Abstract
BACKGROUND
After long-term androgen deprivation therapy, 25–30% prostate cancer (PCa) acquires an aggressive neuroendocrine (NE) phenotype. Dysregulation of YAP1, a key transcription coactivator of the Hippo pathway, has been related to cancer progression. However, its role in neuroendocrine prostate cancer (NEPC) has not been assessed.
METHODS
Immunohistochemistry and bioinformatics analysis were conducted to evaluate YAP1 expression levels during PCa initiation and progression.
RESULTS
YAP1 expression was present in the basal epithelial cells in benign prostatic tissues, lost in low grade PCa, but elevated in high grade prostate adenocarcinomas. Interestingly, the expression of YAP1 was reduced/lost in both human and mouse NEPC.
CONCLUSIONS
The expression of YAP1 is elevated in high grade prostate adenocarcinomas but lost in NEPC.
INTRODUCTION
Prostate cancer (PCa) is the most commonly diagnosed cancer among American men.1 Although androgen deprivation is an effective therapy for advanced and recurrent PCa, most of the prostate tumors eventually become resistant to treatment and progress to castrate-resistant prostate cancer (CRPC).2 In the disease progression, PCa cells may lose the prostatic adenomatous features and trans-differentiate into neuroendocrine prostate cancer (NEPC).3 NEPC is an aggressive pathological type that lacks the responsiveness to androgen deprivation therapy. The survival of the patients with NEPC has been markedly reduced in comparison to adenocarcinomas. Therefore, there is a pressing need for studying the molecular mechanisms that drive the NE differentiation of PCa cells and developing novel agents that could effectively suppress the emergence of this lethal phenotype.
YAP1 and its homologous protein TAZ are key transcriptional coactivators involved in the modulation of several processes in mammalian cells, such as glucose uptake, proliferation, spreading, apoptosis and differentiation.4 The activity of these transcriptional coactivators is mainly modulated by the Hippo pathway, which when activated impairs the translocation of YAP1 and TAZ to the nucleus and promotes their proteasome-mediated degradation.4 Alternatively, when the Hippo pathway is inactivated, YAP1/TAZ can enter the nucleus and activate the transcription of target genes.4 Additionally, their translocation to the nucleus can be modulated by Wnt/β-Catenin signaling in a Hippo-independent manner,5 and the loss of YAP1/TAZ can induce β-Catenin-dependent gene expression,5 suggesting that the Hippo and the Wnt/β-Catenin pathways are interconnected to develop an integrated response.6
Dysregulation of YAP1 has been related to cancer initiation and progression.7,8 In CRPC, YAP1 is associated with cancer proliferation and invasiveness.9,10 However, the significance of YAP1 in NEPC has not been evaluated. In the present study, we showed that the expression of YAP1 was increased in high-grade prostate adenocarcinoma (AdPCa) but was lost in NEPC, suggesting a role of loss of YAP1 in NEPC development.
MATERIAL AND METHODS
Sample collection
De-identified human prostate tissue specimens were obtained from LSU Health-Shreveport Biorepository Core, Overton Brooks VA Medical Center, Ochsner Health System Biorepository and Tissue for Research. These specimens consisted of 22 benign prostate hyperplasia, 4 Gleason score (GS) 6, 24 GS 7, 5 GS 8, 14 GS 9, 7 GS 10 prostate adenocarcinoma, and 12 NEPC primary tissues. Most tissues were from prostatectomies except for five NEPC cases that were from biopsies. All the tissues were used in accordance with LSU Health-Shreveport IRB protocols. TRAMP, 12T-10 LADY, and NE10 archived tumor sections were used for this study.
Immunohistochemistry and Immunofluorescence staining
Immunostaining was performed using Vectastain elite ABC peroxidase kit (Vector Laboratories, Burlingame, CA) as described previously.11 Primary antibodies of YAP1 and Chromogranin A (CHGA) were purchased from Santa Cruz Biotechnology (sc-101199 and sc-1488 respectively, Dallas, TX), Synaptophysin (SYP) (611880, BD biosciences, San Jose, CA), p63 and FOXA2 (ab735 and ab108422, respectively, Abcam, Cambridge, MA). The tissue sections were counterstained, mounted, and imaged with a Zeiss microscope (White Plains, NY). The percentage of cells stained was evaluated on a scale of 1+ (1–25%), 2+ (25–50%), 3+ (50–75%), and 4+ (75–100%) and the intensity of expression, 0 (negative), 1+ (weak), 2+ (moderate), and 3+ (strong). To assess the correlation between the expression of YAP1 and the Gleason score of tumor samples, immunohistochemistry results were further evaluated by a semiquantitative H-score in a blinded manner. For immunofluorescence staining, YAP1 was co-stained with cytokeratin 5 (CK5) or 14 (CK14) (904801 and 905501 respectively, BioLegend, San Diego, CA) and imaged with a Nikon fluorescence microscope (Melville, NY).
Bioinformatics analyses
The mRNA levels of differentially expressed genes were extracted from RNA-seq datasets deposited in GEO and cBioportal, including GSE32967, GSE41192, GSE3325, SU2C, Cancer Cell Line Encyclopedia, and Beltran’s Neuroendocrine Prostate Cancer datasets. The gene expression was analyzed by using RStudio software.12
Gene silencing
PCa cells were transfection with siRNA to knockdown the expression of YAP1. Western blot was conducted to assess the siRNA knockdown efficiency. RNA-seq was conducted using RNA extracted from PC3/Scramble siRNA and PC3/siYAP1 cells (each containing three biological replicates) at the bioinformatic core of Indiana University School of Medicine. Ingenuity Pathway Analysis was conducted to identify the top pathways that are altered using the list of genes that are up- or down- regulated in the YAP1 knockdown cells. ShinyGO was used for Gene Ontology enrichment analysis.13
Luciferase assay
The human PCa cell lines PC3 and 22Rv1 were purchased/authenticated from American type culture collection. TOPFlash is a Wnt/β-Catenin-responsive promoter driven luciferase construct.14 It is used to monitor Wnt/β-Catenin signaling in transfected cells. PCa cells were co-transfected with TOPflash plasmid and scramble siRNA or siRNAs against YAP1 (Santa Cruz Biotechnology) by using X-tremeGENE Transfection Reagent (Sigma Aldrich, St. Louis, MO). Luciferase activity was measured by using Promega Luciferase Assay kit (Promega Biotechnology, Maddison, WI).
