Abstract
Angiotensin Converting Enzyme2 is the cell surface binding site for the coronavirus SARS‐CoV‐2, which causes COVID‐19. We propose that an imbalance in the action of ACE1‐ and ACE2‐derived peptides, thereby enhancing angiotensin II (Ang II) signalling is primary driver of COVID‐19 pathobiology. ACE1/ACE2 imbalance occurs due to the binding of SARS‐CoV‐2 to ACE2, reducing ACE2‐mediated conversion of Ang II to Ang peptides that counteract pathophysiological effects of ACE1‐generated ANG II. This hypothesis suggests several approaches to treat COVID‐19 by restoring ACE1/ACE2 balance: (a) AT receptor antagonists; (b) ACE1 inhibitors (ACEIs); (iii) agonists of receptors activated by ACE2‐derived peptides (e.g. Ang (1–7), which activates MAS1); (d) recombinant human ACE2 or ACE2 peptides as decoys for the virus. Reducing ACE1/ACE2 imbalance is predicted to blunt COVID‐19‐associated morbidity and mortality, especially in vulnerable patients. Importantly, approved AT antagonists and ACEIs can be rapidly repurposed to test their efficacy in treating COVID‐19.
LINKED ARTICLES
This article is part of a themed issue on The Pharmacology of COVID‐19. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v177.21/issuetoc
Abbreviations
- ACEI
ACE inhibitor
- AGTR1
angiotensin II receptor 1
- ALI
acute lung injury
- ARDS
acute respiratory distress syndrome
- EMT
epithelial‐to‐mesenchymal transition
- RAS
renin‐angiotensin signalling
- ROS
reactive oxygen species
1. INTRODUCTION
The SARS‐CoV‐2 virus infects human cells by first binding via its S protein to its target angiotensin‐converting enzyme 2 (ACE2), a 120 kDa integral membrane glycoprotein on the surface of cells in the lungs, heart, kidneys and intestine (Hamming et al., 2004; Wan, Shang, Graham, Baric, & Li, 2020; Zhang, Penninger, Li, Zhong, & Slutsky, 2020; Zhou, Yu, et al., 2020). Binding of SARS coronaviruses to ACE2 is followed by fusion of the viral and plasma membranes, endocytosis and cellular infection of the virus (He, Deng, & Li, 2020). ACE2 expression in respiratory epithelium is important in the pathobiology of COVID‐19 (Xu, Zhong, et al., 2020). The infection typically begins in epithelia in the upper respiratory tract, before spreading to alveoli in the lungs (Xu, Zhong, et al., 2020; Zhou, Yu, et al., 2020); pathological events are more severe in patients with a compromised immune response or the ability to combat the spread of infection (Rothan & Byrareddy, 2020). COVID‐19 typically (in 80% of patients) causes mild symptoms (Wu & McGoogan, 2020) but severe morbidity in a subset of patients, requiring hospitalization, intensive care and, in some cases, causing death. Increased morbidity and mortality occur in patients with co‐morbidities, especially ones associated with aging, including hypertension, cardiac disease, diabetes, chronic lung disease and compromised immunity (Wu & McGoogan, 2020; Zhou, Yu, et al., 2020). Many strategies are being pursued in response to the urgent need for effective therapies of COVID‐19 (e.g. Stebbing et al., 2020).
The SARS‐CoV‐2 virus shares many characteristics with the SARS‐CoV‐1 coronavirus, which caused a pandemic in 2002–2003 (He et al., 2020; Wan et al., 2020). Common features include ~80% shared sequence identity in their viral genomes (He et al., 2020), the range of tissues that are infected, mortality from acute respiratory distress syndrome (ARDS) and their extracellular binding site ACE2 (Wan et al., 2020; Zhang et al., 2020; Zhou, Yang, et al., 2020; Zhou, Yu, et al., 2020). Compared to SAR‐COV‐1, SARS‐CoV‐2 has ~4‐fold higher affinity for ACE2 (Walls et al., 2020). Due to these similarities, information related to SARS‐CoV‐1 can aid in development of hypotheses for treatment of SARS‐CoV‐2, including the repurposing of pharmacological agents approved for use in humans.
2. HOW DOES SARS‐COV‐2 INFECTION CAUSE PATHOLOGY?
Based on published data for both SARS viruses, we propose a pathophysiological chain of events for COVID‐19 (Figure 1). Central to this mechanism is angiotensin II (Ang II) signalling, which we elaborate in sections below.
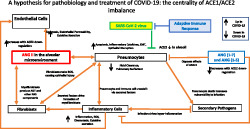
FIGURE 1.
A schema identifying the tissues impacted by SARS‐CoV‐2 infection and COVID‐19 pathobiology. Dashed arrows identify events whose role is as‐yet unclear
SARS‐CoV‐2 infection typically begins in the upper respiratory tract by exposure to the virus in aerosolized droplets or from fomites (Bar‐On, Flamholz, Phillips, & Milo, 2020). Oral epithelial cells and other respiratory tract areas have substantial expression of ACE2, explaining their susceptibility to viral entry (Xu, Zhong, et al., 2020; Zhou, Yu, et al., 2020). The virus spreads into the lower respiratory tract (the lungs) where epithelial cells, in particular Type II pneumocytes, express ACE2 (Hamming et al., 2004; Mossel et al., 2008; Tian et al., 2020; Zhang et al., 2020). Infection in the lungs, and especially damage to the alveoli, is a primary cause of morbidity in COVID‐19 (He et al., 2020; Zhou, Yang, et al., 2020; Zhou, Yu, et al., 2020). In susceptible patients, lesions from viral infection and entry of fluid into alveolar spaces induce respiratory distress. Pulmonary lesions appear to be a hallmark and diagnostic feature of COVID‐19 (Pan et al., 2020). Certain patients have severe inflammation and cytokine storm, with overwhelming immune activation that attacks the host (Pedersen & Ho, 2020). These events can produce respiratory failure and death, especially if critical care support and mechanical ventilation are unavailable and may ultimately be associated with multiple organ failure and death (Du et al., 2020). The pulmonary pathobiology in COVID‐19 is akin to what occurred with SARS‐1 infection (Channappanavar & Perlman, 2017).
In addition to pulmonary injury, COVID‐19 can cause cardiac complications. Most notable is myocarditis from viral infection of the myocardium, perhaps facilitated by ACE2 on cardiac myocytes (Chen, Zhou, & Wang, 2020; Sun, Lu, Xu, Sun, & Pan, 2020). Myocardial infection, myocarditis and cardiomyopathy occurred with SARS‐1 infection (Oudit et al., 2009), but data for their frequency in COVID‐19 are still emerging. Cardiac injury in COVID‐19 patients likely occurs from at least two mechanisms: (a) cytokine release (‘storm’) associated with inflammation in the lung (Pedersen & Ho, 2020) that affects the heart and (b) infection of the myocardium, most likely via viraemia, which has been documented in COVID‐19 patients (Huang et al., 2020). In SARS‐1, a strong association was noted between circulating viral loads and disease severity (Hung et al., 2004). This finding suggests that viraemia results from alveolar damage and access of the virus to the capillary network in the lung, which can occur in more severe cases. The stage of illness during which viraemia occurs in COVID‐19 and if viraemia correlates with severity/injury is not as‐yet well‐defined.
SARS‐1 and COVID‐19 can affect other tissues. A symptom of COVID‐19 is gastric distress, which can occur prior to pulmonary symptoms (Gu, Han, & Wang, 2020; Rothan & Byrareddy, 2020). Faecal transmission of SARS‐CoV‐2 has also been documented (Gu et al., 2020), which confirms the presence of the virus in the gastrointestinal (GI) tract, a site in which ACE2 is expressed (Hamming et al., 2004). The spread of infection to the GI tract may result from viraemia or from the mouth and upper respiratory tract, suggesting that the virus survives passage through the stomach. COVID‐19 can involve other ACE2‐expressing tissues, including the kidneys (Naicker et al., 2020).
The primary causes of COVID‐19 morbidity and mortality are (1) lung injury with associated respiratory distress (and accompanying cytokine storm) and (2) heart failure or other cardiac dysfunction (He et al., 2020; Zhou, Yang, et al., 2020; Zhou, Yu, et al., 2020). Therapeutic strategies for COVID‐19 seek to prevent/slow viral infection or mitigate injury from the infection. In view of the apparent lack of success thus far with antiviral therapies and the time needed to develop vaccines, we propose strategies that focus on blunting the pathobiology that we hypothesize results from ACE1 (angiotensin‐converting enzyme 1)/ACE2 imbalance and enhanced Ang II signalling that occurs with SARS‐CoV‐2 infection.
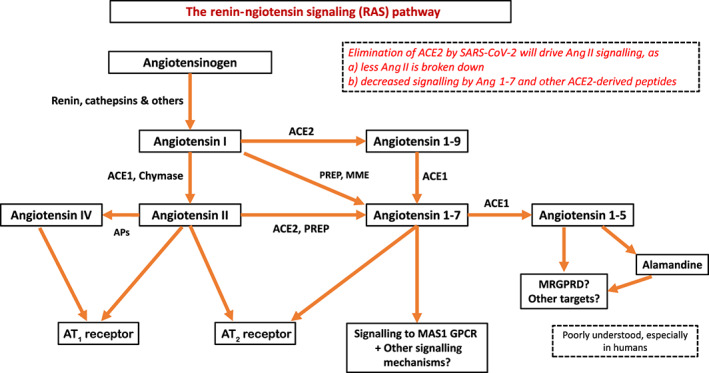
3. THE RENIN‐ANGIOTENSIN SIGNALLING (RAS) PATHWAY
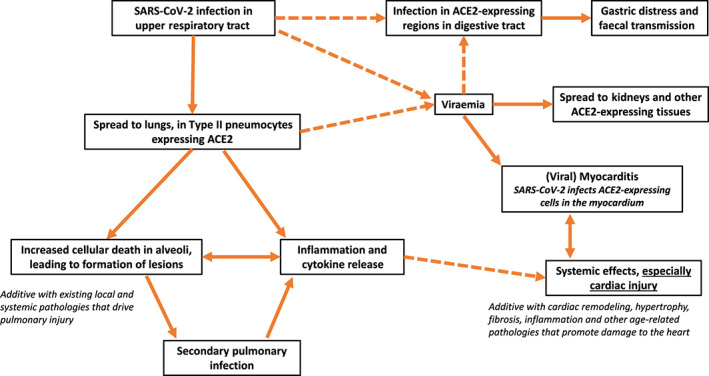
Figure 2 shows a schema of the RAS pathway and is based on data for angiotensin signalling from a range of sources (Forrester et al., 2018; Karnik, Singh, Tirupula, & Unal, 2017; Santos et al., 2019; Tikellis & Thomas, 2012), including the Guide to Pharmacology database (GtoPdb; https://www.guidetopharmacology.org/) (Armstrong et al., 2020). In the ‘traditional’/canonical RAS pathway, renin is secreted from the kidney, and angiotensinogen (AGT) is produced and secreted by the liver. Renin cleaves angiotensinogen to form angiotensin 1 (Ang 1), which in turn generates Ang II or Ang 1–9, primarily from the actions of ACE1 and ACE2, respectively. Ang II is cleaved by multiple enzymes, most importantly ACE2, to form Ang (1–7). Ang II also forms Ang IV via the action of aminopeptidases (APs). Both Ang II and Ang IV act primarily via AT1 receptors (a GPCR). Ang (1–7) acts primarily via the MAS1 (a GPCR) and forms Ang (1–5), which signals via MRGPRD (a GPCR). Ang (1–7) can also act via the AT2 receptor but with much lower affinity than Ang II (GtoPdb entry above).
FIGURE 2.
Renin‐angiotensin signalling (RAS), angiotensin II (Ang II) signalling, the balance between ACE1 and ACE2 and, in red, the impact of COVID‐19. APs, aminopeptidases; MME, membrane metalloendopeptidase; PREP, prolyl endopeptidase
As discussed below, the lung and heart express components of the RAS system, that is, ‘local’ RAS signalling (e.g. Forrester et al., 2018; Uhal, Dang, et al., 2012), that can contribute to tissue injury. For example, activated lung fibroblasts and injured epithelial cells express angiotensinogen and other RAS components (e.g. Uhal, Dang, et al., 2012; Uhal, Li, et al., 2012).
ACE2, a carboxypeptidase (zinc metalloprotease), is the primary enzyme responsible for Ang II degradation, thus regulating signal transduction by Ang II. The conversion by ACE2 of Ang II to Ang (1–7) (and its signalling via MAS1) produces effects that oppose those of Ang II (Karnik et al., 2017; Santos et al., 2018; Santos et al., 2019). ACE2, in particular its catalytic ectodomain, can be shed from cells, an action mediated by the metalloprotease ADAM17, into the circulation as soluble ACE2, levels of which can be increased by Ang II via its ability to increase ADAM17 activity (Lambert et al., 2005).
The RAS signalling pathway (Figure 2) thus relies on a ‘yin/yang relationship’ between ACE1 and ACE2: ACE1 generates angiotensin II (Ang II) and, in turn, signalling by the GPCRs AT1 and AT2 receptors, while ACE2 generates peptides whose receptors act to oppose responses mediated by AT1. AT1 is highly expressed and mediates Ang II signalling and effects (including inflammation, apoptosis, pro‐fibrotic signalling and tissue remodelling) in pulmonary and cardiac tissue (Forrester et al., 2018). By contrast, the role of AT2, which is expressed in the lung and at low levels in the heart, is more controversial (Forrester et al., 2018; Santos et al., 2019; GtoPdb). AT2 receptors can mediate effects that oppose those of AT1, but other data suggest that AT2 promotes effects such as apoptosis. In subsequent section that focus on the pathophysiology of COVID‐19, we emphasize the role of ACE1‐generated AT1 receptors as the key mediator of Ang II actions and the opposing actions of ACE‐derived peptides.
4. SARS VIRUSES AND ANGIOTENSIN ACTION/SIGNALLING
Infection of cells by SARS viruses that bind ACE2 results in two effects: inhibition of ACE2 activity and decrease of ACE2 expression in infected cells (Glowacka et al., 2010; Haga et al., 2008; Kuba et al., 2005; Zhang et al., 2020). Indirect evidence for the latter response in COVID‐19 show elevation in circulating Ang II with viral infection and increased circulating Ang II peptides with higher viral loads (Liu, Yang, et al., 2020).
Our hypothesis is founded on the following ideas that derive from data in reports cited in ensuing sections:
SARS viruses decrease ACE2 activity and expression.
The decrease in ACE activity creates an imbalance in signalling by ACE1 and ACE2 products.
This imbalance increases Ang II/ AT1 signalling and is superimposed on concurrent pathology (e.g. chronic lung disease and cardiac remodelling in the lung and heart, respectively). Ang II is a pivotal mediator of injury in both tissues; enhancement of its effects together with signalling from co‐morbidities can increase severity of COVID‐19.
In patients more susceptible to the damaging effects of Ang II, the decrease in ACE2 activity by SARS viruses can unleash a cascade of injurious effects through a heightened imbalance in the actions of the products of ACE1 versus ACE2.
