Abstract
Introduction
Creating effective programs for cervical cancer prevention is essential to avoid premature deaths from cervical cancer. The Dominican Republic has persistently high rates of cervical cancer, despite the availability of Pap smear screening. This study explored Dominican provider attitudes towards human papillomavirus (HPV) testing and current challenges to effective cervical cancer prevention.
Methods
In this Consolidated Framework for Implementation Research (CFIR)-driven mixed methods study, we conducted in-depth interviews (N=21) and surveys (N=202) with Dominican providers in Santo Domingo and Monte Plata provinces regarding their perspectives on barriers to cervical cancer prevention and their knowledge and attitudes towards HPV testing as an alternative to Pap smear.
Results
Providers believed the main barrier to cervical cancer prevention was lack of cervical cancer awareness and resulting inadequate population screening coverage. Providers felt Pap smear was widely available to women in the Dominican Republic and were unsure how a change to HPV testing for screening would address gaps in current cervical cancer screening programs. A subset of providers felt HPV testing offered important advantages for early detection of cervical cancer and were in favor of more widespread use. Cost of the HPV test and target age for screening with HPV testing were the main barriers to acceptability.
Discussion
Providers had limited knowledge of HPV testing as a screening test. The group was divided in terms of the potential impact of a change in screening test in addressing barriers to cervical cancer prevention in the Dominican Republic. Findings may inform interventions to disseminate global evidence-based recommendations for cervical cancer screening.
Keywords: cervical cancer prevention, Dominican Republic, Latin America and the Caribbean, HPV testing
Introduction
Creating effective programs for cervical cancer prevention is essential to avoid premature deaths from cervical cancer. In Latin America and the Caribbean (LAC) 56,000 women were diagnosed with cervical cancer in 2018, and more than 25,000 women died [1]. Most countries in LAC face the challenge of confronting existing but ineffective cervical cytology (i.e., Pap smear) screening programs [2]. The Dominican Republic is one such country, which has high rates of cervical cancer [age-standardized rate (ASR) 17.1 per 100,000] despite the availability of Pap smear screening [1].
In Latin America generally, Pap smear screening programs have existed for decades, but, with few exceptions, country programs have not succeeded in lowering mortality from cervical cancer [2]. The Pap smear is a fairly subjective test, and it has only moderate (50–65%) sensitivity [3]–[5], requiring frequent screening for accurate detection of cervical pre-cancers. In addition, the infrastructure needed for high quality laboratory services, information systems, and systems for monitoring and quality control, eludes many health systems in low-and middle-income countries (LMICs). As a consequence, countries fail to achieve reliable population-based screening [6], [7]. For women themselves, the necessity of multiple visits for screening, diagnosis, and treatment of precancerous lesions with Pap smear programs creates barriers to care, and may result in women being lost to follow up of abnormal screening (Pap) tests [8], [9][9].
Evidence-based practice guidelines for cervical cancer prevention globally have not been universally defined nor adopted, but mounting evidence supports the use of high-risk human papillomavirus (HPV) testing as a screening modality that more efficiently identifies women at risk for cervical cancer [2], [10]–[12]. An understanding of the role of oncogenic HPV types as a necessary cause of cervical cancer, and of HPV’s slow progression from initial infection to persistent infection, to the development of cervical pre-cancer and cancer, has informed screening practices in terms of initiation of screening, frequency of screening, and mode of testing [13], [14]. With HPV testing, a high negative predictive value (i.e., the very low risk of cervical cancer associated with a negative test) allows women to be screened less frequently, thereby allowing resources to be directed at follow up of women with abnormal tests and at efforts to reach unscreened women [15], [16]. HPV testing via self-collection, in which the woman herself collects a vaginal sample, also reduces sociocultural and access barriers to screening for women who have underutilized existing screening services [17], [18].
Theoretical Framework
The Consolidated Framework for Implementation Research (CFIR) guided the design, data collection tools and analysis for this study. The CFIR is a metatheoretical framework designed to elicit factors that might facilitate or impede successful implementation of public health interventions or practices [19]. The outer setting, inner setting, characteristics of individuals, and intervention characteristics domains were examined; the implementation process domain was not relevant to this pre-adoptive study. A more detailed overview of the use of the CFIR to assess barriers and facilitators to adoption of evidence-based practice for cervical cancer prevention is presented elsewhere (manuscript in preparation). This analysis focused on the knowledge/beliefs regarding the intervention construct, i.e., knowledge/beliefs regarding HPV testing for cervical cancer screening, within the characteristics of individuals domain.
Background: Dominican Republic
The Dominican public health system is divided into nine regions (Region 0-VIII). Region 0 includes the provinces of Santo Domingo and Monte Plata and serves 40% of the population in the public health system [20]. The provinces in Region 0 include the Distrito Nacional of the capital city, Santo Domingo, as well as peri-urban areas outside of the metropolitan area and the neighboring rural province of Monte Plata.
There is very little published literature on cervical cancer prevention in the Dominican Republic. Findings from focus group discussions with women in a previous qualitative study in the Dominican Republic [21], supported by literature from other settings in Latin America and elsewhere, indicate that health care providers play an important role in women’s navigation of the cervical cancer screening and treatment pathway and ultimately in the prevention of cervical cancer [22]–[24]. Providers may also offer insight into health system barriers to cervical cancer screening and treatment in a particular country context [23], [25]. No studies have been identified that focused on Dominican health care provider knowledge regarding current alternatives to Pap smear, including HPV testing. The purpose of this study was 1) to explore Dominican health care providers’ perceptions of current cervical cancer screening systems and what they view as barriers to cervical cancer prevention in the Dominican Republic, and 2) to explore provider knowledge of and attitudes towards HPV testing as a cervical cancer screening test. A mixed method design allowed access to a broader sample of providers, a rich description of the phenomenon of cervical cancer screening and an opportunity for comparison.
Methods
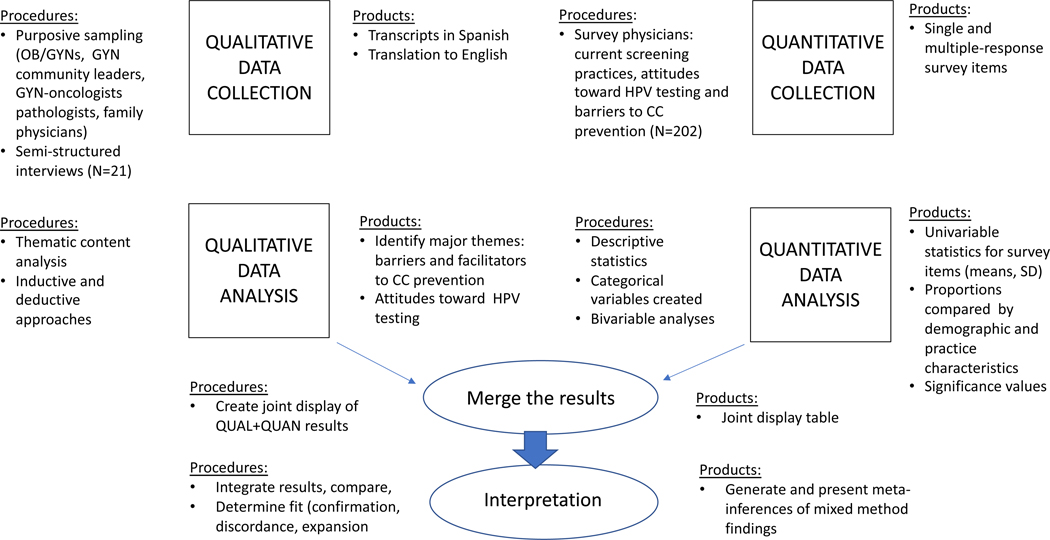
This study used a convergent mixed methods design (Figure 1), with qualitative semi-structured provider interviews and quantitative provider surveys conducted in a single phase [26]. This analysis focused on a subset of findings from the larger mixed methods study examining provider-level barriers and facilitators to adoption of evidence-based practice for cervical cancer prevention in the Dominican Republic. Specifically, provider perceptions of barriers to effective cervical cancer prevention in the existing system, and on attitudes towards HPV testing as an alternative cervical cancer screening modality were examined, using both qualitative and quantitative data sources.