Western blotting
Equal amounts of protein were subjected to SDS-PAGE and then transferred to a PDVF membrane (Bio-Rad, Hercules, CA). Subsequently, membranes were incubated with primary antibodies and HRP-conjugated secondary antibody (Cell Signaling, Beverly, MA). Proteins were revealed by using ProSignal® Dura ECL Reagent (Genesee Scientific, San Diego, CA) and visualized in a Chemidoc™ Touch Imaging System (Bio-Rad).
Statistical analyses
Differential gene expression was evaluated using Chi-Square and two-sided Student’s t-test. The correlation between the expression of YAP1 (H-score) and the Gleason score was assessed using the Spearman correlation test. A p-value of 0.05 was considered statistically significant.
RESULTS
YAP1 expression in normal prostate
We used immunohistochemistry to assess the expression of YAP1 in benign prostatic tissues. YAP1 expression was detected in the nuclei of the prostatic p63-positive basal epithelial cells, as well as stromal cells, but it was absent in the p63-negative luminal glandular epithelial cells (Figs. 1A & 1B). Additionally, dual immunofluorescence staining of YAP1 and CK5 or CK14 that highlights prostatic basal cells was performed to corroborate the YAP1 co-localization pattern in benign basal prostatic epithelium. In concordance with previous data, YAP1 immunostaining was mainly present in the nuclei of the cells that were positive for CK5 and CK14 (Figs. 1C to 1H). These results indicate that the expression of YAP1 is localized in basal epithelial cells.
Fig. 1.
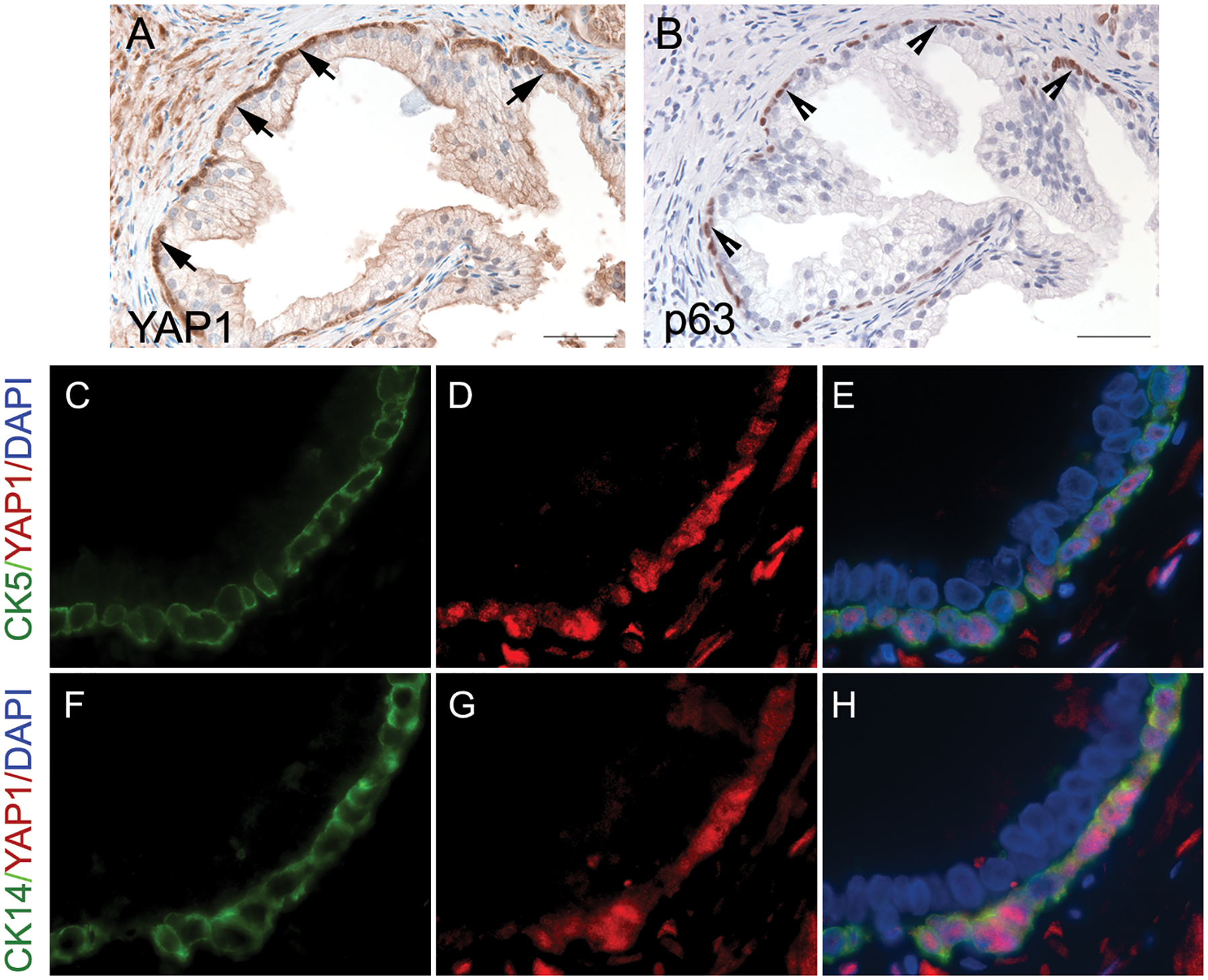
The expression of YAP1 in benign prostatic hyperplasia. (A) YAP1 displayed positive staining in basal cells (positive nuclear staining, indicated by the arrows) and negative staining in the prostatic luminal epithelial cells. (B) Immunohistochemical staining of p63 highlighted basal cells (indicated by the arrowheads) in the periphery of the prostatic gland. (C to H) Dual immunofluorescence staining of YAP1 and cytokeratin 5 (CK5) or cytokeratin 14 (CK14) confirmed the presence of YAP1 expression in prostatic basal epithelial cells.
