Abstract
Background
Hidradenitis suppurativa is an autoinflammatory disorder of keratinization, with dysregulation of T helper type 17 cytokines. Brodalumab is a monoclonal antibody that targets the interleukin (IL) 17 receptor A receptor.
Objectives
To assess the safety and tolerability and clinical response at weeks 12 and 24 of brodalumab in moderate to severe HS. Ten participants with no history of inflammatory bowel disease were administered brodalumab 210 mg/1.5 mL subcutaneously at weeks 0, 1, and 2 and every 2 weeks thereafter until week 24. Participants were assessed for adverse events (grade 2/3 adverse events) and clinical response (Hidradenitis Suppurativa Clinical Response [HiSCR], Sartorius, International Hidradenitis Suppurativa Severity Scoring System [IHS4]), including ultrasonography and skin biopsies.
Results
All 10 participants completed the study. No grade 2/3 adverse events associated with the use of brodalumab were reported. All patients (100%) achieved HiSCR, and 80% achieved IHS4 category change at week 12. HiSCR achievement occurred as early as week 2, likely due to the unique blockade of IL-17A, IL-17C, and IL-17F by brodalumab. Significant improvements were seen in pain, itch, quality of life, and depression.
Conclusions
Brodalumab was well tolerated in this HS cohort, with no serious adverse events and improvement in clinical outcomes. Alterations in dose frequency may be required in those with advanced disease, which requires further exploration.
Keywords: acne inversa, biologics, brodalumab, cohort study, hidradenitis suppurativa, IL-17A, IL-17C, IL-17F, IL-17RA, monoclonal therapeutics, open label, Th17, translational medicine
Hidradenitis suppurativa (HS) is an autoinflammatory disorder of keratinization1; the inflammatory component of the disease involves dysregulation of the T helper (Th) type 17 immune axis.2 Interleukin (IL) 17A, IL-17C, IL-17F, and IL-23 have all been identified in lesional tissue of patients with HS,3,4 and a number of IL-17 therapeutic antibodies are currently undergoing clinical trials in HS. However, because of the differential affinity of these agents against different IL-17 isoforms,5 the relative contributions of each to the inflammatory mechanisms in HS remain unclear.
Brodalumab6 is an IL-17 receptor (IL-17R) antagonist approved by the US Food and Drug Administration for the treatment of moderate to severe psoriasis.7,8 Through binding to IL-17RA (part of the IL-17 receptor dimer), it enables blockade of multiple isoforms of IL-17, most pertinently IL-17A, IL-17C, and IL-17F, which are known contributors to inflammation in psoriasis and atopic dermatitis9 as well as in lesional HS tissue.4
The objectives of this open-label pilot cohort study were to evaluate the safety and tolerability of brodalumab in participants with HS (as graded by the National Cancer Institute’s Common Terminology Criteria for Adverse Events, version 5.0)10 as well as the effect of brodalumab upon clinical disease activity (if any) in participants with HS. Clinical disease activity was assessed through the use of Hidradenitis Suppurativa Clinical Response (HiSCR),11 International Hidradenitis Suppurativa Severity Score (IHS4),12 and Sartorius score.13 This study was approved by the institutional review board of Rockefeller University and prospectively registered on clinicaltrials.gov (NCT03960268). Reporting was conducted in line with the Strengthening Reporting of Observational Studies in Epidemiology (STROBE) checklist.14
METHODS
This study was conducted at The Rockefeller University Hospital, New York, between May 2019 and January 2020. Patients were screened and, if eligible, underwent informed consent discussions in line with the Declaration of Helsinki. Patients older than 18 years with moderate to severe (Hurley stage 2 or 3) HS were eligible for participation. Patients were required to have negative tests results for hepatitis B virus surface antigen, hepatitis C virus antibody, HIV, and tuberculosis (as measured by QuantiFERON Gold) and could not be pregnant or breastfeeding. Individuals with a personal history of inflammatory bowel disease were also excluded from participation.15 Complete inclusion and exclusion criteria for this study are provided in Supplemental Table I (available via Mendeley at https://data.mendeley.com/datasets/98pmyyz67m/1). Individuals taking a biologic or immunomodulating therapy underwent a washout period of 5 half-lives before enrollment in the trial. Clinical assessments, blood work (routine hematologic values including hemoglobin, leucocyte, and platelet count) and skin biopsies were taken at weeks 0, 4, and 12. Biopsies were performed with the assistance of cutaneous ultrasonography (GE [Boston, MA] Logic Q 20-MHz probe), and biopsy sites (lesional, perilesional, and unaffected) were chosen in line with published recommendations for translational research studies in HS.16 Brodalumab 210 mg/1.5 mL was administered via a prefilled syringe at weeks 0, 1, and 2 and every 2 weeks thereafter until week 24.
