Abstract
All meals come to an end. This is because eating and drinking generate feedback signals that communicate to the brain what and how much has been consumed. Here we review our current understanding of how these feedback signals regulate appetite. We first describe classic studies that surgically manipulated the gastrointestinal tract and measured the effects on behavior. We then highlight recent experiments that have used in vivo neural recordings to directly observe how ingestion modulates circuit dynamics in the brain. A general theme emerging from this work is that eating and drinking generate layers of feedback signals, arising sequentially from different tissues in the body, that converge on individual neurons in the forebrain to regulate hunger and thirst.
Introduction
Eating and drinking are fast processes, but their physiologic effects are slow and delayed. For example, drinking can quench thirst in just a few minutes, even though tens of minutes are required for the ingested water to be absorbed into the bloodstream and reestablish fluid balance [1–3]. This phenomenon—loss of appetite before ingested food or water is absorbed—is known as satiation, and implies that the brain uses pre-absorptive signals to dynamically control eating and drinking.
In this review, we summarize recent progress towards understanding how pre-absorptive feedback signals regulate appetite. We discuss the various kinds of sensory information that are encoded by these signals, where and when they are represented in the brain, and how they influence behavior. To organize this discussion, we first describe classic experiments that surgically manipulated the gut and measured the effects on behavior. These experiments led to the hypothesis that an interaction between pre-gastric and gastrointestinal feedback is responsible for the tight control of eating and drinking. We then review recent work that has used techniques for neural recording to observe these feedback signals directly, as they emerge sequentially in the brains of behaving animals.
Sham ingestion revealed the pre-gastric and gastrointestinal controls on appetite
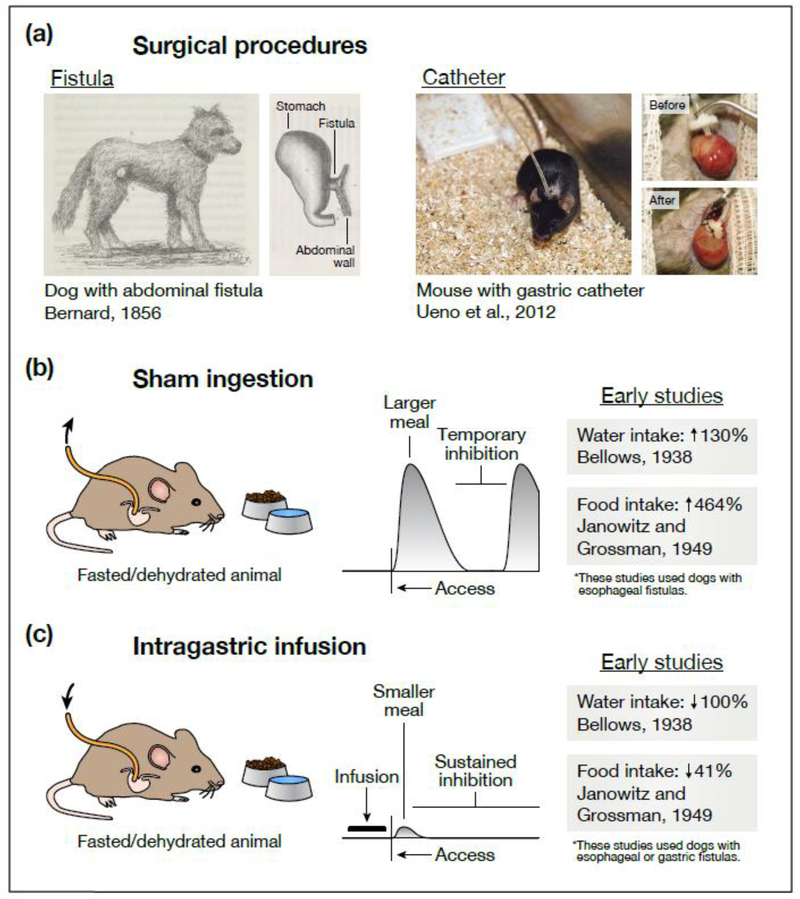
The key role of pre-absorptive feedback signals in satiation was first established by experiments using sham feeding and drinking (Figure 1). In this procedure, an opening (fistula) is surgically created in the esophagus, stomach, or intestines so that any ingested food or water drains from the animal before it can pass further. As early as 1856, Claude Bernard described such a sham drinking experiment in a thirsty dog equipped with a gastric fistula [4]. He reported that the dog drank voraciously when given water, but that “… the thirst was not abated. The animal … drank until it was fatigued. A moment later, when he had rested, he started again, and so on” (p. 50–51, ref. [4]). This revealed that pre-gastric signals alone cannot durably satiate thirst.
Figure 1. Effects of gastrointestinal manipulations on eating and drinking behavior.
(a) Two experimental techniques—sham ingestion and intragastric infusion—allow investigators to manipulate the contents of the gastrointestinal tract during eating and drinking. Today, both techniques frequently rely on a surgical procedure called catheterization, in which one end of a catheter is permanently attached to an animal’s stomach while the other end remains outside the body. Earlier experiments—often employing dogs or other large animals—used a similar procedure called fistulization, in which a portion of the esophagus, stomach, or intestines is ruptured and surgically attached to the animal’s exterior (either directly or via a steel cannula) so that tubing can be temporarily attached during experiments. (b) During sham feeding or drinking, the external end of the catheter is left open so that any ingested food or water drains from the animal before reaching the stomach. This has two striking effects. First, animals consume much larger meals than they would during normal ingestion. Second, while animals will eventually pause eating or drinking, this inhibition is only transient. These observations suggest that pre-gastric signals alone can produce temporary (but not persistent) satiation. (c) During intragastric infusion, the external end of the catheter is used to infuse food or water directly into the stomach, bypassing the mouth and throat altogether. Infusions into hungry or thirsty animals dramatically (sometimes completely) reduce intake when food or water is subsequently presented. This suggests that post-ingestive signals are sufficient to durably satiate hunger and thirst. However, the exact nature of these signals (what sensory information they convey, where in the gut they are generated) cannot be determined from behavioral observations alone. In (b), the listed water intake is the volume sham drunk (fistula open) relative to the volume drunk normally (fistula closed) [2], and the listed food intake is the time spent sham feeding (after fistulization) relative to the time spent feeding normally (before fistulization) [5]. In (c), the listed water intake is the volume sham drunk after intragastric infusion (water was presented 20 min after the dog’s entire fluid deficit was infused; the dog refused to drink) relative to the volume sham drunk without prior infusion [2], and the listed food intake is the amount eaten normally after intragastric infusion (food was presented 20 min after ∼43% of the dogs’ normal meal size was infused) relative to the amount eaten without prior infusion [5]. In the schematics in (b) and (c), the horizontal axis represents time and the vertical axis represents rate of ingestion. These schematics are based on many of the references cited, although it is important to note that they are generalizations and that observations have varied across studies and species. We direct the reader to Chapters 2–4 of ref. [78] and Chapter 19 of ref. [79] for a more thorough discussion. The illustrations in (a) are adapted with permission from Figures 27 and 56 of ref. [4] (public domain, https://gallica.bnf.fr/ark:/12148/bpt6k6214318x) and from Figures 2 and 3 of ref. [80] (copyright 2012, Springer Nature).