The proposed imbalance in the signalling and actions of products of ACE1/ACE2 implies the potential of pharmacological approaches that redress this imbalance via (a) decrease in ACE1 activity, (b) blockade of AT1 and/or (c) increase in ACE2‐mediated signalling by affinity‐trapping the SARs virus, enhancement of ACE2 activity, or agonists for receptors of ACE2‐derived peptides
We provide data from the literature in support of this hypothesis, in particular as related to the lungs and heart, and regarding potential therapeutic approaches that address the imbalance of ACE1/ACE2 signalling and resultant actions involved in the pathobiology and severity of COVID‐19.
5. A MODEL FOR HOW IMBALANCE IN THE RAS PATHWAY PRODUCES COVID‐19 INJURY IN THE LUNGS
ACE2 is highly expressed in the lung parenchyma, particularly in Type II pneumocytes (Type II alveolar cells) (Zou et al., 2020). Type II cells synthesize and release pulmonary surfactant, phospholipids that lower surface tension, which is necessary to maintain alveolar structure (Andreeva, Kutuzov, & Voyno‐Yasenetskaya, 2007). Type II cells also can differentiate to become Type I alveolar cells (which form the structure of alveoli), a mechanism for replacement of Type I cells that are damaged. The SARS‐CoV‐2 and SARS‐CoV‐1 viruses perturb alveoli to produce the major pathology in the lung, with increased fluid entry, cell death and inflammation along with reduction in gas exchange and levels of surfactant (Gralinski & Baric, 2015; Xu, Shi, et al., 2020).
Figure 3 depicts a hypothetical framework for this process and the cell types involved. The SARS‐CoV‐2 virus infects alveolar pneumocytes by binding to ACE2, leading to a decrease in Ang II conversion to ACE2‐derived peptides, for example, a reduction in Ang (1–7) and its actions that counteract effects of Ang II (Figure 2). Hence, Ang II levels increase in the alveolar micro‐environment, with potential effects on multiple cell types. Below, we provide evidence in support of this framework and the role of ACE1/ACE2 imbalance in the lung injury of COVID‐19.
FIGURE 3.
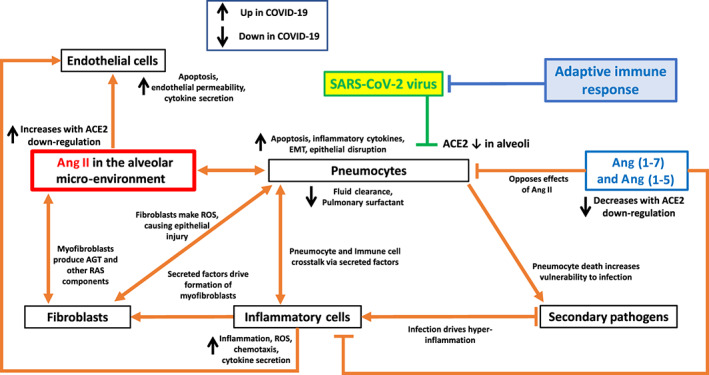
Hypothesized model of cell–cell communication and pathobiology in pulmonary infection from SARS‐CoV‐2 and the role of ACE1‐ and ACE2‐derived peptides in mediating these effects on several different cell types
Ang II has a pro‐apoptotic action on pulmonary epithelial cells (Papp, Li, Zhuang, Wang, & Uhal, 2002; Wang, Ramos, Joshi, et al., 1999; Wang, Zagariya, Ang, et al., 1999; Wang, Zagariya, Ibarra‐Sunga, et al., 1999), a response that is consistent with the pathology from SARS viruses, that is, widespread epithelial damage and alveolar damage and cell death (Zhou, Yang, et al., 2020; Zhou, Yu, et al., 2020). In addition, Ang II promotes and Ang (1–7) suppresses epithelial‐to‐mesenchymal transformation (EMT), whereby epithelial cells acquire a more fibrotic phenotype, a mechanism that may contribute to the formation of pulmonary lesions (Buckley, Medina, & Ehrhardt, 2010; Shao et al., 2019). Ang II also decreases the clearance of alveolar fluid (e.g. Deng, Wang, Deng, Li, & Tong, 2012; Ismael‐Badarneh et al., 2015). Apoptosis and EMT in alveolar epithelial cells are accompanied by an increase in secretion of pro‐inflammatory cytokines (e.g. IL1‐β, IL‐6, MCP‐1(CCL2) and TNF‐α) (e.g. Aumiller, Balsara, Wilhelm, Günther, & Königshoff, 2013; Ma, Xu, Ai, Ming, & Zhao, 2010; Pedersen & Ho, 2020). Epithelial cells engage in crosstalk with immune cells, in particular during infection and apoptosis (Chuquimia et al., 2012; Herold, Ludwig, Pleschka, & Wolff, 2012). These effects are compounded by the secretion of RAS components by activated myofibroblasts and epithelial cells undergoing apoptosis (Wang, Ramos, Joshi, et al., 1999; Wang, Zagariya, Ang, et al., 1999; Uhal, Li, et al., 2012), thereby amplifying Ang II signalling in a positive feedback loop.
Ang II also has pro‐fibrotic effects on fibroblasts that reside in interstitial spaces around alveoli (e.g. Uhal, Kyong Kim, Li, & Molina‐Molina, 2007; Uhal, Li, et al., 2012) and increases apoptosis of endothelial cells and endothelial permeability in the surrounding capillary network (Bodor et al., 2012; Watanabe, Barker, & Berk, 2005), which can increase fluid entry and immune infiltration into regions of the lung. Ang II also affects various types of immune cells (Forrester et al., 2018), increasing macrophage infiltration, ROS production and release of pro‐inflammatory cytokines. Besides immune cells, pathological ROS production occurs in pulmonary fibroblasts, driven by Ang II stimulation and inhibited by the actions of ACE2/Ang (1–7) (Meng et al., 2015). ROS production from activated fibroblasts is a key driver of epithelial injury in models of pulmonary fibrosis (Sakai & Tager, 2013).
Crosstalk among epithelial cells, fibroblasts and immune cells suggests a role for macrophages, which are activated by pulmonary epithelial cells in injury settings and can create positive feedback that promotes inflammation (Chuquimia et al., 2012; Herold et al., 2012; Uhal et al., 2007). Such effects can occur in response to Ang II (Uhal et al., 2007). Ang II can also regulate the function of other immune cells (e.g. dendritic cells and neutrophils) that can promote lung injury (Florez‐Sampedro, Song, & Melgert, 2018; Grommes & Soehnlein, 2011). Importantly, as summarized below, Ang (1–7) has opposing effects that counteract this pathology; these protective effects are blunted by the SARS‐CoV‐2 virus.
Ang II thus promotes a range of pro‐apoptotic, inflammatory, fibrotic and oedema‐associated processes in the alveolar micro‐environment. As cell death occurs, the epithelium, a critical component of innate immunity as a barrier to pathogens and via its formation and release of surfactant (Eisele & Anderson, 2011), becomes compromised, paving the way for secondary infection (e.g. bacterial pneumonia). As a result, immune response is further enhanced, greater inflammation ensues (Figure 3) and alveolar damage is increased, thereby enhancing lung injury and oedema. Patients who recover may have severe tissue damage, potentially with tissue fibrosis. Indeed, studies in patients who recovered from SARS‐1 document the presence of fibrosis and chronic lung damage in severe cases (Ketai, Paul, & Ka‐tak, 2006). Lung tissue from COVID‐19 patients shows evidence of (a) epithelial injury and disruption, in particular of Type 2 pneumocytes, (b) invasion of macrophages and neutrophils and (c) initiation of fibrosis (e.g., He et al., 2020; Luo et al., 2020; Tian et al., 2020).
5.1. Benefits of ACEIs/AT receptor antagonists in reducing pulmonary injury
Numerous studies have assessed the effects of inhibition of Ang II signalling by the administration of ACEIs or AT receptor antagonists as ways to mitigate lung injury in a range of experimental models, including acute respiratory distress syndrome ARDS pulmonary fibrosis. Bleomycin, a natural product‐derived peptide used for cancer chemotherapy, is a widely used model in rodents for the study of pulmonary fibrosis. Bleomycin damages the pulmonary epithelium, which then initiates a fibrotic response; the pulmonary injury and fibrosis can be mitigated by treatment with ACEIs or AT receptor antagonists (e.g. Li, Rayford, & Uhal, 2003; Yao, Zhu, Zhao, & Lu, 2006; Uhal, Dang, et al., 2012). Other models of lung fibrosis, for example, γ‐irradiation, also show deceased injury and fibrosis in response to ACEIs/AT receptor antagonists (e.g. Uhal, Li, et al., 2012). ACEIs/AT receptor antagonists appear to blunt the degree of apoptosis of epithelial and other cell types, indicating that initiating events of lung injury require Ang II signalling to drive a pathological response.
How might this occur? As shown in Figures 2 and 3, increase in local RAS signalling within the lung, that is, injury‐induced production of RAS signalling components by epithelial cells and activated fibroblasts, promotes pulmonary injury and helps explain the efficacy of ACEIs/AT receptor antagonists in blunting this pathology. Further evidence for this mechanism is the reduction in bleomycin‐induced injury and fibrosis by antisense oligonucleotides against angiotensinogen (Li et al., 2007).
Data from other in vivo models support the utility of ACEIs/AT receptor antagonists in blunting lung injury, especially of the epithelium, thus further implicating the role of Ang II in mediating these effects. Examples include acute lung injury induced by oleic acid in rats, in which the use of the ACEI captopril reduced alveolar damage/epithelial disruption, endothelial damage and infiltration of neutrophils He et al., 2007); the protective effect of ACEI/AT receptor antagonist treatment in reducing pneumocyte death in surfactant‐depleted rat lungs (Lukkarinen et al., 2005); and the efficacy of ACEIs in the treatment of radiation‐induced lung injury (Medhora, Gao, Jacobs, & Moulder, 2012). This benefit has also been shown in human studies, for example, a reduction in pulmonary‐related mortality by captopril administered to patients receiving total body irradiation prior to haematopoietic stem cell transplantation (Cohen et al., 2012). ACEI administration also reduces radiation‐induced pneumonitis in lung cancer patients (Kharofa, Cohen, Tomic, Xiang, & Gore, 2012).
Further evidence for effects of sepsis has been obtained from animal models of acute lung injury (ALI) induced by LPS: treatment with the AT receptor antagonist losartan reduced pro‐inflammatory cytokine secretion and lung injury and improved survival, while also reducing Ang II production in the lungs (Shen et al., 2009). The protective effects of losartan in LPS‐induced ALI are associated with reduced contribution by dendritic cells to inflammation (Liu et al., 2012). Captopril was protective in a rat LPS‐induced ALI model, reducing immune cell infiltration, oedema and haemorrhage in alveoli (Li et al., 2015). The latter study also showed that the ratio of ACE1/ACE2 expression is increased in injury and that captopril attenuated injury and decreased that ratio. These findings were replicated in a study in which captopril reduced inflammatory cytokines and monocyte infiltration in bronchoalveolar fluid (Boskabadi, Askari, Hosseini, & Boskabady, 2019). In addition, in a murine H5N1 influenza infection model, treatment with losartan improved survival and reduced oedema, lung injury and immune cell infiltration (Yan et al., 2015). Other studies showed that ACEIs or AT receptor antagonists inhibit ventilator‐induced lung injury (e.g. Chen et al., 2014; Jerng et al., 2007; Wösten‐van Asperen et al., 2008). Also, treatment of rats with losartan in a chronic cigarette smoke‐induced injury model reduced pulmonary remodelling and pulmonary arterial hypertension (Han et al., 2010).
Data from retrospective studies in humans show that ACEIs can prevent or reduce the severity of pneumonia (e.g. Caldeira, Alarcão, Vaz‐Carneiro, & Costa, 2012; Mortensen et al., 2012). In addition, treatment with ACEIs/AT receptor antagonists in COPD (chronic obstructive pulmonary disease) reduces inflammation, co‐morbidities and disease complications (Shrikrishna, Astin, Kemp, & Hopkinson, 2012). Retrospective analyses also suggest that ACEI/AT receptor antagonist treatment may mitigate the effects of radiation pneumonitis (Harder, Park, Nath, Mancini, & Decker, 2015). However, data are not available from prospective clinical studies with ACEIs/AT receptor antagonists in these types of pulmonary injury.
Cell‐based assays provide additional support for the importance of Ang II in mediating lung injury. Ang II induces apoptosis in a human epithelial cell line (A459) and in rat type‐II pneumocytes, effects that are blocked by treatment with ACEIs or AT receptor antagonists (Wang, Zagariya, Ang, et al., 1999; Wang, Zagariya, Ibarra‐Sunga, et al., 1999). Such findings were replicated using losartan in the same cell types, results that confirmed AT1 as the receptor that promotes apoptosis in pulmonary epithelial cells (Papp et al., 2002). Moreover, treatment of human and rat lung epithelial cells with FAS protein (an apoptosis‐inducing ligand) induced apoptosis by increasing angiotensinogen and Ang II expression/secretion, effects blocked by Ang II antibodies (Wang, Zagariya, Ang, et al., 1999). The latter authors further showed that pro‐fibrotic human lung fibroblasts induce epithelial cell apoptosis by producing Ang II and that these myofibroblasts express the components necessary to drive a local RAS signalling cascade (Wang, Ramos, Joshi, et al., 1999; Wang, Zagariya, Ibarra‐Sunga, et al., 1999). In addition, the AT receptor antagonist telmisartan blunts Ang II‐promoted EMT of A549 cells (Buckley et al., 2010).
Thus, the available data provide consistent evidence indicating that Ang II signalling, via AT1, has a central role in lung injury. Blunting this signalling by ACEIs or AT1 receptor antagonists has beneficial effects in modulating such damage, including in the context of acute pulmonary injury caused by infection.
The timescales over which Ang II‐mediated pulmonary injury occurs can be estimated from data in fibrosis models, where an initial injury (e.g. from bleomycin) to the epithelium initiates a series of pathological events. These injuries typically evolve over a 2‐ to 3‐week timeframe (e.g. Li et al., 2003; Yao et al., 2006), with evidence for injury developing within 2–3 days and then progressing over weeks. This is broadly consistent with the timeframes for onset of early symptoms with progression to serious pathology in COVID‐19 patients (e.g. He et al., 2020; Pan et al., 2020; Zhou, Yu, et al., 2020).
5.2. ACE2 and Ang (1–7) reduce pulmonary injury
An extensive literature has emerged describing the protective effects of ACE2 and Ang (1–7) in mitigating pulmonary injury by acting in opposition to the effects of Ang II. The approaches include treatment with soluble ACE2 that ultimately converts Ang II to Ang (1–7), whose protective role is most likely mediated by MAS1.