Figure 1.
Convergent Mixed Methods Study Design
The study was reviewed by the Department of Research and Education (Dirección de Investigación y Gestión del Conocimiento-DIGC) and the Institutional Review Board of the Instituto Nacional del Cáncer Rosa Emilia Sánchez Pérez de Tavares (INCART) in Santo Domingo, and subsequently by the Institutional Review Board at New York University’s Washington Square Campus. All participants read the informed consent document, consented verbally, and were offered a copy of the consent form.
Qualitative
Participants.
Purposive sampling was used to recruit health care providers in Santo Domingo and Monte Plata who represented diverse perspectives and experiences in cervical cancer prevention: obstetrician-gynecologists (OB/GYNS), leaders in the GYN professional community, GYN-oncologists, pathologists, and family physicians, to complete individual key informant interviews (September 2018 and February 2019). We estimated it would take up to 25 qualitative interviews to reach data saturation. Providers in Santo Domingo province represented urban and peri-urban practice settings, and providers in Monte Plata provided the perspective of a rural practice setting [20].
Procedures.
An external advisory board (consisting of two Dominican OB/GYN experts and one health system leader) was formed to provide advice about recruitment and data collection strategies as well as to identify key provider stakeholders. The Dominican co-investigator (NF) at INCART contacted individual key informants and scheduled the in-depth interview at a place and time convenient for the interviewee. Interviews were conducted in Spanish by the principal investigator (EL) and a bilingual research assistant with experience in qualitative research.
Instrument.
Individual interviews were conducted utilizing a semi-structured interview guide, informed by relevant CFIR domains and constructs. Relevant to this analysis were questions such as: “What is working well and what is not working well in current cervical cancer screening systems?” What have you heard about HPV testing as a cervical cancer screening test? What is your opinion about starting HPV testing at age 25–30? How would a change in practice address gaps in existing cervical cancer screening systems? Interviews were audio-recorded on a study-dedicated digital device. Audio-recordings were transcribed verbatim, and translated from Spanish to English, by a native Dominican Spanish speaker.
Data management and analysis.
The process of analysis was both deductive, based on CFIR constructs, and inductive, with themes emerging from the data [27]–[29]. Transcripts were read individually by two members of the research team (EL, NVD) and then reviewed to create a codebook. Initial coding of transcripts was done by two coders and intercoder reliability calculated for 20% of transcripts. Any areas of discrepancy were further discussed by the research team. In-depth content analysis was conducted following coding of the full dataset using Nvivo 12.0 qualitative software (QSR International, Burlington, MA). Content analysis identified themes related to provider perspectives on strengths and challenges of current cervical cancer screening programs and knowledge of HPV testing.
Quantitative
Participants.
In February 2019, we recruited a group of 200 physicians (OB/GYNS, general practice physicians, family practice physicians, and OB/GYN residents) in the provinces of Santo Domingo and Monte Plata who perform cervical cancer screening. According to the external advisory board, though most cervical cancer screening is done by OB/GYNS, family practice and general practice physicians also do screening in some settings. Quota sampling was used to seek balanced representation of specialists (OB/GYNS), non-specialists (family practice and general practice physicians), and OB/GYN residents in Region 0, and participants representing both urban and rural practice settings, and the public and private health sectors.
Procedures.
The director of INCART sent formal letters of invitation to secondary and tertiary hospitals in Santo Domingo and to the four hospitals in Monte Plata. The Dominican co-investigator (NF) followed up to confirm site approval to recruit participants and identify a site contact person. We trained a team of Dominican interviewers on the research topic, survey questionnaire, and use of KoboToolbox for data collection. This team conducted interviewer-administered surveys in participants’ hospital, primary health center or private clinic settings. Providers who practice in both the private and public sectors were asked to respond according to the particular institution in which they were interviewed. Surveys were conducted in settings ranging from rural clinics, to public and private primary level health centers, to secondary level municipal hospitals, to tertiary level large maternity hospitals and private practice gynecology clinics.
Instrument.
The survey was adapted from cervical cancer-related provider surveys used in US and international settings [23], [24], [30], obtained from a question bank compiled by the U.S. Centers for Disease Control and Prevention (CDC). The survey was translated from English to Spanish, back-translated by a bilingual Dominican Spanish speaker, and checked for semantic equivalence [31], [32]. The survey was then piloted with a focus group of Dominican health care providers, and refined by the principal investigator (EL), NF, a GYN-oncologist and expert in cervical cancer prevention in the Dominican Republic, and a Dominican research consultant with extensive experience in survey research in the Dominican Republic. From the 43-question survey, this analysis focused on items in the section on knowledge, attitudes and beliefs regarding cervical cancer screening (knowledge/beliefs regarding the intervention) and the section on attitudes regarding barriers to cervical cancer screening and treatment, using 5-point Likert-type responses. Questions on barriers to cervical cancer prevention were taken from the CDC question bank as well as some specific to the Dominican Republic that emerged from previous focus group discussions with women [21].
Data management and analysis.
Survey responses were entered by the interviewer on a digital device and uploaded directly to the secure KoboToolbox server. Following data cleaning, descriptive statistics were calculated for all survey items. Pearson’s chi-square analyses were used to examine differences in cervical cancer screening knowledge, attitudes and beliefs, as well as perceptions of barriers to cervical cancer prevention, by provider type, and by demographic and practice characteristics. In cases of small cell sizes (i.e., five or fewer observations per cell), fisher’s exact test was used. Values of p ≤ .05 were considered statistically significant. All analyses were done using SPSS 25.0 (IBM Corp., Armonk, NY).
Results
In keeping with the convergent mixed methods design, qualitative and quantitative data were analyzed separately. Mixed methods findings were integrated across concepts rather than individual case level findings, as qualitative and quantitative findings were derived from discrete samples of providers (Figure 1). Following description of the qualitative and quantitative samples, results are presented jointly using the typology of qualitative themes within the overarching categories of provider perspectives on facilitators and barriers to cervical cancer prevention, and attitudes towards HPV testing.
Qualitative Sample
We interviewed 21 health care providers in Santo Domingo (n=17) and Monte Plata (n=4), including nine OB/GYNS, four GYN-oncologists, four pathologists, and four family practice physicians. Among the gynecologists, five were also managers of their departments or organizations. Four were selected as leaders in the GYN community, based on past or present leadership positions in the public or private health sector, or the GYN professional society (Table 1).
Table 1.
