTmC method37. Total genomic DNA was isolated from the selected lines using DNeasy Plant Mini Kit (Qiagen). One microgram of DNA from each sample was used for estimation of genome wide DNA methylation level as described previously37,38.
Transcriptome analysis
To capture the transcriptome changes in the resistant (M4) vs. susceptible (parental) wheat varieties in response to F. graminearum infection, RNA-seq analysis was performed. Two time points, 12 and 48 h post inoculation (hpi), were selected to identify the differentially expressed genes (DEG) of F. graminearum-wheat interaction. These time points were chosen as at 12 hpi fungal spores germinate on the inner surface of lemma and palea whereas, at 48 hpi hyphae start to multiply extensively in the lemma. A total of 18 samples (6 sets of triplicates) were collected and used for RNA-seq analysis.
Plant growth conditions
One best performing M4 line, E.25.10, and a susceptible parent of E.25, Ben, were selected for transcriptome analysis. Seeds for both lines were sown in plastic pots containing 30% soil and 70% Pro-Line C/B Growing Mix (https://jollygardener.com/soil_jg_mixes.htm) and were grown in environment-controlled growth chambers set at 22˚C/20˚C (day/night cycle) with a 16 h photoperiod. Plants were fertilized once, applied two weeks after planting with, 20-20-20 (N-P-K).
Fusarium inoculations
Strain PH-1 of F. graminearum (provided by Dr. H Corby Kistler, USDA-ARS Cereal Disease Laboratory) was used for the inoculations of the plants used in the transcriptome analysis. Conidia were produced in CarboxyMethyl Cellulose (CMC) liquid medium by incubating in a shaker at 28 °C, 180 rpm for 2 days and harvested as described elsewhere39. Eight E.25.10 plants and eight Ben plants were inoculated with macroconidial inoculum and four E.25.10 plants and four Ben plants, were mock-inoculated with water. At mid-anthesis, 2–3 florets in a single spike of a biological replicate were inoculated by pipetting conidial suspension between the palea and lemma. Immediately following the inoculation the inoculated spikes were misted with water and covered with a plastic bag to promote disease development. Mock-inoculated plants were also misted with water and covered with a plastic bag. Of the eight E.25.10 and eight Ben plants, four plants were sampled at 12 hpi and four at 48 hpi. Infected spikelets from each of the four biological replicates were harvested separately at 12 and 48 hpi. Inoculated spikelets were also collected from the each of the four biological replicates of the mock-inoculated plants at 48 hpi. Harvested spikelets were frozen immediately in liquid nitrogen and stored in − 80 °C freezer for further analysis.
RNA sequencing and data processing
Three biological replicates of each genotype and treatment were used for transcriptome analysis. Total RNA was harvested from the infected spikelets at 12 and 48 hpi and also from the mock-inoculated spikelets using Spectrum Plant Total RNA Kit (Millipore Sigma) following manufacturer’s instructions. On column DNase digestion protocol (Millipore Sigma) was performed to remove any residual DNA during the RNA isolation. Three biological replicates for each genotype and treatment were used for transcriptome analysis. TruSeq dual indexed stranded RNA libraries were prepared following the manufacturers guidelines (Illumina). RNA quality and library size were analyzed on a Bioanalyzer (Agilent Technologies). Libraries were sequenced on an Illumina Genome Analyzer using HiSeq 2500 High Output, 50 bp PE flow cell and v4 chemistry at the University of Minnesota Genomics Center. The sequencing files were submitted to NCBI following preliminary analysis (SRA accession PRJNA595999).
Raw data was processed, using the rnaseq2 pipeline available at the gopher-pipelines (https://bitbucket.org/jgarbe/gopher-pipelines/wiki/rnaseq2-pipeline), for quality filtration, adapter trimming and reads mapping using Kallisto. The mapping result output was used for downstream analysis.
Differential gene expression analysis
Normalization and differential expression analyses were performed using DESeq240. After calling for differentially expressed genes (DEGs), the normalized data along with log2 fold changes and p-values ≤ 0.01, were used for downstream analysis. Genes differentially expressed with log2 fold change ≥ 2 or ≤ − 2 and a P ≤ 0.01 were considered significant. For the identification of DEGs, the DESeq cutoff was set to 0.5 RPKM while the DESeq parameters for dispersion estimation were set with method “pooled” and sharing Mode “fitOnly”. The false discovery rate (FDR) threshold for DEG calling was set to 0.05. DEGs common to the mock-inoculated and Fusarium-inoculated were removed from the further analysis. Common and unique DEGs between the inoculated treatment samples harvested at 12 and 48 hpi were used for various annotation purposes.
Gene Ontology annotations and enrichment analysis
For the Gene Ontology (GO) analysis, gene ids for each DEGs were identified using the blastP similarity search with e-value of 10–5 in the corresponding Triticum aestivum database (https://phytozome.jgi.doe.gov/pz/portal.html#!info?alias=Org_Taestivum_er). GO enrichment analysis was performed using agriGO41. MapMan analysis was performed with default parameters to assign MapMan bins for the differentially expressed transcripts (https://MapMan.gabipd.org/). Common and unique DEGs with log2 fold changes that were obtained from DESeq2 output were used in MapMan.
For pathway analyses the KO ids were assigned to the significant DEGs using the blastKOALA (https://www.kegg.jp/blastkoala/) option at the Kyoto Encyclopedia of Genes and Genomes (KEGG) server. KEGG database integrates genomic information with functional information by collecting manually drawn pathway maps on cellular processes and gene annotations42. The assigned KO ids were used to perform pathway analysis using KEGG pathway tool.
Quantitative real-time polymerase chain reaction
Fourteen genes with various expression values were selected for validation of gene expression using quantitative RT-PCR (qRT-PCR). The selected genes and their primers are listed in Supplementary Table S1 with the corresponding Ensembl Gene IDs. The default parameters of a template protocol, SYBR Green I 96-II, of the Roche LightCycler 480 was used for the qRT-PCR.
Results
Screening of the treated lines
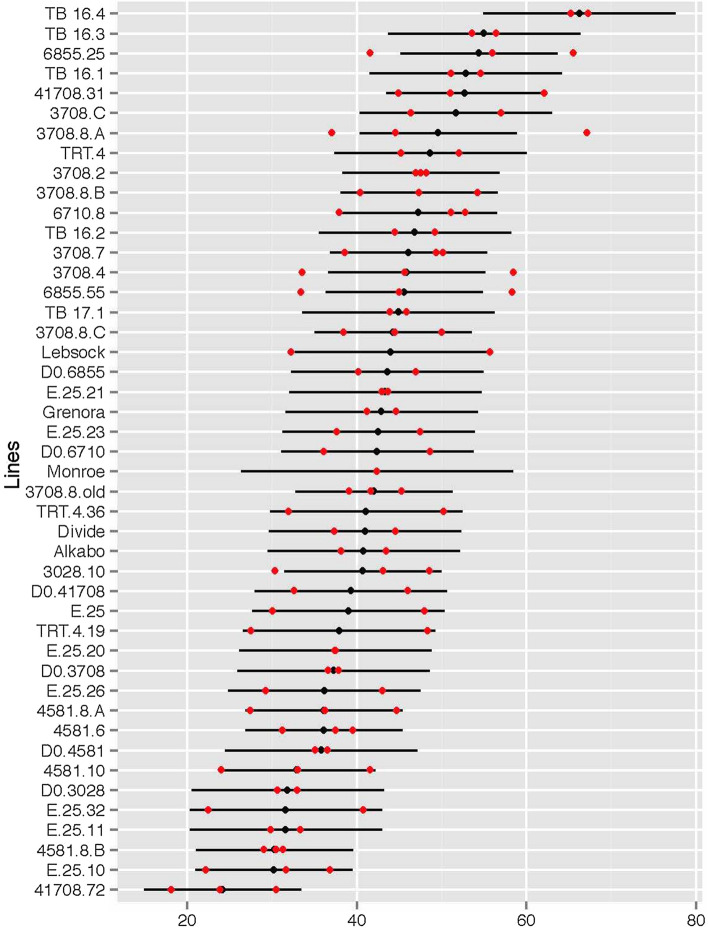
The germination rate following the 5-azacytidine treatments was approximately 50%, which was likely due to lethality of the treatment. A total of 415 treated seeds, of the 800 treated seeds, germinated, matured and grew into plants that produced seed. Of the 415, 32 lines were selected following elimination screening and were used in more rigorous testing. The scores for individual lines over different field experiments varied, but five, of the 32, lines showed promising result by having a lower FHB severity, FDK and DON values (Fig. 1, Supplementary Tables S2–S6). The data from the greenhouse inoculation experiments further supported the field data and showed a lower disease severity in the selected M4 lines (Table 1, Fig. 2). The selected M4 lines (E.25.10, E.25.11, E.25.23, E.25.32 and 41,708.72) had higher level of FHB resistance as compared with the parental lines and susceptible check (Figs. 1 and 2, Table 1, Supplementary Tables S2–S6).