Data from rodent models indicate that ACE2/Ang (1–7) can mitigate fibrosis induced by agents such as bleomycin. For example, in a mouse bleomycin model, treatment with recombinant ACE2 reduced epithelial injury, pro‐fibrotic cytokine release, activation of fibroblasts and inflammatory cell infiltration, thereby prominently reducing the extent of lung injury (Wang, Wang, Yang, Guo, & Sun, 2015). Studies in a bleomycin model in rats have revealed that the protective effects by Ang (1–7) may occur by inhibition of signalling cascades that involve MAP Kinase and NF‐κB (Meng et al., 2014). The authors also found that the Ang (1–7) or ACE2 overexpression had antifibrotic effects via inhibition of MAPK and NF‐κB in human lung fibroblasts. Other studies confirm a protective role by ACE2/Ang (1–7) in bleomycin‐treated rats (Wu et al., 2014). Lung samples from patients with idiopathic pulmonary fibrosis (IPF) or from bleomycin‐treated mice and rats have decreased ACE2 expression in association with lung injury and fibrosis (Li et al., 2007). Fibrosis (collagen accumulation) was also enhanced in mice treated with ACE2 siRNA or ACE2 inhibition, but treatment with recombinant ACE2 reduced bleomycin‐induced fibrosis (Li, Molina‐Molina, et al., 2008). In a cigarette smoke‐induced model of lung injury in mice, Zhang et al. (2018) showed that treatment with Ang 1–7 reduced lung inflammation and fibrosis.
ACE2/Ang (1–7) also have a protective effect in ALI/ARDS models induced by LPS stimulation. In LPS‐induced acute respiratory distress syndrome models in rats, treatment with Ang (1–7) or an AT antagonist reduced lung injury and inflammation and improved lung function (Chen et al., 2014; Wösten‐van Asperen et al., 2011). He et al. (2015) reported that mesenchymal stem cells (MSCs) engineered to overexpress ACE2 had strong protective effects in an ALI model, improving endothelial barrier integrity and reducing lung injury and inflammation. Inhibition of ACE2 increased lung injury, IL‐17 signalling and inflammation with infiltration by neutrophils in a murine model of bacterial (Pseudomonas) lung infection, but the converse occurred in mice treated with recombinant ACE2 (Sodhi et al., 2019). ACE2 not only mitigated lung injury but also improved response to the infection. Ex vivo experiments with mouse lung organoids confirmed the effects of ACE2 in limiting IL‐17 signalling.
In vitro data with human cells support the idea that ACE2/Ang (1–7) can protect cells from Ang II or bleomycin‐induced apoptosis. It has been suggested that endoplasmic‐reticulum (ER) stress induces apoptosis, which can be eliminated by treatment with Ang (1–7) via MAS1 receptor activation (Uhal et al., 2013; Uhal, Li, Xue, Gao, & Abdul‐Hafez, 2011). TGF‐β1 treatment promotes EMT in human airway epithelial cells, a response associated with a reduction of ACE2 expression and elevation of migration and expression of myofibroblast markers; treatment with ANG (1–7) blocked TGF‐β1‐induced EMT and activation of targets downstream of TGF‐β1 (Shao et al., 2019).
Effects of the MAS1 receptor in modulating immune response have been reported in a number of studies. These include effects of MAS1 on neutrophil influx in models of arthritis in mice and rats (da Silveira et al., 2010) and on the ability of macrophages to phagocytose neutrophils that have undergone apoptosis (Barroso et al., 2017). MAS1 knockout mice have greater inflammatory cell infiltration, lung remodelling and inflammatory cytokine production in models of allergic pulmonary inflammation (Magalhães et al., 2016) and altered macrophage function that contributes to a range of inflammatory pathology (Hammer et al., 2016).
Thus, considerable data document a protective role for ACE2/Ang (1–7) by opposing effects of Ang II in lung injury. ACE2/Ang (1–7) have many such actions, which include blunting of alveolar epithelial apoptosis, infiltration of inflammatory cells, activation of fibroblasts and endothelial disruption.
5.3. ACE2 in SARS‐CoV infections of the lung
In a landmark study, using mouse models of acid‐respiration or LPS, the Penninger group initially showed a protective role of ACE2 in acute respiratory distress syndrome (Imai et al., 2005) and demonstrated that ACE1‐mediated signalling promotes ARDS, but ACE2 exerts strong protective effects. ACE2 knockout (KO) mice had dramatically increased lung injury, effects that were reduced by treating with recombinant human ACE2. Conversely, ACE1‐deficient mice had reduced severity of injury, supporting the concept that ACE1/ACE2 balance is a central mediator of lung injury.
The Penninger group subsequently used ACE2 KO mice and found that ACE2 expression is necessary for SARS infection (Kuba et al., 2005). The authors hypothesized that the RAS signalling blockade or treatment with recombinant ACE2 would protect from SARS‐induced injury. A crucial observation was that SARS‐CoV‐infected mice resembled ACE2 KO mice in their susceptibility to lung injury (Kuba, Imai, Rao, Jiang, & Penninger, 2006). Penninger and co‐workers have extended these ideas to COVID‐19, advocating for the use of ACE2 as a target in SARS‐CoV‐2, including by providing soluble ACE2 (Zhang et al., 2020). The Penninger group recently showed the efficacy of recombinant human ACE2 in drastically reducing the infectivity of SARS‐CoV‐2 in ex vivo models, including organoids (Monteil et al., 2020).
5.4. What is the contribution of AT2 receptors to pathobiology in the lung and COVID‐19?
Few studies have assessed the actions of AT2 receptors in the lung. A protective role for AT2 was implied by findings indicating that AT2‐null mice and those treated with an AT2 antagonist were more vulnerable to ARDS (Imai et al., 2005). However, subsequent studies related to the lungs using selective antagonists of AT2 to distinguish its effects from AT1 reached the opposite conclusion: In general, it appears that AT2 drives pathology synergistically with AT1, in particular in cell types relevant to the hypothesis presented above.
Examples of such studies include animal models of fibrosis (Konigshoff et al., 2007; Waseda et al., 2008) and in vitro data from human lung fibroblasts (Konigshoff et al., 2007). AT2 also promotes apoptosis in pulmonary endothelial cells (Lee, Mungunsukh, Tutino, Marquez, & Day, 2010), rat alveolar epithelial cells (Bechara et al., 2003) and human alveolar epithelial cell lines (Pickel et al., 2010). Consistent with animal data on fibrosis are histological data from patients with systemic sclerosis and lung fibrosis, which show elevated alveolar AT2 receptor expression with disease; higher AT2 expression is associated with increased mortality Parra et al. (2014).
Reviews that discuss AT2 action in other tissues (e.g. Forrester et al., 2018; Lemarié & Schiffrin, 2010; Jones, Vinh, McCarthy, Gaspari, & Widdop, 2008) also note disagreements regarding AT2 action (e.g. effects on vasodilation and protection from ischaemic injury), but all concur that AT2 exerts pro‐apoptotic effects in various cell types. A pro‐fibrotic role for AT2 that promotes injury in concert with AT1 has also been noted in lung fibroblasts, e.g., Uhal, Li, et al. (2012). We conclude that AT2 may act in parallel with AT1 in driving cell death and tissue damage in the schema depicted in Figure 3.
5.5. How is the pathobiology of lung injury related to ACE1/ACE2 imbalance counteracted?
The data described above lead us to propose a model for pulmonary pathology based on imbalance in RAS signalling that influences multiple cell types (Figure 4) and has feedback loops that amplify pathobiology. Most COVID‐19 patients suffer minor illness and avoid severe tissue injury. We propose that this occurs via an effective adaptive immune response that eliminates infection and prevents the damage induced by the RAS pathway and associated feedback mechanisms. By contrast, patients with underlying pathologies suffer more severe disease because of amplified RAS‐driven mechanisms. These mechanisms cause acute injury that exceed the capacity of the protective immune response. The damaging effects of the virus via RAS/Ang II are thus competing with the effectiveness of the immune response (Figure 4).Early insights into the immune response to COVID‐19 have been reported (To et al., 2020, Thevarajan et al., 2020; Zhou, Yu, et al., 2020) along with the proposed models of this response (Li et al., 2020; Prompetchara, Ketloy, & Palaga, 2020). Analysis of the immunopathology of SARS‐1 and MERS (Channappanavar & Perlman, 2017) is consistent with such data and models. Prior reviews describe the importance of T cell‐mediated adaptive immune response to coronaviruses, especially SARS (Channappanavar, Zhao, & Perlman, 2014; Li, Wu, et al., 2008) and provided insight into the roles of macrophages, dendritic cells, B cells and T cells in SARS‐CoV‐1 infection (Yasui et al., 2014; Zhao, Zhao, Van Rooijen, & Perlman, 2009).
FIGURE 4.
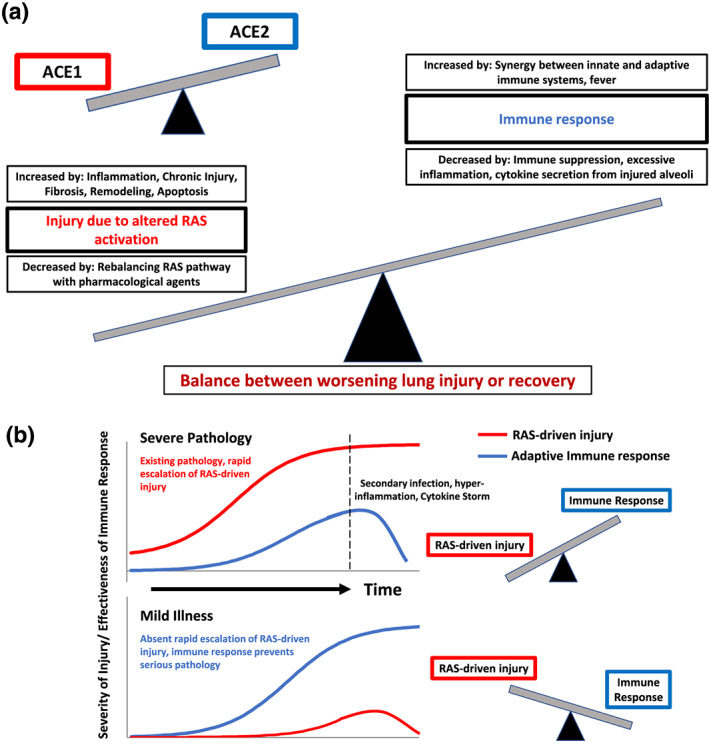
ACE1/ACE2 imbalance, lung injury and factors that determine severe pathology or mild illness. (a) A model for the course of COVID‐19 that links ACE1/ACE2 imbalance to lung injury via the RAS pathway and the protective role of the immune response. (b) An illustration of the predicted course of COVID‐19 in two settings. Top: Severe pathology in patients who lack appropriate immune response or who have prior conditions that enhance ACE1/ACE imbalance (and RAS‐induced injury). The result is increased injury that overwhelms the adaptive immune response. Bottom: Mild illness in which patients lack underlying conditions and can mount an appropriate immune response, so that RAS‐induced injury is less serious, an effective immune response occurs and the infection is resolved
Based on these studies, the following framework describes the interplay between pathological—as opposed to protective—immune response and acute pulmonary injury in COVID‐19, in part extrapolating from information and ideas related to SARS‐CoV‐1 (Channappanavar & Perlman, 2017).
During early stages of infection, inflammatory cells (macrophages, neutrophils and dendritic cells) infiltrate into the alveoli in response to cytokine secretion by alveolar cells. Immune infiltration is greater in subjects who experience more rapid RAS‐induced injury and epithelial cell death (i.e. a more severe imbalance between ACE1 and ACE2), thereby establishing a positive feedback loop: accumulation of large numbers of inflammatory cells further promotes cell death in alveoli.
Human coronaviruses express proteins that suppress the production by immune cells of interferons (IFNs), which suppress viral replication. This interferon response is exacerbated, and injury is increased in conditions of ‘hyper‐inflammation’ that undermine protective innate immune responses. Such hyper‐inflammation is more likely to occur in conditions of escalated RAS‐driven injury, due to greater ACE1/ACE2 imbalance.
Seroconversion begins early, within ~5 days from the onset of symptoms, with increasing titres of IgM antibodies, and promotes an adaptive immune response, mediated by T helper cells, cytotoxic T cells and antibody production by B cells, recruited by T helper cells. Hyper‐inflammation results from extensive injury that overwhelms this adaptive response, with accumulation of activated macrophages that can suppress effective antigen presentation by dendritic cells and the recruitment of T cells.
In patients with mild pulmonary injury, effective recruitment of T cells leads to clearance of the virus, followed by tissue repair and restoration of homeostasis. Transition occurs from IgM to IgG antibodies. In the case of severe pathology, a vicious cycle of inflammation and cell death leads to widespread epithelial disruption and onset of pneumonia. Such patients are more vulnerable to bacterial infection with the decrease in surfactant production and loss of an intact epithelial barrier.
Mild disease (with less severe RAS‐driven effects) shows a progressive decline in viral loads and improvement in symptoms and signs of infection, generally by ~7 days from initial symptoms. In progressive disease (with more severe RAS‐driven effects), greater pulmonary injury (perhaps with secondary pneumonia) necessitates critical care, including assisted ventilation. ARDS can develop if inflammation, cell death and infection continue. Cardiac complications may exacerbate this, especially if myocarditis and further pulmonary oedema occur. The cytokine storm, perhaps stemming from lung injury, ARDS, enodthelial injury, and/or viraemia, also has the potential to exert systemic effects, raising the risk of multiple organ failure (Pedersen & Ho, 2020).
The outcome of COVID‐19 infection is thus determined by the competing actions of different elements that promote (the RAS pathway) or blunt (immune response) lung injury (Figure 4). In severe cases, a series of injurious effects unfold and overwhelm protective immune responses. Greater imbalance in the effects if ACE1 and ACE2 in the RAS pathway is predicted to exacerbate pathology, making it more likely that the immune response will be overcome. Factors/co‐morbidities that increase the ACE1/ACE2 imbalance are discussed below.
6. A MODEL FOR HOW IMBALANCE IN THE RAS PATHWAY PRODUCES COVID‐19 INJURY IN THE HEART
By analogy with our model for lung injury from COVID‐19 (Figure 3) and based on data in the literature, Figure 5 describes a model for COVID‐19 pathology in the heart. Below, we discuss details of this model.
FIGURE 5.