The primary safety evaluation was the number of grade 2/3 adverse events during the 24 weeks of the clinical study. Change in clinical disease activity was assessed by the number of patients achieving HiSCR11 (defined as a ≥50% reduction in inflammatory lesion count—abscesses plus inflammatory nodules—and no increase in abscesses or draining fistulas) at week 12 and week 24 compared with baseline; as well as change in the Sartorius score13 and the IHS412 at weeks 12 and 24 compared with baseline. Patient-reported outcomes including Visual Analogue Scales of pain, itch, and global disease assessment; Dermatology Life Quality Index17 (DLQI); Beck Depression Inventory18 (BDI); and Patient Health Questionnaire-919 were administered at weeks 0, 12, and 24.
Statistical analyses of safety and tolerability were analyzed descriptively. Changes in clinical outcomes and patient-reported outcomes were analyzed using nonparametric assessments for paired assessments (Wilcoxon’s matched-pairs signed rank test) with correction for multiple comparisons. Missing data was handled using nonresponder imputation.
RESULTS
Demographic characteristics of included patients are presented in Supplemental Table II (available via Mendeley at https://data.mendeley.com/datasets/98pmyyz67m/1). Overall, 50% of participants were male, and 50% were female, with 6 out of 10 patients being active smokers. Eight of the 10 participants had Hurley stage 2 disease with a median abscess and nodule count (AN count) of 10 (range, 4–16) and median draining fistula count of 3 (range, 0–35). Six of the 10 patients were obese (body mass index, >30.0 kg/m2), and previous therapies included oral antibiotic therapies (n = 10), adalimumab (n = 7), infliximab (n = 4), secukinumab (n = 2), ixekizumab (n = 2), and large surgical excisions with HS recurrence (n = 6).
All 10 patients completed the 2 primary timepoints of week 12 and week 24. A 12-week observation period was designed after the completion of the 24-week treatment period to assess response after treatment withdrawal; however, all participants withdrew from the study at week 24 to avoid the 12-week treatment withdrawal period. All patients elected to continue with brodalumab therapy after the completion of the study.
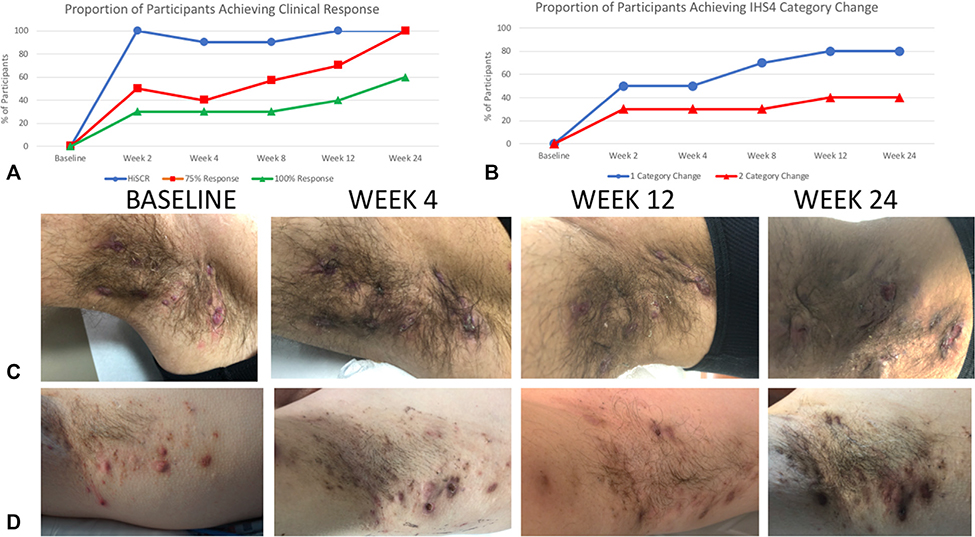
All patients (100%) achieved HiSCR at week 2 compared with baseline (Fig 1), with 5 of 10 patients achieving a 75% reduction in AN count and 3 of 10 patients achieving a 100% reduction in AN count. All 10 patients (100%) had achieved HiSCR at week 12, with 7 of 10 patients achieving a 75% reduction in AN count, and 40% of patients achieving a 100% reduction in AN count (Fig 1). This continued to increase, with 100% patients having a 75% reduction in AN count at week 24 and 40% of patients having a 100% response in AN count. At week 2, 50% patients achieved IHS4 category change, increasing to 80% at week 12, which was maintained until week 24. At week 12, 40% of patients had a 2-category change in IHS4 score that was maintained until week 24.