Expression of YAP1 increased in PIN but lost in mouse prostate NEPC
We used TRAMP and LADY, the two widely used PCa mouse models,15,16 to evaluate YAP1 expression during PCa progression. TRAMP mice develop PIN and a subset of tumors progress into NEPC.17 We found that YAP1 expression was absent in the luminal epithelial cells in wild-type prostates (Fig. 2A, n=3) but present in PIN of TRAMP mice (Fig. 2B, n = 3). Rare clusters of PIN cells stained positive for FOXA2, a marker of NEPC (Fig. 2C). In the TRAMP tumors that developed NEPC (n=5), YAP1 was expressed in PIN lesions within the NEPC tumors (Fig. 2D). However, YAP1 was undetectable in the NEPC cells (Fig. 2D) which were highlighted by NEPC marker chromogranin A (CHGA) (Fig. 2E). The NEPC marker FOXA2 showed diffuse immunoreactivity in NEPC cells in contrast to its rare-to-negative stain in PIN tissues (Figs. 2C & 2F).
Fig. 2.
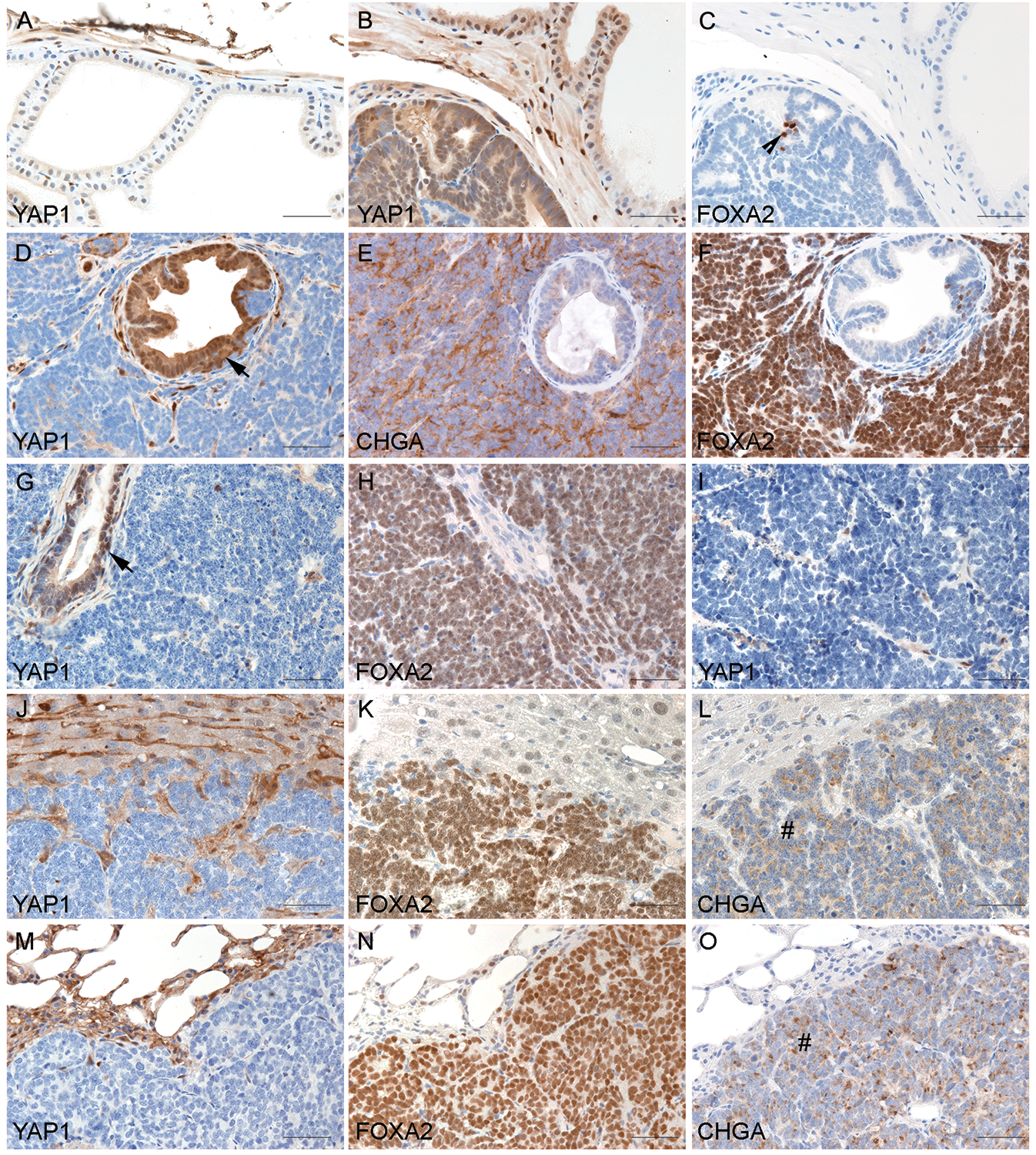
Microscopic examination of the prostate of TRAMP and LADY mice revealed that the expression of YAP1 increased in PIN but decreased in NEPC. (A) Wild-type prostate demonstrating positive staining of YAP1 in the occasional basal epithelia but negative staining in luminal epithelia. (B&C) Serial sections of a PIN tumor of TRAMP demonstrating diffuse positive staining of YAP1 and the rare FOXA2-positive cells in PIN (indicated by the arrowhead). (D to F) Serial sections of a NEPC tumor of TRAMP demonstrating diffuse positive staining of YAP1 in PIN components (indicated by the arrow) but negative in NEPC cells. NEPC markers chromogranin A (CHGA) and FOXA2 were stained positive in NEPC cells but negative in PIN. (G & H) Serial sections of a 12T-10 NEPC tumor demonstrating negativity of YAP1 in NEPC cells in contrast to the positive staining in focal PIN (indicated by the arrow). Positive staining of FOXA2 was seen in the NEPC cells. (I) NE10 tumor showed negative YAP1 staining. (J to O) The expression of YAP1 in NEPC metastases. Sections of the liver (J to L) and lung (M to O) with metastasis of NEPC demonstrated negative YAP1 staining in metastatic NEPC. In contrast, FOXA2 and CHGA were positive in NEPC metastases (indicated by the # signs).