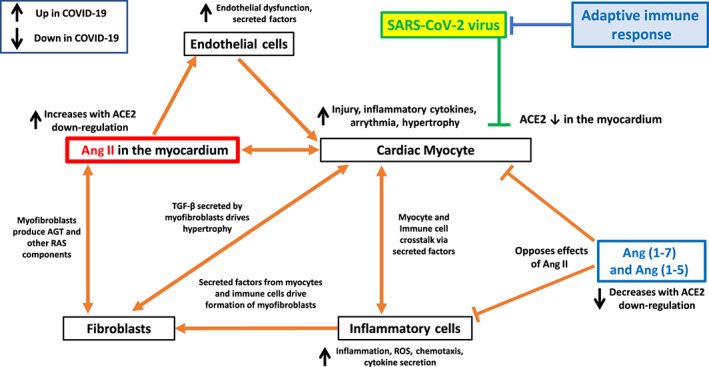
Hypothesized model of cell–cell communication in myocardial infection from SARS‐CoV‐2 and the influence of ACE1/ACE2 imbalance on a variety of cell types involved in cardiac injury
ACE2 is highly expressed in the myocardium in particular on cardiac myocytes but also in endothelial cells and fibroblasts (Patel, Zhong, Grant, & Oudit, 2016; Santos et al., 2018). The binding of SARS‐COV‐2 to ACE2 from infection of the myocardium decreases ACE2 activity (Oudit et al., 2009) and results in increased ACE1/Ang II driven signalling and a decrease in effects of ACE2‐derived peptides, including Ang (1–7). Ang II alters the function of multiple cell types in the heart, including cardiomyocytes, fibroblasts, endothelial cells and inflammatory cells, in particular macrophages. Ang (1–7) action occurs on cardiomyocytes, inflammatory cells and cardiac fibroblasts.
Akin to the lung, the heart has a local RAS that can contribute to cardiac pathology (De Mello & Danser, 2000; Forrester et al., 2018; Reyes et al., 2017). Ang II has direct effects on cardiomyocytes, increasing hypertrophy and contractility, altering heart rate and rhythm and enhancing secretion of cytokines that help facilitate cardiac remodelling (Forrester et al., 2018; Reyes et al., 2017). Ang II converts cardiac fibroblasts to a more pro‐fibrotic myofibroblast phenotype and enhances secretion of factors (in particular TGF‐β and RAS signalling components) that promote hypertrophy via crosstalk with cardiomyocytes (Frieler & Mortensen, 2015; Forrester et al., 2018). Ang II also exerts effects on endothelial cells, inducing endothelial dysfunction and cytokine secretion, in particular of endothelin‐1, which enhances myocyte hypertrophy (Forrester et al., 2018). RAS signalling can promote pro‐inflammatory effects on inflammatory cells, in particular cardiac macrophages, which can further increase myocyte hypertrophy and fibroblast activation (Frieler & Mortensen, 2015; Forrester et al., 2018). Ang II signalling in multiple cardiac cell types increases oxidative stress and ROS (Forrester et al., 2018).
Signalling in the heart by ACE2‐derived peptides, in particular Ang (1–7), opposes effects of Ang II (Patel et al., 2016; Santos et al., 2018). Via those actions, the ACE2‐Ang (1–7)‐MAS1 axis mitigates hypertrophic, fibrotic, oxidative stress and remodelling effects of Ang II. Protective effects via ACE2 on cardiac myocytes are likely particularly important in cardiomyopathy and heart failure (e.g. Flores‐Muñoz, Smith, Haggerty, Milligan, & Nicklin, 2011).
Ang II thus alters multiple cell types in the heart and promotes a pro‐inflammatory, hypertrophic state via a range of mechanisms. These mechanisms are counteracted by ACE2‐derived products, in particular Ang (1–7), implying an alteration in ACE1/ACE2 balance as a contributor to the resultant cardiac phenotype. The SARS‐CoV‐2‐promoted increase in ACE1/Ang II actions resulting from a decrease in ACE2‐derived peptides is predicted to unleash inflammatory, oxidative stress and remodelling events and potentially myocyte apoptosis, depressed myocardial function, heart failure, arrhythmia and cardiac fibrosis.
7. ENHANCED ACE1/ACE2 IMBALANCE IN CO‐MORBIDITIES THAT INFLUENCE COVID‐19 MORBIDITY AND MORTALITY
Why do some patients with co‐morbidities have increased susceptibility to morbidity and mortality from COVID‐19? Our model for pulmonary and cardiac injury implies a role for crosstalk between ACE2‐expressing cells infected by SARS viruses and other cell types, especially inflammatory cells (e.g. macrophages and neutrophils) and fibroblasts. In patients with underlying disease, one or more of these cell types may be dysfunctional. Superimposed SARS‐CoV‐2 infection further amplifies Ang II signalling (which promotes injury) and suppresses Ang (1–7) signalling (which opposes injurious effects), thus increasing pathobiology. Multiple clinical conditions may be associated with elevated vulnerability to COVID‐19. Examples include:
Chronic lung injury/disease. Lung injury (e.g. from fibrotic disease, smoking or radiation) is associated with increased local inflammatory signalling, predisposing the epithelium to Ang II‐promoted injury. In these settings, epithelial cells and fibroblasts can have elevated pro‐inflammatory Ang‐mediated responses. The addition of SARS‐CoV‐2 further increases this imbalance, thereby enhancing lung injury.
Cardiac hypertrophy and remodelling. Ang II regulates cardiac remodelling in multiple settings, including hypertension. The elevation in Ang II signalling, derived in part from cardiac RAS, increases effects of Ang II in the heart (Forrester et al., 2018). Patients with cardiac pathologies associated with remodelling are thus particularly susceptible to the imbalance in the RAS pathway caused by myocardial SARS‐CoV‐2 infection. Decreased cardiac function, especially in patients with left heart failure, may also increase the likelihood of pulmonary oedema, accompanying pulmonary infection and complications.
Diabetes, obesity, metabolic syndrome and chronic inflammatory disease. Advances in the understanding of the immune system and chronic inflammation have led to the concept of ‘inflammageing’, whereby aging is associated with the advent of chronic inflammation and the presence of inflammation‐associated illnesses, including Type 2 diabetes (Ferrucci & Fabbri, 2018; Fulop et al., 2018). Chronic inflammation is also predicted to rise with obesity (Ferrucci & Fabbri, 2018), a risk factor for COVID‐19 morbidity. Inflammation is a key mechanism by which elevated Ang II signalling and ACE1/ACE2 imbalance causes injury (Figures 3, 4, 5). Certain patients with Type 2 diabetes and obesity also have hypertension and hypercholesterolaemia; together, these features characterize the metabolic syndrome. The metabolic syndrome is associated with chronic inflammation, which may be a causative feature of this syndrome (Monteiro & Azevedo, 2010). Increased RAS activity appears to be a pathogenic factor in metabolic syndrome (Skov, Persson, Frøkiær, & Christiansen, 2014). Patients with the metabolic syndrome are thus ‘pre‐sensitized’ to RAS‐mediated effects and, hence, potentially more vulnerable to dysregulation of RAS by COVID‐19.
Weakened adaptive immune response. Within the “inflammageing” paradigm, aging‐associated chronic inflammation via the innate immune system is coupled with a weakened adaptive immune response. The adaptive immune system thus has a reduced ability to establish a defence against SARS‐CoV‐2, allowing greater viral infectivity and tissue injury. Immune‐compromised subjects from other causes (e.g. those on immune‐suppressive medications) would also be more vulnerable to SARS‐CoV‐2/COVID‐19.
>ACE polymorphisms. ACE1 insertion/deletion polymorphisms (I/D) have been widely studied. The D‐allele is associated with higher ACE1 activity. Patients with the D‐allele, especially those with the D/D genotype, are at higher risk of morbidity and mortality from ARDS (Adamzik et al., 2007) and certain cardiac, pulmonary and inflammatory conditions (e.g. Gard, 2010). The ACE1/ACE2 imbalance hypothesis predicts that patients with the D‐allele of ACE1, in particular the D/D genotype, will have elevated severity of COVID‐19, as was seen in patients with SARS‐1 (Itoyama et al., 2004). In addition, other genetic variants in ACE1 or ACE2 activity or components involved in the actions of the peptides that they generate might also contribute to differences in severity of SARS‐Cov‐2 infections; assessment of expression of such genetic variants might aid in personalized therapeutic approaches (Tan, Liao, Zhou, Mei, & Wong, 2018).
Therefore, the ACE1/ACE imbalance model for SARS‐CoV‐2 pathobiology can help explain how/why patients with certain underlying conditions/co‐morbidities are at greater risk if infected with SARS‐CoV‐2/COVID‐19. Elderly individuals are at particular risk, since many of the co‐morbidities are age‐associated. Those with health conditions such as immune deficiencies, diabetes or cardiac disease will likely be at greater risk for more severe COVID‐19 infections and ACE1/Ang II‐mediated pathology. By contrast, children (who lack co‐morbidities associated with ACE1/ACE2 imbalance) are predicted to have less morbidity and mortality from COVID‐19.
8. OPPORTUNITIES TO TARGET THE ANGIOTENSIN PATHWAY
The concepts and data discussed above suggest a variety of approaches that may decrease the pathobiology of SARS‐CoV‐2 infection resulting from the imbalance in signalling by peptides generated by ACE1 and ACE2 (Figure 6). Several complementary strategies might improve the clinical status and outcome of patients with COVID‐19. The goal of these approaches is to restore ACE1/ACE2 balance in favour of ACE2‐derived peptides. The proposed therapies, discussed below, are directed at (a) inhibition of ACE1; (b) inhibition of the binding of SARS‐Cov‐2 to ACE2; (c) agonism of receptors activated by ACE2‐derived peptides; and (e) inhibition of AT1 receptors (Table 1).
FIGURE 6.
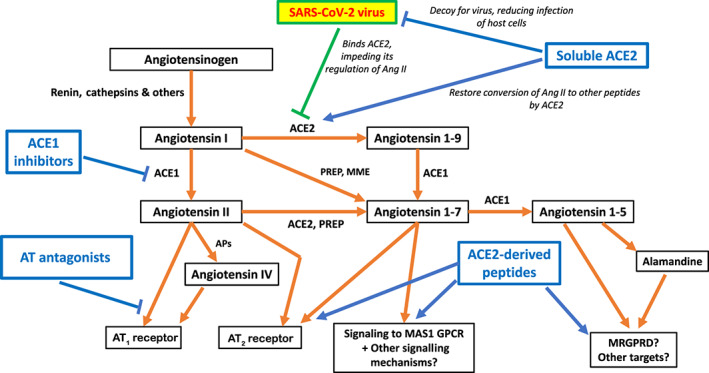
Pharmacological agents that target the RAS pathway and counteract effects of SARS‐CoV‐2 infection
TABLE 1.
The pros and cons of different tools for targeting the RAS pathway
| Therapeutic approach | Pros | Cons | Comments |
|---|---|---|---|
| ACE inhibitors (ACEIs) | • Mitigates effects of Ang II in relevant cell types/tissues involved in COVID‐19 pathobiology | • Does not salvage the protective effects of ACE2 inhibited by SARS‐CoV‐2 | Potential for rapid (compassionate) use in at‐risk patients and for rapid (repurposing) clinical trials |
| • Dry cough is common side effect | |||
| • Well‐established clinical utility | |||
| • Well‐defined PK and PD | • May not be effective in tissues with high Chymase expression (e.g. heart) | ||
| • Large amount of preclinical animal data | |||
| • Oral administration. Possible inhalable administration | |||
| • Challenges with drug interactions, hypotensive patients and other side effects | |||
| • Generic drugs, with established production, supply chain and handling | |||
| AT receptor antagonists | • Mitigates effects of Ang II (via AT1) in relevant cell types/tissues involved in COVID‐19 pathobiology | • Does not salvage the protective effects of ACE2 inhibited by SARS‐CoV‐2 | Potential for rapid (compassionate) use in at‐risk patients and for rapid (repurposing) clinical trials |
| • Challenges with drug interactions, hypotensive patients and other side effects | |||
| • Long track record of clinical use | |||
| • Will not impede possible pathological effects of AT2 receptor | |||
| • Well‐defined PK and PD | |||
| • Large amount of preclinical animal data | |||
| • Oral administration. Possible inhalable administration | |||
| • Generic drugs, with established production, supply chain and handling | |||
| Recombinant soluble ACE2 | • ‘Rescues’ ACE2 activity inhibited by SARS‐CoV‐2 infection via action as a ‘decoy’ for the virus | • Limited data from human studies; unknowns regarding safety | Additional preclinical studies needed, in parallel with early‐phase trials |
| • Limited information regarding dosing, target engagement, PK, PD and so forth | |||
| • Mitigates effects of Ang II in relevant cell types/tissues involved in COVID‐19 pathobiology | |||
| • Higher costs + more complicated handling, new supply chain | |||
| • Substantial preclinical data in animals | • Infusion is required | ||
| Ang (1–7) | • Engages protective MAS1 signalling, mitigating harmful effects of ACE2 inhibition by SARS‐CoV‐2 | • Very limited data from human studies | Additional preclinical studies needed; unclear that human trials are justified |
| • Less available preclinical data than for the other options | |||
| • Limited information regarding dosing, target engagement, PK, PD and so forth | |||
| • Indirectly inhibits pathological Ang II signalling | |||
| • Higher costs + more complicated handling, new supply chain | |||
| • Infusion is required |
Abbreviations: PD, pharmacodynamics; PK, pharmacokinetics.
8.1. Inhibition of ACE1
The ability of ACE1 to generate Ang II is a critical step in providing the ‘driver’ for multiple features of the pathophysiology of COVID‐19. ACE1 (also known as dipeptidyl carboxypeptidase 1) is a zinc metalloenzyme that removes a dipeptide from the C‐terminus of certain peptides, including His‐Leu from the inactive decapeptide Ang I to generate Ang II, the active octapeptide. ACE1 inhibitors approved for clinical use are competitive peptide antagonists that blunt Ang II formation but also the cleavage by ACE1 of the peptide bradykinin. We have found no studies that assessed the efficacy of ACE1 inhibitors in SARS‐1 infections. The decrease in Ang II formation would likely be beneficial in such infections. However, since a substantial proportion (~20%) of patients administered ACEIs develop cough as a side effect (generally attributed to an increase in bradykinin) and dry cough is a feature of COVID‐19 infection, this is a potential drawback of this approach.
8.2. Inhibition of the binding of SARS‐Cov‐2 to ACE2
As ACE2 is the key cellular receptor for SARS‐Cov‐2, approaches that block its interaction with the virus have the potential to maintain ACE2 activity and its generation of products, such as Ang(1–7), that counteract pathological actions of Ang II. One such approach is to use soluble ACE2 as a decoy to bind the virus and spare cellular ACE2 (Zhang et al., 2020). A recombinant human ACE2, GSK2586881, has been tested and was well‐tolerated in Phase 1 studies and a Phase 2 trial with ARDS patients (Khan et al., 2017). GSK2586881 was shown to reduce circulating levels of Ang II and IL‐6. However, this study was not powered to verify effects on clinical endpoints and direct therapeutic effects on the lungs were unclear. A clinical trial investigating the use of recombinant human ACE2 in COVID‐19 was initiated in China, but enrolment was subsequently withdrawn (ClinicalTrials.gov identifier NCT04287686). Linkage of the extracellular domain of the ACE2 protein to human IgG Fc domain is a strategy that could have advantages for treatment of COVID‐19, including prolongation of the half‐life of ACE2 (Kruse, 2020; P. Liu et al., 2018). A related ‘decoy approach’ is the use of the receptor binding domain (RBD) of the SARS S protein that interacts with ACE2 (e.g. Wong, Li, Moore, Choe, & Farzan, 2004); this approach has not yet been tested in patients. Small molecules have been identified as inhibitors of coronavirus binding to ACE2 (Adedeji et al., 2013), but their efficacy has only been evaluated in limited preclinical studies. All these approaches, which block viral entry into cells, maintain ACE2 activity and thus would be expected to blunt disease pathobiology. By contrast, agents that block later steps in viral infectivity (e.g. cellular entry and endosomal inhibition) will likely be associated with decreases in ACE2 activity and thus predicted to be counterproductive for the generation of beneficial ACE2‐derived peptides.