In the 1930s, Roland Bellows and Edward Adolph repeated Bernard’s experiment, this time using quantitative measurements of behavior [1,2]. They found that dogs engaging in sham drinking exhibited a distinctive behavior, in which they would rapidly drink a large volume of water and then pause for 10–30 minutes before recommencing drinking. This cycle of drinking and pausing led Bellows to hypothesize that there are “two factors concerned in the satisfaction of thirst”: a fast signal that arises from the mouth or throat and temporarily quenches thirst when water is drunk, and a slower signal that arises from elsewhere in the body and enables the durable suppression of thirst (p. 96–97, ref. [2]).
A decade later, Henry Janowitz and Morton Grossman [5] made strikingly similar observations with sham feeding in dogs. They showed that dogs with open esophageal fistulas could temporarily suppress their hunger by simply chewing and swallowing food, even though the food would drain out of the fistula. However, the dogs ate much less food if the fistula was closed and the food allowed to enter the stomach. Later experiments showed that this pre-gastric inhibition of feeding underwent extinction if animals sham fed on successive days [6], implying that it is learned based on post-ingestive feedback. These and subsequent behavioral studies [7–14] suggested that the satiation of hunger and thirst follow a similar logic. First, temporary satiation is produced during ingestion by rapid pre-gastric (oropharyngeal or exterosensory) signals. Later, more durable satiety is produced by signals arising from the stomach, intestines, or post-absorptive tissues.
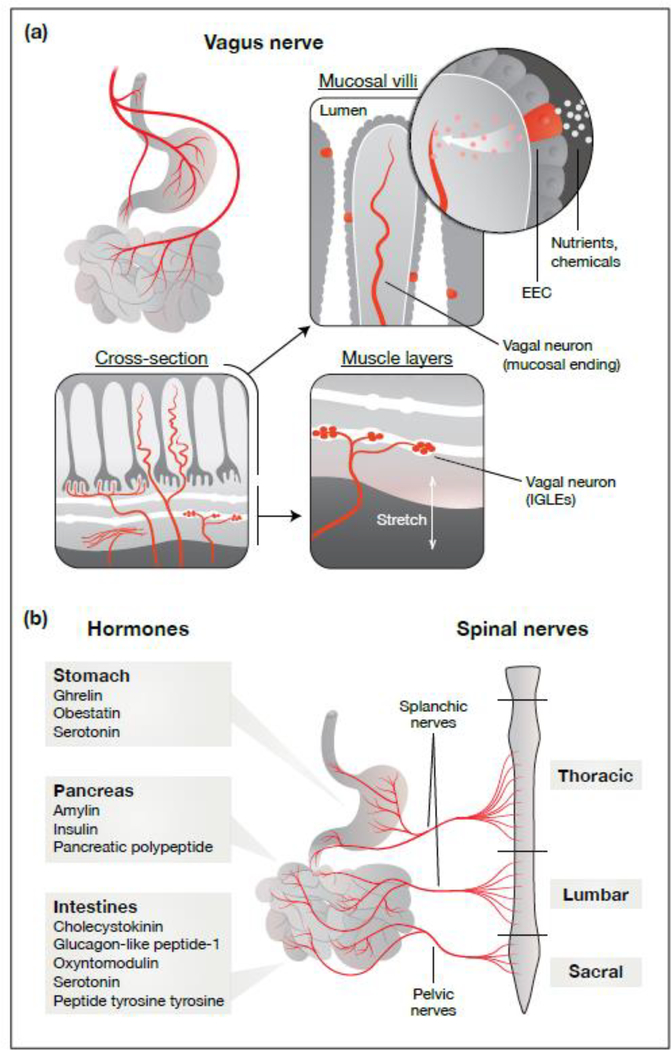
The precise identity of the pre-gastric mechanisms that drive satiation remains unclear, although gustatory, olfactory, somatosensory, and visual cues are all likely involved. For gastrointestinal feedback, two mechanisms have received the most attention [15] (Figure 2). The first centers around enteroendocrine cells (EECs), which are rare epithelial cells that reside in the mucosal layer of the gastrointestinal tract [16]. EECs express on their surface a variety of transporters and receptors that sample the chemical composition of the gut lumen and, upon detection of nutrients or other substances, trigger the release of hormones such as cholecystokinin (CCK), glucagon-like peptide-1 (GLP1), peptide tyrosine tyrosine (PYY), and serotonin [17]. These hormones feedback to inhibit food intake and modulate autonomic reflexes such as gastric emptying. This feedback is mediated by a combination of endocrine effects on the brain, paracrine effects on nearby tissues and nerve fibers, and direct synaptic connections between EECs and the adjacent axon terminals of sensory neurons [18–20].
Figure 2. Pathways for gut–brain communication.
(a) The vagus nerve densely innervates the gastrointestinal tract and transmits sensory information to the brain. Vagal afferents are characterized by the types of sensory endings that they form in the gut, as well as by their gene expression profiles and response properties. Mechanosensitive vagal afferents innervate the muscle layers of the stomach and intestines, where they form IGLEs and IMAs to directly detect distension. In contrast, chemosensitive vagal afferents predominantly innervate the mucosa, where they monitor nutrients and other chemical signals via communication with nearby EECs. (b) Ingestion triggers the release of hormones by sensory cells, such as EECs, throughout the gastrointestinal tract. These hormones are released in response to specific nutrient, chemical, and other stimuli, and can directly influence local tissues and nerve fibers (paracrine effects) as well as distant tissues like the brain (endocrine effects). In addition to the vagus nerve and the endocrine action of hormones, information from the gut is also transmitted to the brain by spinal nerves. However, the gastrointestinal signals that are encoded by subtypes of spinal afferents—and how those signals might influence ingestion—have not been well characterized. Abbreviations: EECs, enteroendocrine cells; IGLEs, intraganglionic laminar endings; IMAs, intramuscular arrays.