We also examined the expression of YAP1 in LADY mice including 12T-10, a NEPC model,18 and NE10, a xenograft line derived from 12T-10 NEPC.19 In 12T-10 LADY tumors, YAP1 expression was present in focal PIN lesions but was absent in the FOXA2-positive NEPC cells (Figs. 2G & 2H). YAP1 expression was lost in NE10 tumors (Fig. 2I). Additionally, YAP1 immunostaining was absent in NEPC metastases to the liver and lung, which expressed NEPC markers FOXA2 and CHGA (Figs. 2J to 2O). These results indicate that mouse-derived NEPC tumors exhibit a marked reduction in YAP1 expression compared to the non-NE tumors.
The expression of YAP1 increased in human high-grade prostate adenocarcinomas but decreased in NEPC
YAP1 expression levels were also evaluated in human prostatic tissues including benign prostatic hyperplasia, low grade adenocarcinomas (AdPCa), high grade AdPCa, and NEPC. The results were summarized in Fig. 3 and suppl. Table 1. YAP1 expression was detected in fibromuscular stromal cells in most cases. However, YAP1 expression in PCa was altered during progression of the disease (Figs. 3A to 3J). YAP1 was expressed in basal epithelial cells as well as fibromuscular stromal cells in benign prostatic tissues but absent from the luminal epithelial cells except of one case of benign prostatic glandular tissues (Fig. 3A, n=22). YAP1 expression was also absent in human high-grade PIN (2 of 2 cases), which was in contrast to the positive expression in mouse prostatic PIN. Of the 4 low-grade AdPCas with GS 6, 3 cases demonstrated absence of YAP1 expression in the acini of AdPCa (Fig. 3B) and only one tumor showed weak (1+) positive nuclear staining of YAP1. In AdPCa with GS 7, a subset of tumors (10 of 24 cases, 42%) exhibited positive YAP1 nuclear localization. Approximately 1–25% of cancer cells demonstrated various degrees of intensity (from 1+ weak to 3+ strong) of YAP1 staining. Of the 5 AdPCa with GS 8, 4 cases (80%) were positive, with one tumor showing strong YAP1 immunostain of 3+ in 50–75% of cancer cells and 2+ in the remaining cells. In AdPCa with GS 9, 10 of 14 (71%) tumors showed ~ 50–75% of cells with moderate (2+) to strong (3+) YAP1 immunostaining while 4 of 7 (57%) AdPCas with GS 10 exhibited as many as 3+ (50–75%) of cancer cells with 2+ (moderate) to 3+ (strong) YAP1 expression. Overall, the expression of YAP1 (H-score) was associated with the Gleason score of AdPCa (Spearman correlation test, r=0.4962, p<0.0001).
Fig. 3.
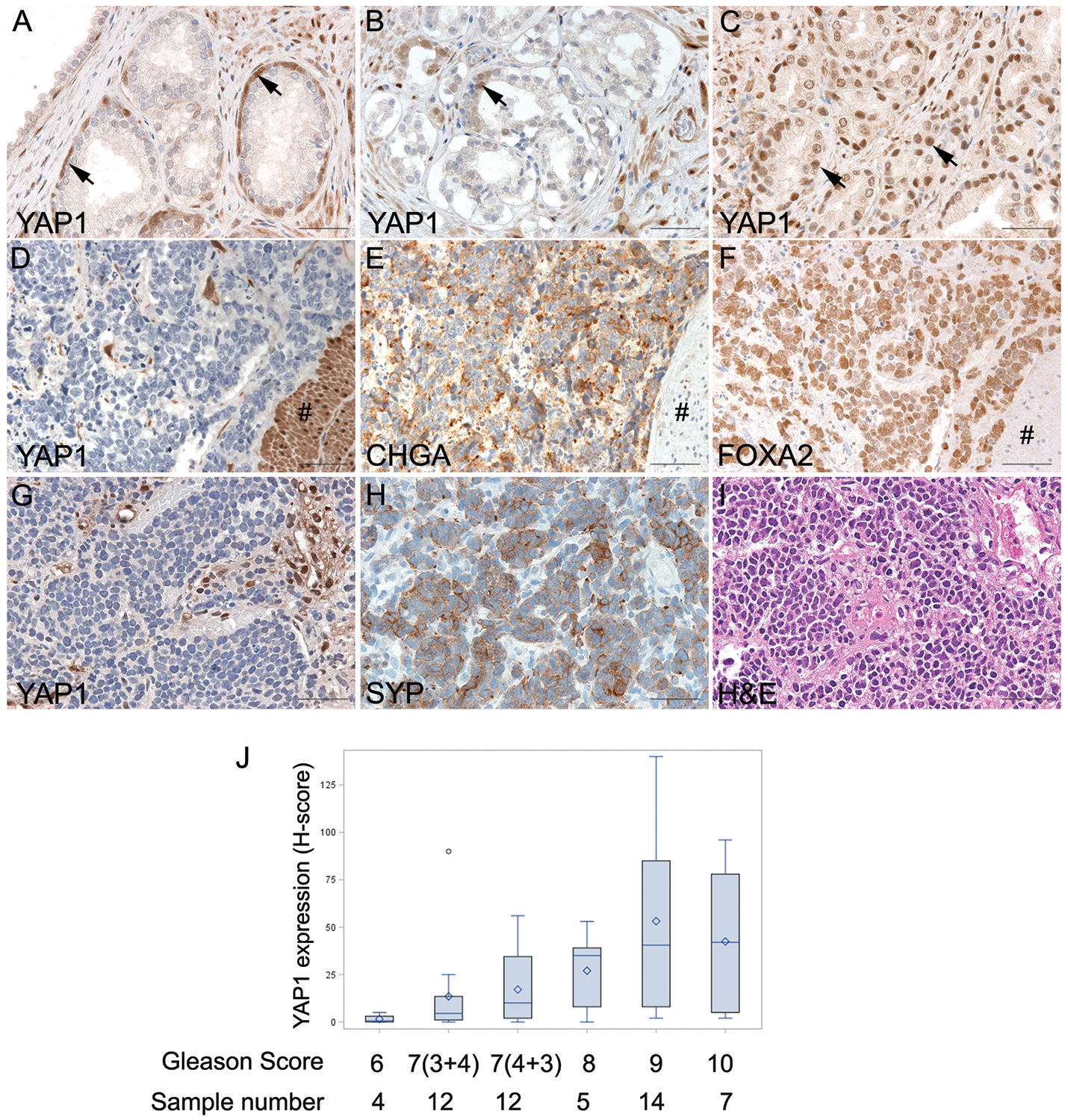
The expression of YAP1 in human prostatic tissues. (A) Benign prostatic tissue demonstrated positive YAP1 staining in the stroma cells and basal cells (indicated by the arrows). (B) Low-grade prostate adenocarcinoma. YAP1 expression was present in stromal cells and occasional basal cells (indicated by the arrow) but was absent in adenocarcinoma cells. (C) High-grade prostate adenocarcinoma. YAP1 was expressed in prostate adenocarcinomas (indicated by the arrows) with a Gleason score of 8 or higher. (D to F) Serial sections of a NEPC with focal adenocarcinoma. YAP1 protein was expressed in high-grade adenocarcinoma components (indicated by the # sign), but the expression was lost in NEPC cells. CHGA and FOXA2 were diffusely expressed in NEPC but not in adenocarcinoma cells. (G to I) Serial sections of a small cell carcinoma demonstrating negative YAP1 and positive synaptophysin (SYP) staining in NEPC cells. (J) Distribution of YAP1 expression (H-score) among PCa with various Gleason grade. The expression of YAP1 was associated with Gleason score (Spearman correlation test, r=0.4962, p<0.0001).