8.3. Agonism of receptors activated by ACE2‐derived peptides
Ang (1–7) is considered the major ACE2‐derived peptide but ACE2 can also generate other peptides, including Ang (1–9) and the heptapeptide alamandine. Ang (1–7) primarily acts via the MAS1 receptor (Figure 2) while response to Ang (1–9) may also involve AT2 receptor; alamandine has effects similar to Ang (1–7) via interactions with the Mas‐related GPCR, member D (MRGPRD) (Alexander et al., 2019). The actions of the ACE2‐Ang (1–7)‐MAS1 receptor, ACE2‐Ang (1–9)‐AT2 receptor and ACE2‐alamandine‐MRGPRD pathways generally oppose those of the Ang II‐AT1 pathway, though this remains controversial for AT2, as discussed earlier in this text. Activation of the former pathways, in particular via GPCRs that mediate their effects, is thus predicted to blunt COVID‐19 pathobiology. Signalling by Ang (1–7) via MAS1 can increase activity of MAPK, phosphoinositide 3‐kinase/Akt and NADPH oxidase and subsequently activate effectors that include forkhead box protein 01 (FOXO1), COX2 and generation of prostanoids and NO (Povlsen, Grimm, Wehland, Infanger, & Krüger, 2020; Santos et al., 2019). Less is currently known regarding alamandine signalling and action. Additional peptides may be generated as part of non‐canonical (‘non‐classic’) Ang II signalling (Santos et al., 2019). Efforts are underway to discover and develop drugs that mimic the physiological peptides in their actions on MAS1 and MRGPRD receptors. Initial clinical studies have tested an oral formulation of hydroxypropyl‐β‐cyclodextrin‐Ang‐(1−7) on muscle injury (Becker et al., 2018). As recently reviewed (Wang et al., 2019), targeting of non‐canonical angiotensin II signalling pathways has also been initiated in studies of ARDS. Such approaches should be highly suitable for testing in patients with COVID‐19.
8.4. Inhibition of AT1 receptor and potentially post‐AT1 signalling mechanisms
Antagonism of the AT1 receptor , the primary mediator of Ang II‐promoted tissue injury in SARS infections, is an attractive means to improve the course/outcome of patients with COVID‐19 by preventing and/or decreasing such injury. This prediction is supported by data from studies of ventilator‐induced lung injury and ARDS (Wang et al., 2019) and other settings of tissue injury (e.g. Arumugam et al., 2016). A recent preprint reported less morbidity and mortality in elderly COVID‐19 patients with hypertension treated with AT1 receptor antagonists prior to hospitalization (Y. Liu, Huang, et al., 2020). Since numerous AT1 receptor antagonists are approved drugs for other indications (e.g. hypertension, heart failure and renal disease), such receptor antagonists could be rapidly tested as therapeutics on a compassionate use basis and in trials to assess their efficacy for COVID‐19. Although the use of AT antagonists is potentially effective in decreasing lung and cardiac injury from COVID‐19, possible side effects, including systemic hypotension, may occur in patients receiving those drugs.
Table 1 summarizes information (including the pros and cons) of each of those types of therapeutic approaches. Systemic administration of drugs will likely be the focus of therapies for COVID‐19 but should administration by inhalation be considered for direct delivery to the lungs (albeit collapsed alveoli in COVID‐19 patients might limit such delivery)? An important advantage of such an approach is to reduce effects/complications from systemic administration. We speculate that various inhaled agents might offer benefit in the lungs by increasing cellular cAMP (e.g. agonists of β‐adrenoceptors or other Gs‐linked GPCRs), cGMP (e.g. guanylyl cyclase activators) or both cyclic nucleotides (PDE inhibitors). Given the importance of inflammation to COVID‐19 pathobiology, as discussed above and in Pedersen and Ho (2020), inhalational administration of other immunomodulatory/anti‐inflammatory drugs may also have utility. Multiple ACEIs and AT1 receptor antagonists are available in solution and thus are potential candidates for use with nebulizers. Such an approach might minimize complications from systemic administration of those agents, of particular importance in hypotensive patients or those at risk for hypotension. Of note, both ACEIs and AT1 receptor antagonists have been administered experimentally via inhalational methods (e.g. Godugu et al., 2013; Suk & Ensign‐Hodges, 2019).
Much recent debate has occurred regarding the use of ACEIs and AT1 receptor antagonists in COVID‐19 patients (e.g. discussed in Sriram & Insel, 2020; Vaduganathan et al., 2020), with some authors emphasizing potential harms of these medications while others argue against this idea or hypothesize benefits of these drugs. Most of such articles are brief correspondences with limited supporting evidence. As a result, health providers and patients have been confused regarding the administration of ACEIs and AT antagonists to patients with COVID‐19.
Articles with concern regarding the potential harms of ACEI/AT1 receptor antagonist use in COVID‐19 generally cite articles that report administration of ACEI/AT1 receptor antagonists may increase ACE2 expression, thereby possibly increasing the risk and spread of infection. We recently analysed results from animal and human studies and concluded that no consistent, replicable data provide evidence of a relationship between ACEI/AT1 receptor antagonist use and ACE2 expression (Sriram & Insel, 2020). Here, we focus on the potential benefits of ACEI/AT1 receptor antagonist and other ways to target the RAS pathway in COVID‐19, an idea that others share (e.g., Zhang et al., 2020), who suggested ACE2 as a target.
Below, we list several clinical trials for COVID‐19 that target the RAS pathway. These studies are mostly of small size and, to our knowledge, have not begun enrolment. The targeting of the RAS for therapeutic benefit has thus as‐yet not received the attention given to other approaches.
NCT04335123: Study of Open Label Losartan in COVID‐19. Phase‐1 trial to evaluate losartan safety in COVID‐19; University of Kansas Medical Center
NCT04312009: Losartan for Patients With COVID‐19 Requiring Hospitalization; University of Minnesota
NCT04335136: Recombinant Human Angiotensin‐converting Enzyme 2 (rhACE2) as a Treatment for Patients With COVID‐19 [APN01‐COVID‐19]; Apeiron Biologics and multiple academic collaborators in Europe
NCT04332666: Angiotensin‐(1,7) Treatment in COVID‐19: the ATCO Trial (ATCO); Erasme University Hospital in Belgium
9. SUMMARY AND CONCLUSIONS
The COVID‐19 pandemic has sparked an urgent search for effective therapeutics, with little clear success at the time we have prepared this article (early April 2020). Targeting the RAS pathway has received limited attention even though it is an important component of COVID‐19 pathobiology with implications for therapeutics that could ameliorate tissue injury, disease progression and improve morbidity and mortality. A key reason for the limited attention on the RAS system as a therapeutic target may stem from the lack of mechanistic insight regarding the potential benefit of targeting this pathway.
To address this gap, we propose an overriding hypothesis: imbalance of ACE1‐ and ACE2‐mediated signalling as a primary driver of tissue pathobiology in COVID‐19, impacting the phenotypes of multiple interacting cell types in infected tissue, leading to feedback loops that promote inflammation and injury. Tissue damage from the infection is a consequence of enhanced Ang II/AT1 signalling and decreased signalling by Ang(1–7) and perhaps other ACE2‐derived peptides. In the lungs and heart (Figures 3 and 5), the imbalance in the RAS pathway and positive feedback loops can establish a vicious cycle of events mediated by communication among cell types that produce COVID‐19 pathology. Similar mechanisms may also occur in other organs.
Numerous studies and findings corroborate this hypothesis, including results from animal models, clinical data in humans and in vitro findings with human and rodent cells. Moreover, it provides a parsimonious explanation for key features of the disease and for the contribution of co‐morbidities to adverse outcomes in COVID‐19.
The hypothesis also leads to several testable predictions for COVID‐19: (a) susceptibility for adverse outcomes in those with specific ACE1 genotypes and perhaps other genetic variants in RAS pathway elements; (b) potential for patients with a range of illnesses to have worse outcomes; (c) adjusting for other clinical variables, patients administered AT1 receptor antagonists (and perhaps ACEIs) should have improved outcomes; (d) counteracting the imbalance in RAS signalling with agents discussed above should modulate clinicopathological effects of the SARS‐CoV‐2 virus; (e) early administration of RAS‐targeted agents may yield maximum benefit, preventing acute lung injury and acute respiratory distress syndrome by mitigating the damage from the imbalance in ACE1‐ and ACE2‐derived peptides and their signalling.
Given the severity of the COVID‐19 crisis, what types of studies (besides clinical trials) might help test our hypothesis? We envisage several possibilities: (a) in vitro studies using human alveolar epithelial cells, perhaps in 3‐D organoid models in co‐cultures with other key cell types (e.g. fibroblasts, endothelial cells and immune cells) to determine if cellular injury by SARS‐CoV‐2 infection is inhibited by ACEIs/AT1 receptor antagonists/soluble ACE2. Monteil et al. (2020) have demonstrated efficacy of recombinant ACE2 in blunting infectivity of SARS‐CoV‐2 in such models but more detailed analyses and further studies are needed. (b) In vivo studies to test efficacy of ACEIs/AT1 receptor antagonists/soluble ACE2 in animal models (e.g. mice, ferrets and rhesus monkeys, with preference for primate models, e.g. Sutton & Subbarao, 2015) of SARS‐1 infection. Ferrets may be useful to assess SARS‐CoV‐2 infectivity, but infected animals appear to have few pathological features (Shi et al., 2020); rhesus monkeys with SARS‐CoV‐2 infection show signs of clinical disease (Bao et al., 2020). (c) Epidemiological data from COVID‐19 patients to define associations between ACEI/AT1 receptor antagonist use/dose and disease severity/mortality. Certain data of this type have been obtained (Liu, Huang, et al., 2020), but more are needed, especially because of confounding factors in such analyses, including co‐morbidities relevant to COVID‐19. A multi‐country database that assessed usage of AT1 receptorantagonists and ACEIs prior to hospital admission with clinical outcomes from COVID‐19 might be very helpful for such analyses and to define possible differences from patients in different countries.
The hypothesis thus implies that those being treated for approved indications with ACEIs and AT antagonists should maintain their use of these drugs and, in addition, that those drugs may have therapeutic utility in treating patients who develop COVID‐19, in particular those most vulnerable to this viral infection (e.g., those >70 years old and/or with co‐morbidities). ACEIs and AT1 receptor antagonists have well‐known safety profiles, making these drugs well‐suited for repurposing. In addition, a rationale exists for testing the possible therapeutic effects of soluble ACE2 or perhaps ACE2 peptides. The current and likely future challenge of treating seriously ill COVID‐19 patients argues for aggressive approaches. We urge that these approaches include ones that seek to restore ACE1/ACE2 imbalance.
9.1. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2019/20 (Alexander, Christopoulas al., 2019; Alexander, Fabbro et al., 2019). The information of Slo3 and Slo1 channels were referred to the Concise Guide to PHARMACOLOGY 2019/20: Ion channels (Alexander, Mathie et al., 2019).
CONFLICT OF INTEREST
The authors have no conflicts of interest.
ACKNOWLEDGEMENT
Support was provided by Academic Senate of the University of California, San Diego. We thank Drs. Gordon Gill, Jeffrey Jasper and Richard A. Insel for providing helpful comments prior to submission of this manuscript.