Figure 1.
ANOVA plot showing the Fusarium head blight (FHB) disease severity estimates with 95% confidence intervals as black lines and dots. The lines at top and bottom have highest and lowest severity, respectively. Red dots indicate 80% trimmed mean of visual scoring data of disease severity, collected during the summer 2015 field season at Saint Paul, MN. Each red dot indicates one replicate. One replicate is an 80% trimmed mean of scores from 20 spikes. X-axis shows disease severity percentage and Y-axis show the name of the lines tested during the study.
Table 1.
Visual sore of FHB disease severity, Fusarium damaged kernel (FDK) percentage and deoxynivalenol (DON) values from the greenhouse inoculations.
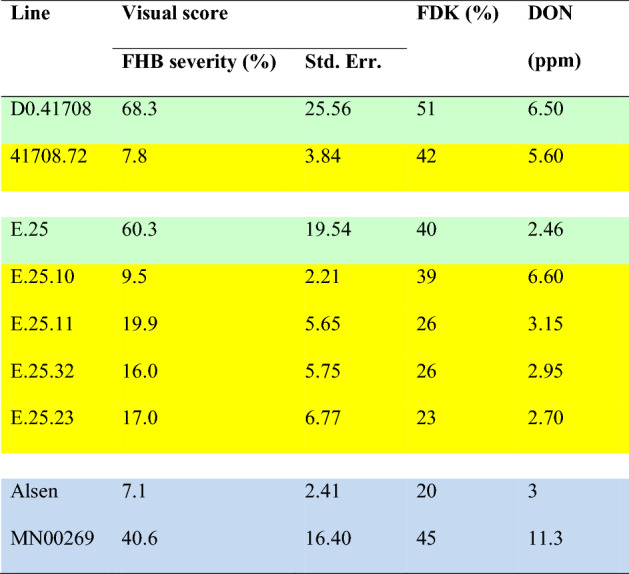
*Five best performing M4 lines are highlighted in yellow and the parents in light green.
Checks, Alsen (FHB resistant) and MN00269 (FHB susceptible), are highlighted in light blue.
The values are mean of three biological replicates.
For visual score, each biological replicates are mean of 15 spikes.
Figure 2.
Fusarium-inoculated and control spikes of wheat showing various degree of disease severity. Uninoculated (A) and water inoculated (B) spikes as a control, susceptible check (C), resistant check (D), parental line (E) and M4 line showing disease severity upon point inoculations.
Stability and inheritance of resistance
The BC1:F3 families, derived by crossing E.25.11 and 41,708.72 separately with Ben, were tested for inheritance and stability of the resistance. A total of 388 BC1:F3 families were tested in the summer 2017 field season together with the parental lines. Some of BC1:F3 families showed resistance similar to or better than the parental lines. Selected BC1:F3 families (50 best and 50 worst performing from the field data) were tested again in greenhouse, which supported the findings of the field study. The select BC1:F3 families performed on par with their parental lines. This preliminary data supports stable inheritance of resistance; however, additional testing is required to validate the number of resistant families to better estimate the number of genes underlying the trait.
Estimation of genome wide DNA methylation level
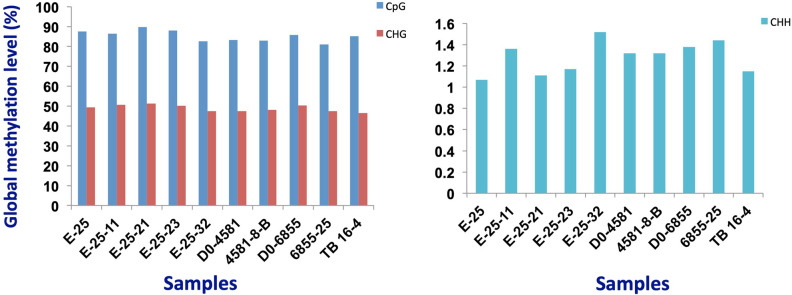
Methylome analysis using FASTmC estimates genome-wide DNA methylation levels at all cytosine sequences37, which indicated no significant difference at the global level between parental and M4 lines. Some of the best performing M4 lines, E.25.11, E.25.23 and E.25.32 did not show a significant difference from their parental line, E.25 (Fig. 3). This suggests that M1 lines may have had significant level of demethylation that was unstable and which was lost during advancement, M1 to M4.
Figure 3.
Global methylation level profiling using FASTmC method for M4 and parental lines. CpG islands are sites of transcription initiation. Methylation in the CpG site leads to transcriptional silencing of the genes. CHG: where H is A, C or T and CHH: where H is A, C or T.
Transcriptome analysis
On average, 40 million reads (range 31.51–49.62 million) were obtained for each sample, which were used to capture the dynamic transcriptome changes in resistant vs. susceptible line.
Differential expression of genes
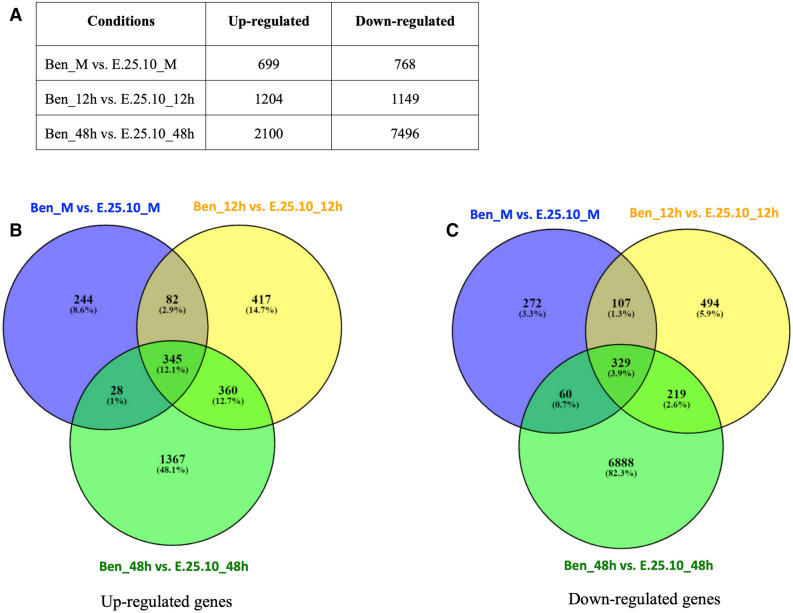
The total number of DEGs (log2 fold change ≥ 2 or ≤ -2 and a P < 0.01) at the two time points, 12 and 48 hpi, and mock treatment (M) are shown in Fig. 4A. A total of 699 and 768 genes were up- and down-regulated, respectively, in the Ben_M vs. E.25.10_M (mock-inoculated); 1204 and 1149 up- and down-regulated genes, respectively, in the Ben_12h vs. E.25.10_12h (12 hpi); and 2100 and 7496 up- and down-regulated genes, respectively, in the Ben_48h vs. E.25.10_48h (48 hpi) treatments (Fig. 4A). A total of 360 and 219 genes were commonly up- and down-regulated in the Ben_12h vs. E.25.10_12h and Ben_48h vs. E.25.10_48h (Fig. 4). A total of 417 and 1367 genes were up-regulated at 12 and 48 hpi, whereas, a total of 494 and 6888 genes were down-regulated at 12 and 48 hpi, respectively, and were unique to the provided conditions (Fig. 4). The genes, which were present commonly in mock-inoculated and the Fusarium-inoculated wheat at 12 and 48 hpi, were considered wound responsive, or plant growth and development related genes and were eliminated from further analysis (Fig. 4B,C). Genes that were common between the 12 hpi and 48 hpi samplings (360 and 219; Fig. 4B,C) were handled separately from the genes that were unique to either the 12 hpi (417 and 494; Fig. 4B,C) or 48 hpi sampling (1367 and 6888; Fig. 4B,C) and used in further analysis to examine early and late host responses.