Demographic/Practice Characteristics of Providers in Santo Domingo and Monte Plata
| QUALITATIVE SAMPLE (N=21) | |
|---|---|
| N (%)1 | |
| Sex | |
| Male | 9 (43) |
| Female | 12 (57) |
| Practice setting | |
| Public | 11 (52) |
| Private | 10 (48) |
| Medical Specialty | |
| Obstetrician-gynecologist | 9 (43) |
| GYN-oncologist | 4 (19) |
| Pathologist | 4 (19) |
| Family practice physician | 4 (19) |
| Practice Location | |
| Santo Domingo | 17 (81) |
| Monte Plata | 4 (19) |
| GYN leaders | |
| OB/GYN department manager | 4 (19) |
| Past/present GYN society leader | 4 (19) |
| Leader of organization | 1 (5) |
|
| |
| QUANTITATIVE SAMPLE (N=202) | |
|
| |
| Mean age (SD, range) | 41.73 (10.97, 19-72) |
| Median age (IQR) | 40 (19, 32-51) |
| Sex | |
| Male | 66 (33) |
| Female | 136 (67) |
| Country of medical training | |
| Dominican Republic | 201 (99.5) |
| Other | 1 (0.5) |
| Country of residency/specialty training | |
| Dominican Republic | 185 (91.6) |
| Other | 4 (2) |
| Mean years practicing medicine (SD, range) | 14.46 (9.80, 0–46) |
| Median years practicing medicine (IQR) | 12 (16, 6–22) |
| Practice setting | |
| Public | 154 (76.2) |
| Private | 48 (23.8) |
| Medical Specialty | |
| Obstetrician-gynecologist | 101 (50.0) |
| Family practice physician | 23 (11.4) |
| General practice physician | 27 (13.4) |
| OB/GYN resident | 51 (25.2) |
| Practice Location | |
| Santo Domingo | 172 (85.1) |
| Monte Plata | 30 (14.9) |
| Patients generally come from… | |
| Rural areas | 16 (7.9) |
| Urban areas | 98 (48.5) |
| Both | 88 (43.6) |
Unless otherwise indicated
SD = standard deviation OB/GYN = obstetrician-gynecologist
IQR = interquartile range
Quantitative Sample
Survey participants included 101 OB/GYNs, 23 family physicians, 27 general practice physicians, and 51 OB/GYN residents, from four of the five municipalities of Santo Domingo province, and all of the four municipalities of Monte Plata province. Providers had a mean age of 42, were majority female (67%), and had almost all trained in the Dominican Republic (99.5% for medical training, 91.6% for residency). Distribution by practice setting, medical specialty, and practice location was determined by quota sampling design. Most participants reported their patients came from either strictly urban areas (48.5%) or a combination of urban and rural areas (43.6%) (Table 1).
Perceptions of Strengths in Current Screening System
Qualitative and quantitative findings regarding provider-perceived facilitators and barriers to cervical cancer prevention are displayed jointly in Table 2.
Table 2.
Dominican Provider Perspectives on “What is Working Well and What is Not Working Well in Current Cervical Cancer Screening System?”
| Domains | Themes | Summary | Illustrative Quotes | Quantitative | Metainferences |
|---|---|---|---|---|---|
| Facilitators To Cervical Cancer Prevention | Pap testing widely available | Pap smear screening readily available in all health sectors and levels of care (primary, secondary, tertiary) Pap (conventional cytology) is cheap and therefore accessible |
“In all areas of the DR a woman of reproductive age and also in menopause has the opportunity to have a Pap done. I think the country has succeeded in that.” (pathologist, private, Santo Domingo) | 56.9% disagreed that cost was a barrier to Pap screening. Providers >40, with >10 years in practice, GYN specialists more likely to agree cost of screening is a barrier screening. | CONFIRMATION: Providers felt pap smear screening is readily available to Dominican women Some provider differences in whether cost might be a barrier to screening |
| Pap is an accurate method to screen for cervical cancer | Opinions about Pap smear differed by practice role, e.g., some pathologists and GYN-oncologists acknowledged limitation of false negative Pap results. Most did not mention this as a concern. Several thought Liquid-based cytology more accurate than conventional cytology | “ if costs were reduced for liquid-based Pap patients could have it done more than the conventional Pap. it has been shown to be more sensitive and specific for early detection of these lesions.” (OB/GYN, public, Santo Domingo) “There is also a group that have normal Paps and then come back with an advanced cancer.” (pathologist, public, Santo Domingo) |
79.2% agreed “Pap is an accurate method for cervical cancer screening,” with no significant demographic or practice differences | CONFIRMATION: most providers feel Pap test is an accurate cervical cancer screening method, Partial discordance among pathologists and GYN-oncologists in QUAL | |
| Barriers to Cervical Cancer Prevention | Lack of population coverage | Not all women are screened • Two extremes discussed: portion of population over-screened; others have never had Pap • Urban vs. rural populations • Socioeconomic differences |
“Many women don’t go have the Pap test done. When it’s done it’s already late.” (OB/GYN, dept manager, public, Monte Plata) “Here we have women that have their Pap every 6 months, and then you have women coming from rural areas and they are diagnosed with cancer already in an advanced stage. You ask them when their last pap was and they tell you they have never had a pap done or it’s been 10 years or…‘when my last child was born.’ That’s the story here with all those who have cancer. It’s a failure of the system.” (OB/GYN, dept manager, public, Santo Domingo) |
Population screening coverage not measured | QUAL data only, though subsequent theme of lack of public awareness (QUAL+QUAN) relates to women not being screened |
| Lack of cervical cancer awareness | Lack of public awareness (of cervical cancer and availability of screening) is a barrier to screening |
“Patient education is not the most effective because there are women who have never had a Pap done. There are women who don’t know what the process is. There are women who still are afraid to have a Pap done…those are the reasons that we have so much cervical cancer.” (pathologist, public, Santo Domingo) “It’s rare that you encounter a cervical cancer in patients of higher incomes. Because these are patients that go more frequently, that have access to information. They have more access to health care providers. Our problem is really with patients with scarce resources, because of lack of access to care, and lack of access to information.” (OB/GYN, public, Santo Domingo) |
92.1% of providers agreed lack of public awareness is barrier to early detection of cervical cancer. CONTRAST: 67.3% of providers agreed “My patients are aware of the need for cervical cancer screening tests.” Providers in private sector were more likely to agree (79.2% vs. 63.6%. p=.05). Non-specialists were less likely to agree than GYN specialists and ob/gyn residents (56% vs. 69.3% and 74.5%, respectively, p=.03) |
CONFIRMATION: lack of public awareness of cervical cancer is perceived to be a barrier to cervical cancer prevention However, women who see a provider are aware of the need for cervical cancer screening; EXPANSION: women of higher socioeconomic status and education more likely to see providers |
|
| Individual level barriers | A few providers thought some women do not take responsibility for their own health |
“There is a problem of..self-care…Because you can offer a service but if women don’t come…” (family physician, private, Santo Domingo) “They don’t see it as necessary, that nothing will happen to me, or basically a lack of awareness that my health is my responsibility.” (family physician, private, Santo Domingo) |
Not measured, emerged in qualitative interviews | N/A | |
| Provider/health system barriers | • Inconsistent service delivery and/or supply availability limits access to screening in some settings (especially primary and secondary health centers) • Practice-style barriers • Insurers restrict family physicians • Patient not always aware they can see a GP or family MD vs a GYN for screening |
“A lot of times the woman waits to go to a gynecologist to have the Pap done, because she thinks only gynecologists do Pap smears. They don’t understand that also general medical doctors and family doctors, all types of doctors are trained to do it.” (family physician, private, Santo Domingo) | Provider-level barriers not measured | N/A | |
| Challenges with follow up | • Long waiting times for results (Monte Plata) • Women do not come for Pap results • Women have difficulty obtaining follow-up care |
“The further away a patient is, the more things become more difficult…the [Pap] slides travel to another place where they are read and the diagnostic chain can get lost.” (pathologist, public, Santo Domingo) “The cost of follow up testing, because sometimes patients don’t have the economic means to travel from the villages to the city…the majority of the failures are because the patient doesn’t have the resources. They can come to the cancer hospital and have everything done free, but [can’t] get there…patients are lost to follow up that way.” (OB/GYN, dept manager, public, Monte Plata) |
50.5% overall agreed cost of follow-up evaluations was a barrier to care. Providers >40 (59.4% vs. 40.6%, p=.002), with>10 yrs experience (58.9% vs. 37.2%, p=.001) more likely to agree. Residents less likely than GYN-specialists and non-specialists to agree (35.3% vs. 57.4% and 52.0% respectively, p=.02.). Lack of availability of colposcopy a barrier in Monte Plata (73.3%) but not in Santo Domingo (73.3% vs. 44.2%, p=.01) | CONFIRMATION barriers exist (cost and availability) to follow up after abnormal screening tests, but not universal. Women in rural province of Monte Plata perceived to face greater barriers to follow up of abnormal screening tests due to less availability of colposcopy and services. EXPANSION: lower quality and efficiency of lab results for rural women create barriers to follow up |
Abbreviations: Dept: department; GP: general practice provider; family MD: family practice physician; HPV: human papillomavirus; OB/GYN: obstetrician-gynecologist; QUAL: qualitative; QUAN: quantitative
Pap testing available.