Sriram K, Insel PA. A hypothesis for pathobiology and treatment of COVID‐19: The centrality of ACE1/ACE2 imbalance. Br J Pharmacol. 2020;177:4825–4844. 10.1111/bph.15082
REFERENCES
- Adamzik, M. , Frey, U. , Sixt, S. , Knemeyer, L. , Beiderlinden, M. , Peters, J. , & Siffert, W. (2007). ACE I/D but not AGT (−6) A/G polymorphism is a risk factor for mortality in ARDS. European Respiratory Journal, 29(3), 482–488. 10.1183/09031936.00046106 [DOI] [PubMed] [Google Scholar]
- Adedeji, A. O. , Severson, W. , Jonsson, C. , Singh, K. , Weiss, S. R. , & Sarafianos, S. G. (2013). Novel inhibitors of severe acute respiratory syndrome coronavirus entry that act by three distinct mechanisms. Journal of Virology, 87(14), 8017–8028. 10.1128/JVI.00998-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Christopoulos, A. , Davenport, A. P. , Kelly, E. , Mathie, A. , Peter, J. A. , … CGTP Collaborators . (2019). The Concise Guide to PHARMACOLOGY 2019/2020: G protein‐coupled receptors. British Journal of Pharmacology, 176, S21–S141. 10.1111/bph.14748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Mathie, A. , Peters, J. A. , Veale, E. L. , Striessnig, J. , Kelly, E. , … CGTP Collaborator . (2019). The Concise Guide to PHARMACOLOGY 2018/19: ion channels. British Journal of Pharmacology, 176, S142–S228. 10.1111/bph.14749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Fabbro, D. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , … CGTP collaborators . (2019). The concise guide to PHARMACOLOGY 2019/20: Enzymes. British Journal of Pharmacology, 176, S297–S396. 10.1111/bph.14572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreeva, A. V. , Kutuzov, M. A. , & Voyno‐Yasenetskaya, T. A. (2007). Regulation of surfactant secretion in alveolar type II cells. American Journal of Physiology‐Lung Cellular and Molecular Physiology, 293(2), L259–L271. [DOI] [PubMed] [Google Scholar]
- Armstrong, J. F. , Faccenda, E. , Harding, S. D. , Pawson, A. J. , Southan, C. , Sharman, J. L. , … Spedding, M. (2020). The IUPHAR/BPS guide to pharmacology in 2020: Extending immunopharmacology content and introducing the IUPHAR/MMV guide to malaria pharmacology. Nucleic Acids Research, 48(D1), D1006–D1021. 10.1093/nar/gkz951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arumugam, S. , Sreedhar, R. , Thandavarayan, R. A. , Karuppagounder, V. , Krishnamurthy, P. , Suzuki, K. , … Watanabe, K. (2016). Angiotensin receptor blockers: Focus on cardiac and renal injury. Trends in Cardiovascular Medicine, 26(3), 221–228. 10.1016/j.tcm.2015.06.004 [DOI] [PubMed] [Google Scholar]
- Aumiller, V. , Balsara, N. , Wilhelm, J. , Günther, A. , & Königshoff, M. (2013). WNT/β‐catenin signaling induces IL‐1β expression by alveolar epithelial cells in pulmonary fibrosis. American Journal of Respiratory Cell and Molecular Biology, 49(1), 96–104. 10.1165/rcmb.2012-0524OC [DOI] [PubMed] [Google Scholar]
- Bao, L. , Deng, W. , Gao, H. , Xiao, C. , Liu, J. , Xue, J. , … Qi, F. (2020). Reinfection could not occur in SARS‐CoV‐2 infected rhesus macaques. bioRxiv. 10.1101/2020.03.13.990226 [DOI] [Google Scholar]
- Bar‐On, Y. M. , Flamholz, A. , Phillips, R. , & Milo, R. (2020). SARS‐CoV‐2 (COVID‐19) by the numbers. eLife, 9, e57309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barroso, L. C. , Magalhaes, G. S. , Galvão, I. , Reis, A. C. , Souza, D. G. , Sousa, L. P. , … Teixeira, M. M. (2017). Angiotensin‐(1‐7) promotes resolution of neutrophilic inflammation in a model of antigen‐induced arthritis in mice. Frontiers in Immunology, 8, 1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara, R. I. , Brown, L. A. S. , Eaton, D. C. , Roman, J. , & Guidot, D. M. (2003). Chronic ethanol ingestion increases expression of the angiotensin II type 2 (AT2) receptor and enhances tumor necrosis factor‐α‐and angiotensin II‐induced cytotoxicity via AT2 signaling in rat alveolar epithelial cells. Alcoholism: Clinical and Experimental Research, 27(6), 1006–1014. [DOI] [PubMed] [Google Scholar]
- Becker, L. K. , Totou, N. , Moura, S. , Kangussu, L. , Millán, R. D. S. , Campagnole‐Santos, M. J. , … Santos, R. A. S. (2018). Eccentric overload muscle damage is attenuated by a novel angiotensin‐(1‐7) treatment. International Journal of Sports Medicine, 39(10), 743–748. 10.1055/a-0633-8892 [DOI] [PubMed] [Google Scholar]
- Bodor, C. , Nagy, J. P. , Végh, B. , Németh, A. , Jenei, A. , MirzaHosseini, S. , … Rosivall, L. (2012). Angiotensin II increases the permeability and PV‐1 expression of endothelial cells. American Journal of Physiology‐Cell Physiology, 302(1), C267–C276. 10.1152/ajpcell.00138.2011 [DOI] [PubMed] [Google Scholar]
- Boskabadi, J. , Askari, V. R. , Hosseini, M. , & Boskabady, M. H. (2019). Immunomodulatory properties of captopril, an ACE inhibitor, on LPS‐induced lung inflammation and fibrosis as well as oxidative stress. Inflammopharmacology, 27(3), 639–647. 10.1007/s10787-018-0535-4 [DOI] [PubMed] [Google Scholar]
- Buckley, S. T. , Medina, C. , & Ehrhardt, C. (2010). Differential susceptibility to epithelial‐mesenchymal transition (EMT) of alveolar, bronchial and intestinal epithelial cells in vitro and the effect of angiotensin II receptor inhibition. Cell and Tissue Research, 342(1), 39–51. 10.1007/s00441-010-1029-x [DOI] [PubMed] [Google Scholar]
- Caldeira, D. , Alarcão, J. , Vaz‐Carneiro, A. , & Costa, J. (2012). Risk of pneumonia associated with use of angiotensin converting enzyme inhibitors and angiotensin receptor blockers: Systematic review and meta‐analysis. BMJ, 345, e4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Channappanavar, R. , & Perlman, S. (2017, July). Pathogenic human coronavirus infections: Causes and consequences of cytokine storm and immunopathology. In Seminars in Immunopathology (Vol. 39, No. 5, pp. 529–539). Springer Berlin Heidelberg. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Channappanavar, R. , Zhao, J. , & Perlman, S. (2014). T cell‐mediated immune response to respiratory coronaviruses. Immunologic Research, 59(1–3), 118–128. 10.1007/s12026-014-8534-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, C. , Zhang, Z. , Li, Z. , Zhang, F. , Peng, M. , Chen, Y. , & Wang, Y. (2014). Losartan attenuates microvascular permeability in mechanical ventilator‐induced lung injury in diabetic mice. Molecular Biology Reports, 41(2), 809–814. 10.1007/s11033-013-2920-9 [DOI] [PubMed] [Google Scholar]
- Chen, C. , Zhou, Y. , & Wang, D. W. (2020). SARS‐CoV‐2: A potential novel etiology of fulminant myocarditis. Herz, 45(3)230–232. 10.1007/s00059-020-04909-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuquimia, O. D. , Petursdottir, D. H. , Rahman, M. J. , Hartl, K. , Singh, M. , & Fernández, C. (2012). The role of alveolar epithelial cells in initiating and shaping pulmonary immune responses: Communication between innate and adaptive immune systems. PLoS ONE, 7(2), e32125 10.1371/journal.pone.0032125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen, E. P. , Bedi, M. , Irving, A. A. , Jacobs, E. , Tomic, R. , Klein, J. , … Moulder, J. E. (2012). Mitigation of late renal and pulmonary injury after hematopoietic stem cell transplantation. International Journal of Radiation Oncology* Biology* Physics, 83(1), 292–296. 10.1016/j.ijrobp.2011.05.081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silveira, K. D. , Coelho, F. M. , Vieira, A. T. , Sachs, D. , Barroso, L. C. , Costa, V. V. , … dos Santos, R. A. S. (2010). Anti‐inflammatory effects of the activation of the angiotensin‐(1–7) receptor, MAS, in experimental models of arthritis. The Journal of Immunology, 185(9), 5569–5576. 10.4049/jimmunol.1000314 [DOI] [PubMed] [Google Scholar]
- De Mello, W. C. , & Danser, A. J. (2000). Angiotensin II and the heart: On the intracrine renin‐angiotensin system. Hypertension, 35(6), 1183–1188. 10.1161/01.hyp.35.6.1183 [DOI] [PubMed] [Google Scholar]
- Deng, J. , Wang, D. X. , Deng, W. , Li, C. Y. , & Tong, J. (2012). The effect of endogenous angiotensin II on alveolar fluid clearance in rats with acute lung injury. Canadian Respiratory Journal, 19(5), 311–318. 10.1155/2012/951025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du, Y. , Tu, L. , Zhu, P. , Mu, M. , Wang, R. , Yang, P. , … Li, T. (2020). Clinical features of 85 fatal cases of COVID‐19 from Wuhan: A retrospective observational study. American Journal of Respiratory and Critical Care Medicine. Online ahead of print. 10.1164/rccm.202003-0543OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisele, N. A. , & Anderson, D. M. (2011). Host defense and the airway epithelium: Frontline responses that protect against bacterial invasion and pneumonia. Journal of Pathogens, 2011, 249802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrucci, L. , & Fabbri, E. (2018). Inflammageing: Chronic inflammation in ageing, cardiovascular disease, and frailty. Nature Reviews Cardiology, 15(9), 505–522. 10.1038/s41569-018-0064-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores‐Muñoz, M. , Smith, N. J. , Haggerty, C. , Milligan, G. , & Nicklin, S. A. (2011). Angiotensin1‐9 antagonises pro‐hypertrophic signalling in cardiomyocytes via the angiotensin type 2 receptor. The Journal of Physiology, 589(4), 939–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florez‐Sampedro, L. , Song, S. , & Melgert, B. N. (2018). The diversity of myeloid immune cells shaping wound repair and fibrosis in the lung. Regeneration, 5(1), 3–25. 10.1002/reg2.97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester, S. J. , Booz, G. W. , Sigmund, C. D. , Coffman, T. M. , Kawai, T. , Rizzo, V. , … Eguchi, S. (2018). Angiotensin II signal transduction: An update on mechanisms of physiology and pathophysiology. Physiological Reviews, 98(3), 1627–1738. 10.1152/physrev.00038.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frieler, R. A. , & Mortensen, R. M. (2015). Immune cell and other noncardiomyocyte regulation of cardiac hypertrophy and remodeling. Circulation, 131(11), 1019–1030. 10.1161/CIRCULATIONAHA.114.008788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulop, T. , Larbi, A. , Dupuis, G. , Le Page, A. , Frost, E. H. , Cohen, A. A. , … Franceschi, C. (2018). Immunosenescence and inflamm‐aging as two sides of the same coin: Friends or foes? Frontiers in Immunology, 8, 1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gard, P. R. (2010). Implications of the angiotensin converting enzyme gene insertion/deletion polymorphism in health and disease: A snapshot review. International Journal of Molecular Epidemiology and Genetics, 1(2), 145–157. [PMC free article] [PubMed] [Google Scholar]
- Glowacka, I. , Bertram, S. , Herzog, P. , Pfefferle, S. , Steffen, I. , Muench, M. O. , … Eichler, J. (2010). Differential downregulation of ACE2 by the spike proteins of severe acute respiratory syndrome coronavirus and human coronavirus NL63. Journal of Virology, 84(2), 1198–1205. 10.1128/JVI.01248-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godugu, C. , Patel, A. R. , Doddapaneni, R. , Marepally, S. , Jackson, T. , & Singh, M. (2013). Inhalation delivery of Telmisartan enhances intratumoral distribution of nanoparticles in lung cancer models. Journal of Controlled Release, 172(1), 86–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gralinski, L. E. , & Baric, R. S. (2015). Molecular pathology of emerging coronavirus infections. The Journal of Pathology, 235(2), 185–195. 10.1002/path.4454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grommes, J. , & Soehnlein, O. (2011). Contribution of neutrophils to acute lung injury. Molecular Medicine, 17(3), 293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu, J. , Han, B. , & Wang, J. (2020). COVID‐19: Gastrointestinal manifestations and potential fecal‐oral transmission. Gastroenterology. 10.1053/j.gastro.2020.02.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haga, S. , Yamamoto, N. , Nakai‐Murakami, C. , Osawa, Y. , Tokunaga, K. , Sata, T. , … Ishizaka, Y. (2008). Modulation of TNF‐α‐converting enzyme by the spike protein of SARS‐CoV and ACE2 induces TNF‐α production and facilitates viral entry. Proceedings of the National Academy of Sciences, 105(22), 7809–7814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer, A. , Yang, G. , Friedrich, J. , Kovacs, A. , Lee, D. H. , Grave, K. , … Gold, R. (2016). Role of the receptor Mas in macrophage‐mediated inflammation in vivo. Proceedings of the National Academy of Sciences, 113(49), 14109–14114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamming, I. , Timens, W. , Bulthuis, M. L. C. , Lely, A. T. , Navis, G. J. , & van Goor, H. (2004). Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. The Journal of Pathology: A Journal of the Pathological Society of Great Britain and Ireland, 203(2), 631–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, S. X. , He, G. M. , Wang, T. , Chen, L. , Ning, Y. Y. , Luo, F. , … Xu, D. (2010). Losartan attenuates chronic cigarette smoke exposure‐induced pulmonary arterial hypertension in rats: Possible involvement of angiotensin‐converting enzyme‐2. Toxicology and Applied Pharmacology, 245(1), 100–107. 10.1016/j.taap.2010.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder, E. M. , Park, H. S. , Nath, S. K. , Mancini, B. R. , & Decker, R. H. (2015). Angiotensin‐converting enzyme inhibitors decrease the risk of radiation pneumonitis after stereotactic body radiation therapy. Practical Radiation Oncology, 5(6), e643–e649. 10.1016/j.prro.2015.07.003 [DOI] [PubMed] [Google Scholar]
- Harding, S. D. , Sharman, J. L. , Faccenda, E. , Southan, C. , Pawson, A. J. , Ireland, S. , … NC‐IUPHAR (2018). The IUPHAR/BPS Guide to pharmacology in 2018: Updates and expansion to encompass the new guide to immunopharmacology. Nucleic Acids Research, 46, D1091–D1106. 10.1093/nar/gkx1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, F. , Deng, Y. , & Li, W. (2020). Coronavirus disease 2019 (COVID‐19): What we know? Journal of Medical Virology. 10.1002/jmv.25766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, H. , Liu, L. , Chen, Q. , Liu, A. , Cai, S. , Yang, Y. , … Qiu, H. (2015). Mesenchymal stem cells overexpressing angiotensin‐converting enzyme 2 rescue lipopolysaccharide‐induced lung injury. Cell Transplantation, 24(9), 1699–1715. 10.3727/096368914X685087 [DOI] [PubMed] [Google Scholar]
- He, X. , Han, B. , Mura, M. , Xia, S. , Wang, S. , Ma, T. , … Liu, Z. (2007). Angiotensin‐converting enzyme inhibitor captopril prevents oleic acid‐induced severe acute lung injury in rats. Shock, 28(1), 106–111. 10.1097/SHK.0b013e3180310f3a [DOI] [PubMed] [Google Scholar]
- Herold, S. , Ludwig, S. , Pleschka, S. , & Wolff, T. (2012). Apoptosis signaling in influenza virus propagation, innate host defense, and lung injury. Journal of Leukocyte Biology, 92(1), 75–82. 10.1189/jlb.1011530 [DOI] [PubMed] [Google Scholar]
- Huang, C. , Wang, Y. , Li, X. , Ren, L. , Zhao, J. , Hu, Y. , … Cheng, Z. (2020). Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet, 395(10223), 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung, I. F. N. , Cheng, V. C. C. , Wu, A. K. L. , Tang, B. S. F. , Chan, K. H. , Chu, C. M. , … Chan, K. S. (2004). Viral loads in clinical specimens and SARS manifestations. Emerging Infectious Diseases, 10(9), 1550–1557. 10.3201/eid1009.040058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai, Y. , Kuba, K. , Rao, S. , Huan, Y. , Guo, F. , Guan, B. , … Crackower, M. A. (2005). Angiotensin‐converting enzyme 2 protects from severe acute lung failure. Nature, 436(7047), 112–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismael‐Badarneh, R. , Guetta, J. , Klorin, G. , Berger, G. , Abu‐saleh, N. , Abassi, Z. , & Azzam, Z. S. (2015). The role of angiotensin II and cyclic AMP in alveolar active sodium transport. PLoS ONE, 10(8): e0137118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoyama, S. , Keicho, N. , Quy, T. , Phi, N. C. , Long, H. T. , Van Ban, V. , … Yanai, H. (2004). ACE1 polymorphism and progression of SARS. Biochemical and Biophysical Research Communications, 323(3), 1124–1129. 10.1016/j.bbrc.2004.08.208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerng, J. S. , Hsu, Y. C. , Wu, H. D. , Pan, H. Z. , Wang, H. C. , Shun, C. T. , … Yang, P. C. (2007). Role of the renin‐angiotensin system in ventilator‐induced lung injury: An in vivo study in a rat model. Thorax, 62(6), 527–535. 10.1136/thx.2006.061945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, E. S. , Vinh, A. , McCarthy, C. A. , Gaspari, T. A. , & Widdop, R. E. (2008). AT2 receptors: Functional relevance in cardiovascular disease. Pharmacology & Therapeutics, 120(3), 292–316. 10.1016/j.pharmthera.2008.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnik, S. S. , Singh, K. D. , Tirupula, K. , & Unal, H. (2017). Significance of angiotensin 1–7 coupling with MAS1 receptor and other GPCRs to the renin‐angiotensin system: IUPHAR review 22. British Journal of Pharmacology, 174(9), 737–753. 10.1111/bph.13742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketai, L. , Paul, N. S. , & Ka‐tak, T. W. (2006). Radiology of severe acute respiratory syndrome (SARS): The emerging pathologic‐radiologic correlates of an emerging disease. Journal of Thoracic Imaging, 21(4), 276–283. 10.1097/01.rti.0000213581.14225.f1 [DOI] [PubMed] [Google Scholar]