However, YAP1 expression was absent in 6/12 NEPC (Figs. 3D & 3G, suppl. Table 1, n=12), including one AdPCa with Paneth-like NE differentiation, one large cell NEPC, one unspecified NEPC and three small cell NEPC. In the NEPC with focal adenocarcinoma (Fig. 3D), YAP1 expression was present in the adenocarcinoma cells but absent in NEPC components. Chromogranin A (CHGA) (Fig. 3E), FOXA2 (Fig. 3F), and Synaptophysin (SYP) (Fig. 3H) were used as markers for NEPC. These results demonstrate that YAP expression is lost in NEPC.
To further validate the reduction of YAP1 expression in NEPC, we analyzed mRNA levels of YAP1 in an RNA-seq dataset of 46 LuCaP, PCa patient-derived xenograft models (PDXs). The expression levels of YAP1, TAZ, AR, and NEPC markers including FOXA2, SOX2, SYP and CHGA were displayed as a heatmap. As shown in Fig. 4A, YAP1 expression was lost in all 8 NEPC LuCaP PDXs as well as the amphicrine LuCaP 77CR line that is ARpositive NEpositive. However, loss of YAP1 expression only occurred in 1 of 35 ARpositive AdPCa. YAP1 expression was detectable in the double negative LuCaP 173.2 as well as the ARlow/NEnegative LuCaP 176 PDX. In contrast to the reduced YAP1 expression in NEPC, TAZ expression in these LuCaP PDXs did not show any consistent patterns (Fig. 4A). Statistical analysis revealed that the loss of YAP1 expression correlated with NE phenotype (Chi-Square test, p<0.001). Taken together, these data further support the lack of expression of YAP1 in NEPC.
Fig. 4.
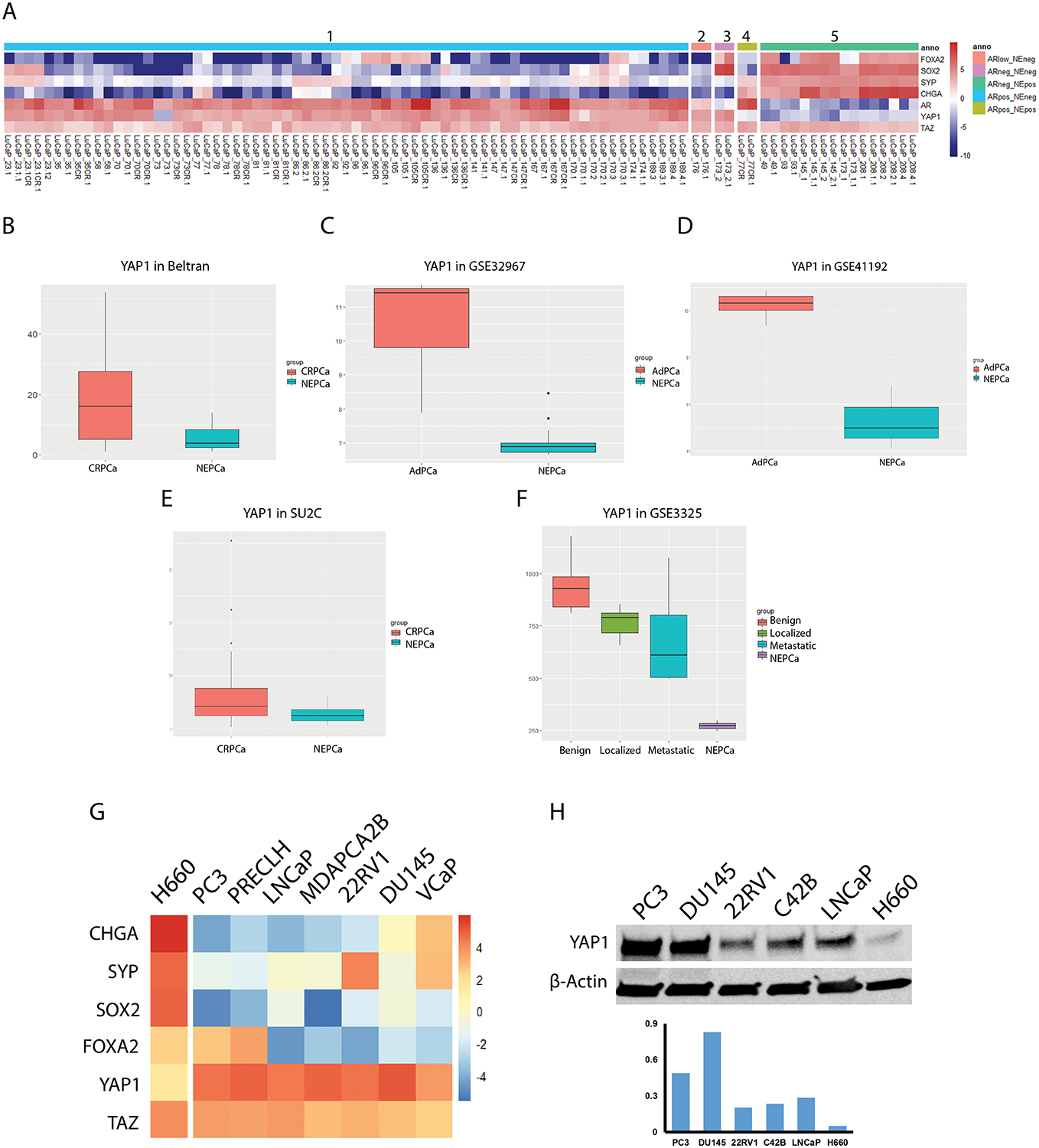
The differential expression of YAP1 in PCa. (A) The expression of YAP1 in LuCaP PDXs. The expression levels of YAP1, TAZ, AR, and NEPC markers (FOXA2, SOX2, CHGA, and SYP) were extracted from RNAseq data derived from 46 LuCaP PDXs including (from left to right) AR+/NE− (group 1, n=35), ARlow/NE− (group 2, n=1), AR−/NE− (group 3, n=1), AR+/NE+ (group 4, n=1), and AR−/NE+ tumors (group 5, n=8). YAP1 expression was lost in all the NE positive LuCaP PDXs. Whereas, YAP1 was expressed in all but one prostate adenocarcinoma cases. (B - F) Expression of YAP1 in CRPC vs NEPC. The mRNA levels of YAP1 were extracted from RNA-seq/RNA microarray data of Neuroendocrine Prostate Cancer. The levels of YAP1 was decreased in NEPC. (G) Heatmap of differentially expressed genes in PCa cell lines. The mRNA levels of YAP1, TAZ, and NEPC markers (CHGA, SYP, SOX2, and FOXA2) were extracted from RNA-seq data of Cancer Cell Line Encyclopedia. The mRNA level of YAP1 was the lowest in NEPC H660 cells. But TAZ displayed no consistent patterns among PCa cell lines. (H) Western blot in assessing the protein levels of YAP1 in PCa cell lines. Lower panel is the quantification of Western blot result.
Results of analysis of the RNA-seq of human tumor dataset published by Beltran et al20 corroborated that NEPC tumors exhibited reduced YAP1 expression (Fig. 4B, t-test, p<0.01). However, this trend was not followed by TAZ (data not shown). Similar results were also observed in additional datasets including GSE32967, GSE41192, GSE3325, and the SU2C datasets (Figs. 4C–Figs. 4F).21