A few providers reported that slight improvements had been made in cervical cancer prevention in recent years. As a whole, providers felt that opportunities for Pap smear screening were readily available in both the private and public health sectors and at the primary, secondary and tertiary care levels. Nonetheless, all qualitative participants felt that improvements are needed and that current opportunistic screening (rather than an organized national program) did not reach all Dominican women, leading to persistently high rates of cervical cancer mortality despite the availability of Pap smear screening at all levels of the health care system. In the quantitative survey fewer than half of providers (39.1%) thought the cost of cervical cancer screening was a barrier to care for their patients (Table 3.), but there were some differences by age, years in practice and medical specialty. Providers age 40 or older were more likely to agree that cost of screening was a barrier (48.1% vs 29.2%, p=.02), as were those with more than ten years of practice (45.2% vs. 29.5%, p=.05). GYN-specialists were also more likely than non-specialists or residents (47.5% vs. 30.0% and 31.4%, respectively, p=.03) to agree (Table 4).
Table 3.
Dominican Provider Opinions on Barriers to Cervical Cancer Prevention in Santo Domingo (N=172) and Monte Plata Provinces (N=30)
| Santo Domingo | Monte Plata | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| PERCEIVED BARRIERS | Agree n (%) | Disagree n (%) | Neither agree nor disagree n (%) | Agree n (%) | Disagree n (%) | Neither agree nor disagree n (%) | p-value1 |
| “Lack of public awareness about cervical cancer is a barrier to early detection in Dominican women.” | 158 (91.9) | 10 (5.8) | 4 (2.3) | 28 (93.3) | 1 (3.3) | 1 (3.3) | 0.82 |
| “The cost of cervical cancer screening for patients is a barrier to care for my patients.” | 70 (40.7) | 94 (54.7) | 8 (4.7) | 9 (30.0) | 21 (70.0) | - | 0.20 |
| “The cost of evaluations recommended after an abnormal cervical cancer screening test is a barrier to care for my patients.” | 89 (51.7) | 72 (41.9) | 3 (6.4) | 13 (43.3) | 14 (46.7) | 3 (10.0) | 0.61 |
| “Lack of availability of colposcopy services is a barrier to care for my patient.” | 76 (44.2) | 89 (51.7) | 7 (4.1) | 22 (73.3) | 8 (26.7) | - | 0.01 |
| “Lack of availability of treatment for precancerous lesions (cryotherapy, LEEP) is a barrier to care for my patients.” | 70 (40.7) | 92 (53.3) | 10 (5.8) | 18 (60.0) | 10 (33.3) | 2 (6.7) | 0.12 |
Pearson’s χ2 unless otherwise indicated
Table 4.
Demographic/Practice Correlates of Provider Perceptions of Barriers to Cervical Cancer Prevention in the Dominican Republic
| Lack of public awareness about cervical cancer is a barrier to early detection in Dominican women | Cost of cervical cancer screening is a barrier to care for my patients | Cost of evaluations recommended after abnormal screening test is a barrier to care for my patients | Lack of availability of colposcopy services is a barrier to care for my patients | Lack of availability of LEEP or cryotherapy (precancer treatment) is a barrier to care for my patients | |
|---|---|---|---|---|---|
| Percentage of sample overall who agree | 92.1% | 39.1% | 50.5% | 48.5% | 43.6% |
| Age, Years in practice | NS | Higher agreement among providers ≥40 and with ≥10 years in practice | Higher agreement among providers ≥40 and with ≥10 years in practice | NS | NS |
| Public vs. private sector | NS | NS | NS | NS | NS |
| Medical Specialty | NS | Higher agreement among GYN-specialists | Higher agreement among GYN-specialists and non-specialists, more disagreement or neutral responses among residents | NS | NS |
| Practice location; Patients generally come from rural, urban or both | NS | NS | NS | Higher agreement among providers in Monte Plata | NS |
Pap accuracy.
Most providers viewed the Pap test as an effective screening test. A few providers, particularly the pathologists in the qualitative sample, discussed limitations of cytology, in terms of high false negative (i.e., low test sensitivity) rates. In the quantitative sample, the majority (79.2%) of providers agreed that Pap smear is an accurate screening method for detecting cervical cancer (Table 5), and there were no statistically significant differences according to demographic and practice characteristics. Agreement was lower among GYN-specialists and residents compared to non-specialists (Table 6), but the difference was not statistically significant (p=.08).
Table 5.
Dominican Provider Knowledge and Attitudes Regarding Cervical Cancer Screening and HPV Testing (N=202)
| STATEMENT | Agree n (%) | Disagree n (%) | Neither agree nor disagree n (%) |
|---|---|---|---|
| “The Pap smear is an accurate method to screen for cervical cancer.” | 160 (79.2) | 23 (11.4) | 19(9.4) |
| “High-risk HPV testing and Pap smear done together are more accurate than the Pap smear alone for evaluating the risk of cervical cancer.” | 189 (93.6) | 9 (4.5) | 4(2.0) |
| “Doing high-risk HPV testing alone, followed by Pap smear for those patients with a positive high-risk HPV test is an accurate method for evaluating the risk of cervical cancer.” | 148 (73.3) | 44 (21.8) | 10(5) |
| “I am aware of the option of self-collection of the high-risk HPV test for cervical cancer screening” | 43 (21.3) | 137 (67.8) | 22(10.9) |
| “In my medical opinion, I consider self-collection of the HPV test to be a valid method.” | 34 (16.8) | 144 (71.3) | 24(11.9) |
| “I believe that, in general, self-collection of the HPV test would be acceptable to Dominican women” | 84 (41.6) | 92 (45.6) | 26(12.9) |
Table 6.
Demographic/Practice Correlates of Provider Knowledge/Attitudes Towards HPV Testing for Cervical Cancer Screening
| Agree the Pap smear is an accurate method for detecting cervical cancer | Agree the HPV test and Pap performed together are more accurate than Pap alone for evaluating cervical cancer risk | Agree HPV testing alone followed by Pap for abnormal is an accurate method for CC screening | Aware of self-collection as option for HPV testing | Agree HPV self-collection is a valid method of screening | Agree Dominican women would accept HPV self-collection | |
|---|---|---|---|---|---|---|
| Percentage of sample overall who agree | 79.2% | 93.6% | 73.3% | 21.3% | 16.8% | 41.6% |
| Age, Years in practice | NS | NS | Higher for providers ≥40 | Higher for providers with ≥10 years in practice | NS | Lower for providers with ≥10 years in practice |
| Public vs. private sector | NS | NS | NS | NS | NS | Higher among public sector providers |
| Medical Specialty | NS (but lower levels of agreement among GYN specialists and residents vs non-specialists) | NS | NS | NS | NS | Higher among non-specialists |
| Practice location; Patients generally come from rural, urban or both | NS | NS | NS (but higher levels of agreement in Santo Domingo than Monte Plata) | NS | NS | More neutral responses from those whose patients come from urban areas |
Perceptions of Challenges in Current Screening System
Lack of population coverage.