- Khan, A. , Benthin, C. , Zeno, B. , Albertson, T. E. , Boyd, J. , Christie, J. D. , … Hardes, K. (2017). A pilot clinical trial of recombinant human angiotensin‐converting enzyme 2 in acute respiratory distress syndrome. Critical Care, 21(1), 234 10.1186/s13054-017-1823-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharofa, J. , Cohen, E. P. , Tomic, R. , Xiang, Q. , & Gore, E. (2012). Decreased risk of radiation pneumonitis with incidental concurrent use of angiotensin‐converting enzyme inhibitors and thoracic radiation therapy. International Journal of Radiation Oncology* Biology* Physics, 84(1), 238–243. 10.1016/j.ijrobp.2011.11.013 [DOI] [PubMed] [Google Scholar]
- Königshoff, M. , Wilhelm, A. , Jahn, A. , Sedding, D. , Amarie, O. V. , Eul, B. , … Rose, F. (2007). The angiotensin II receptor 2 is expressed and mediates angiotensin II signaling in lung fibrosis. American Journal of Respiratory Cell and Molecular Biology, 37(6), 640–650. 10.1165/rcmb.2006-0379TR [DOI] [PubMed] [Google Scholar]
- Kruse, R. L. (2020). Therapeutic strategies in an outbreak scenario to treat the novel coronavirus originating in Wuhan, China. F1000Research, 9, 72 10.12688/f1000research.22211.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuba, K. , Imai, Y. , Rao, S. , Gao, H. , Guo, F. , Guan, B. , … Bao, L. (2005). A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus–induced lung injury. Nature Medicine, 11(8), 875–879. 10.1038/nm1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuba, K. , Imai, Y. , Rao, S. , Jiang, C. , & Penninger, J. M. (2006). Lessons from SARS: Control of acute lung failure by the SARS receptor ACE2. Journal of Molecular Medicine, 84(10), 814–820. 10.1007/s00109-006-0094-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert, D. W. , Yarski, M. , Warner, F. J. , Thornhill, P. , Parkin, E. T. , Smith, A. I. , … Turner, A. J. (2005). Tumor necrosis factor‐α convertase (ADAM17) mediates regulated ectodomain shedding of the severe‐acute respiratory syndrome‐coronavirus (SARS‐CoV) receptor, angiotensin‐converting enzyme‐2 (ACE2). Journal of Biological Chemistry, 280(34), 30113–30119. 10.1074/jbc.M505111200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, Y. H. , Mungunsukh, O. , Tutino, R. L. , Marquez, A. P. , & Day, R. M. (2010). Angiotensin‐II‐induced apoptosis requires regulation of nucleolin and Bcl‐xL by SHP‐2 in primary lung endothelial cells. Journal of Cell Science, 123(10), 1634–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemarié, C. A. , & Schiffrin, E. L. (2010). The angiotensin II type 2 receptor in cardiovascular disease. Journal of the Renin‐Angiotensin‐Aldosterone System, 11(1), 19–31. 10.1177/1470320309347785 [DOI] [PubMed] [Google Scholar]
- Li, C. K. F. , Wu, H. , Yan, H. , Ma, S. , Wang, L. , Zhang, M. , … Douek, D. C. (2008). T cell responses to whole SARS coronavirus in humans. The Journal of Immunology, 181(8), 5490–5500. 10.4049/jimmunol.181.8.5490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, G. , Fan, Y. , Lai, Y. , Han, T. , Li, Z. , Zhou, P. , … Zhang, Q. (2020). Coronavirus infections and immune responses. Journal of Medical Virology, 92(4), 424–432. 10.1002/jmv.25685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, X. , Molina‐Molina, M. , Abdul‐Hafez, A. , Uhal, V. , Xaubet, A. , & Uhal, B. D. (2008). Angiotensin converting enzyme‐2 is protective but downregulated in human and experimental lung fibrosis. American Journal of Physiology‐Lung Cellular and Molecular Physiology, 295(1), L178–L185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, X. , Rayford, H. , & Uhal, B. D. (2003). Essential roles for angiotensin receptor AT1a in bleomycin‐induced apoptosis and lung fibrosis in mice. The American Journal of Pathology, 163(6), 2523–2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, X. , Zhuang, J. , Rayford, H. , Zhang, H. , Shu, R. , & Uhal, B. D. (2007). Attenuation of bleomycin‐induced pulmonary fibrosis by intratracheal administration of antisense oligonucleotides against angiotensinogen mRNA. Current Pharmaceutical Design, 13(12), 1257–1268. 10.2174/138161207780618867 [DOI] [PubMed] [Google Scholar]
- Li, Y. , Zeng, Z. , Li, Y. , Huang, W. , Zhou, M. , Zhang, X. , & Jiang, W. (2015). Angiotensin‐converting enzyme inhibition attenuates lipopolysaccharide‐induced lung injury by regulating the balance between angiotensin‐converting enzyme and angiotensin‐converting enzyme 2 and inhibiting mitogen‐activated protein kinase activation. Shock, 43(4), 395–404. 10.1097/SHK.0000000000000302 [DOI] [PubMed] [Google Scholar]
- Liu, J. , Zhang, P. S. , Yu, Q. , Liu, L. , Yang, Y. , Guo, F. M. , & Qiu, H. B. (2012). Losartan inhibits conventional dendritic cell maturation and Th1 and Th17 polarization responses: Νovel mechanisms of preventive effects on lipopolysaccharide‐induced acute lung injury. International Journal of Molecular Medicine, 29(2), 269–276. 10.3892/ijmm.2011.818 [DOI] [PubMed] [Google Scholar]
- Liu, P. , Wysocki, J. , Souma, T. , Ye, M. , Ramirez, V. , Zhou, B. , … Jin, J. (2018). Novel ACE2‐Fc chimeric fusion provides long‐lasting hypertension control and organ protection in mouse models of systemic renin angiotensin system activation. Kidney International, 94(1), 114–125. 10.1016/j.kint.2018.01.029 [DOI] [PubMed] [Google Scholar]
- Liu, Y. , Huang, F. , Xu, J. , Yang, P. , Qin, Y. , Cao, M. , … Lv, J. (2020). Anti‐hypertensive Angiotensin II receptor blockers associated to mitigation of disease severity in elderly COVID‐19 patients. medRxiv. 10.1101/2020.03.20.20039586 [DOI] [Google Scholar]
- Liu, Y. , Yang, Y. , Zhang, C. , Huang, F. , Wang, F. , Yuan, J. , … Zhang, Z. (2020). Clinical and biochemical indexes from 2019‐nCoV infected patients linked to viral loads and lung injury. Science China Life Sciences, 63(3), 364–374. 10.1007/s11427-020-1643-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukkarinen, H. P. , Laine, J. , Aho, H. , Zagariya, A. , Vidyasagar, D. , & Kääpä, P. O. (2005). Angiotensin II receptor inhibition prevents pneumocyte apoptosis in surfactant‐depleted rat lungs. Pediatric Pulmonology, 39(4), 349–358. 10.1002/ppul.20187 [DOI] [PubMed] [Google Scholar]
- Luo, W. , Yu, H. , Gou, J. , Li, X. , Sun, Y. , Li, J. , & Liu, L. (2020). Clinical pathology of critical patient with novel coronavirus pneumonia (COVID‐19). Pathology & Pathobiology. 2020020407 [Google Scholar]
- Ma, X. , Xu, D. , Ai, Y. , Ming, G. , & Zhao, S. (2010). Fas inhibition attenuates lipopolysaccharide‐induced apoptosis and cytokine release of rat type II alveolar epithelial cells. Molecular Biology Reports, 37(7), 3051–3056. 10.1007/s11033-009-9876-9 [DOI] [PubMed] [Google Scholar]
- Magalhães, G. S. , Rodrigues‐Machado, M. G. , Motta‐Santos, D. , Alenina, N. , Bader, M. , Santos, R. A. , … Campagnole‐Santos, M. J. (2016). Chronic allergic pulmonary inflammation is aggravated in angiotensin‐(1–7) Mas receptor knockout mice. American Journal of Physiology‐Lung Cellular and Molecular Physiology, 311(6), L1141–L1148. [DOI] [PubMed] [Google Scholar]
- Medhora, M. , Gao, F. , Jacobs, E. R. , & Moulder, J. E. (2012). Radiation damage to the lung: Mitigation by angiotensin‐converting enzyme (ACE) inhibitors. Respirology, 17(1), 66–71. 10.1111/j.1440-1843.2011.02092.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng, Y. , Li, T. , Zhou, G. S. , Chen, Y. , Yu, C. H. , Pang, M. X. , … Li, X. (2015). The angiotensin‐converting enzyme 2/angiotensin (1–7)/Mas axis protects against lung fibroblast migration and lung fibrosis by inhibiting the NOX4‐derived ROS‐mediated RhoA/Rho kinase pathway. Antioxidants & Redox Signaling, 22(3), 241–258. 10.1089/ars.2013.5818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng, Y. , Yu, C. H. , Li, W. , Li, T. , Luo, W. , Huang, S. , … Li, X. (2014). Angiotensin‐converting enzyme 2/angiotensin‐(1‐7)/Mas axis protects against lung fibrosis by inhibiting the MAPK/NF‐κB pathway. American Journal of Respiratory Cell and Molecular Biology, 50(4), 723–736. 10.1165/rcmb.2012-0451OC [DOI] [PubMed] [Google Scholar]
- Monteil, V. , Kwon, H. , Prado, P. , Hagelkrüys, A. , Wimmer, R. A. , Stahl, M. , … Romero, J. P. (2020). Inhibition of SARS‐CoV‐2 infections in engineered human tissues using clinical‐grade soluble human ACE2. Cell. 10.1016/j.cell.2020.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro, R. , & Azevedo, I. (2010). Chronic inflammation in obesity and the metabolic syndrome. Mediators of Inflammation, 2010, 1–10. 10.1155/2010/289645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen, E. M. , Nakashima, B. , Cornell, J. , Copeland, L. A. , Pugh, M. J. , Anzueto, A. , … Fine, M. J. (2012). Population‐based study of statins, angiotensin II receptor blockers, and angiotensin‐converting enzyme inhibitors on pneumonia‐related outcomes. Clinical Infectious Diseases, 55(11), 1466–1473. 10.1093/cid/cis733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mossel, E. C. , Wang, J. , Jeffers, S. , Edeen, K. E. , Wang, S. , Cosgrove, G. P. , … Holmes, K. V. (2008). SARS‐CoV replicates in primary human alveolar type II cell cultures but not in type I‐like cells. Virology, 372(1), 127–135. 10.1016/j.virol.2007.09.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naicker, S. , Yang, C. W. , Hwang, S. J. , Liu, B. C. , Chen, J. H. , & Jha, V. (2020). The novel coronavirus 2019 epidemic and kidneys. Kidney International, 97, 824–828. 10.1016/j.kint.2020.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oudit, G. Y. , Kassiri, Z. , Jiang, C. , Liu, P. P. , Poutanen, S. M. , Penninger, J. M. , & Butany, J. (2009). SARS‐coronavirus modulation of myocardial ACE2 expression and inflammation in patients with SARS. European Journal of Clinical Investigation, 39(7), 618–625. 10.1111/j.1365-2362.2009.02153.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan, F. , Ye, T. , Sun, P. , Gui, S. , Liang, B. , Li, L. , … Zheng, C. (2020). Time course of lung changes on chest CT during recovery from 2019 novel coronavirus (COVID‐19) pneumonia. Radiology, 295(3): 715–721. 10.1148/radiol.2020200370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papp, M. , Li, X. , Zhuang, J. , Wang, R. , & Uhal, B. D. (2002). Angiotensin receptor subtype AT1 mediates alveolar epithelial cell apoptosis in response to ANG II. American Journal of Physiology‐Lung Cellular and Molecular Physiology, 282(4), L713–L718. [DOI] [PubMed] [Google Scholar]
- Parra, E. R. , Ruppert, A. D. P. , & Capelozzi, V. L. (2014). Angiotensin II type 1 and 2 receptors and lymphatic vessels modulate lung remodeling and fibrosis in systemic sclerosis and idiopathic pulmonary fibrosis. Clinics, 69(1), 47–54. 10.6061/clinics/2014(01)07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel, V. B. , Zhong, J. C. , Grant, M. B. , & Oudit, G. Y. (2016). Role of the ACE2/angiotensin 1–7 axis of the renin–angiotensin system in heart failure. Circulation Research, 118(8), 1313–1326. 10.1161/CIRCRESAHA.116.307708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen, S. F. , & Ho, Y. C. (2020). SARS‐CoV‐2: A storm is raging. The Journal of Clinical Investigation, 130(5): 2202–2205. 10.1172/JCI137647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickel, L. , Matsuzuka, T. , Doi, C. , Ayuzawa, R. , Maurya, D. K. , Xie, S. X. , … Tamura, M. (2010). Over‐expression of angiotensin II type 2 receptor gene induces cell death in lung adenocarcinoma cells. Cancer Biology & Therapy, 9(4), 277–285. 10.4161/cbt.9.4.10643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Povlsen, A. L. , Grimm, D. , Wehland, M. , Infanger, M. , & Krüger, M. (2020). The vasoactive Mas receptor in essential hypertension. Journal of Clinical Medicine, 9(1), 267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prompetchara, E. , Ketloy, C. , & Palaga, T. (2020). Immune responses in COVID‐19 and potential vaccines: Lessons learned from SARS and MERS epidemic. Asian Pacific Journal of Allergy and Immunology, 38, 1–9. 10.12932/AP-200220-0772 [DOI] [PubMed] [Google Scholar]
- Reyes, S. , Varagic, J. , Ahmad, S. , VonCannon, J. , Kon, N. D. , Wang, H. , … Ferrario, C. M. (2017). Novel cardiac intracrine mechanisms based on Ang‐(1‐12)/chymase axis require a revision of therapeutic approaches in human heart disease. Current Hypertension Reports, 19(2), 16 10.1007/s11906-017-0708-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothan, H. A. , & Byrareddy, S. N. (2020). The epidemiology and pathogenesis of coronavirus disease (COVID‐19) outbreak. Journal of Autoimmunity, 109, 102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai, N. , & Tager, A. M. (2013). Fibrosis of two: Epithelial cell‐fibroblast interactions in pulmonary fibrosis. Biochimica et Biophysica Acta (BBA)‐Molecular Basis of Disease, 1832(7), 911–921. 10.1016/j.bbadis.2013.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos, R. A. S. , Oudit, G. Y. , Verano‐Braga, T. , Canta, G. , Steckelings, U. M. , & Bader, M. (2019). The renin‐angiotensin system: Going beyond the classical paradigms. American Journal of Physiology‐Heart and Circulatory Physiology, 316(5), H958–H970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos, R. A. S. , Sampaio, W. O. , Alzamora, A. C. , Motta‐Santos, D. , Alenina, N. , Bader, M. , & Campagnole‐Santos, M. J. (2018). The ACE2/angiotensin‐(1–7)/MAS axis of the renin‐angiotensin system: focus on angiotensin‐(1–7). Physiological Reviews, 98(1), 505–553. 10.1152/physrev.00023.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao, M. , Wen, Z. B. , Yang, H. H. , Zhang, C. Y. , Xiong, J. B. , Guan, X. X. , … He, X. F. (2019). Exogenous angiotensin (1‐7) directly inhibits epithelial‐mesenchymal transformation induced by transforming growth factor‐β1 in alveolar epithelial cells. Biomedicine & Pharmacotherapy, 117, 109193. [DOI] [PubMed] [Google Scholar]
- Shen, L. , Mo, H. , Cai, L. , Kong, T. , Zheng, W. , Ye, J. , … Xiao, Z. (2009). Losartan prevents sepsis‐induced acute lung injury and decreases activation of nuclear factor‐κB and mitogen‐activated protein kinases. Shock, 31(5), 500–506. 10.1097/SHK.0b013e318189017a [DOI] [PubMed] [Google Scholar]
- Shi, J. , Wen, Z. , Zhong, G. , Yang, H. , Wang, C. , Huang, B. , … Zhao, Y. (2020). Susceptibility of ferrets, cats, dogs, and other domesticated animals to SARS–coronavirus 2. Science. eabb7015 Online ahead of print. 10.1126/science.abb7015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrikrishna, D. , Astin, R. , Kemp, P. R. , & Hopkinson, N. S. (2012). Renin–angiotensin system blockade: A novel therapeutic approach in chronic obstructive pulmonary disease. Clinical Science, 123(8), 487–498. 10.1042/CS20120081 [DOI] [PubMed] [Google Scholar]
- Skov, J. , Persson, F. , Frøkiær, J. , & Christiansen, J. S. (2014). Tissue renin–angiotensin systems: A unifying hypothesis of metabolic disease. Frontiers in Endocrinology, 5, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodhi, C. P. , Nguyen, J. , Yamaguchi, Y. , Werts, A. D. , Lu, P. , Ladd, M. R. , … Zhang, Y. (2019). A dynamic variation of pulmonary ACE2 is required to modulate neutrophilic inflammation in response to pseudomonas aeruginosa lung infection in mice. The Journal of Immunology, 203(11), 3000–3012. 10.4049/jimmunol.1900579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sriram, K. , & Insel, P. A. (2020). Risks of ACE inhibitor and ARB usage in COVID‐19: evaluating the evidence. Clinical Pharmacology & Therapeutics. 10.1002/cpt.1863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stebbing, J. , Phelan, A. , Griffin, I. , Tucker, C. , Oechsle, O. , Smith, D. , & Richardson, P. (2020). COVID‐19: Combining antiviral and anti‐inflammatory treatments. The Lancet Infectious Diseases, 20(4): 400–402. 10.1016/S1473-3099(20)30132-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suk, J. S. , & Ensign‐Hodges, L. (2019). Development of angiotensin II receptor blocker nanoparticles for an inhaled therapeutic treatment of COPD via TGF‐β antagonism (Doctoral dissertation, Johns Hopkins University).