In order to provide additional supportive data, we analyzed the mRNA transcript levels of YAP1 and TAZ in PCa cell lines by using publicly available RNA-seq data.22 The expression level of YAP1 was the lowest in H660 cells, the only NEPC cell line, among all the PCa cell lines tested (Fig. 4G). The reduced expression of YAP1 in H660 was confirmed by Western blot (Fig. 4H). However, TAZ displayed a different expression pattern and its mRNA level did not show consistent decrease in H660 cells (Fig. 4G).
YAP1 loss in AdPCa cells induces Wnt/β-Catenin responsive promoter
Hippo/YAP1 pathway has a close crosstalk with Wnt/β-Catenin signaling,5,6 and previous studies have linked the activation of Wnt/β-Catenin signaling with NE differentiation of PCa.23,24 Therefore, we assessed the effects of YAP1 knockdown on the Wnt/β-Catenin activity. The activity of Wnt/β-Catenin increased in PCa PC3 cells as well as in 22Rv1 cells after YAP1 knockdown (sFig. 1). To further explore the downstream events mediated by the loss of YAP1 expression, we conducted RNA-Seq experiments followed by IPA analysis of the genes that are up- or down- regulated in PC3/siYAP1 cells. The results indicated that the top pathways changed following YAP1 knockdown include axonal guidance, hepatic fibrosis, protein kinase A, RhoA, and PI3K/AKT signaling. However, Wnt/β-Catenin signaling was not enriched in YAP1 knockdown cells. YAP1 knockdown did not cause significant changes in the expression of NEPC markers either (sFig. 2 and sTable2).
DISCUSSION
The development of NEPC is a clinically important issue. We need to elucidate the pathogenesis of NEPC and develop an effective therapy for this aggressive disease.3 In this study, we reported that the Hippo pathway coactivator YAP1 was reduced/lost in NEPC, and a reduction of YAP1 provided a mechanism to activate Wnt/β-Catenin signaling in PCa cells. These results suggest a role of loss of YAP1 in the pathogenesis of NEPC.
In cancer, YAP1 usually functions as an oncogene, promoting cell proliferation and invasion.4 However, YAP1 also exerts tumor suppressive role in certain cellular contexts. For example, YAP1 overexpression reduces small cell lung carcinoma (SCLC) growth.25 Inversely, its loss stimulates breast cancer cell proliferation and mobility, protecting them from anoikis.26 These data suggest that YAP1 exerts a dual-role during tumorigenesis. Therefore, it would be interesting to monitor the changes in its expression during PCa progression. In this study, we demonstrated that the expression of YAP1 was limited to the basal cells in benign prostatic glandular epithelia and was absent in the luminal glandular cells, supporting the previous proposal that YAP1 may serve as a biomarker for prostatic basal epithelial cells.27 YAP1 expression was absent in the cancer cells of early stage AdPCa where there were no basal cells in the glandular epithelium. Its expression was increased; however, in advanced stages of AdPCa. The increased levels of YAP1 expression was associated with Gleason score of AdPCa (Fig. 3J) and advanced tumor-node-metastasis (TNM) stages.28,29 Additionally, the inhibition of this protein in high-grade PCa cells not only impaired cellular proliferation, but also enhanced cellular sensitivity to ADT,9,30 supporting that inhibition of YAP1 could control AdPCa progression.