Figure 4.
Table and Venn diagrams displaying differentially expressed genes in M4 line as compared to Ben. A total number of up- and down-regulated genes (log2 fold change ≥ 2 or ≤ -2 and a P < 0.01) in M4 line as compared to Ben in mock-inoculated at 48 h and Fusarium inoculated at 12 h and 48 h (A). Venn diagrams displaying up-regulated (B) and down-regulated (C) common (overlapping) and unique (non-overlapping) genes upon mock-inoculation at 48 h and Fusarium inoculation at 12 h and 48 h. Genes from panel (A) were used to display in Venn diagrams (B,C).
Gene expression levels of fourteen genes, with a range of expression values, were validated using qRT-PCR. The relative fold change in the expression of selected genes was in agreement with the transcriptome results and validated its findings (Supplementary Fig. S2).
Functional assessment of differentially expressed genes
The agriGO categorization of differentially expressed common genes at the 12 and 48 hpi samplings revealed elevated expression of genes related to ion binding, cation binding, metal ion binding, defense response, cell wall organization and modification, hydrolase activity, enzyme regulator activity and other activities in the E.25.10 line, as compared to Ben (Supplementary Fig. S3, Supplementary Table S7), that could play essential roles in conferring FHB resistance. In addition, genes related to binding, metabolic processes and several other categories, which could also play roles in conferring FHB resistance, were down-regulated in the M4 line as compared to Ben (Supplementary Fig. S4; Supplementary Table S8). The genes, which were uniquely up- or down-regulated at 12 hpi or 48 hpi, were also categorized using agriGO. The genes, unique to the 12 hpi sampling time, were involved with cell wall synthesis, cell wall organization, oxidative stress, signal transmission, sequence specific DNA binding, and transcription factor activity (Supplementary Figs. S5 and S6, Supplementary Tables S9 and S10). The genes unique to the 48 hpi sampling time were involved in several other activities such as cell wall macromolecule catabolic process, protein ubiquitination, protein amino acid phosphorylation, regulation of gene expression, cell signaling and communication, multidrug transport, photosynthesis, negative regulation of catalytic activity and response to oxidative stress (Supplementary Figs. S7 and S8, Supplementary Tables S11 and S12).
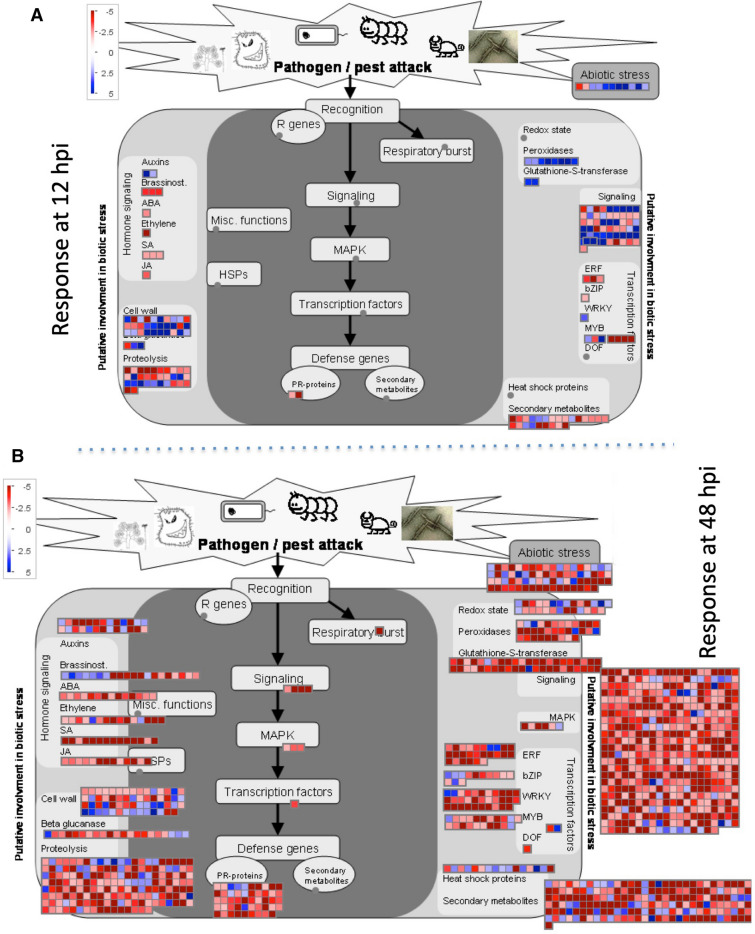
MapMan categorization revealed a significant level of DEGs, common to the 12 and 48 hpi sampling times, related to abiotic and biotic stresses, signaling, secondary metabolism, proteolysis, signaling, hormone signaling, and PR-proteins as differentially expressed in M4 line as compared to Ben (Supplementary Fig. S9). The transcriptional pattern of the same genes at 12 and 48 hpi differed significantly (Supplementary Fig. S9). MapMan categorization of the genes unique to the 12 or 48 hpi sampling times showed differentially expressed genes for abiotic stress, signaling, transcription factors, secondary metabolites, PR-proteins, proteolysis, cell wall, and hormone signaling (Fig. 5). As expected a large number of genes related to biotic stress were found differentially expressed at 48 hpi, as compared to 12 hpi, which coincides with fungal growth and accumulation from 12 to 48 hpi and suggests their role in the FHB resistance of the M4 line.
Figure 5.
MapMan overview showing differentially expressed unique genes related to biotic and abiotic stress in M4 line at 12 hpi (A) and 48 hpi (B). Up-regulated genes are shown by light to deep blue color boxes and the down-regulated genes are shown by light to deep red color boxes. Color intensity show the level of expressions as indicated by the intensity bar on top left of each panel. Genes involved in same functions are clubbed together. Grey dots indicate that there were no significant expressions of such genes.