Fig 1.
Measures of clinical response to brodalumab therapy in hidradenitis suppurativa. A, The proportion of patients achieving HiSCR at weeks 12 and 24 was 100%, with 75% reduction in AN counts (red) and 100% reduction in AN counts (green) in 60% and 40% of participants, respectively. B, Measurement of clinical outcomes using IHS4 category change shows 80% and 40% of patients achieving 1-category and 2-category change respectively. C and D, Representative clinical photos show a rapid reduction in the inflammatory nature of nodules at week 4 compared to baseline and continued improvement at week 12. HiSCR was maintained at Week 24 despite external triggers initiating limited flares of disease. AN, Abscess and nodule count; HiSCR, Hidradenitis Suppurativa Clinical Response; IHS4, International Hidradenitis Suppurativa Severity Score.
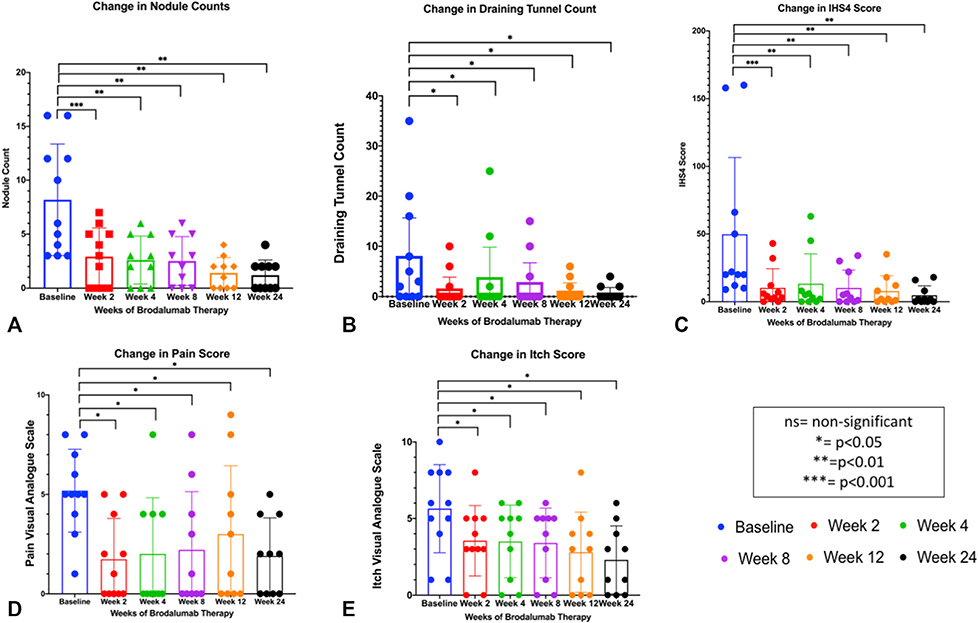
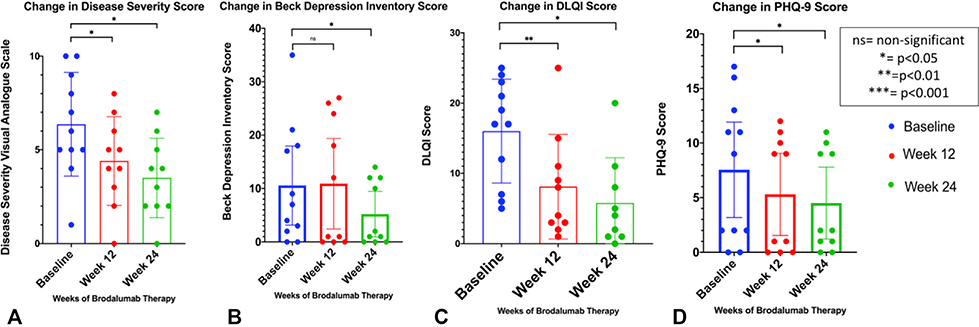
Average nodule counts, draining tunnel counts, and IHS4 scores all reduced dramatically within the first 2 weeks of therapy (Fig 2), with an increase in draining tunnel counts at week 4, which then continued to decrease over time. Pain and Itch Visual Analogue Scale scores steadily decreased (Fig 2), which mirrored the steady decreases in patient-reported outcomes such as overall disease severity, DLQI, BDI, and PHQ-9 scores (Fig 3). Total Sartorius scores significantly decreased at weeks 12 and 24 compared to baseline in all participants. The changes in nodule, draining tunnel, IHS4, and pain and itch scores were statistically significant compared to baseline at all timepoints (Fig 2). The changes in disease severity, DLQI, and PHQ-9 scores were statistically significant from baseline at weeks 12 and 24, with the changes in BDI scores significant from baseline only at the week 24 timepoint (Fig 3).