The main barrier to effective cervical prevention identified by participants was lack of population screening coverage, i.e., some groups of Dominican women have never been screened or are under-screened and thereby at increased risk for cervical cancer. Differences between women who did and did not access screening were identified in education, socioeconomic status, and geographic location. Women of lower socioeconomic status, lower levels of education and women from rural areas were considered less likely to be screened. In fact, two extremes were commonly discussed, particularly as a difference between women in urban areas and women in rural areas: a certain population of women is screened more often than necessary while other women are never screened at all or have not been screened in many years.
Lack of public awareness of cervical cancer prevention.
The main explanation given for women not utilizing screening services was lack of awareness of cervical cancer and of the availability or purpose of screening. Similar provider perceptions were found in the quantitative survey, in which almost all providers (92%) agreed that lack of awareness of cervical cancer at the level of the population was a barrier to effective cervical cancer prevention in the Dominican Republic (Table 3).
Provider and health system barriers.
A few providers focused on individual barriers to screening, saying that women did not take responsibility for their health. Others acknowledged health system or provider-level barriers to screening. Problems such as inconsistent availability of supplies for speculum exams was mentioned in some settings, or limited number of hours in the day or days during the week that screening services were offered. Three of the family physicians mentioned the practice-style barrier that some physicians will not do gynecologic exams if the woman comes to the office wearing pants (as opposed to a skirt that could cover her during the exam). They expressed concern that these were important missed opportunities for preventive care. Several family physicians in Santo Domingo also mentioned that both insurer-driven practice restrictions and patient misconceptions about who can perform cervical cancer screening may limit access to screening.
Follow-up barriers.
Finally, participants discussed problems of follow up after screening tests. Several providers in Monte Plata complained about long waiting time for Pap results. Providers reported that many women do not come to get their Pap smear results, and in some settings face economic or transportation barriers to follow-up care for colposcopy (diagnostic evaluation following an abnormal screening test) or treatment. In the smaller municipal hospitals in Monte Plata for instance, colposcopy services were not available, and women were referred to the larger provincial hospital for colposcopy or were in some cases referred to the capital of Santo Domingo to see specialists in the cancer hospitals.
Quantitative survey results showed that half of providers overall agreed that the cost of follow-up evaluations recommended after an abnormal screening test was a barrier to care for their patients. Providers age 40 or older (59.4% vs. 40.6%, p=.002) and those with more than ten years of practice experience (58.9% vs. 37.2%, p=.001) were more likely to agree with this statement. Residents were significantly less likely than the GYN-specialists or non-specialists (35.3% vs. 57.4% and 52.0% respectively, p=.02) to agree that cost of follow-up presented a barrier to care. With regard to availability (vs. cost) of follow up testing and treatment, 73.3% of providers from Monte Plata agreed that lack of availability of colposcopy was a barrier to care for their patients, and 60% agreed that lack of availability of treatment for precancerous lesions was a barrier to care for their patients. This contrasted with providers in Santo Domingo among whom only 44.2% (p=.01) felt their patients faced barriers to colposcopy and 40.7% (not statistically significant) to precancerous treatments.
In the qualitative interviews, following the discussion of strengths and challenges of existing cervical cancer screening systems, providers’ knowledge and beliefs about HPV testing as an alternative screening test was explored with questions that went from broad to more specific targeted questions. Level of discussion and the extent to which more detailed questions were asked depended on the interviewee’s familiarity with HPV testing as a screening modality (Table 7).
Table 7.
Mixed Method Findings of Dominican Provider Knowledge and Attitudes Toward HPV testing for Cervical Cancer Screening
| Domains | Themes | Summary | Illustrative Quotes | Quantitative | Metainferences |
|---|---|---|---|---|---|
| Knowledge of and Attitudes Toward HPV testing for Cervical Cancer screening | HPV testing as stand-alone screening test | • Limited knowledge of HPV test as stand-alone screening test • A few pathologists, OB/GYNs and GYN-oncologists knew more through conferences/literature • Most aware of HPV test as complement to Pap and in favor of it being available to all patients (public sector); private sector using already • One pathologists and several gynecologists skeptical, valuing the Pap’s assessment of cellular changes |
“The Ministry has not authorized routine use of [the HPV test]…I would focus first on taking the normal sample, the conventional Pap. And then from there, depending on the patient’s risk factors I would do the HPV test.” (OB/GYN, dept manager, Monte Plata) “it can be used as a screening test but it is not yet available here in our country as a screening test. Rather it’s an auxiliary test that we use for those who can afford it.” (OB/GYN, private, Santo Domingo) |
93.6% of providers agreed “HPV testing and Pap done together is more accurate than Pap alone for evaluating cervical cancer risk.” 73.3% agreed “HPV testing alone, followed by Pap smear for follow up of patients with a positive HPV test is an accurate method for cervical cancer screening”. Providers age 40 or older more likely to agree (81.1% vs. 64.6%, p=.03). Only 21.3% of providers aware of option of self-collection for HPV testing. 16.8% agreed self-collection of HPV test is valid method 41.6% agreed self-collection would be acceptable to Dominican women. | CONFIRMATION: limited provider knowledge of HPV as stand-alone test or discussions of self-collection. Providers supported use of HPV testing with Pap and felt combination to be more accurate than Pap alone. EXPANSION: QUAL offered details from some providers on what they knew of advantages of HPV testing: higher sensitivity. On the other side, skepticism on part of some QUAL providers, insisting on need for cellular reading of Pap smear |
| Subtheme: Starting Screening with HPV test at age 25-30 | • Almost all providers in QUAL felt too late • Based on early age of onset of sexual activity, believe Dominican women need to be screened earlier. |
“No, that’s very late. Because these days women start having sex at age 15. As soon as they start their sexual life, they should start screening…because we have had cervical cancers associated with HPV in 23 year-old patients.” (pathologist, private, Santo Domingo) “I think at 25, not to wait until 30 as has been said, because we have a lot of early cancer. Because they start their sexual life very early, at age 12 or 11.” (GYN-oncologist, private, Santo Domingo) |
Provider opinions about specific parameters for HPV testing not measured. | N/A | |
| Subtheme: Acceptability to patients | Most providers felt HPV testing would be acceptable to patients • Women generally follow physician recommendations • Women want what is “best” • Extended screening interval might be appealing One GYN-oncologist did not think women would accept waiting until age 25-30 to be screened |
“There would have to be a campaign to raise awareness in the population that this test is more accurate for this disease than the Pap.” (GYN-oncologist, public, Santo Domingo) “Here all patients want to do their liquid-based Pap. They want to be ok. They want the best for themselves. It would not be difficult to introduce [this test].” (family physician, private, Santo Domingo) |
41.6% of providers agreed “Dominican women would accept HPV self-test.” Providers with >10 years of practice more likely to disagree with this statement (52.4% vs. 34.6%, p=.009). Providers in public sector (46.8% vs. 25%, p=.02) and non-specialists (54.0% vs. 34.7% and 43.1% for gyn-specialists and residents respectively, p=.01) more likely to agree | QUAN/QUAL asked different questions, cannot fully compare QUAL: Providers thought HPV testing would be acceptable to patients QUAN: Only 41.6% of providers agreed self-collection of HPV test would be acceptable to women. |
|
| Subtheme: Cost of HPV testing | Main obstacle providers saw to adoption of HPV testing was cost • Currently use HPV test for triage of abnormal Paps, patients pay • Providers doubtful MOH could pay for HPV test • One provider mentioned HPV testing beginning to be covered by insurance • Widespread adoption dependent on HPV testing being available at no cost |
“In the private sector, it’s being used a lot. it’s really too expensive for the majority of the population. Not all of the population has access to it.” (OB/GYN, dept manager, public, Monte Plata) “For our patients in the public sector, the problem is cost. If we could somehow get the test, subsidize the costs and be able to offer it, we would be offering it yesterday.” (pathologist, public, Santo Domingo) |
Not measured, emerged in qualitative interviews | N/A | |
| Potential Impact of Change in Screening Practice | How HPV testing could address gaps in existing cervical cancer screening systems | Opinions somewhat split • Some (pathologists and GYN-oncologists) felt change to HPV testing would address shortcomings of Pap • Others felt change in test would not address system problems or reach unscreened women • One participant feared change to more expensive test would widen gap between screened and unscreened women. |
“We need…better sensitivity, better specificity, better quality of our diagnostic tests, better screening. Right now we have false negatives and false positives with conventional cytology and those patients can either get lost to follow up and treatment or are overtreated for a cervical lesion, that in the end after going to consults and having biopsies done, is determined to be nothing.” (pathologist, public, Santo Domingo) “I don’t think the problem will be fixed with a new test; it’s more a matter of a change in attitude and in the components of the system.” (family physician, private, Santo Domingo) “What we need to have is an organized system that reaches all women at risk. … The current programs don’t reach 50% of the population at risk…Even with the conventional pap, which is an inexpensive test, we don’t reach the whole population.” (OB/GYN, dept manager, public, Santo Domingo) |
Majority (55.9%) of providers said “no” they would NOT agree with replacing Pap with HPV testing for cervical cancer screening | CONFIRMATION: Providers not fully convinced of value brought by change to HPV testing for cervical cancer screening. DISCORDANCE: Subgroup of providers in QUAL focused on limitations of Pap and potential impact of a change to a more sensitive test |
Abbreviations: Dept: department; HPV: human papillomavirus; OB/GYN: obstetrician-gynecologist; QUAL: qualitative; QUAN: quantitative;
Provider Knowledge and Attitudes Regarding HPV testing as a Screening Test
HPV testing as stand-alone screening test.