- Sun, P. , Lu, X. , Xu, C. , Sun, W. , & Pan, B. (2020. Feb 25). Understanding of COVID‐19 based on current evidence. Journal of Medical Virology. 10.1002/jmv.25722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton, T. C. , & Subbarao, K. (2015). Development of animal models against emerging coronaviruses: From SARS to MERS coronavirus. Virology, 479, 247–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan, W. S. D. , Liao, W. , Zhou, S. , Mei, D. , & Wong, W. F. (2018). Targeting the renin‐angiotensin system as novel therapeutic strategy for pulmonary diseases. Curr Opin Pharamacol, 40, 9–17. [DOI] [PubMed] [Google Scholar]
- Thevarajan, I. , Nguyen, T. H. , Koutsakos, M. , Druce, J. , Caly, L. , van de Sandt, C. E. , … Tong, S. Y. (2020). Breadth of concomitant immune responses prior to patient recovery: A case report of non‐severe COVID‐19. Nature Medicine, 26(4): 453–455. 10.1038/s41591-020-0819-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian, S. , Hu, W. , Niu, L. , Liu, H. , Xu, H. , & Xiao, S. Y. (2020). Pulmonary pathology of early phase 2019 novel coronavirus (COVID‐19) pneumonia in two patients with lung cancer. Journal of Thoracic Oncology, 15(5): 700–704. 10.1016/j.jtho.2020.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tikellis, C. , & Thomas, M. C. (2012). Angiotensin‐converting enzyme 2 (ACE2) is a key modulator of the renin angiotensin system in health and disease. International Journal of Peptides, 2012, 256294 10.1155/2012/256294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- To, K. K. W. , Tsang, O. T. Y. , Leung, W. S. , Tam, A. R. , Wu, T. C. , Lung, D. C. , … Lau, D. P. L. (2020). Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS‐CoV‐2: An observational cohort study. The Lancet Infectious Diseases, 20(5): 565–574. 10.1016/S1473-3099(20)30196-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhal, B. D. , Dang, M. T. T. , Li, X. , & Abdul‐Hafez, A. (2012). Angiotensinogen gene transcription in pulmonary fibrosis. International Journal of Peptides, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhal, B. D. , Kyong Kim, J. , Li, X. , & Molina‐Molina, M. (2007). Angiotensin‐TGF‐β1 crosstalk in human idiopathic pulmonary fibrosis: Autocrine mechanisms in myofibroblasts and macrophages. Current Pharmaceutical Design, 13(12), 1247–1256. [DOI] [PubMed] [Google Scholar]
- Uhal, B. D. , Li, X. , Piasecki, C. C. , & Molina‐Molina, M. (2012). Angiotensin signalling in pulmonary fibrosis. The International Journal of Biochemistry & Cell Biology, 44(3), 465–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhal, B. D. , Li, X. , Xue, A. , Gao, X. , & Abdul‐Hafez, A. (2011). Regulation of alveolar epithelial cell survival by the ACE‐2/angiotensin 1–7/Mas axis. American Journal of Physiology‐Lung Cellular and Molecular Physiology, 301(3), L269–L274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhal, B. D. , Nguyen, H. , Dang, M. , Gopallawa, I. , Jiang, J. , Dang, V. , … Morimoto, K. (2013). Abrogation of ER stress‐induced apoptosis of alveolar epithelial cells by angiotensin 1–7. American Journal of Physiology‐Lung Cellular and Molecular Physiology, 305(1), L33–L41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaduganathan, M. , Vardeny, O. , Michel, T. , McMurray, J. J. , Pfeffer, M. A. , & Solomon, S. D. (2020). Renin–angiotensin–aldosterone system inhibitors in patients with COVID‐19. New England Journal of Medicine. 10.1056/NEJMsr2005760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walls, A. C. , Park, Y. J. , Tortorici, M. A. , Wall, A. , McGuire, A. T. , & Veesler, D. (2020). Structure, function, and antigenicity of the SARS‐CoV‐2 spike glycoprotein. Cell. 10.1016/j.cell.2020.02.058. in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan, Y. , Shang, J. , Graham, R. , Baric, R. S. , & Li, F. (2020). Receptor recognition by the novel coronavirus from Wuhan: An analysis based on decade‐long structural studies of SARS coronavirus. Journal of Virology, 94(7). 10.1128/JVI.00127-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, D. , Chai, X. Q. , Magnnusen, C. G. , Zosky, G. R. , Shu, S. H. , Wei, X. , & Hu, S. S. (2019). Renin‐angiotensin‐system, a potential pharmacological candidate, in acute respiratory distress syndrome during mechanical ventilation. Pulmonary Pharmacology & Therapeutics, 58, 101833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, L. , Wang, Y. , Yang, T. , Guo, Y. , & Sun, T. (2015). Angiotensin‐converting enzyme 2 attenuates bleomycin‐induced lung fibrosis in mice. Cellular Physiology and Biochemistry, 36(2), 697–711. 10.1159/000430131 [DOI] [PubMed] [Google Scholar]
- Wang, R. , Ramos, C. , Joshi, I. , Zagariya, A. , Pardo, A. , Selman, M. , & Uhal, B. D. (1999). Human lung myofibroblast‐derived inducers of alveolar epithelial apoptosis identified as angiotensin peptides. American Journal of Physiology‐Lung Cellular and Molecular Physiology, 277(6), L1158–L1164. [DOI] [PubMed] [Google Scholar]
- Wang, R. , Zagariya, A. , Ang, E. , Ibarra‐Sunga, O. , & Uhal, B. D. (1999). Fas‐induced apoptosis of alveolar epithelial cells requires ANG II generation and receptor interaction. American Journal of Physiology‐Lung Cellular and Molecular Physiology, 277(6), L1245–L1250. [DOI] [PubMed] [Google Scholar]
- Wang, R. , Zagariya, A. , Ibarra‐Sunga, O. , Gidea, C. , Ang, E. , Deshmukh, S. , … Uhal, B. D. (1999). Angiotensin II induces apoptosis in human and rat alveolar epithelial cells. American Journal of Physiology‐Lung Cellular and Molecular Physiology, 276(5), L885–L889. [DOI] [PubMed] [Google Scholar]
- Waseda, Y. , Yasui, M. , Nishizawa, Y. , Inuzuka, K. , Takato, H. , Ichikawa, Y. , … Nakao, S. (2008). Angiotensin II type 2 receptor antagonist reduces bleomycin‐induced pulmonary fibrosis in mice. Respiratory Research, 9(1), 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe, T. , Barker, T. A. , & Berk, B. C. (2005). Angiotensin II and the endothelium: Diverse signals and effects. Hypertension, 45(2), 163–169. 10.1161/01.HYP.0000153321.13792.b9 [DOI] [PubMed] [Google Scholar]
- Wong, S. K. , Li, W. , Moore, M. J. , Choe, H. , & Farzan, M. (2004). A 193‐amino acid fragment of the SARS coronavirus S protein efficiently binds angiotensin‐converting enzyme 2. Journal of Biological Chemistry, 279(5), 3197–3201. 10.1074/jbc.C300520200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wösten‐van Asperen, R. M. , Lutter, R. , Haitsma, J. J. , Merkus, M. P. , van Woensel, J. B. , Van Der Loos, C. M. , … Bos, A. P. (2008). ACE mediates ventilator‐induced lung injury in rats via angiotensin II but not bradykinin. European Respiratory Journal, 31(2), 363–371. 10.1183/09031936.00060207 [DOI] [PubMed] [Google Scholar]
- Wösten‐van Asperen, R. M. , Lutter, R. , Specht, P. A. , Moll, G. N. , van Woensel, J. B. , van der Loos, C. M. , … Bos, A. P. (2011). Acute respiratory distress syndrome leads to reduced ratio of ACE/ACE2 activities and is prevented by angiotensin‐(1–7) or an angiotensin II receptor antagonist. The Journal of Pathology, 225(4), 618–627. 10.1002/path.2987 [DOI] [PubMed] [Google Scholar]
- Wu, H. , Li, Y. , Wang, Y. , Xu, D. , Li, C. , Liu, M. , … Li, Z. (2014). Tanshinone IIA attenuates bleomycin‐induced pulmonary fibrosis via modulating angiotensin‐converting enzyme 2/angiotensin‐(1‐7) axis in rats. International Journal of Medical Sciences, 11(6), 578–586. 10.7150/ijms.8365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, Z. , & McGoogan, J. M. (2020). Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China: Summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 10.1001/jama.2020.2648. In press [DOI] [PubMed] [Google Scholar]
- Xu, H. , Zhong, L. , Deng, J. , Peng, J. , Dan, H. , Zeng, X. , … Chen, Q. (2020). High expression of ACE2 receptor of 2019‐nCoV on the epithelial cells of oral mucosa. International Journal of Oral Science, 12(1), 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, Z. , Shi, L. , Wang, Y. , Zhang, J. , Huang, L. , Zhang, C. , … Tai, Y. (2020). Pathological findings of COVID‐19 associated with acute respiratory distress syndrome. The Lancet Respiratory Medicine., 8(4), 420–422. 10.1016/S2213-2600(20)30076-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan, Y. , Liu, Q. , Li, N. , Du, J. , Li, X. , Li, C. , … Jiang, C. (2015). Angiotensin II receptor blocker as a novel therapy in acute lung injury induced by avian influenza A H5N1 virus infection in mouse. Science China Life Sciences, 58(2), 208–211. 10.1007/s11427-015-4814-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao, H. W. , Zhu, J. P. , Zhao, M. H. , & Lu, Y. (2006). Losartan attenuates bleomycin‐induced pulmonary fibrosis in rats. Respiration, 73(2), 236–242. 10.1159/000090140 [DOI] [PubMed] [Google Scholar]
- Yasui, F. , Kohara, M. , Kitabatake, M. , Nishiwaki, T. , Fujii, H. , Tateno, C. , … Kai, C. (2014). Phagocytic cells contribute to the antibody‐mediated elimination of pulmonary‐infected SARS coronavirus. Virology, 454, 157–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, H. , Penninger, J. M. , Li, Y. , Zhong, N. , & Slutsky, A. S. (2020). Angiotensin‐converting enzyme 2 (ACE2) as a SARS‐CoV‐2 receptor: Molecular mechanisms and potential therapeutic target. Intensive Care Medicine, 46(4): 586–590. 10.1007/s00134-020-05985-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y. , Li, Y. , Shi, C. , Fu, X. , Zhao, L. , & Song, Y. (2018). Angiotensin‐(1‐7)‐mediated Mas1 receptor/NF‐κB‐p65 signaling is involved in a cigarette smoke‐induced chronic obstructive pulmonary disease mouse model. Environmental Toxicology, 33(1), 5–15. 10.1002/tox.22454 [DOI] [PubMed] [Google Scholar]
- Zhao, J. , Zhao, J. , Van Rooijen, N. , & Perlman, S. (2009). Evasion by stealth: Inefficient immune activation underlies poor T cell response and severe disease in SARS‐CoV‐infected mice. PLoS Pathogens, 5(10), e1000636 10.1371/journal.ppat.1000636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, F. , Yu, T. , Du, R. , Fan, G. , Liu, Y. , Liu, Z. , … Guan, L. (2020). Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: A retrospective cohort study. The Lancet., 395(10229), P1054–P1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, P. , Yang, X. L. , Wang, X. G. , Hu, B. , Zhang, L. , Zhang, W. , … Chen, H. D. (2020). A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature, 579(7798), 270–273. 10.1038/s41586-020-2012-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou, X. , Chen, K. , Zou, J. , Han, P. , Hao, J. , & Han, Z. (2020). Single‐cell RNA‐Seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019‐nCoV infection. Frontiers of Medicine, 14(2): 185–192. 10.1007/s11684-020-0754-0 [DOI] [PMC free article] [PubMed] [Google Scholar]