Fig 2.
Brodalumab therapy shows significant reductions in (A) mean nodule count, (B) mean draining tunnel count, (C) mean IHS4 score, (D) mean pain numerical score, and (E) mean itching numerical score. Each outcome was statistically significant from baseline at weeks 12 and 24 with average nodule count, average IHS4 score, average pain score, and average itch score being statistically significant at week 2 of therapy. IHS4, International Hidradenitis Suppurativa Severity Score.
Fig 3.
Patient-based outcome measures were significantly reduced at weeks 12 and 24 of brodalumab therapy compared with baseline scores. Patient-rated disease severity (A) Visual Analogue Scale scores, (B) mean DLQI scores, (C) mean Beck Depression Inventory scores, and (D) Mean PHQ-9 scores were reduced significantly at weeks 12 and 24. DLQI, Dermatology Life Quality Index; PHQ-9, Patient Health Questionnaire-9.
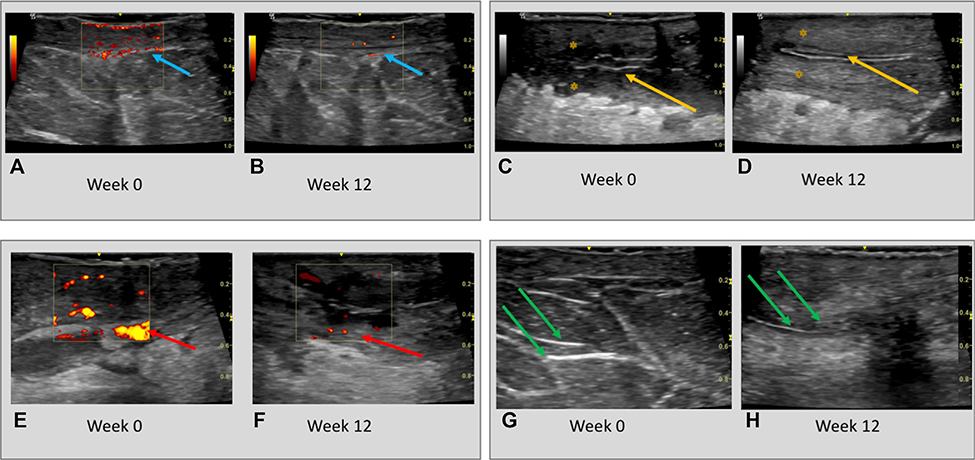
Significant decreases in vascularity and inflammation, as measured by cutaneous Doppler ultrasonography, were seen at weeks 4, 12, and 24 compared with baseline in all 10 patients (Fig 4). Doppler signal reductions were particularly apparent in the superficial dermis and surrounding parallel hyperechoic structures of the dermis, indicative of epithelialized tunnels (Fig 4, A, B, E, and F).
Fig 4.
A, C, E, and G, Ultrasonographic changes at baseline and (B, D, F, H) after 12 weeks of treatment with brodalumab. Dermal Doppler ultrasonography intensity in lesional dermal tissues at (A) baseline (blue arrows) is significantly attenuated at (B) week 12 (blue arrows) of therapy. The diameter of draining tunnels (yellow arrows) and surrounding edema (yellow asterisk) at (C) baseline is also reduced at (D) week 12. Similarly, Doppler intensity (red arrows) surrounding (E) epithelialized dermal tunnels (F) reduces at week 12 compared with baseline. The diameter and echogenicity of dermal tunnels (green arrows) is also (H) attenuated at week 12 compared with (G) baseline.