Pathways expressed or suppressed
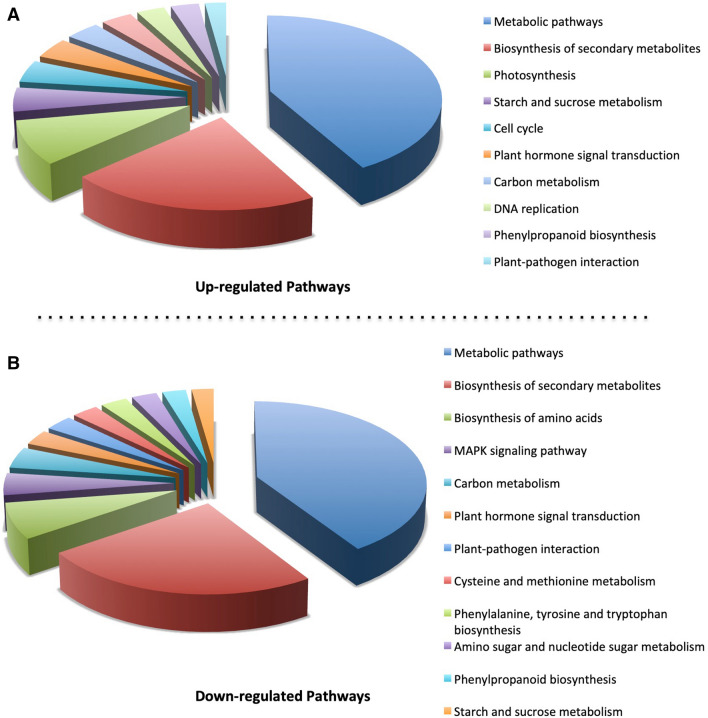
KEGG analysis demonstrated that diverse defense mechanisms were expressed more intensely or suppressed in the resistant M4 line at both 12 and 48 hpi as compared to the susceptible parental line (Fig. 6). A large number of genes representing metabolic pathways and biosynthesis of secondary metabolites were up- and down-regulated (Fig. 6). In addition, pathways involved in plant pathogen interaction, MAPK signaling, plant hormone signal transduction, starch and sucrose metabolism, photosynthesis and biosynthesis of secondary metabolites were significantly differentially expressed (Fig. 6).
Figure 6.
Classification of Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways (42) detected in the present analysis. The differentially expressed genes showing up- and down-regulated pathways in M4 lines in response to Fusarium infection are shown here.
Discussion
In this study, we explored the possibility of generating a novel source of FHB resistance using DNA demethylation in durum wheat seeds that may be useful for future breeding efforts. Seeds of eight advanced durum lines were treated with 5-azacytidine, allowed to germinate, grow into plants, set seed and were propagated through to the M4 generation. This was done to assure that the epigenetic modification is stable and heritable. Five best performing lines were selected following a multi-year, multi-location and field vs. green house testing. Except a few variations, the performances of selected lines were consistent and the results were reproducible. The variation in FHB resistance in the M4 lines or in the parental lines observed during this study can be attributed to variability in FHB reactions, unfavorable environmental conditions for disease development or escape from inoculation which commonly occur in field FHB evaluation as documented by other researchers43–45. Stability and inheritance of resistance of M4 lines were further tested by crossing two M4 lines (E.25.11 and 41,708.72) with a susceptible parental line, Ben and developing BC1:F3 families and testing them by Fusarium inoculation, which confirmed that the modification is stable, genetic and heritable.
The M4 lines together with parental lines were subjected to global methylome level analysis to see if an overall difference in methylation level is evident. There were slight non-significant differences in methylation level in the M4 vs. parental lines. This suggests that unstable demethylation were lost in the process of advancement of M1 to M4 generation. Only the stable demethylations are present in M4 generation and contributing to resistance against FHB. The underlying mechanism was investigated by transcriptome analysis of a M4 line and a susceptible parent at two time points, 12 and 48 hpi, and including a mock inoculation. The transcriptome analyses showed that distinct groups of genes were activated at different stages (12 and 48 hpi) in the M4 line and the susceptible parent in response to Fusarium infection.
Genes associated with Fusarium infection and plant response
To filter the genes associated with Fusarium infection, we eliminated the common genes that were shared between “Ben_M vs. E.25.10_M” and “Ben_12h vs. E.25.10_12h” and “Ben_M vs. E.25.10_M” and “Ben_48h vs. E.25.10_48h” (Fig. 4) and moved forward with the reminder. This was also done to remove the genes related to genetic background of Ben and E.25.10 lines. The macroconidia of Fusarium are reported to germinate within 6–12 h, and thus it was expected that the plants would elicit a defense response by 12 hpi. There were 360 up-regulated and 219 down-regulated genes common between 12 and 48 hpi. In addition, there were 417 up-regulated and 494 down-regulated unique genes at 12 hpi, which indicates an early response to infection. However, a more intense response was documented at 48 hpi as a total of 1367 up- and 6888 down-regulated unique genes were found. The common and unique genes represented several defense related pathways and were documented by Gene Ontology and MapMan analyses (Supplementary Figs. S3–S9 and Fig. 5). Numerous genes that were related to PR-proteins, hormone signaling, signaling, transcription factors, secondary metabolism, cell wall and oxidative stress were found differentially expressed in response to Fusarium infections.
Hormone signaling
Genes related to auxins, ABA, brassinosteroids, ethylene, jasmonic acid and salicylic acid were found differentially expressed at the 12 and 48 hpi samplings. However, there were significant differences in the number of genes between the two sampling times. Elevated levels of auxin in plant tissues have been observed previously following pathogen infection46. In the current analysis, at least 26 genes associated with auxin related pathways were found differentially expressed. Brassinosteroids are plant-specific steroidal hormones, which are involved in signal transduction that results in the regulation of expression of several hundred genes including stress related genes47. Twenty genes related to brassinosteroids were found differentially expressed in the current study, which is significant considering that these are known to play a role in signal transduction. Studies of the co-application of exogenous ABA or jasmonic acid with F. graminearum demonstrated increased wheat susceptibility, suggesting a major negative role of ABA and jasmonic acid in FHB resistance48,49. As all 14 genes related to ABA and 13 genes related to jasmonic acid were found down regulated in the current analysis, our data would support the role of ABA and jasmonic acid in increased wheat susceptibility. Ethylene is induced in plant tissues upon pathogen challenge; however, the role of ET in plant defense is ambiguous due to both positive and negative effects observed during host–pathogen interactions50. The reported studies match our findings where 13 out of the 15 ethylene related genes were down-regulated in the resistant line. SA pathway has been reported to play critical roles in resistance against F. graminearum and genes related to the SA pathway have been reported to be up-regulated in previous studies51,52. However, all of the 14 genes associated with SA pathway in the current analysis were found down regulated. This may indicate a genotype-dependent response or the importance of the timing of activation of the SA pathway, as has been reported in wheat by others53,54.
Pathogenesis related proteins
Induction of pathogenesis related (PR) proteins, PR-1, PR-2, PR-3, PR-4, and PR-5, have been documented in Fusarium infected wheat 6–12 hpi, reaching the highest levels at 36–48 h55, as was in our study (Fig. 5). Forty nine PR proteins were found differentially expressed during the current analysis and were categorized as; NB-ARC domain-containing disease resistance proteins, receptor like proteins 46 (RLP46), kinase family proteins with leucine-rich repeat (LRR) domains, receptor like proteins 27 (RLP27), leucine-rich repeat (LRR) family proteins, leucine-rich repeat serine/threonine protein kinases such as FLS2, receptor like proteins 33 (RLP33), acidic endochitinase precursors, LRR and NB-ARC domains-containing disease resistance proteins, PR-protein 1 precursors, PR-protein PRMS precursors, proteins Z (Z4), or serine protease inhibitor (SERPIN) family proteins. Two LRR receptor like kinases (LRR-RLK), one from barley (HvLRRK-6H) and one from wheat (TaLRRK-6D), were found highly induced upon F. graminearum infection. Virus-induced gene silencing (VIGS) of these genes resulted in susceptibility of the host plants56.