However, the expression of YAP1 was decreased/lost in cell line, human, and mouse NEPC tumor samples (Figs. 2 to 4). These findings were consistent with that demonstrated in SCLC cells, where the YAP1 expression is reduced in SCLC and its downregulation is essential to NE differentiation.8,31 However, the molecular mechanism that relates the loss of YAP1 with the acquisition of NE phenotype has not been elucidated. Prior studies in lung cancer have demonstrated that YAP1 knockdown induces the expression of several neuroendocrine markers such as Rab3A to achieve NE differentiation.8 In this study, we showed that downregulation of YAP1 activated Wnt/β-Catenin responsive promoter. The activation of Wnt/β-Catenin signaling has been previously related to the induction of NE differentiation of PCa.23,24 Additionally, previous research has shown that YAP1/TAZ inhibit the activation of Wnt/β-Catenin signaling by recruiting β-TrCP to the β-Catenin destruction complex, an essential step for inducing β-Catenin’s degradation by the ubiquitin-proteasome system.5 Consequently, the downregulation of YAP1 could induce the nuclear translocation of β-Catenin resulting in the activation of Wnt/β-Catenin signaling.6 In consistent with the published findings,32,33 our studies demonstrated that Wnt/β-Catenin activity increased after YAP1 knockdown, supporting the idea that loss of YAP1 provides a mechanism to activate Wnt/β-Catenin signaling in AdPCa cells. However, RNAseq analysis indicated that transient YAP1 knockdown was not sufficient to activate endogenous Wnt/β-Catenin signaling or induce the expression of NEPC markers. More studies would follow to determine whether long-term loss of YAP1 could promote Wnt/β-Catenin signaling or induce NE differentiation of AdPCa cells.
Although the levels of YAP1 decreased/lost in NEPC, the closely-related TAZ did not display consistent changes in prostatic tissues (Figs. 4A & 4G). Despite the high homology of YAP1 and TAZ proteins, they have different structures, are involved in differential protein-protein interactions, and have different regulation and downstream functions.34,35 Although YAP1 and TAZ were thought to be functionally redundant, inactivation of YAP1 or TAZ alone can result in significant changes in multiple cellular processes and it has been shown that YAP1 knockout has a greater functional impact.34,35 We show here that YAP1 and TAZ display different expression patterns in PCa and RNAseq results indicate that the relative mRNA abundance for YAP1 is higher than TAZ in non-NE PCa cells (Fig. 4G). These results indicate that they may have distinct roles in NEPC and that YAP1 is more important than TAZ. This concept is also supported by the observation that YAP1 knockdown alone is sufficient to activate the Wnt/β-Catenin responsive promoter in PCa cells.
Taken together, our results highlighted the alteration of YAP1 expression during PCa progression. Although previous studies have linked YAP1’s overexpression with increased AdPCa malignancy, we showed here YAP1 is down regulated in NEPC. We also have evidence supporting that YAP1 downregulation activates the Wnt/β-Catenin signaling, which could lead to NE differentiation. However, more studies would be necessary to determine how the loss of YAP1 affects NE differentiation, as well as if YAP1 inhibitors could be safely used as therapeutic adjuvants for treating AdPCa.
Supplementary Material
ACKNOWLEDGMENT
We thank Dr. Robert Matusik at Vanderbilt University for providing tissues of LADY mice and advice on this research. This research was supported by NIH R03 CA212567, R01 CA226285, U54 GM104940, DOD W81XWH-12-1-0212, and LSUHSC FWCC and Office of Research funding to Yu, X.
Footnotes
CONFLICT OF INTEREST
We declare no conflicts of interest.
REFERENCES
- 1.Debes JD, Tindall DJ. Mechanisms of androgen-refractory prostate cancer. The New England journal of medicine 2004; 351(15): 1488–1490. [DOI] [PubMed] [Google Scholar]
- 2.Dehm SM, Tindall DJ. Molecular regulation of androgen action in prostate cancer. J Cell Biochem 2006; 99(2): 333–344. [DOI] [PubMed] [Google Scholar]
- 3.Beltran H, Tomlins S, Aparicio A, Arora V, Rickman D, Ayala G et al. Aggressive variants of castration-resistant prostate cancer. Clinical cancer research : an official journal of the American Association for Cancer Research 2014; 20(11): 2846–2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ma S, Meng Z, Chen R, Guan KL. The Hippo Pathway: Biology and Pathophysiology. Annual review of biochemistry 2019; 88: 577–604. [DOI] [PubMed] [Google Scholar]
- 5.Azzolin L, Panciera T, Soligo S, Enzo E, Bicciato S, Dupont S et al. YAP/TAZ incorporation in the β-catenin destruction complex orchestrates the Wnt response. Cell 2014; 158(1): 157–170. [DOI] [PubMed] [Google Scholar]
- 6.Varelas X, Miller BW, Sopko R, Song S, Gregorieff A, Fellouse FA et al. The Hippo pathway regulates Wnt/beta-catenin signaling. Developmental cell 2010; 18(4): 579–591. [DOI] [PubMed] [Google Scholar]
- 7.Yu FX, Zhao B, Guan KL. Hippo Pathway in Organ Size Control, Tissue Homeostasis, and Cancer. Cell 2015; 163(4): 811–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ito T, Matsubara D, Tanaka I, Makiya K, Tanei ZI, Kumagai Y et al. Loss of YAP1 defines neuroendocrine differentiation of lung tumors. Cancer science 2016; 107(10): 1527–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang L, Yang S, Chen X, Stauffer S, Yu F, Lele SM et al. The hippo pathway effector YAP regulates motility, invasion, and castration-resistant growth of prostate cancer cells. Molecular and cellular biology 2015; 35(8): 1350–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuser-Abali G, Alptekin A, Lewis M, Garraway IP, Cinar B. YAP1 and AR interactions contribute to the switch from androgen-dependent to castration-resistant growth in prostate cancer. Nat Commun 2015; 6: 8126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Connelly ZM, Yang S, Chen F, Yeh Y, Khater N, Jin R et al. Foxa2 activates the transcription of androgen receptor target genes in castrate resistant prostatic tumors. American journal of clinical and experimental urology 2018; 6(5): 172–181. [PMC free article] [PubMed] [Google Scholar]