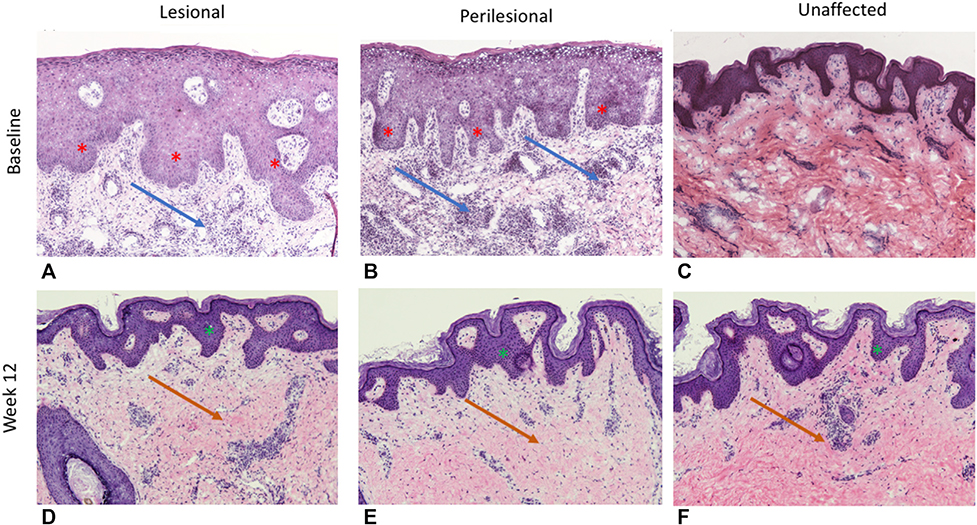
Reduction in psoriasiform epidermal hyperplasia, with re-establishment of a granular layer, reduction in parakeratosis, and CD3+ and CD11c dermal infiltrates were also noted at weeks 4, 12, and 24 compared with baseline (Fig 5). These changes were seen in lesional, perilesional, and unaffected skin samples across all participants.
Fig 5.
Representative histology of (A, D) lesional, (B, E) perilesional, and (C, F) unaffected tissue at (A-C) baseline (week 0) and (D-F) week 12 of treatment with brodalumab 210 mg/1.5 mg every 2 weeks. Baseline lesional and perilesional samples illustrate significant psoriasiform epidermal hyperplasia (red asterisk) with diffuse mixed dermal inflammatory infiltrate (blue arrows). Unaffected tissue illustrates milder manifestations of inflammatory infiltrates and psoriasiform epidermal hyperplasia. After 12 weeks of brodalumab therapy, psoriasiform epidermal hyperplasia is much less pronounced (green asterisk), with a noticeable reduction in dermal inflammatory infiltrates (orange arrows).
DISCUSSION
No serious adverse events were noted in the setting of brodalumab therapy in this HS cohort. The 4 adverse events reported (Supplemental Table III, available via Mendeley at https://data.mendeley.com/datasets/98pmyyz67m/1) were minor, with 3 of the 4 events unlikely to be associated and the 1 self-resolving upper respiratory tract infection possibly associated, given the reports of upper respiratory tract infection in the phase 3 randomized controlled trial of brodalumab in psoriasis.8
The rapid reduction in inflammatory lesion count (AN count) was unexpected but consistent with the mechanism of action of brodalumab as an IL-17RA antagonist acting on a number of different cell types, including neutrophils, dendritic cells, keratinocytes, and other inflammatory leucocytes.5 The ability of brodalumab to block IL-17A, IL-17C, and IL-17F may be important because each of these cytokines can drive neutrophilic inflammation,7 with the blockade of all 3 isoforms a potential benefit above other agents blocking only IL-17A or IL-17A/IL-17F. Other IL-17 monoclonal antibodies trialed in HS do not have the ability to block the range of IL-17 isoforms possible with brodalumab. The pharmacodynamic properties of brodalumab in suppressing the downstream cascade of keratinocyte-derived CXCL cytokines and other inflammatory mediators has been observed in psoriasis,7 and verification of brodalumab’s similar mechanism of action in HS is required. If verified, this would lend further credence to the suggestion of HS being an autoinflammatory disease of keratinocytes.1
The pronounced reduction in cutaneous inflammation is visible upon clinical examination (Fig 1), although larger nodules and deeper abscesses may take longer than 12 weeks to resolve (along with any residual postinflammatory hyperpigmentation). The rapid reduction in pain (Fig 2) and steady improvement in patient-reported outcomes (Fig 3) such as the DLQI are consistent with the known PD properties of this drug in suppressing Th17-mediated inflammatory pathways.7 Two participants with severe, widespread Hurley stage 3 disease had greater pain levels during the 24 weeks of treatment, despite clinical improvement, and this may be explained by a degree of central pain sensitization in the setting of severe disease.20
What is not able to be appreciated from the clinical photographs is the reduction in dermal tunnel drainage after administration of brodalumab. Lesional edema, tenderness, and drainage were reduced significantly during the loading doses of brodalumab (weeks 0, 1, and 2) and after drug administration throughout the remainder of the 24 weeks (Supplemental Fig 4, available via Mendeley at https://data.mendeley.com/datasets/98pmyyz67m/1). This suggests that the action of brodalumab not only targets the development of cutaneous nodules and abscesses but also the purulent discharge from epithelialized dermal tunnels. This is supported by the results of decreased dermal Doppler ultrasonographic intensity surrounding tunnels and the reduction in the diameter and hyperechoic intensity of tunnels after Brodalumab therapy. The suggestion that epithelialized tunnels may be active contributors to inflammation is a concept that requires further mechanistic evaluation.