Providers in the qualitative sample overall had limited knowledge of HPV testing as a stand-alone screening test. Only one third of providers had learned more about the use of HPV testing for screening from international journals or meetings. Most providers were aware of the HPV test as a complement to Pap and in favor of it being available to all patients. Those practicing in the private sector were already using it for their patients who could afford to pay the out-of-pocket expense for it. Demographic and practice correlates of knowledge/attitudes towards HPV testing are shown in Table 6 and integrated in Table 7. As mentioned, most providers (79.2%) thought Pap smear was an accurate method to screen for cervical cancer, but 93.6% of providers thought HPV testing and Pap done together was more accurate than Pap alone for evaluating cervical cancer risk. A smaller number, but still the majority (73.3%), thought HPV testing alone, followed by Pap smear for follow up of patients with a positive HPV test was an accurate method for cervical cancer screening. Providers age 40 or older were significantly more likely than those under age 40 to agree with HPV testing alone being an accurate screening method (81.1% vs. 64.6%, p=.03). Knowledge of the use of self-collection for HPV testing was limited among both samples of providers. Only one provider in the qualitative sample briefly mentioned self-collection as a component (and potential advantage) of HPV testing. Quantitative findings showed that only 21.3% of providers were aware of the option of self-collection for HPV testing. When asked whether, in their medical opinion, self-collection of the HPV test would be a valid method, only 16.8% agreed. A much higher proportion of providers (41.6%) agreed that self-collection of the HPV test would be acceptable to Dominican women.
Starting screening at age 25–30.
Almost all providers in the qualitative sample were uncomfortable with the idea of starting HPV testing at age 25–30, as is recommended by WHO and other international guidelines [10], [11], fearing that this was “too late.” Providers felt that based on the early age at which Dominican women begin their sexual activity women needed to begin screening earlier. In the qualitative sample age of onset of sexual activity mentioned ranged from age 9 to 16. Similarly, in the quantitative sample, the mean of the estimated average age of onset of sexual activity was 14.2 (standard deviation, 1.78), with a range from 10–19 years. In the qualitative sample, those that thought starting screening at age 25–30 could be effective in the Dominican Republic were the minority. In addition, more than one third of providers relayed personal experience with patients who had had invasive cancer before age 25. In their direct clinical experience, starting screening at age 25–30 was therefore unacceptable.
Acceptability to patients.
Most providers in the qualitative sample thought that HPV testing as a screening test would be acceptable to patients. It was thought that women would generally follow their doctor’s recommendations and that women were interested in what was the “best” test, if they could afford it. One pathologist acknowledged that less frequent screening associated with HPV testing would likely be preferable to women. On the other hand, one GYN-oncologist did not think women would find it acceptable to wait until age 25 or 30 to have screening, as they have been acculturated to screening earlier with Pap smears. Providers felt there would be a need to educate the public about HPV testing, including one provider who said there was a need to combat the myth that HPV testing is a test for sexually transmitted infections rather than a cervical cancer screening test.
There were some differences in the quantitative sample as well. Overall 41.6% of providers agreed that “in general Dominican women would accept HPV self-test.” Providers who had practiced for 10 years or more were more likely to disagree with this statement (52.4% vs. 34.6%, p=.009). Providers in the public sector (46.8% vs. 25%, p=.02) and non-specialists (54.0% vs. 34.7% and 43.1% for GYN-specialists and residents respectively, p=.01) were more likely to agree that Dominican women would accept self-testing for HPV tests.
Cost of HPV testing.
The main obstacle providers saw to adoption of HPV testing was cost of the HPV test. Currently most providers offer the HPV test with Pap smear screening to their patients, when indicated for triage of abnormal Pap tests, but many patients cannot afford to pay for the HPV test. Providers were doubtful that the Ministry of Health would have the resources to pay for HPV testing in the public sector and so providers perceived there would continue to be a public and private sector division in terms of accessibility of HPV testing to patients. One provider who has been using HPV testing extensively in the private sector mentioned that HPV testing is beginning to be covered by insurance and that this may change its accessibility for patients.
Potential Impact of Change in Screening Practice
Returning to the qualitative questions of what is working and not working well in the current cervical cancer prevention system in the Dominican Republic, providers were asked in what way a change in screening practice (i.e., the test itself) would address some of the gaps identified. These opinions were somewhat divided. Some providers, particularly some of the pathologists and GYN-oncologists, felt a change in test would address the shortcomings of Pap testing. Other providers felt a change in screening test would not address system problems in cervical cancer screening, nor reach women who were not screened. In fact, one participant feared that a more expensive test would widen the gap even further between screened and unscreened women in the Dominican Republic.
In the quantitative sample, when asked a yes/no question as to whether they would agree with replacing Pap smear with HPV testing for cervical cancer screening, if it were available in all health sectors, slightly more than half (55.9%) of providers said they would not.
Mixed Methods Integration and Interpretation
Joint displays of mixed methods findings and interpretation are presented in Tables 2 and 7. Metainferences regarding provider perspectives on barriers and facilitators to cervical cancer prevention in the Dominican Republic, knowledge/attitudes towards HPV testing, and opinions about the potential impact of a change in screening practice are shown. An assessment of fit (i.e., confirmation, discordance, expansion) of the compared qualitative and quantitative data is also indicated [33]. In most instances, comparison of data sources yielded confirmation or expansion of findings. Regarding attitudes towards HPV testing, there were a few areas of discordance or incomplete comparison.