Transcription factors
In response to external stimuli, transcription factors generate primary responses by up- or down-regulating downstream gens as a part of plant defense mechanism57. Thirteen bZIP transcription factor family proteins were found differentially expressed (4 up- and 9 down-regulated) in the current analysis. The present study also documented differential expression of at least 26 genes encoding for AP2/EREBP (ERF) transcription factor family proteins. The WRKY transcription factor family had 32 genes differentially expressed during the present analysis. The types of WRKY proteins were WRKY35, WRKY11, WRKY65, WRKY51, WRKY50, WRKY33, WRKY30, WRKY41, WRKY71, WRKY55, WRKY3, WRKY11, WRKY40, WRKY46 and WRKY9. WRKY, Myb and bZIP transcription factors have been shown to paly important roles in FHB resistance in multiple studies58,59, which suggests why they were differentially expressed in the current analysis. A bZIP transcription factor, Fgap1, has been reported to mediate oxidative stress response in Fusarium infection60. TaWRKY45 expression was enhanced in response to Fusarium infection and a transgenic wheat plant constitutively expressing TaWRKY45 showed increased resistance to FHB61. Another WRKY protein, TaWRKY70, was found to have higher expression and regulate downstream genes in response to Fusarium infection. VIGS silencing of the TaWRKY70 resulted in a higher fungal biomass and susceptibility62.
Signaling
Four hundred and seventy seven genes related to signaling pathways were found differentially expressed in response to Fusarium infection in the M4 line in this study (Fig. 5). Of the 477 genes identified, 123 genes belonged to the LRR receptor kinases family, 55 to S-locus glycoprotein-like receptor kinases, 41 to calcium signaling, 39 to legume-lectin receptor kinases, 37 to DUF 26 receptor kinases, 37 to unclassified receptor kinases, 35 to cell wall associated receptor kinases, 17 to G-proteins, 15 to Catharanthus roseus-like RLK1 receptor kinases, 14 to thaumatin-like receptor kinases, 14 to sugar and nutrient physiology, 7 to light reactions and 6 to MAP kinases. LRR receptor kinases have been extensively studied because of their role in host response and have been discussed previously (See “Pathogenesis related proteins” on PR-proteins). S-locus glycoprotein-like receptor kinases has been reported to play a negative role in plant defense responses63, which supports the finding that 54, of the 55 S-locus glycoprotein like receptor kinases genes identified in the current analysis, were down-regulated. Ca2 + signaling is crucial for plant innate immunity as it mediates signaling process by variation in cytosolic Ca2 + concentration64. Differential expression of 41 Ca2 + signaling genes found in this study is in accordance with their previously reported role in plant defense. Overexpression of a L-type lectin-like protein kinase 1 (AtLPK1) was demonstrated to confer resistance against Botrytis cinerea in Arabidopsis thaliana by inducing stronger expression of a group of defense-related genes65. Significant differential expression of 39 legume-lectin genes in the present study further supports the role of legume-lectin genes in resistance to fungal pathogens in durum. G-protein coupled receptors (GPCRs) transmit signals, from extracellular challenges to intracellular G-proteins, that ultimately direct the appropriate biological response in host cells66. Significant differential expression of 17 G-proteins in the present study is well aligned with its reported role in biotic and abiotic stresses.
Cell wall
Fifty-nine proteins with a role in cell wall synthesis, organization or degradation were found to be differentially expressed in the current analysis. In addition to the 59 proteins with a role in cell wall synthesis, organization or degradation, we observed the differential expression of 21 glucanases and 4 chitinase genes in our dataset, which is in line with the previous findings57,67.
Of the 59 genes, 8 genes were associated with cell wall modifications, 6 were cellulose synthase genes, 5 were glucuronoxylan genes, 16 were cell wall degradation related genes (cellulases and beta-1,4-glucanases, mannan-xylose-arabinose-fucose and pectate lyases and polygalacturonases), 10 genes were related to cell wall precursor synthesis, 9 genes were related to cell wall hemicellulose synthesis and 5 genes represented cell wall pectin esterases. Cell wall composition and lignification has been reported to play important roles in conferring host resistance to FHB67. Cell wall features transferred from common wheat to durum wheat, by generating recombinant inbred lines (RILs) obtained by crossing the hexaploid resistant wheat with the susceptible durum wheat, were reported to improve FHB resistance significantly68.
Secondary metabolites
Plant deal with Fusarium infection and mycotoxin accumulation through the inhibition of toxin biosynthesis or converting the mycotoxin into less toxic compounds. The inhibition of toxin biosynthesis is handled through antioxidant properties of secondary metabolites. The main secondary metabolites with antioxidant activity belong to phenolic compounds, carotenoids and tocopherols in cereals69. A total of 181 genes representing for secondary metabolites biosynthesis were differentially expressed in the M4 line as compared to the susceptible line. Of the 181 genes, 45 genes were involved in lignin biosynthesis and belonged to phenylpropanoids, 23 were anthocyanins, 7 were chalcones, 21 were dihydroflavonols, 16 were flavonols and all of them belonged to flavonoids, 20 belonged to isoprenoids and were involved in mevalonate pathway, carotenoids pathway or terpenoids pathway and 7 were simple phenols. Phenolics are the major contributors to total antioxidant capacity of cereals and are divided into two groups: flavonoid phenylpropanoids and non-flavonoid phenylpropanoids which includes anthocyanins, chalcones, flavones, flavonols, flavanones, flavanols, stilbenes, lignans, and phenolic acids70. We documented differentially expressed gens, representing lignins, chalcones, flavonols, and phenols, which is in agreement to the previous reports by providing FHB resistance to M4 plants.
Oxidative stress
An FHB-resistant wheat variety has been reported to show rapid induction of ascorbate peroxidase (APX) and polyphenol oxidase (PPO) activity, correlated with the activity of antioxidative enzymes71. In the current study, 27 genes related to redox (thioredoxin, glutaredoxins, ascorbate and glutathione), 33 to peroxidases and 44 to glutathione S transferases were recorded as being differentially expressed. The significant differential expression of glutathione S transferases in the M4 line is in accordance with several previous studies, which have identified a role of glutathione S transferases in FHB resistance, with the resistance conferred by detoxifying the DON and thus reducing the aggressiveness of the pathogen72,73.
Abiotic stress
Plants are exposed to a wide range of biotic and abiotic stresses at the same time, or consecutively. Studies have also reported stress specific or independent commonalities in the response to stresses in different plant systems74,75. The crosstalk between the biotic and abiotic stress signaling pathways becomes synergistic and may lead to a cross-tolerance and enhancement of the plant’s resistance to pathogens76. The significant differential expression of 70 abiotic stress (heat, cold, drought and salinity) related genes in response to Fusarium infection in the current analysis also supports the commonalities between abiotic and biotic stress response as reported by other researchers. Based on the previous reports and findings of this study, some or all 70 abiotic stress related genes may also have contributed to the FHB resistance in the M4 line included in this study.
Altered pathways and mechanism of resistance in M4 line
In this study we documented at least 10 pathways that had 10 or more genes up-regulated and 23 pathways that had 10 or more genes down-regulated in response to Fusarium infection. Metabolic pathways and the pathways involved in the biosynthesis of secondary metabolites recorded the highest number of genes, which were up- or down-regulated (Fig. 6). The high number of DEGs in metabolic and secondary metabolite pathways suggests that the Fusarium-infected M4 plants need both energy and antioxidants in order to fight the Fusarium infections as reported by Gorinstein et al.70 and Erayman et al.77. A large number, 29, of genes from the MAPK signaling pathway were found to be differentially expressed in this study and these genes might have helped the plant in a late defense response to pathogen, or through stomatal development or maintaining the homeostasis of reactive oxygen species (Supplementary Fig. S14). A significant number of up-regulated genes were also found in association with photosynthesis and starch and sucrose metabolism pathways (Supplementary Figs. S10 and S11). Fifteen genes were documented to be up-regulated in photosynthesis pathways including photosystem I and II and the photosynthetic electron transport system (Supplementary Fig. S10). Twenty-six DEGs were recorded as being up-regulated in starch and sucrose metabolism pathway (Supplementary Fig. S11). Starch and sucrose metabolism pathway is reported to play critical role in FHB resistance in previous studies77,78. This suggests that the M4 plants in this study were capable of generating more energy, as compared to susceptible plants, and therefore could respond better to Fusarium infection. Genes related to plant pathogen interactions and plant hormone signal transduction pathways were found significantly differentially expressed (Fig. 6). Eighteen genes, representing the plant-pathogen interaction pathway, had significant differential expression in response to Fusarium infection, and may contribute to resistance in the M4 line via hypersensitive response, cell wall reinforcement, induction of defense related genes and stomatal closure (Supplementary Fig. S12). In addition, twenty-four genes from plant hormone signal transduction pathways were also recorded as being differentially expressed in this study. These genes regulate cell enlargement, cell division, shoot initiation, stomatal closure and senescence (Supplementary Fig. S13).