A black box warning exists in the United States regarding the association between brodalumab and suicidality, although the causation has been disputed in the literature.21 In the design of this pilot study, we astutely monitored each patient’s mental state at each visit and via telephone contact in between visits and had no occurrences of suicidal thoughts or behaviors. In fact, patient-reported depression scores (BDI and PHQ-9) were significantly reduced at week 24 compared to baseline, with PHQ-9 also significantly reduced at week 12. Despite the small sample size, this lends credence to the suggestion of Lebwohl et al21 (supported by observational evidence22) that untreated or insufficiently treated cutaneous disease is a large contributor to depression and suicidality.
The fact that 100% of patients achieved HiSCR at week 2 of this study emphasizes the response of this cohort to brodalumab therapy. Given the documented concerns regarding elevated placebo response rates in HS clinical trials23,24 and the results of this and other cohort studies of IL-17 and IL-23 inhibitors in HS,25–28 we undertook a deeper analysis of clinical response through evaluation of the 75% and 100% response rates in this cohort, showing a progressive reduction in clinical disease throughout the 24 weeks of the study. In a similar way to how reductions in Psoriasis Area and Severity Index scores of 50%,75%, and 90% have been surpassed in psoriasis, more comprehensive measures of clinical response may need to be adopted in HS. The additional complicating factor in HS, however, is that the morphologic heterogeneity of disease (nodules, tunnels, etc), means that the IHS4 may have advantages over nodule counts, given the inclusion of draining tunnels in its scoring.24 The response rate in this study may be influenced by the fact that 8 of the 10 participants had Hurley stage 2 disease, with 5 participants with dermal tunnels on ultrasonography and histology. Given the influence of tunnels on clinical response rates with adalimumab,24 it is not inconceivable that stratification of severity by the presence of tunnels may be required in larger trials to accurately assess the impact of Brodalumab on Hurley stage 3 disease.
Two patients had widespread, severe Hurley stage 3 disease (patients 2 and 6) (Supplementary Table II), and although these patients achieved HiSCR, the response of their draining tunnels was cyclic. Tunnels would reduce and/or cease draining within 24 hours of dose administration, and the greatest sustained improvement was seen during the loading dose period (weeks 0–3). Once on the every-2-week dosing regimen, however, 5t o 7 days after dosing, the patient would report extensive painful drainage, suggesting an insufficient frequency of dosing. Although overall the trend over 24 weeks was toward improvement, these patients represent anecdotal evidence that in severe disease with extensive dermal tunnels, an increased frequency of dosing (as in recalcitrant psoriasis29) may need to be explored.
This study is limited by its lack of placebo control arm, size (N = 10), and short duration of therapy (24 weeks). It is comparable in size to studies involving anakinra30 and infliximab,31 but larger cohorts, longer trials, and a variety of dosing frequencies are suggested as considerations for future studies, given the positive response of this pilot cohort.
CONCLUSION
Brodalumab administered over 24 weeks in this pilot cohort did not result in any severe adverse events. Clinical response to brodalumab therapy was rapid, with 100% of the cohort achieving HiSCR at weeks 12 and 24. No safety signals regarding depression or suicidality were identified. Alterations in dosing frequency may be required in future studies to provide sustained effect in participants with extensive draining tunnels. Larger placebo-controlled studies are required to establish the true potential of brodalumab as an effective treatment for HS.
All brodalumab used in this study was provided under an Investigator Initiated Study Agreement with Valeant Pharmaceuticals. Valeant Pharmaceuticals had no input into the conduct of the study, analysis of the data, or composition of the manuscript.
CAPSULE SUMMARTY.
In this open-label cohort study (N = 10) investigating the effect of subcutaneous brodalumab 210 mg/1.5 mL every 2 weeks on disease activity in hidradenitis suppurativa (HS), no serious adverse effects were reported; 100% of participants (N = 10) achieved Hidradenitis Suppurativa Clinical Response at week 12. Significant improvements were seen in pain, itch, quality of life, and depression, with no episodes of self-harm or suicidality.
Brodalumab was well tolerated and showed a high rate of clinical response in this cohort. Larger placebo-controlled studies stratifying by disease severity are encouraged to further explore the safety and efficacy of brodalumab in HS.