Discussion
The mixed methods findings of this study indicate that Dominican providers in Region 0 believe that Pap smear screening for cervical cancer is widely available and accessible to women, and that the principal barrier to cervical cancer prevention is a matter of screening utilization. They believe that at the population level many women–particularly women from rural areas and lower socioeconomic strata–remain unscreened because they are not aware of cervical cancer and the need for screening, and if they eventually present to care it is at an advanced stage of disease. A few providers mentioned circumstances in which women might be turned away for screening due to provider schedule or supply constraints, but most focused on the issue of inadequate public education regarding cervical cancer. Cost and availability of follow up after abnormal screening tests are an additional barrier to cervical cancer prevention, mostly in rural areas. On the question of knowledge and attitudes regarding HPV testing, there was limited knowledge among this group of providers as a whole about HPV testing as a stand-alone screening test. Though most did not think a change in testing modality would address current gaps in cervical cancer screening, a minority of providers in the qualitative portion, particularly pathologists and GYN-oncologists who had more extensive knowledge of HPV testing, did think a change in screening was needed in order to impact cervical cancer mortality (CFIR constructs such as relative advantage of HPV testing are explored in more detail elsewhere, manuscript in preparation)
Qualitative findings that socioeconomic, geographic and educational factors affect access to information and utilization of cervical cancer screening, are supported indirectly by the quantitative findings that the majority of providers thought their own patients were aware of the need for screening (in contrast to opinions about the general population). Previous studies have found that having a regular health care provider is an important determinant of whether women are up to date on screening [34], [35].
A focus on lack of knowledge of cervical cancer as the reason women do not get screened underestimates the multi-layered demographic, social and cultural barriers women face in accessing screening services [8], [35]. Women must not only understand the need for screening. They must also balance competing work and childcare priorities with their own health care needs. They must trust the health system and health care providers, and overcome fears regarding the gynecologic exam, the Pap test, and potential abnormal results or cancer diagnosis [8], [34]. If test results are abnormal, women must again negotiate some of the same barriers to seek care for further diagnostic and treatment services. Many demographic and social barriers, such as poverty, lack of education, and racial/ethnic minority status are not readily modifiable. Factors related to health services delivery, including knowledge, attitudes, and practices of health care providers, that influence health care access and utilization, can be addressed.
Limited provider knowledge regarding the HPV test as a cervical cancer screening test limited providers’ ability to comment on what advantages HPV testing would offer. Most providers seemed largely unaware of the potential benefits of HPV testing: the possibility of reaching unscreened women through self-collected samples [18], and the superior test performance allowing an extended interval following a negative screening test and decreasing the volume of screening tests to be done within an individual and the population as a whole [11].
This study was strengthened by use of the CFIR to understand not only provider perspectives on cervical cancer prevention in the Dominican Republic but also provider-level barriers and facilitators to any future change in cervical cancer screening. The study was limited by small sample size in a single region of the Dominican Republic. In addition, a single phase of data collection meant that survey adaptation could not be informed by qualitative findings, which might have created more country-specific items for the closed-ended questions on barriers to cervical cancer prevention. Mixed method findings and initial trial of the survey questionnaire could be used to refine the survey instrument for a future study of providers at the national level in the Dominican Republic. Despite these limitations, this study complements previous findings on patient-perceived barriers to cervical cancer prevention in the Dominican Republic and provides important information regarding provider perspectives on existing systems and potential innovations for cervical cancer screening in the Dominican Republic. These findings may inform the development of physician education activities regarding evolving global guidelines for improving cervical cancer prevention efforts.
Acknowledgements:
INCART for study support, Marija Miric from O&M Medical School for consultation regarding recruitment, Mildred Martinez for fieldwork consultation and coordination
Funding: This research was supported in part by the NYU CTSA grant TL1 TR001447 from the National Center for Advancing Translational Sciences, National Institutes of Health.
Additional support was received through New York University Rory Meyers College of Nursing Fred Schmidt and Paula Greenidge Scholarships and the Sigma Theta Tau Upsilon Chapter Research Grant
Footnotes
Conflict of Interest: The authors declare that they have no conflict of interest
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Contributor Information
Erica Liebermann, New York University Rory Meyers College of Nursing 433 First Avenue, 6th floor, New York, NY 10010.
Nancy Van Devanter, New York University Rory Meyers College of Nursing, 433 First Avenue, Rm 670, New York, NY 10010.
Natalia Frías Gúzman, Instituto Nacional de Cáncer Rosa Emilia Sánchez Pérez de Tavares (INCART) Avenida Correa y Cidrón Santo Domingo, Dominican Republic 10103.
Marilyn J Hammer, The Phyllis F. Cantor Center for Research in Nursing and Patient Care Services Dana-Farber Cancer Institute 450 Brookline Avenue, LW523 Boston, MA 02215.
Danielle Ompad, New York University College of Global Public Health, 715 Broadway, Rm 1011, New York, NY 10003.
References
- [1].Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, and Jemal A, “Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries,” CA. Cancer J. Clin, vol. 68, no. 6, pp. 394–424, 2018, doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- [2].Jeronimo J, Holme F, Slavkovsky R, and Camel C, “Implementation of HPV testing in Latin America,” J. Clin. Virol. Off. Publ. Pan Am. Soc. Clin. Virol, vol. 76 Suppl 1, pp. S69–73, March. 2016, doi: 10.1016/j.jcv.2015.11.035. [DOI] [PubMed] [Google Scholar]
- [3].Herrero R. et al. , “New Approaches to Cervical Cancer Screening in Latin America and the Caribbean,” Vaccine, vol. 26, Supplement 11, pp. L49–L58, August. 2008, doi: 10.1016/j.vaccine.2008.05.025. [DOI] [PubMed] [Google Scholar]
- [4].Ogilvie G, Nakisige C, Huh WK, Mehrotra R, Franco EL, and Jeronimo J, “Optimizing secondary prevention of cervical cancer: Recent advances and future challenges,” Int. J. Gynecol. Obstet, vol. 138, pp. 15–19, July. 2017, doi: 10.1002/ijgo.12187. [DOI] [PubMed] [Google Scholar]
- [5].Sankaranarayanan R, Thara S, Esmy PO, and Basu P, “Cervical cancer: screening and therapeutic perspectives,” Med. Princ. Pract. Int. J. Kuwait Univ. Health Sci. Cent, vol. 17, no. 5, pp. 351–364, 2008, doi: 10.1159/000141498. [DOI] [PubMed] [Google Scholar]
- [6].Murillo R, “[Cervical cancer control in Colombia: achievements and challenges of cytology based programs],” Bioméd. Rev. Inst. Nac. Salud, vol. 28, no. 4, pp. 467–470, December. 2008. [PubMed] [Google Scholar]