In addition to EECs, vagal afferents comprise a second important class of sensory cells that regulate satiation. Vagal afferents have cell bodies located in the nodose ganglion and axons that bifurcate into two branches, one of which innervates the abdominal viscera and the other of which terminates in the nucleus of the solitary tract and area postrema of the brainstem [21]. Vagal sensory neurons are robustly activated by both mechanical and chemical signals associated with ingestion [22], and stimulation of vagal afferents can inhibit food intake [23–25]. Mechanical signals, such as distension of the stomach or intestines, directly activate vagal neurons that have specialized sensory endings known as intraganglionic laminar endings (IGLEs) and intramuscular arrays (IMAs), while chemical signals are thought to activate vagal neurons primarily indirectly via communication with EECs in the mucosal villi [21]. Recent work has begun to identify genetic markers for subtypes of vagal neurons that detect specific chemical and mechanical signals and have distinct patterns of visceral innervation [25,26].
Like the vagus nerve, spinal afferents innervate the abdominal viscera and therefore represent a third potential mechanism for gastrointestinal feedback during eating and drinking. However, the diversity of gut-innervating spinal afferents is not well characterized, and key questions about how they might regulate appetite—including what signals they encode and how that information is transmitted to the brain to influence behavior—remain largely unexplored.
Neural control of appetite: from inference to observation
Our understanding of eating and drinking has been deeply influenced by experiments that measured the effects of gastrointestinal manipulations on behavior (Figure 1). Yet this strategy cannot teach us everything we want to understand about the regulation of appetite, because behavior is many steps removed from the underlying neural processes. This creates three challenges.
The first challenge relates to specificity. When a manipulation inhibits food intake, we often do not know why an animal has decided not to eat. This could be due to a specific change in a satiation-promoting feedback signal or have a more general cause (such as malaise or fatigue), and behavior alone cannot always distinguish between these possibilities. A second challenge relates to redundancy. Ablation of most gastrointestinal hormones has little or no behavioral phenotype [27], presumably because eating and drinking are robust to loss of individual feedback signals. How do we determine what information these individual signals are conveying under physiologic conditions? A third challenge relates to timing. Animals eat and drink at variable rates, and for this reason ingestive behavior must often be measured over tens of minutes to detect a meaningful effect of a perturbation. Yet on this timescale eating and drinking can generate numerous different feedback signals that arise in close succession. How can we disentangle these layers of rapid feedback?
One way to address these challenges is to monitor the activity of the neurons that control appetite, and thereby observe directly the information that the brain receives during eating and drinking. Traditionally, Fos immunohistochemistry has been an important method to do this, enabling brain-wide visualization of neural responses to gastrointestinal manipulations and identification of relevant brain regions [28–30]. However, Fos histology is limited by the fact that it is slow (integrates neural activity over tens of minutes), unidirectional (only detects activation), and enables only one measurement per animal. For this reason, there is the potential for considerable additional insight by using methods that record neural activity in real-time and during behavior.
Microdialysis measurements of dopamine release
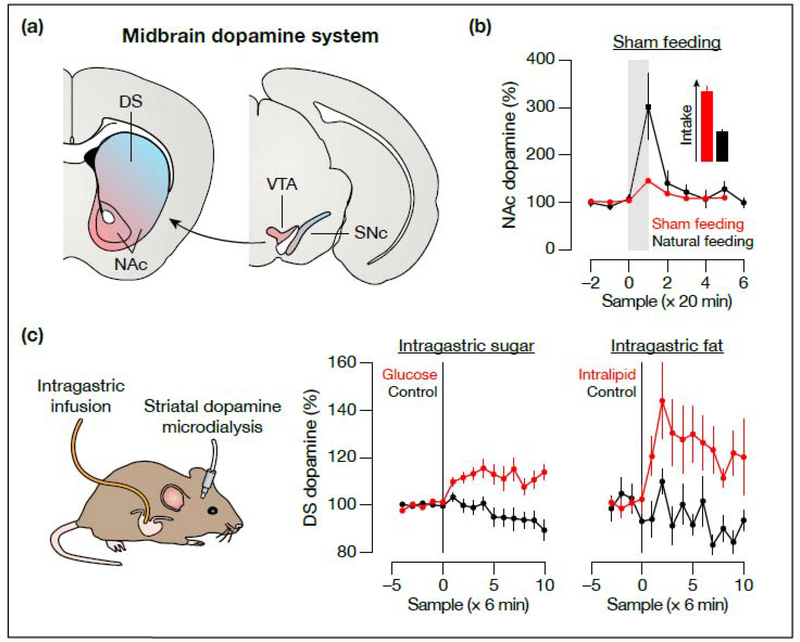
Eating [31,32] and drinking [33,34] stimulate the release of dopamine in the striatum. This dopamine release is mediated by exterosensory cues, such as the sight and smell of food [35,36], and oropharyngeal signals, such as sweet taste [37,38] or mouth wetness [39]. However, the fact that sham feeding stimulates less dopamine release than natural feeding [37,40,41] implies that post-ingestive signals are also involved.
To investigate how gastrointestinal signals modulate the dopamine system, several studies have monitored striatal dopamine levels by microdialysis while infusing nutrients into the stomachs of behaving mice [42–4 5] (Figure 3). Although microdialysis is slow (temporal resolution of several minute), it has the important advantage relative to Fos in that it enables continuous recordings in a single animal [46]. These experiments showed that intragastric infusion of sugars [42,45] or fats [43,44] causes a rapid increase in dopamine in the dorsal striatum (DS). In contrast, a broader array of signals—including exterosensory [35,36], oral [37,38,41], and post-ingestive sugar [38,42,45] cues—appears to drive dopamine release in the adjacent nucleus accumbens (NAc) during feeding, suggesting that the DS may be more specifically involved in gastrointestinal nutrient responses. These effects of nutrients on dopamine release are proposed to be transmitted by the vagus nerve and to mediate the rewarding effects of post-ingestive nutrient detection [24]. Recent work using optical dopamine sensors [47,48] has reproduced some of these microdialysis findings at higher temporal resolution [49].
Figure 3. Microdialysis measurements of striatal dopamine release.