Acknowledgments
Funding sources: Dr Frew was supported in part by grant UL1 TR001866 from the National Center for Advancing Translational Sciences, National Institutes of Health (NIH) Clinical and Translational Science Award program. Author Navrazhina was supported by a Medical Scientist Training Program grant from the National Institute of General Medical Sciences of the NIH under award number T32GM007739 to the Weill Cornell/-Rockefeller/Sloan Kettering Tri-institutional MD-PhD Program.
Disclosure: Dr Krueger has received research support (grants paid to institution) from AbbVie, Amgen, Bristol Myers Squibb, Boehringer, EMD Serono, Innovaderm, Kineta, LEO Pharma, Novan, Novartis, Paraxel, Pfizer, Regeneron, and Vitae and personal fees from AbbVie, Acros, Allergan, Aurigne, BiogenIdec, Boehringer, Escalier, Janssen, Lilly, Novartis, Pfizer, Roche, and Valeant. Dr Frew; Authors Navrazhina, Grand, Sullivan-Whalen, and Gilleaudeau; and Drs Garcet and Ungar have no conflicts of interest to declare.
Abbreviations used
- AN
abscess and nodule count
- BDI
Beck Depression Inventory
- DLQI
Dermatology Life Quality Index
- HiSCR
Hidradenitis Suppurativa Clinical Response
- HS
hidradenitis suppurativa
- IHS4
International Hidradenitis Suppurativa Severity Score
- IL
interleukin
- IL-17R
interleukin 17 receptor
- Th
T helper
Footnotes
IRB approval status: Reviewed and approved by Rockefeller University Institutional Review Board.
REFERENCES
- 1.De Vita V, McGonagle D. Hidradenitis suppurativa as an autoinflammatory keratinization disease. J Allergy Clin Immunol. 2018;141(56):1953. [DOI] [PubMed] [Google Scholar]
- 2.Moran B, Sweeney CM, Hughes R, et al. Hidradenitis suppurativa is characterized by dysregulation of the Th17:Treg cell axis which is corrected by anti-TNF therapy. J Invest Dermatol. 2017;137(11):2389–2395. [DOI] [PubMed] [Google Scholar]
- 3.Witte-Handel E, Wolk K, Tsaousi A, et al. The IL-1 pathway is hyperactive in hidradenitis suppurativa and contributes to skin infiltration and destruction. J Invest Dermatol. 2019;139(6): 1294–1305. [DOI] [PubMed] [Google Scholar]
- 4.Navrazhina K, Frew JW, Krueger JG. Interleukin 17C is elevated in lesional tissue of hidradenitis suppurativa. Br J Dermatol. 2020;182:1045–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaffen S IL-7 receptor composition. Nat Rev Immunol. 2016;16: 4. [DOI] [PubMed] [Google Scholar]
- 6.Papp K, Leonardi C, Menter A, et al. Brodalumab, an anti-interleukin-17-receptor antibody for psoriasis. N Engl J Med. 2012;366:1181–1189. [DOI] [PubMed] [Google Scholar]
- 7.Russell CB, Rand H, Bigler J, Kerkof K, Timour M, Bautista E. Gene expression profiles normalized in psoriatic skin by treatment with brodalumab, a human anti-il-17 receptor monoclonal antibody. J Immunol. 2014;192:3828–3836. [DOI] [PubMed] [Google Scholar]
- 8.Papp K, Reich K, Paul C, et al. A prospective phase III, randomized, double-blind, placebo controlled study of brodalumab in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2016;175:273–286. [DOI] [PubMed] [Google Scholar]
- 9.Guttman-Yassky E, Krueger JG. atopic dermatitis and psoriasis: two different immune diseases or one spectrum? Curr Opin Immunol. 2017;48:68–73. [DOI] [PubMed] [Google Scholar]
- 10.US Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE v5.0). November 27, 2017. Available at: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_50. Accessed June 8, 2020.