Conclusion
This study explored an alternative method for generating FHB resistance in durum wheat, as most of the durum wheat cultivars are susceptible to FHB. Eight of the advanced durum lines were treated with 5-azacytidine to remove methylation and allow the expression of probable candidate genes. Treated lines were advanced four generations (M4) and tested for the FHB resistance over multiple years and locations. Five of the treated lines showed promising resistance to FHB and were selected for methylome level analysis and transcriptome analysis. Methylome level/percentage analysis did not show a significant difference; however, transcriptome analysis indicated significant differences between the parental and M4 line. Genes that were differentially expressed more than two fold in the M4 line were filtered by eliminating the genes expressed in the mock-inoculated control plants. Differential gene expression patterns associated with the M4 line indicated multi-facetted defense responses.
We performed transcriptome profiling of wheat spikes against the Fusarium infection at 12 and 48 hpi to explore the early and late response. The M4 line activated defense systems by differentially expressing transcripts related to PR proteins, transcription factors, signaling, secondary metabolites, proteolysis, cell wall, oxidative stress and hormone signaling. The transcripts related to binding activity were the processes most affected by Fusarium infection in the M4 lines. In addition, signaling, metabolic processes, PR-proteins and oxidative stress associated transcripts were higher in the M4 lines. The KEGG pathway enrichment indicated that genes involved in metabolic pathways and the biosynthesis of secondary metabolites were highly affected. Our results demonstrate how a new approach to generating resistance to a notorious pathogen, Fusarium, can help with developing new durum wheat varieties with improved FHB resistance. The findings of the study may assist in breeders in the development of new varieties with improved FHB resistance by utilizing M4 lines as a parent. In future work we plan to undertake deep RNA sequencing of multiple M4 and parental lines to locate individual genes or combinations of genes conferring FHB resistance in durum wheat.
Supplementary Information
Acknowledgements
Funding for the project was provided by the U.S. Department of Agriculture, Agricultural Research Service, under Agreement No. 58-3640-3-008, and the Minnesota State Agricultural Experiment Station. This is a cooperative project with the U.S. Wheat and Barley Scab Initiative. Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the view of the U.S. Department of Agriculture. We thank Drs. Yanhong Dong (University of Minnesota) for assistance in DON analysis, Robert J Schmitz, University of Georgia and Katie Liberatore (Caylaxt inc.) for assistance in methylome analysis, and Marisa Miller (Pairwise Genomics) for assistance in transcriptome analysis. We thank Roger Casper (USDA-ARS Cereal Disease Lab) and Amar Elakkad (University of Minnesota) for excellent technical and field assistance. Mention of trade names or commercial products in this publication is solely for purpose of providing specific information and does not imply recommendation or endorsement by the US Department of Agriculture. USDA is an equal opportunity provider and employer.
Author contributions
S.F.K. and J.K. planned and organized the study. J.K., S.P., E.M.E., S.X. and R.D.M. developed and/or screened the M4 lines. S.F.K., R.D.M. and J.K. supervised the project. J.K. and S.F.K. analyzed the screening data. J.K. and K.M.R. performed the transcriptome analysis. J.K. and S.F.K. prepared the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-73521-2.
References
- 1.Schroeder HW, Christensen JJ. Factors affecting resistance of wheat to scab caused by Gibberella zeae. Phytopathol. 1963;53:831–838. [Google Scholar]
- 2.Cuthbert, P. A., Somers, D. J. & Brulé-Babel, A. Mapping of Fhb2 on chromosome 6BS: a gene controlling Fusarium head blight field resistance in bread wheat (Triticum aestivum L.). Theor. Appl. Genet. 114, 429–437, (2007). [DOI] [PubMed]
- 3.Zhang H, et al. Population analysis of the Fusarium graminearum species complex from wheat in China show a shift to more aggressive isolates. PLoS ONE. 2012;7:e31722. doi: 10.1371/journal.pone.0031722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McMullen M, et al. A unified effort to fight an enemy of wheat and barley: Fusarium head blight. Plant Dis. 2012;96:1712–1728. doi: 10.1094/PDIS-03-12-0291-FE. [DOI] [PubMed] [Google Scholar]
- 5.McMullen M, Jones R, Gallenberg D. Scab of wheat and barley: a re-emerging disease of devastating impact. Plant Dis. 1997;81:1340–1348. doi: 10.1094/PDIS.1997.81.12.1340. [DOI] [PubMed] [Google Scholar]
- 6.Wilson W, Dahl B, Nganje W. Economic costs of Fusarium head blight, scab and deoxynivalenol. World Mycotoxin J. 2018;11:291–302. doi: 10.3920/WMJ2017.2204. [DOI] [Google Scholar]
- 7.Koch A, et al. An RNAi-Based control of Fusarium graminearum infections through spraying of long dsRNAs involves a plant passage and is controlled by the fungal silencing machinery. PLoS Pathog. 2016;12(10):e1005901. doi: 10.1371/journal.ppat.1005901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kazan K, Gardiner DM, Manners JM. On the trail of a cereal killer: recent advances in Fusarium graminearum pathogenomics and host resistance. Mol. Plant Pathol. 2012;13(4):399–413. doi: 10.1111/j.1364-3703.2011.00762.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bai G, Shaner G. Scab of wheat: prospects for control. Plant Dis. 1994;78:760–766. doi: 10.1094/PD-78-0760. [DOI] [Google Scholar]
- 10.Buerstmayr H, Ban T, Anderson JA. QTL mapping and marker-assisted selection for Fusarium head blight resistance in wheat: a review. Plant Breed. 2009;128:1–26. doi: 10.1111/j.1439-0523.2008.01550.x. [DOI] [Google Scholar]
- 11.Clarke JM, Clarke FR, Pozniak CJ. Forty-six years of genetic improvement in Canadian durum wheat cultivars. Can. J. Plant Sci. 2010;90:791–801. doi: 10.4141/cjps10091. [DOI] [Google Scholar]
- 12.Miedaner T, Longin CFH. Genetic variation for resistance to Fusarium head blight in winter durum material. Crop Pasture Sci. 2014;65:46–51. doi: 10.1071/CP13170. [DOI] [Google Scholar]
- 13.Prat N, Buerstmayr M, Steiner B, Robert O, Buerstmayr H. Current knowledge on resistance to Fusarium head blight in tetraploid wheat. Mol Breed. 2014;34:1689–1699. doi: 10.1007/s11032-014-0184-2. [DOI] [Google Scholar]
- 14.Ghavami, F. et al. Mixed model association mapping for fusarium head blight resistance in tunisian-derived durum wheat populations. G3 (Bethesda). 1(3), 209–218 (2011). [DOI] [PMC free article] [PubMed]
- 15.Garvin DF, Stack RW, Hansen JM. Quantitative trait locus mapping of increased Fusarium head blight susceptibility associated with a wild emmer wheat chromosome. Phytopathol. 2009;99:447–452. doi: 10.1094/PHYTO-99-4-0447. [DOI] [PubMed] [Google Scholar]
- 16.Jauhar PP, Peterson TS. Chromosome engineering of durum wheat with alien chromatin of diploid wheatgrass. J. Crop Improv. 2009;23:319–331. doi: 10.1080/15427520902838648. [DOI] [Google Scholar]