- 12.Team R RStudio: Integrated Development Environment for R. RStudio, Inc.: Boston, MA, 2015. [Google Scholar]
- 13.Ge SX, Jung D, Yao R. ShinyGO: a graphical enrichment tool for animals and plants. Bioinformatics 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Veeman MT, Slusarski DC, Kaykas A, Louie SH, Moon RT. Zebrafish prickle, a modulator of noncanonical Wnt/Fz signaling, regulates gastrulation movements. Curr Biol 2003; 13(8): 680–685. [DOI] [PubMed] [Google Scholar]
- 15.Greenberg NM, DeMayo F, Finegold MJ, Medina D, Tilley WD, Aspinall JO et al. Prostate cancer in a transgenic mouse. Proc Natl Acad Sci U S A 1995; 92(8): 3439–3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kasper S, Sheppard PC, Yan Y, Pettigrew N, Borowsky AD, Prins GS et al. Development, progression, and androgen-dependence of prostate tumors in probasin-large T antigen transgenic mice: a model for prostate cancer. Lab Invest 1998; 78(6): i–xv. [PubMed] [Google Scholar]
- 17.Huss WJ, Gray DR, Tavakoli K, Marmillion ME, Durham LE, Johnson MA et al. Origin of androgen-insensitive poorly differentiated tumors in the transgenic adenocarcinoma of mouse prostate model. Neoplasia 2007; 9(11): 938–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Masumori N, Thomas TZ, Chaurand P, Case T, Paul M, Kasper S et al. A probasin-large T antigen transgenic mouse line develops prostate adenocarcinoma and neuroendocrine carcinoma with metastatic potential. Cancer Res 2001; 61(5): 2239–2249. [PubMed] [Google Scholar]
- 19.Masumori N, Tsuchiya K, Tu WH, Lee C, Kasper S, Tsukamoto T et al. An allograft model of androgen independent prostatic neuroendocrine carcinoma derived from a large probasin promoter-T antigen transgenic mouse line. The Journal of urology 2004; 171(1): 439–442. [DOI] [PubMed] [Google Scholar]
- 20.Beltran H, Prandi D, Mosquera JM, Benelli M, Puca L, Cyrta J et al. Divergent clonal evolution of castration-resistant neuroendocrine prostate cancer. Nature medicine 2016; 22(3): 298–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng S, Yu X. Bioinformatics analyses of publicly available NEPCa datasets. Am J Clin Exp Urol 2019; 7(5): 327–340. [PMC free article] [PubMed] [Google Scholar]
- 22.Barretina J, Caponigro G, Stransky N, Venkatesan K, Margolin AA, Kim S et al. The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature 2012; 483(7391): 603–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang X, Chen MW, Terry S, Vacherot F, Chopin DK, Bemis DL et al. A human- and male-specific protocadherin that acts through the wnt signaling pathway to induce neuroendocrine transdifferentiation of prostate cancer cells. Cancer research 2005; 65(12): 5263–5271. [DOI] [PubMed] [Google Scholar]
- 24.Yu X, Wang Y, DeGraff DJ, Wills ML, Matusik RJ. Wnt/beta-catenin activation promotes prostate tumor progression in a mouse model. Oncogene 2011; 30(16): 1868–1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nishikawa E, Osada H, Okazaki Y, Arima C, Tomida S, Tatematsu Y et al. miR-375 is activated by ASH1 and inhibits YAP1 in a lineage-dependent manner in lung cancer. Cancer research 2011; 71(19): 6165–6173. [DOI] [PubMed] [Google Scholar]
- 26.Yuan M, Tomlinson V, Lara R, Holliday D, Chelala C, Harada T et al. Yes-associated protein (YAP) functions as a tumor suppressor in breast. Cell death and differentiation 2008; 15(11): 1752–1759. [DOI] [PubMed] [Google Scholar]
- 27.Liu CY, Yu T, Huang Y, Cui L, Hong W. ETS (E26 transformation-specific) up-regulation of the transcriptional co-activator TAZ promotes cell migration and metastasis in prostate cancer. The Journal of biological chemistry 2017; 292(22): 9420–9430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feng J, Ren P, Gou J, Li Z. Prognostic significance of TAZ expression in various cancers: a meta-analysis. OncoTargets and therapy 2016; 9: 5235–5244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun Z, Xu R, Li X, Ren W, Ou C, Wang Q et al. Prognostic Value of Yes-Associated Protein 1 (YAP1) in Various Cancers: A Meta-Analysis. PloS one 2015; 10(8): e0135119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jiang N, Ke B, Hjort-Jensen K, Iglesias-Gato D, Wang Z, Chang P et al. YAP1 regulates prostate cancer stem cell-like characteristics to promote castration resistant growth. Oncotarget 2017; 8(70): 115054–115067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Horie M, Saito A, Ohshima M, Suzuki HI, Nagase T. YAP and TAZ modulate cell phenotype in a subset of small cell lung cancer. Cancer science 2016; 107(12): 1755–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Imajo M, Miyatake K, Limura A, Miyamoto A, Nishida E. A molecular mechanism that links Hippo signalling to the inhibition of Wnt/beta-catenin signalling. The EMBO journal 2012; 31(5): 1109–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barry ER, Morikawa T, Butler BL, Shrestha K, de la Rosa R, Yan KS et al. Restriction of intestinal stem cell expansion and the regenerative response by YAP. Nature 2013; 493(7430): 106–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Plouffe SW, Lin KC, Moore JL 3rd, Tan FE, Ma S, Ye Z et al. The Hippo pathway effector proteins YAP and TAZ have both distinct and overlapping functions in the cell. The Journal of biological chemistry 2018; 293(28): 11230–11240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Callus BA, Finch-Edmondson ML, Fletcher S, Wilton SD. YAPping about and not forgetting TAZ. FEBS letters 2019; 593(3): 253–276. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