- 11.Kimball AB, Sobell JM, Zouboulis CC, et al. HiSCR (Hidradenitis Suppurativa Clinical Response): a novel clinical endpoint to evaluate therapeutic outcomes in patients with hidradenitis suppurativa from the placebo-controlled portion of a phase 2 adalimumab study. J Eur Acad Dermatol Venereol. 2016;30(6): 989–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zouboulis CC, Tzellos T, Kyrgidis A, et al. Development and validation of the International Hidradenitis Suppurativa Severity Score System (IHS4), a novel dynamic scoring system to assess HS severity. Br J Dermatol. 2017;177(5):1401–1409. [DOI] [PubMed] [Google Scholar]
- 13.Sartorius K, Emtestam L, Jamac GB, Lapins J. Objective scoring of hidradenitis suppurativa reflecting the role of tobacco smoking and obesity. Br J Dermatol. 2009;161(4):831–839. [DOI] [PubMed] [Google Scholar]
- 14.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol 2008;61(4):344–349. [DOI] [PubMed] [Google Scholar]
- 15.Targan SR, Feagan B, Vermeire S, et al. A randomized, double blind, placebo-controlled phase 2 study of brodalumab in patients with moderate-to-severe Crohn’s disease. Am J Gastroenterol. 2016;111(11):1599–1607. [DOI] [PubMed] [Google Scholar]
- 16.Frew JW, Navrazhina K, Byrd AS, Garg A, Ingram JR, Kirby JS. Defining lesional, perilesional and unaffected skin in hidradenitis suppurativa: proposed recommendations for clinical trials and translational research studies. Br J Dermatol. 2019;181(6):1339–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)—a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19(3):210–216. [DOI] [PubMed] [Google Scholar]
- 18.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961; 4:561–571. [DOI] [PubMed] [Google Scholar]
- 19.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001; 16(9):606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neilsen RM, Andersen PL, Sigsgaard V, Mikkelsen PR, Jemec GBE. Pain perception in patients with hidradenitis suppurativa. Br J Dermatol. 2020;182:166–174. [DOI] [PubMed] [Google Scholar]
- 21.Lebwohl MG, Papp KA, Marangell LB, Koo J, Blauvelt A, Gooderham M. Psychiatric adverse events during treatment with brodalumab: analysis of psoriasis clinical trials. J Am Acad Dermatol. 2018;78:81–89. [DOI] [PubMed] [Google Scholar]
- 22.Machado MO, Stergiopoulos V, Maes M, Kurdyak PA, Lin PY, Wang LJ. Depression and anxiety in adults with hidradenitis suppurativa: a systematic review and meta-analysis. JAMA Dermatol. 2019;155(8):939–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ali AA, Seng EK, Alavi A, Lowes MA. Exploring changes in placebo treatment arms in hidradenitis suppurativa randomized clinical trials: a systematic review. J Am Acad Dermatol. 2020;82(1):45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frew JW, Jiang CS, Singh N, et al. Clinical response rates, placebo response rates and significantly associated covariates are dependent upon choice of outcome measure in hidradenitis suppurativa: a post-hoc analysis of PIONEER 1 and 2 individual patient data. J Am Acad Dermatol. 2020;82: 1150–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kovacs M, Podda M. Guselkumab in the treatment of severe hidradenitis suppurativa. J Eur Acad Dermatol Venereol. 2019; 33(3):e140–e141. [DOI] [PubMed] [Google Scholar]
- 26.Casseres RG, Kahn JS, Her MJ, Rosmarin D. Guselkumab in the treatment of hidradenitis suppurativa: a retrospective chart review. J Am Acad Dermatol. 2019;81(1):265–267. [DOI] [PubMed] [Google Scholar]
- 27.Marasca C, Megna M, Balato A, Balato N, Napolitano M, Fabbrocini G. Secukinumab and hidradenitis suppurativa: friends or foes? JAAD Case Rep. 2019;5(2):184–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Odorici G, Pellacani G, Conti A. Ixekizumab in hidradenitis suppurativa: a case report in a psoriatic patient [e-pub ahead of print]. G Ital Dermatol Venereol. 10.23736/S0392-0488.18.06135-7. Accessed June 8, 2020. [DOI] [PubMed] [Google Scholar]
- 29.Yeung J, Ladda M, Piguet V. Dose optimisation of brodalumab in moderate-to-severe plaque psoriasis. Abstract presented at: 24th World Congress of Dermatology; June 10–15, 2019 Milan, Italy. [Google Scholar]
- 30.Tzanetakou V, Kanni T, Giatrakou S, Katoulis A, Papdavid E, Netea MG. Safety and efficacy of anakinra in severe hidradenitis suppurativa: a randomized clinical trial. JAMA Dermatol. 2016;152(1):52–59. [DOI] [PubMed] [Google Scholar]
- 31.Grant A, Gonzales T, Montgomery MO, Cardenas V, Kerdel FA. Infliximab therapy for patients with moderate to severe hidradenitis suppurativa:a randomized, double-blind, placebo-controlled crossover trial. J Am Acad Dermatol. 2020; 62(2):205–217. [DOI] [PubMed] [Google Scholar]