(a) Coronal representation of the midbrain dopamine system. The colormap highlights the broad anatomical organization of mesostriatal dopamine projections, although it is noteworthy that dopamine neurons—including their inputs, outputs, and response properties—are very heterogeneous. (b) In this experiment, hungry rats were given access to a sugar (sucrose) solution for 20 min while dopamine release was measured by microdialysis. Natural feeding stimulates intense dopamine release [40], while sham feeding results in a much smaller increase (despite greater intake) [37]. Similar results were obtained with rats sham feeding a fat (corn oil) solution [41]. These observations suggest that post-ingestive signals must contribute to dopamine release during feeding. (c) Left: In hungry mice, intragastric infusion of sugar rapidly stimulates dopamine release [42,45]. Right: Intragastric infusion of fat has a similar effect [43,44]. These experiments showed the first gut-to-brain sensory responses in behaving animals, and demonstrated that nutrient signals in particular drive dopamine release in the DS. The gray shaded area in (b) indicates the availability of food, and the black vertical lines in (c) indicate the start of intragastric infusion. The data in (b) are adapted with permission from Figure 1 of ref. [40] (copyright 2001, Elsevier) and from Figure 3 of ref. [37] (copyright 2004, American Physiological Society). The data in (c) are adapted with permission from Figure S3 of ref. [44] (copyright 2013, American Association for the Advancement of Science) and from Figure S1 of ref. [45] (copyright 2016, Springer Nature). Abbreviations: DS, dorsal striatum; NAc, nucleus accumbens; SNc, substantia nigra pars compacta; VTA, ventral tegmental area.
Dopamine is critical for helping animals learn to associate foods with their post-ingestive consequences [50,51], and in this way dopamine microdialysis has provided important insight into how animals develop preferences for foods and bias feeding choices across meals. On the other hand, dopamine does not appear to play a critical role in the generation of hunger or thirst or in the suppression of those drives by eating and drinking [52]. Investigation of these processes requires monitoring neural circuits with a more specific role in appetite.
Optical recordings of hypothalamic hunger neurons
Early electrophysiological recordings found neurons in several hypothalamic nuclei that respond to gastrointestinal signals [53–55], but interpretation of these findings was limited by an inability to determine the identity or function of the recorded neurons, or to monitor their activity in awake animals. A breakthrough came with the development of fiber photometry [56,57] and microendoscope imaging [58], two approaches that made it possible for the first time to monitor the activity of genetically-defined neurons deep in the brains of awake, behaving mice.
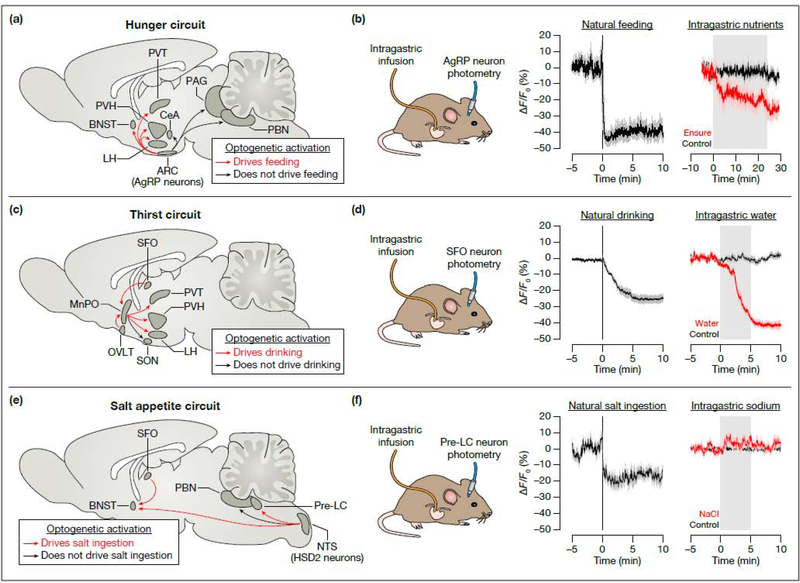
These tools were first applied to agouti-related peptide (AgRP) neurons, a small population of hypothalamic neurons that are activated by food deprivation and critical for hunger [59,60]. Based on their sensitivity to hormones such as leptin, it was long assumed that the activity of AgRP neurons would gradually fluctuate in unison with changes in circulating hormones and nutrients. However, in vivo recordings revealed that these neurons are instead rapidly inhibited the moment that a hungry animal sees and smells food [61–63]. This rapid, pre-gastric inhibition predicts how much food an animal will eat in the forthcoming meal [64], suggesting that AgRP neurons can anticipate the physiologic effects of impending behavior.
The rapid inhibition of AgRP neurons by the sight and smell of food is reversed if the food is not consumed [61–63], implying it is contingent on subsequent post-ingestive signals. To test this directly, AgRP neurons were monitored while nutrients were infused directly into the stomachs of behaving mice, thereby bypassing any oral or exterosensory cues [64,65] (Figure 4a,b). This revealed that intragastric nutrients inhibit AgRP neurons on a timescale of minutes as they are progressively infused into the stomach, in a manner proportional to their caloric content but largely independent of their macronutrient identity. On longer timescales (tens-of-minutes to hours) AgRP neurons are modulated by the hormone leptin [64], which circulates at levels proportional to body fat stores. Thus, AgRP neurons receive layers of signals that report on future, current, and past ingestive behavior, which they integrate to estimate the animal’s need for energy.
Figure 4. Optical recordings of neurons that control hunger, thirst, and salt appetite.
(a) Sagittal representation of the brain’s hunger circuit, highlighting the projections of AgRP neurons. (b) Left: AgRP neurons are rapidly inhibited when hungry mice are presented with food, before feeding begins [61]. Right: AgRP neurons are rapidly inhibited by intragastric infusion of the liquid diet Ensure [64], which is a complex mixture of fats, proteins, and sugars. (c) Sagittal representation of the brain’s thirst circuit, highlighting the projections from the lamina terminalis (SFO, MnPO, and OVLT). (d) Left: SFO thirst neurons are rapidly inhibited when thirsty mice drink water, in a manner that is time-locked to ingestion [3]. Right: SFO thirst neurons are rapidly inhibited by intragastric infusion of water, and are activated by infusion of hypertonic solutions (not shown) [70]. (e) Sagittal representation of the brain’s salt appetite circuit, highlighting the projections of aldosterone-sensitive HSD2 neurons. The pre-LC neurons that drive salt appetite project broadly throughout the brain (including to the BNST), although the functions of these projections have not yet been annotated. (f) Left: Pre-LC neurons are rapidly inhibited when salt-deprived mice drink sodium solutions [73]. Right: In contrast, pre-LC neurons are not modulated by intragastric infusion of NaCl [73]. The black vertical lines in (b), (d), and (f) indicate the start of food, water, or salt (0.15 M NaCl) availability, and the gray shaded areas indicate the duration of intragastric infusion. The data in (b) are adapted with permission from Figure 5 of ref. [61] (copyright 2015, Elsevier) and from Figure 1 of ref. [64] (copyright 2017, Elsevier). The data in (d) are adapted with permission from Figure 2 of ref. [3] (copyright 2016, Springer Nature) and from Figure 1 of ref. [70] (copyright 2019, Springer Nature). The data in (f) are adapted with permission from Figure 4 of ref. [73] (copyright 2019, Springer Nature). Abbreviations: AgRP, agouti-related peptide; ARC, arcuate nucleus; BNST, bed nucleus of the stria terminalis; CeA, central amygdala; HSD2, 11β-hydroxysteriod dehydrogenase type 2; LH, lateral hypothalamus; MnPO, median preoptic nucleus; NTS, nucleus of the solitary tract; OVLT, organum vasculosum of the lamina terminalis; PAG, periaqueductal gray; PBN, parabrachial nucleus; pre-LC, pre-locus coeruleus; PVH, paraventricular hypothalamus; PVT, paraventricular thalamus; SFO, subfornical organ; SON, supraoptic nucleus.