- [7].Villa LL, “Cervical Cancer in Latin America and the Caribbean: The Problem and the Way to Solutions,” Cancer Epidemiol. Biomarkers Prev, vol. 21, no. 9, pp. 1409–1413, September. 2012, doi: 10.1158/1055-9965.EPI-12-0147. [DOI] [PubMed] [Google Scholar]
- [8].Agurto I, Bishop A, Sánchez G, Betancourt Z, and Robles S, “Perceived barriers and benefits to cervical cancer screening in Latin America.,” Prev. Med, vol. 39, no. 1, pp. 91–98 8p, July. 2004, [Online]. Available: http://search.ebscohost.com/login.aspx?direct=true&db=rzh&AN=106673491&site=ehost-live. [DOI] [PubMed] [Google Scholar]
- [9].Paolino M, Sankaranarayanan R, and Arrossi S, “[Social determinants of dropout from diagnosis and treatment by women with abnormal Pap smears in Buenos Aires, Argentina],” Rev. Panam. Salud Pública Pan Am. J. Public Health, vol. 34, no. 6, pp. 437–445, December. 2013. [PubMed] [Google Scholar]
- [10].World Health Organization, “WHO | Guidelines for screening and treatment of precancerous lesions for cervical cancer prevention,” WHO, 2014. http://apps.who.int/rhl/guidelines/screening_and_treatment_of_precancerous_lesions/en/(accessed May 30, 2016). [PubMed] [Google Scholar]
- [11].Jeronimo J. et al. , “Secondary Prevention of Cervical Cancer: ASCO Resource-Stratified Clinical Practice Guideline,” J. Glob. Oncol, p. JGO006577, 2016, Accessed: Oct. 25, 2016. [Online]. Available: http://jgo.ascopubs.org/content/early/2016/10/08/JGO.2016.006577.abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Denny L. et al. , “Series: Interventions to close the divide for women with breast and cervical cancer between low-income and middle-income countries and high-income countries,” The Lancet, vol. 389, pp. 861–870, February. 2017, doi: 10.1016/S0140-6736(16)31795-0. [DOI] [PubMed] [Google Scholar]
- [13].Alemany L. et al. , “Time trends of human papillomavirus types in invasive cervical cancer, from 1940 to 2007,” Int. J. Cancer, vol. 135, no. 1, pp. 88–95, July. 2014, doi: 10.1002/ijc.28636. [DOI] [PubMed] [Google Scholar]
- [14].Brotherton JML et al. , “Eurogin Roadmap 2015: How has HPV knowledge changed our practice: vaccines,” Int. J. Cancer J. Int. Cancer, February. 2016, doi: 10.1002/ijc.30063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Dillner J. et al. , “Long term predictive values of cytology and human papillomavirus testing in cervical cancer screening: joint European cohort study,” BMJ, vol. 337, no. oct13 1, pp. a1754–a1754, October. 2008, doi: 10.1136/bmj.a1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Cuzick J. et al. , “Overview of Human Papillomavirus-Based and Other Novel Options for Cervical Cancer Screening in Developed and Developing Countries,” Vaccine, vol. 26, pp. K29–K41, August. 2008, doi: 10.1016/j.vaccine.2008.06.019. [DOI] [PubMed] [Google Scholar]
- [17].Arrossi S. et al. , “Effect of self-collection of HPV DNA offered by community health workers at home visits on uptake of screening for cervical cancer (the EMA study): a population-based cluster-randomised trial,” Lancet Glob. Health, vol. 3, no. 2, pp. e85–94, February. 2015, doi: 10.1016/S2214-109X(14)70354-7. [DOI] [PubMed] [Google Scholar]
- [18].Arrossi S, Paolino M, Thouyaret L, Laudi R, and Campanera A, “Evaluation of scaling-up of HPV self-collection offered by community health workers at home visits to increase screening among socially vulnerable under-screened women in Jujuy Province, Argentina,” Implement. Sci, vol. 12, no. 1, December. 2017, doi: 10.1186/s13012-017-0548-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, and Lowery JC, “Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science,” Implement. Sci, vol. 4, pp. 50–50 1p, January. 2009, doi: 10.1186/1748-5908-4-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].ENDESA, “República Dominicana Encuesta Demográfica y de Salud 2013 [FR292]-FR292.pdf,” 2013. http://dhsprogram.com/pubs/pdf/FR292/FR292.pdf (accessed Mar. 31, 2016).
- [21].Liebermann EJ et al. , “Barriers to Cervical Cancer Screening and Treatment in the Dominican Republic: Perspectives of Focus Group Participants in the Santo Domingo Area,” J. Transcult. Nurs, p. 104365961984624, May 2019, doi: 10.1177/1043659619846247. [DOI] [PubMed] [Google Scholar]
- [22].Stormo AR et al. , “Findings and lessons learned from a multi-partner collaboration to increase cervical cancer prevention efforts in Bolivia,” Rural Remote Health, vol. 13, no. 4, p. 2595, December. 2013. [PMC free article] [PubMed] [Google Scholar]
- [23].Stormo AR, de Moura L, and Saraiya M, “Cervical cancer-related knowledge, attitudes, and practices of health professionals working in brazil’s network of primary care units,” The Oncologist, vol. 19, no. 4, pp. 375–382, April. 2014, doi: 10.1634/theoncologist.2013-0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Townsend J, Stormo A, Roland K, Buenconsejo-Lum L, White S, and Saraiya M, “Current Cervical Cancer Screening Knowledge, Awareness, and Practices Among US Affiliated Pacific Island Providers: Opportunities and Challenges,” ONCOLOGIST, vol. 19, no. 4, pp. 383–393, April. 2014, Accessed: Nov. 21, 2016. [Online]. Available: http://ezproxy.library.nyu.edu:2048/login?url=http://search.ebscohost.com/login.aspx?direct=true&db=edswsc&AN=000334401300016&site=eds-live. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Aldrich T, “Mexican physicians’ knowledge and attitudes about the human papillomavirus and cervical cancer: a national survey,” Sex. Transm. Infect, vol. 81, no. 2, pp. 135–141, April. 2005, doi: 10.1136/sti.2003.008557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Creswell JW and Plano Clark VL, Designing and conducting mixed methods research, 2nd ed. Los Angeles: SAGE Publications, 2011. [Google Scholar]
- [27].Hsieh H-F and Shannon SE, “Three Approaches to Qualitative Content Analysis,” Qual. Health Res, vol. 15, no. 9, pp. 1277–1288, November. 2005, doi: 10.1177/1049732305276687. [DOI] [PubMed] [Google Scholar]
- [28].Miles MB, Huberman AM, and Saldaña J, Qualitative data analysis: a methods sourcebook, Third edition. Thousand Oaks, Califorinia: SAGE Publications, Inc, 2014. [Google Scholar]
- [29].Patton MQ, Qualitative research & evaluation methods: integrating theory and practice, Fourth edition. Thousand Oaks, California: SAGE Publications, Inc, 2015. [Google Scholar]
- [30].Stormo AR et al. , “Bolivian health providers’ attitudes toward alternative technologies for cervical cancer prevention: a focus on visual inspection with acetic acid and cryotherapy,” J. Womens Health 2002, vol. 21, no. 8, pp. 801–808, August. 2012, doi: 10.1089/jwh.2012.3796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Hilton A. and Skrutkowski M, “Translating instruments into other languages: development and testing processes,” Cancer Nurs., vol. 25, no. 1, pp. 1–7, 2002. [DOI] [PubMed] [Google Scholar]
- [32].Maneesriwongul W. and Dixon JK, “Instrument translation process: a methods review,” J. Adv. Nurs, vol. 48, no. 2, pp. 175–186, October. 2004, doi: 10.1111/j.1365-2648.2004.03185.x. [DOI] [PubMed] [Google Scholar]
- [33].Fetters MD, Curry LA, and Creswell JW, “Achieving Integration in Mixed Methods Designs-Principles and Practices,” Health Serv. Res, vol. 48, no. 6pt2, pp. 2134–2156, December. 2013, doi: 10.1111/1475-6773.12117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Liebermann EJ, VanDevanter N, Hammer MJ, and Fu MR, “Social and Cultural Barriers to Women’s Participation in Pap Smear Screening Programs in Low-and Middle-Income Latin American and Caribbean Countries: An Integrative Review,” J. Transcult. Nurs, p. 1043659618755424, 2018. [DOI] [PubMed] [Google Scholar]
- [35].Soneji S. and Fukui N, “Socioeconomic determinants of cervical cancer screening in Latin America.,” Determinantes Socioeconómicos Las Pruebas Detección Sist. Cáncer Cervicouterino En América Lat., vol. 33, no. 3, pp. 174–182, March. 2013, [Online]. Available: http://ezproxy.library.nyu.edu:2048/login?url=http://search.ebscohost.com/login.aspx?direct=true&db=her&AN=91095653&site=eds-live. [DOI] [PMC free article] [PubMed] [Google Scholar]