- 17.Bender J. DNA methylation and epigenetics. Annu. Rev. Plant Biol. 2004;55:41–68. doi: 10.1146/annurev.arplant.55.031903.141641. [DOI] [PubMed] [Google Scholar]
- 18.Kenneth M, et al. A naturally occurring epigenetic mutation in a gene encoding an SBP-box transcription factor inhibits tomato fruit ripening. Nat. Genet. 2006;38(8):948–952. doi: 10.1038/ng1841. [DOI] [PubMed] [Google Scholar]
- 19.Marfil CF, Camadro EL, Masuelli RW. Phenotypic instability and epigenetic variability in a diploid potato of hybrid origin Solanum ruiz-lealii. BMC Plant Biol. 2009;9:21. doi: 10.1186/1471-2229-9-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chinnusamy V, Zhu J. Epigenetic regulation of stress responses in plants. Curr. Opin. Plant Biol. 2009;12(2):133–139. doi: 10.1016/j.pbi.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choi C, Sano H. Abiotic-stress induces demethylation and transcriptional activation of a gene encoding a glycerophosphodiesterase-like protein in tobacco plants. Mol Genet Genomics. 2007;277(5):589–600. doi: 10.1007/s00438-007-0209-1. [DOI] [PubMed] [Google Scholar]
- 22.Yu A, et al. Dynamics and biological relevance of DNA demethylation in Arabidopsis antibacterial defense. Proc Natl Acad Sci U S A. 2013;110:2389–2394. doi: 10.1073/pnas.1211757110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wada Y, Miyamoto K, Kusano T, Sano H. Association between up-regulation of stress-responsive genes and hypomethylation of genomic DNA in tobacco plants. Mol. Gen. Genomics. 2004;271:658–666. doi: 10.1007/s00438-004-1018-4. [DOI] [PubMed] [Google Scholar]
- 24.López Sánchez, A., Stassen, J. H., Furci, L., Smith, L. M. & Ton, J. The role of DNA (de)methylation in immune responsiveness of Arabidopsis. Plant J. 88(3), 361–374 (2016). [DOI] [PMC free article] [PubMed]
- 25.Le TN, et al. DNA demethylases target promoter transposable elements to positively regulate stress responsive genes in Arabidopsis. Genome Biol. 2014;15:458. doi: 10.1186/s13059-014-0458-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang X, et al. Genome-wide high-resolution mapping and functional analysis of DNA methylation in Arabidopsis. Cell. 2006;126:1189–1201. doi: 10.1016/j.cell.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 27.Vanyushin BF, Ashapkin VV. DNA methylation in higher plants: past, present and future. Biochim Biophys Acta. 2011;1809:360–368. doi: 10.1016/j.bbagrm.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 28.Tatra GS, Miranda J, Chinnappa CC, Reid DM. Effect of light quality and 5-azacytidine on genomic methylation and stem elongation in two ecotypes of Stellaria longipes. Physiol. Plant. 2000;109(3):313–321. doi: 10.1034/j.1399-3054.2000.100313.x. [DOI] [Google Scholar]
- 29.Christman JK. 5-Azacytidine and 5-aza-2′-deoxycytidine as inhibitors of DNA methylation: mechanistic studies and their implications for cancer therapy. Oncogene. 2002;21(35):5483–5495. doi: 10.1038/sj.onc.1205699. [DOI] [PubMed] [Google Scholar]
- 30.Akimoto K, et al. Epigenetic inheritance in rice plants. Ann Bot. 2007;100(2):205–217. doi: 10.1093/aob/mcm110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Elias EM, Manthey FA, AbuHammad W. Registration of ‘Carpio’ Durum Wheat. J. Plant Reg. 2015;9:78–82. doi: 10.3198/jpr2014.05.0030crc. [DOI] [Google Scholar]
- 32.Elias EM, Manthey FA. Registration of ‘Joppa’ Durum Wheat. J. Plant Reg. 2016;10:139–144. doi: 10.3198/jpr2015.11.0071crc. [DOI] [Google Scholar]
- 33.Gautam P, Dill-Macky R. Impact of moisture, host genetics and Fusarium graminearum isolates on Fusarium head blight development and trichothecene accumulation in spring wheat. Mycotoxin Res. 2012;28(1):45–58. doi: 10.1007/s12550-011-0115-6. [DOI] [PubMed] [Google Scholar]
- 34.Puri KD, Zhong S. The 3ADON population of Fusarium graminearum found in North Dakota is more aggressive and produces a higher level of DON than the prevalent 15ADON population in spring wheat. Phytopathol. 2010;100:1007–1014. doi: 10.1094/PHYTO-12-09-0332. [DOI] [PubMed] [Google Scholar]
- 35.Fuentes RG, et al. Resource allocation and cultivar stability in breeding for Fusarium head blight resistance in spring wheat. Crop Sci. 2005;45:1965–1972. doi: 10.2135/cropsci2004.0589. [DOI] [Google Scholar]
- 36.Elias EM, Miller JD. Registration of ‘Ben’ durum Wheat. Crop Sci. 1998;38:895. doi: 10.2135/cropsci1998.0011183X003800030070x. [DOI] [Google Scholar]
- 37.Bewick, A. J. et al. FASTmC: A Suite of Predictive Models for Nonreference-Based Estimations of DNA Methylation. G3 (Bethesda).6(2), 447–52 (2015). [DOI] [PMC free article] [PubMed]
- 38.Urich MA, Nery JR, Lister R, Schmitz RJ, Ecker JR. MethylC-seq library preparation for base-resolution whole-genome bisulfite sequencing. Nat Protoc. 2015;10(3):475–483. doi: 10.1038/nprot.2014.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Menke J, Dong YH, Kistler HC. Fusarium graminearum Tri12p influences virulence to wheat and trichothecene accumulation. Mol. Plant-Microbe Interact. 2012;25:1408–1418. doi: 10.1094/MPMI-04-12-0081-R. [DOI] [PubMed] [Google Scholar]
- 40.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12):550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tian, T. et al. agriGO v2.0: a GO analysis toolkit for the agricultural community, 2017 update. Nucleic Acids Res. 45(W1), W122-W129, (2017). [DOI] [PMC free article] [PubMed]
- 42.Kanehisa M, Goto S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28:27–30. doi: 10.1093/nar/28.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miedaner T, Schilling AG. Genetic variation of aggressiveness in individual field populations of Fusarium graminearum and Fusarium culmorum tested on young plants of winter rye. Eur. J. Plant Pathol. 1996;102:823–830. doi: 10.1007/BF01877051. [DOI] [Google Scholar]
- 44.Šíp V, et al. Effects of genotype, environment and fungicide treatment on development of Fusarium head blight and accumulation of DON in winter wheat grain. Czech J. Genet. Plant Breed. 2007;43:16–31. doi: 10.17221/1905-CJGPB. [DOI] [Google Scholar]
- 45.Szabo-Hever A, et al. Genetic diversity and resistance to Fusarium head blight in synthetic hexaploid wheat derived from Aegilops tauschii and diverse Triticum turgidum subspecies. Front. Plant Sci. 2018;9:1829. doi: 10.3389/fpls.2018.01829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fu J, Wang S. Insights into auxin signaling in plant-pathogen interactions. Front. Plant Sci. 2011;2:74. doi: 10.3389/fpls.2011.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sharma I, Kaur N, Pati PK. Brassinosteroids: a promising option in deciphering remedial strategies for abiotic stress tolerance in rice. Front. Plant Sci. 2017;8:2151. doi: 10.3389/fpls.2017.02151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Buhrow LM, Cram D, Tulpan D, Foroud NA, Loewen MC. Exogenous abscisic acid and gibberellic acid elicit opposing effects on Fusarium graminearum infection in wheat. Phytopathol. 2016;106(9):986–996. doi: 10.1094/PHYTO-01-16-0033-R. [DOI] [PubMed] [Google Scholar]