The pathway by which nutrient signals from the gut are transmitted to AgRP neurons is unclear but likely involves the enteroendocrine–vagal system. The evidence for this includes the fact that AgRP neurons are inhibited by administration of hormones that are naturally released by EECs (CCK, PYY, serotonin) [64,65] as well as by infusion of nutrients directly into the duodenum (which stimulates release of these same hormones) [25]. Moreover, the inhibition of AgRP neurons by dietary fat requires CCK [64], and surgical vagotomy blocks the ability of CCK or intragastric fat to inhibit AgRP neuron activity [49]. Recently, the role of specific vagal cell types in modulating AgRP neurons has been investigated by stimulating vagal neurons using DREADDs while simultaneously monitoring AgRP neuron activity by fiber photometry [25]. This revealed that AgRP neurons are inhibited by CCK A receptor (CCKAR)-expressing vagal mechanoreceptors innervating the stomach or intestines, but surprisingly not by putative chemosensory vagal afferents that innervate the intestinal mucosa (and express even higher levels of CCKAR) [25]. This revealed that AgRP neurons are sensitive to mechanical signals from the gut. It also suggested that information about nutrients (e.g., through CCK) and meal volume (from distension) that is transmitted to the forebrain may already be integrated at the level of vagal afferents [22].
Thirst circuits integrate signals from the oropharynx, gut, and blood
Thirst is triggered by activation of glutamatergic neurons in the subfornical organ (SFO) and organum vasculosum of the lamina terminalis (OVLT) [66,67]. Because these neurons lie outside the blood–brain barrier and are directly activated by increases in blood osmolarity, it was long assumed that their activity would gradually fluctuate in unison with changes in the blood. However, in vivo neural recordings revealed that thirst-promoting SFO neurons [3] and their downstream targets [68,69] also receive rapid anticipatory signals from the oropharynx during eating and drinking. For example, SFO thirst neurons are progressively inhibited each time a mouse takes a lick of water, in a way that tracks the cumulative volume of water consumed [3]. This pre-gastric modulation allows thirst neurons to anticipate changes in blood osmolarity before ingested water is absorbed and thereby terminate drinking pre-emptively.
The oropharyngeal signal described above allows thirst neurons to track the volume of fluids consumed, but provides no information about their composition [3]. Recent work has shown that this missing information is provided by a signal from the gut that tracks fluid osmolarity [70,71] (Figure 4c,d). For example, SFO thirst neurons and their downstream targets are rapidly inhibited when water is infused into the stomach and are rapidly activated when hypertonic fluids are infused, in direct proportion to the osmolarity of the infused solution [70]. This gastrointestinal modulation functions to control thirst satiation, by either stabilizing the transient inhibition of thirst neurons produced by oropharyngeal signals when water is drunk or by causing thirst neuron activity to rebound when hypertonic fluids are consumed [70]. While the specific cell types and pathways mediating this gut–brain communication are largely unknown, the vagus nerve [70] and key forebrain GABAergic interneurons that suppress thirst [70,71] have been shown to be involved. On longer timescales (tens-of-minutes to hours) ingested fluids can modulate SFO thirst neurons directly, through their effects on the volume and osmolarity of the blood. Thus, forebrain thirst neurons integrate layers of signals that report on ingestion and its physiologic effects in order to generate a running estimate of the body’s need for water.
In contrast to the regulation of thirst, gut osmosensing does not appear to play a major role in salt appetite. Early behavioral studies showed that sodium detection in the mouth, but not the gastrointestinal tract, satiates the innate desire for sodium in salt-deprived animals [72], and fiber photometry recordings have confirmed that salt appetite-promoting neurons in the pre-locus coeruleus (pre-LC) do not respond to intragastric sodium [73] (Figure 4e,f). Recently, key neurons in two other brain regions that control ingestive behavior—the parabrachial nucleus [74,75] and insular cortex [76,77]—have also been observed by calcium imaging, demonstrating that these cells respond to both interoceptive and exterosensory cues. Investigation of the specific gastrointestinal signals that regulate these hindbrain and cortical cell types should generate important new insight in the near future.
Layers of feedback converge on forebrain neurons that control appetite
The experiments described above have made it possible to observe how eating and drinking modulate key neurons that control appetite. A major finding has been that these cells receive layers of feedback signals, which begin the moment that food or water is detected and then emerge sequentially as ingestion proceeds (Figure 4). These signals are remarkably diverse—spanning, for example, the smell of food, the nutrient content of the gut, and the level of body fat reserves—yet are seamlessly integrated within individual neurons to track changes in bodily state. This reveals how the feedback control of appetite is represented in the key circuits that control eating and drinking. Our ability to monitor these cells while systematically manipulating their inputs, whether they arise from within the body or from the outside world, creates an exciting new opportunity to probe the logic underlying ingestive behavior.
Highlights.
Eating and drinking trigger layers of pre-gastric and gastrointestinal feedback
These signals are generated by molecularly-distinct cell types in peripheral tissues
Vagal feedback from the gut drives dopamine release and reinforcement learning
AgRP neurons receive signals reflecting future, current, and past feeding behavior
Thirst neurons integrate feedback signals from the oropharynx, gut, and blood
Acknowledgements
We thank members of the Knight laboratory for comments on the manuscript and Julia Kuhl for illustrations. C.A.Z. acknowledges support from the NSF Graduate Research Fellowship (DGE-1144247), UCSF Discovery Fellowship, Genentech Foundation Predoctoral Fellowship, and NIH National Research Service Award (F31-HL137383). Z.A.K. is a Howard Hughes Medical Institute Investigator and acknowledges support from the New York Stem Cell Foundation, American Diabetes Association Pathway Program, Rita Allen Foundation, McKnight Foundation, Alfred P. Sloan Foundation, Brain and Behavior Research Foundation, Esther A. and Joseph Klingenstein Foundation, UCSF Program for Breakthrough Biomedical Research, and UCSF Nutrition Obesity Research Center. This work was also supported by an NIH Director’s New Innovator Award (DP2-DK109533), R01-DK106399, and R01-NS094781.
Footnotes
Conflict of interest statement
Nothing declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
- 1.Adolph EF: Measurements of water drinking in dogs. Am J Physiol 1938, 125:75–86. [Google Scholar]
- 2.Bellows RT: Time factors in water drinking in dogs. Am J Physiol 1938, 125:87–97. [Google Scholar]
- 3.Zimmerman CA, Lin Y-C, Leib DE, Guo L, Huey EL, Daly GE, Chen Y, Knight ZA: Thirst neurons anticipate the homeostatic consequences of eating and drinking. Nature 2016, 537:680–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bernard C: Leçons de physiologie expérimentale appliquée à la médecine [Lectures on Experimental Physiology Applied to Medicine]. Paris: Baillière; 1856. [Google Scholar]
- 5.Janowitz HD, Grossman MI: Some factors affecting the food intake of normal dogs and dogs with esophagostomy and gastric fistula. Am J Physiol 1949, 159:143–148. [DOI] [PubMed] [Google Scholar]
- 6.Davis JD, Campbell CS: Peripheral control of meal size in the rat: effect of sham feeding on meal size and drinking rate. J Comp Physiol Psychol 1973, 83:379–387. [DOI] [PubMed] [Google Scholar]
- 7.Towbin EJ: Gastric distension as a factor in the satiation of thirst in esophagostamized dogs. Am J Physiol 1949, 159:533–541. [DOI] [PubMed] [Google Scholar]
- 8.Adolph EF: Thirst and its inhibition in the stomach. Am J Physiol 1950, 161:374–386. [DOI] [PubMed] [Google Scholar]
- 9.Mook DG: Oral and postingestional determinants of the intake of various solutions in rats with esophageal fistulas. J Comp Physiol Psychol 1963, 56:645–659. [Google Scholar]
- 10.Young RC, Gibbs J, Antin J, Holt J, Smith GP: Absence of satiety during sham feeding in the rat. J Comp Physiol Psychol 1974, 87:795–800. [DOI] [PubMed] [Google Scholar]
- 11.Blass EM, Hall WG: Drinking termination: interactions among hydrational, orogastric, and behavioral controls in rats. Psychol Rev 1976, 83:356–374. [PubMed] [Google Scholar]
- 12.Deutsch JA, Young WG, Kalogeris TJ: The stomach signals satiety. Science 1978, 201:165–167. [DOI] [PubMed] [Google Scholar]
- 13.Maddison S, Wood RJ, Rolls ET, Rolls BJ, Gibbs J: Drinking in the rhesus monkey: peripheral factors. J Comp Physiol Psychol 1980, 94:365–374. [DOI] [PubMed] [Google Scholar]
- 14.Gibbs J, Maddison SP, Rolls ET: Satiety role of the small intestine examined in sham-feeding rhesus monkeys. J Comp Physiol Psychol 1989, 95:1003–1015. [DOI] [PubMed] [Google Scholar]
- 15.Kim K-S, Seeley RJ, Sandoval DA: Signalling from the periphery to the brain that regulates energy homeostasis. Nat Rev Neurosci 2018, 19:185–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gribble FM, Reimann F: Enteroendocrine cells: chemosensors in the intestinal epithelium. Annu Rev Physiol 2016, 78:277–299. [DOI] [PubMed] [Google Scholar]
- 17.Haber AL, Biton M, Rogel N, Herbst RH, Shekhar K, Smillie C, Burgin G, Delorey TM, Howitt MR, Katz Y, et al. : A single-cell survey of the small intestinal epithelium. Nature 2017, 551:333–339.* This study reported single-cell sequencing of the intestinal epithelium and identified eight genetically distinct subtypes of mature EECs.
- 18.Bohórquez DV, Shahid RA, Erdmann A, Kreger AM, Wang Y, Calakos N, Wang F, Liddle RA: Neuroepithelial circuit formed by innervation of sensory enteroendocrine cells. J Clin Invest 2015, 125:782–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bellono NW, Bayrer JR, Leitch DB, Castro J, Zhang C, O’Donnell TA, Brierley SM, Ingraham HA, Julius D: that couple to sensory neural pathways. Cell 2017, 170:185–198.** This study characterized the chemosensory properties of enterochromaffin cells (an EEC subtype) in the small intestine and colon and showed that these cells form serotonergic synapses with peripheral sensory neurons.
- 20.Kaelberer MM, Buchanan KL, Klein ME, Barth BB, Montoya MM, Shen X, Bohórquez DV: A gut-brain neural circuit for nutrient sensory transduction. Science 2018, 361:eaat5236.** This study showed that nutrient-sensory EECs (CCK cells in the small intestine and PYY cells in the colon) form synapses with vagal and spinal sensory neurons.
- 21.Berthoud H-R, Neuhuber WL: Functional and chemical anatomy of the afferent vagal system. Auton Neurosci 2000, 85:1–17. [DOI] [PubMed] [Google Scholar]
- 22.Schwartz GJ, Moran TH: Sub-diaphragmatic vagal afferent integration of meal-related gastrointestinal signals. Neurosci Biobehav Rev 1996, 20:47–56. [DOI] [PubMed] [Google Scholar]
- 23.Yao G, Kang L, Li J, Long Y, Wei H, Ferreira CA, Jeffery JJ, Lin Y, Cai W, Wang X: Effective weight control via an implanted self-powered vagus nerve stimulation device. Nat Commun 2018, 9:5349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Han W, Tellez LA, Perkins MH, Perez IO, Qu T, Ferreira J, Ferreira TL, Quinn D, Liu Z-W, Gao X-B, et al. : A neural circuit for gut-induced reward. Cell 2018, 175:665–678.* This study showed that activation of vagal sensory neurons broadly innervating the upper gastrointestinal tract suppresses feeding and intragastric fat administration, and drives dopamine release and reinforcement.
- 25.Bai L, Mesgarzadeh S, Ramesh KS, Huey EL, Liu Y, Gray LA, Aitken TJ, Chen Y, Beutler LR, Ahn JS, et al. : Genetic identification of vagal sensory neurons that control feeding. Cell 2019, 179:1129–143.** This study reported a cellular atlas of the vagus nerve that connects the genetic identity of vagal cell types to their anatomy and function. It further showed that stimulation of a vagal subtype that forms IGLE mechanoreceptors innervating the intestines potently suppresses feeding and inhibits AgRP neurons. This revealed a role for intestinal mechanosensation in the regulation of hunger.