- 49.Qi P-F, et al. Jasmonic acid and abscisic acid play important roles in host–pathogen interaction between Fusarium graminearum and wheat during the early stages of fusarium head blight. Physiol. Mol. Plant Pathol. 2016;93:39–48. doi: 10.1016/j.pmpp.2015.12.004. [DOI] [Google Scholar]
- 50.Broekaert WF, Delaure SL, De Bolle MF, Cammue BP. The role of ethylene in host-pathogen interactions. Annu. Rev. Phytopathol. 2006;44:393–416. doi: 10.1146/annurev.phyto.44.070505.143440. [DOI] [PubMed] [Google Scholar]
- 51.Makandar R, et al. Salicylic acid regulates basal resistance to Fusarium head blight in wheat. Mol. Plant. Microbe. Interact. 2012;25(3):431–439. doi: 10.1094/MPMI-09-11-0232. [DOI] [PubMed] [Google Scholar]
- 52.Wang L, et al. Integrated transcriptome and hormone profiling highlight the role of multiple phytohormone pathways in wheat resistance against fusarium head blight. PLoS ONE. 2018;13(11):e0207036. doi: 10.1371/journal.pone.0207036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Makandar R, et al. Involvement of salicylate and jasmonate signaling pathways in Arabidopsis interaction with Fusarium graminearum. Mol. Plant-Microbe. Interact. 2010;23:861–870. doi: 10.1094/MPMI-23-7-0861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ding L, et al. Resistance to hemibiotrophic F graminearum infection is associated with coordinated and ordered expression of diverse defense signaling pathways. PLoS ONE. 2011;6:e19008. doi: 10.1371/journal.pone.0019008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pritsch C, et al. Systemic expression of defense response genes in wheat spikes as a response to Fusarium graminearum infection. Physiol. Mol. Plant Pathol. 2001;58(1):1–12. doi: 10.1006/pmpp.2000.0308. [DOI] [Google Scholar]
- 56.Thapa G, et al. A pathogen-responsive leucine rich receptor like kinase contributes to Fusarium resistance in cereals. Front. Plant Sci. 2018;9:867. doi: 10.3389/fpls.2018.00867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen C, Chen Z. Potentiation of developmentally regulated plant defense response by AtWRKY18, a pathogen-induced Arabidopsis transcription factor. Plant Physiol. 2002;129:706–716. doi: 10.1104/pp.001057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pan Y, et al. Transcriptome dynamics associated with resistance and susceptibility against fusarium head blight in four wheat genotypes. BMC Genomics. 2018;19(1):642. doi: 10.1186/s12864-018-5012-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Steiner B, Kurz H, Lemmens M, Buerstmayr H. Differential gene expression of related wheat lines with contrasting levels of head blight resistance after Fusarium graminearum inoculation. Theor Appl Genet. 2009;118(4):753–764. doi: 10.1007/s00122-008-0935-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Montibus, M. et al. Is the Fgap1 mediated response to oxidative stress chemotype dependent in Fusarium graminearum? FEMS Microbiol Lett. 363(2), fnv232 (2016). [DOI] [PubMed]
- 61.Bahrini I, et al. Characterization of a wheat transcription factor, TaWRKY45, and its effect on Fusarium head blight resistance in transgenic wheat plants. Breed. Sci. 2011;61:121–129. doi: 10.1270/jsbbs.61.121. [DOI] [Google Scholar]
- 62.Kage U, Yogendra KN, Kushalappa AC. TaWRKY70 transcription factor in wheat QTL-2DL regulates downstream metabolite biosynthetic genes to resist Fusarium graminearum infection spread within spike. Sci. Rep. 2017;7:42596. doi: 10.1038/srep42596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim HS, et al. An S-locus receptor-like kinase plays a role as a negative regulator in plant defense responses. Biochem Biophys Res Commun. 2009;381(3):424–428. doi: 10.1016/j.bbrc.2009.02.050. [DOI] [PubMed] [Google Scholar]
- 64.Boudsocq M, et al. Differential innate immune signaling via Ca (2+) sensor protein kinases. Nature. 2010;464:418–422. doi: 10.1038/nature08794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Huang P, et al. Overexpression of L-type lectin-like protein kinase 1 confers pathogen resistance and regulates salinity response in Arabidopsis thaliana. Plant Sci. 2013;203–204:98–106. doi: 10.1016/j.plantsci.2012.12.019. [DOI] [PubMed] [Google Scholar]
- 66.Dilks T, Halsey K, De Vos RP, Hammond-Kosack KE, Brown NA. Non-canonical fungal G-protein coupled receptors promote Fusarium head blight on wheat. PLoS Pathog. 2019;15(4):e1007666. doi: 10.1371/journal.ppat.1007666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lahlali R, et al. Cell wall biomolecular composition plays a potential role in the host type II resistance to Fusarium head blight in wheat. Front. Microbiol. 2016;7:910. doi: 10.3389/fmicb.2016.00910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Giancaspro A, et al. Cell wall features transferred from common into durum wheat to improve Fusarium Head Blight resistance. Plant Sci. 2018;274:121–128. doi: 10.1016/j.plantsci.2018.05.016. [DOI] [PubMed] [Google Scholar]
- 69.Boutigny A-L, Richard-Forget F, Barreau C. Natural mechanisms for cereal resistance to the accumulation of Fusarium trichothecenes. Eur. J. Plant Pathol. 2008;121:411–423. doi: 10.1007/s10658-007-9266-x. [DOI] [Google Scholar]
- 70.Gorinstein S, et al. Comparison of composition and antioxidant capacity of some cereals and pseudocereals. Int. J. Food Sci. Tech. 2008;43:629–637. doi: 10.1111/j.1365-2621.2007.01498.x. [DOI] [Google Scholar]
- 71.Spanic, V., Viljevac Vuletic, M., Abicic, I. & Marcek, T. Early response of wheat antioxidant system with special reference to Fusarium head blight stress. Plant Physiol. Biochem.115, 34–43 (2017). [DOI] [PubMed]
- 72.Han Q, et al. A glutathione S-transferase gene from Lilium regale Wilson confers transgenic tobacco resistance to Fusarium oxysporum. Sci. Hortic. (Amsterdam) 2016;198:370–378. doi: 10.1016/j.scienta.2015.11.047. [DOI] [Google Scholar]
- 73.Dhokane D, Karre S, Kushalappa AC, McCartney C. Integrated metabolo-transcriptomics reveals Fusarium head blight candidate resistance genes in wheat QTL-Fhb2. PLoS ONE. 2016;11:e0155851. doi: 10.1371/journal.pone.0155851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang H, Sonnewald U. Differences and commonalities of plant responses to single and combined stresses. Plant J. 2017;90(5):839–855. doi: 10.1111/tpj.13557. [DOI] [PubMed] [Google Scholar]
- 75.Nejat N, Mantri N. Plant immune system: crosstalk between responses to biotic and abiotic stresses the missing link in understanding plant defence. Curr Issues Mol Biol. 2017;23:1–16. doi: 10.21775/cimb.023.001. [DOI] [PubMed] [Google Scholar]
- 76.Rejeb IB, Pastor V, Mauch-Mani B. Plant responses to simultaneous biotic and abiotic stress: molecular mechanisms. Plants (Basel). 2014;3(4):458–475. doi: 10.3390/plants3040458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Erayman M, et al. Transcriptome analysis of wheat inoculated with Fusarium graminearum. Front Plant Sci. 2015;6:867. doi: 10.3389/fpls.2015.00867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhou W, Kolb FL, Bai GH, Shaner G, Domier LL. Genetic analysis of scab resistance QTL in wheat with microsatellite and AFLP markers. Genome. 2002;45:719–727. doi: 10.1139/g02-034. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.