- 26.Williams EK, Chang RB, Strochlic DE, Umans BD, Lowell BB, Liberles SD: Sensory neurons that detect stretch and nutrients in the digestive system. Cell 2016, 166:209–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Woods SC, May-Zhang AA, Begg DP: How and why do gastrointestinal peptides influence food intake? Physiol Behav 2018, 193:218–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olson BR, Freilino M, Hoffman GE, Stricker EM, Sved AF, Verbalis JG: c-Fos expression in rat brain and brainstem nuclei in response to treatments that alter food intake and gastric motility. Mol Cell Neurosci 1993, 4:93–106. [DOI] [PubMed] [Google Scholar]
- 29.Carlson SH, Beitz A, Osborn JW: Intragastric hypertonic saline increases vasopressin and central Fos immunoreactivity in conscious rats. Am J Physiol Regul Integr Comp Physiol 1997, 272:R750–R758. [DOI] [PubMed] [Google Scholar]
- 30.Mönnikes H, Lauer G, Bauer C, Tebbe J, Zittel TT, Arnold R: Pathways of Fos expression in locus ceruleus, dorsal vagal complex, and PVN in response to intestinal lipid. Am J Physiol Regul Integr Comp Physiol 1997, 273:R2059–R2071. [DOI] [PubMed] [Google Scholar]
- 31.Hernandez L, Hoebel BG: Feeding and hypothalamic stimulation increase dopamine turnover in the accumbens. Physiol Behav 1988, 44:599–606. [DOI] [PubMed] [Google Scholar]
- 32.Radhakishun FS, van Ree JM, Westerink BHC: Scheduled eating increases dopamine release in the nucleus accumbens of food-deprived rats as assessed with on-line brain dialysis. Neurosci Lett 1988, 85:351–356. [DOI] [PubMed] [Google Scholar]
- 33.Yoshida M, Yokoo H, Mizoguchi K, Kawahara H, Tsuda A, Nishikawa T, Tanaka M: Eating and drinking cause increased dopamine release in the nucleus accumbens and ventral tegmental area in the rat: measurement by in vivo microdialysis. Neurosci Lett 1992, 139:73–76. [DOI] [PubMed] [Google Scholar]
- 34.Young AMJ, Joseph MH, Gray JA: Increased dopamine release in vivo in nucleus accumbens and caudate nucleus of the rat during drinking: a microdialysis study. Neuroscience 1992, 48:871–876. [DOI] [PubMed] [Google Scholar]
- 35.Wilson C, Nomikos GG, Collu M, Fibiger HC: Dopaminergic correlates of motivated behavior: importance of drive. J Neurosci 1995, 15:5169–5178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roitman MF, Stuber GD, Phillips PEM, Wightman RM, Carelli RM: Dopamine operates as a subsecond modulator of food seeking. J Neurosci 2004, 24:1265–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hajnal A, Smith GP, Norgren R: Oral sucrose stimulation increases accumbens dopamine in the rat. Am J Physiol Regul Integr Comp Physiol 2004, 286:R31–R37. [DOI] [PubMed] [Google Scholar]
- 38.de Araujo IE, Oliveira-Maia AJ, Sotnikova TD, Gainetdinov RR, Caron MG, Nicolelis MAL, Simon SA: Food reward in the absence of taste receptor signaling. Neuron 2008, 57:930–941. [DOI] [PubMed] [Google Scholar]
- 39.Fortin SM, Roitman MF: Challenges to body fluid homeostasis differentially recruit phasic dopamine signaling in a taste-selective manner. J Neurosci 2018, 38:6841–6853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hajnal A, Norgren R: Accumbens dopamine mechanisms in sucrose intake. Brain Res 2001, 904:76–84. [DOI] [PubMed] [Google Scholar]
- 41.Liang N-C, Hajnal A, Norgren R: Sham feeding corn oil increases accumbens dopamine in the rat. Am J Physiol Regul Integr Comp Physiol 2006, 291:R1236–R1239. [DOI] [PubMed] [Google Scholar]
- 42.Ren X, Ferreira JG, Zhou L, Shammah-Lagnado SJ, Yeckel CW, de Araujo IE: Nutrient selection in the absence of taste receptor signaling. J Neurosci 2010, 30:8012–8023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ferreira JG, Tellez LA, Ren X, Yeckel CW, de Araujo IE: Regulation of fat intake in the absence of flavour signalling. J Physiol 2012, 590:953–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tellez LA, Medina S, Han W, Ferreira JG, Licona-Limόn P, Ren X, Lam TT, Schwartz GJ, de Araujo IE: A gut lipid messenger links excess dietary fat to dopamine deficiency. Science 2013, 341:800–802. [DOI] [PubMed] [Google Scholar]
- 45.Tellez LA, Han W, Zhang X, Ferreira TL, Perez IO, Shammah-Lagnado SJ, van den Pol AN, de Araujo IE: Separate circuitries encode the hedonic and nutritional values of sugar. Nat Neurosci 2016, 19:465–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zetterström T, Sharp T, Marsden CA, Ungerstedt U: In vivo measurement of dopamine and its metabolites by intracerebral dialysis: changes after d-amphetamine. J Neurochem 1983, 41:1769–1773. [DOI] [PubMed] [Google Scholar]
- 47.Patriarchi T, Cho JR, Merten K, Howe MW, Marley A, Xiong W-H, Folk RW, Broussard GJ, Liang R, Jang MJ, et al. : Ultrafast neuronal imaging of dopamine dynamics with designed genetically encoded sensors. Science 2018, 360:eaat4422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sun F, Zeng J, Jing M, Zhou J, Feng J, Owen SF, Luo Y, Li F, Wang H, Yamaguchi T, et al. : A genetically encoded fluorescent sensor enables rapid and specific detection of dopamine in flies, fish, and mice. Cell 2018, 174:481–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alhadeff AL, Goldstein N, Park O, Klima ML, Vargas A, Betley JN: Natural and drug rewards engage distinct pathways that converge on coordinated hypothalamic and reward circuits. Neuron 2019, 103:891–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sclafani A, Touzani K, Bodnar RJ: Dopamine and learned food preferences. Physiol Behav 2011, 104:64–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.de Araujo IE, Ferreira JG, Tellez LA, Ren X, Yeckel CW: The gut–brain dopamine axis: a regulatory system for caloric intake. Physiol Behav 2012, 106:394–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Palmiter RD: Is dopamine a physiologically relevant mediator of feeding behavior? Trends Neurosci 2007, 30:375–381. [DOI] [PubMed] [Google Scholar]
- 53.Anand BK, Pillai RV: Activity of single neurones in the hypothalamic feeding centres: effect of gastric distension. J Physiol 1967, 192:63–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brimble MJ, Dyball REJ: Characterization of the responses of oxytocin- and vasopressin-secreting neurones in the supraoptic nucleus to osmotic stimulation. J Physiol 1977, 271:253–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Maddison S, Horrell RI: Hypothalamic unit responses to alimentary perfusions in the anesthetised rat. Brain Res Bull 1979, 4:259–266. [DOI] [PubMed] [Google Scholar]
- 56.Cui G, Jun SB, Jin X, Pham MD, Vogel SS, Lovinger DM, Costa RM: Concurrent activation of striatal direct and indirect pathways during action initiation. Nature 2013, 494:238–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gunaydin LA, Grosenick L, Finkelstein JC, Kauvar IV, Fenno LE, Adhikari A, Lammel S, Mirzabekov JJ, Airan RD, Zalocusky KA, et al. : Natural neural projection dynamics underlying social behavior. Cell 2014, 157:1535–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ghosh KK, Burns LD, Cocker ED, Nimmerjahn A, Ziv Y, Gamal AE, Schnitzer MJ: Miniaturized integration of a fluorescence microscope. Nat Methods 2011, 8:871–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sternson SM, Eiselt A-K: Three pillars for the neural control of appetite. Annu Rev Physiol 2017, 79:401–423. [DOI] [PubMed] [Google Scholar]
- 60.Andermann ML, Lowell BB: Toward a wiring diagram understanding of appetite control. Neuron 2017, 95:757–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen Y, Lin Y-C, Kuo T-W, Knight ZA: Sensory detection of food rapidly modulates arcuate feeding circuits. Cell 2015, 160:829–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Betley JN, Xu S, Cao ZFH, Gong R, Magnus CJ, Yu Y, Sternson SM: Neurons for hunger and thirst transmit a negative-valence teaching signal. Nature 2015, 521:180–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mandelblat-Cerf Y, Ramesh RN, Burgess CR, Patella P, Yang Z, Lowell BB, Andermann ML: Arcuate hypothalamic AgRP and putative POMC neurons show opposite changes in spiking across multiple timescales. eLife 2015, 4:e07122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Beutler LR, Chen Y, Ahn JS, Lin Y-C, Essner RA, Knight ZA: Dynamics of gut-brain communication underlying hunger. Neuron 2017, 96:461–475.* This study showed that AgRP neurons are inhibited on a minutes-timescale by gastrointestinal nutrients and EEC-released hormones and on an hours-timescale by leptin. It further showed that CCK is specifically required for the effect of dietary fat on these neurons.
- 65.Su Z, Alhadeff AL, Betley JN: Nutritive, post-ingestive signals are the primary regulators of AgRP neuron activity. Cell Rep 2017, 21:2724–2736.* This study showed that eating caloric (but not non-caloric) foods entrains anticipatory inhibition of AgRP neurons to associated sensory cues like sight and smell.
- 66.Zimmerman CA, Leib DE, Knight ZA: Neural circuits underlying thirst and fluid homeostasis. Nat Rev Neurosci 2017, 18:459–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gizowski C, Bourque CW: The neural basis of homeostatic and anticipatory thirst. Nat Rev Nephrol 2018, 14:11–25. [DOI] [PubMed] [Google Scholar]
- 68.Mandelblat-Cerf Y, Kim A, Burgess CR, Subramanian S, Tannous BA, Lowell BB, Andermann ML: Bidirectional anticipation of future osmotic challenges by vasopressin neurons. Neuron 2017, 93:57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Allen WE, DeNardo LA, Chen MZ, Liu CD, Loh KM, Fenno LE, Ramakrishnan C, Deisseroth K, Luo L: Thirst-associated preoptic neurons encode an aversive motivational drive. Science 2017, 357:1149–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zimmerman CA, Huey EL, Ahn JS, Beutler LR, Tan CL, Kosar S, Bai L, Chen Y, Corpuz TV, Madisen L, et al. : A gut-to-brain signal of fluid osmolarity controls thirst satiation. Nature 2019, 568:98–102..** This study showed that thirst and vasopressin neurons are bidirectionally modulated by gastrointestinal osmolarity, and that this signal controls thirst satiation and involves the vagus nerve. It further showed that individual thirst neurons integrate feedback signals arising from the oropharynx, gut, and blood.
- 71.Augustine V, Ebisu H, Zhao Y, Lee S, Ho B, Mizuno GO, Tian L, Oka Y: Temporally and spatially distinct thirst satiation signals. Neuron 2019, 103:242–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nachman M, Valentino DA: Roles of taste and post-ingestional factors in the satiation of sodium appetite in rats. J Comp Physiol Psychol 1966, 62:280–283. [DOI] [PubMed] [Google Scholar]
- 73.Lee S, Augustine V, Zhao Y, Ebisu H, Ho B, Kong D, Oka Y: Chemosensory modulation of neural circuits for sodium appetite. Nature 2019, 568:93–97.* This study showed that hindbrain salt appetite neurons are inhibited by oral sodium taste but are not modulated by gastrointestinal signals.
- 74.Ryan PJ, Ross SI, Campos CA, Derkach VA, Palmiter RD: Oxytocin-receptor-expressing neurons in the parabrachial nucleus regulate fluid intake. Nat Neurosci 2017, 20:1722–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Campos CA, Bowen AJ, Roman CW, Palmiter RD: Encoding of danger by parabrachial CGRP neurons. Nature 2018, 555:617–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Livneh Y, Ramesh RN, Burgess CR, Levandowski KM, Madara JC, Fenselau H, Goldey GJ, Diaz VE, Jikomes N, Resch JM, et al. : Homeostatic circuits selectively gate food cue responses in insular cortex. Nature 2017, 546:611–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Livneh Y, Sugden AU, Madara JC, Essner RA, Flores VI, Sugden LA, Resch JM, Lowell BB, Andermann ML: Estimation of current and future physiological states in insular cortex. Neuron 2020, 105:1–18.** This study showed that neurons in insular cortex not only respond to food- and water-predicting cues but also track slower changes in internal state. It further showed that the state-dependence of food and water cue responses involves a hypothalamus→amygdala→cortex pathway.
- 78.Smith GP (Ed): Satiation: From Gut to Brain. New York: Oxford University Press; 1998. [Google Scholar]
- 79.Ramsay DJ, Booth DA (Eds): Thirst: Physiological & Psychological Aspects. London: Springer-Verlag; 1991. [Google Scholar]
- 80.Ueno A, Lazaro R, Wang P-Y, Higashiyama R, Machida K, Tsukamoto H: Mouse intragastric infusion (iG) model. Nat Protoc 2012, 7:771–781. [DOI] [PMC free article] [PubMed] [Google Scholar]