Abstract
Achieving military mission objectives requires high levels of performance from Airmen who operate under extreme physical and cognitive demands. Thus, there is a critical need to establish scientific interventions to enhance physical fitness and cognitive performance—promoting the resilience of Airmen and aiding in mission success. We therefore conducted a comprehensive, 12-week randomized controlled trial in active-duty Air Force Airmen (n = 148) to compare the efficacy of a multimodal intervention comprised of high-intensity interval aerobic fitness and strength training paired with a novel nutritional supplement [comprised of β-hydroxy β-methylbutyrate (HMB), lutein, phospholipids, DHA and selected micronutrients including B12 and folic acid] to high-intensity interval aerobic fitness and strength training paired with a standard of care placebo beverage. The exercise intervention alone improved several dimensions of physical fitness [strength and endurance (+ 8.3%), power (+ 0.85%), mobility and stability (+ 22%), heart rate (− 1.1%) and lean muscle mass (+ 1.4%)] and cognitive function [(episodic memory (+ 9.5%), processing efficiency (+ 7.5%), executive function reaction time (− 4.8%) and fluid intelligence accuracy (+ 19.5%)]. Relative to exercise training alone, the multimodal fitness and nutritional intervention further improved working memory (+ 9.0%), fluid intelligence reaction time (− 7.7%), processing efficiency (+ 1.8%), heart rate (− 2.4%) and lean muscle mass (+ 1.5%). These findings establish the efficacy of a multimodal intervention that incorporates aerobic fitness and strength training with a novel nutritional supplement to enhance military performance objectives and to provide optimal exercise training and nutritional support for the modern warfighter.
Subject terms: Nutrition, Human behaviour, Physiology
Introduction
Adaptive military operations are a hallmark of modern warfare and depend on the capacity for flexible, resilient behavior. Airmen face extreme physical and mental demands that require the capacity to overcome the negative physical and psychological effects of operating in challenging field environments characterized by “volatile, uncertain, complex, and ambiguous” events1. A primary aim of research in the nutritional sciences is therefore to establish and validate nutritional interventions for the modern warfighter that are designed to provide optimal nutritional support and supplementation for body and mind. There is growing interest within the United States military to understand the effects of nutritional factors on warfighter resilience examined with respect to both physical and cognitive performance2,3.
Challenging field environments can prevent warfighters from realizing their optimal physical and cognitive performance. Participants with higher levels of stress and anxiety in military survival training exercises have lower scores on the Army Physical Readiness Test4. Acute stress in special operation soldiers may impair visuo-spatial capacity and working memory due to high dopamine and norepinephrine turnover in the prefrontal cortex, which is known to impair cognition and spatial working memory5. Surveys among military populations indicate that most warfighters use multivitamins, protein supplements or sports bars/drinks with a perception that these supplements improve their performance6,7. However, the efficacy of many of these supplements is not supported by randomized controlled trials8,9. Caffeine benefits executive function in sleep-deprived individuals; but the effects are acute and have negative side effects in some individuals10. Recent evidence further suggests that some supplements may contain dangerous or illegal substances that can have unknown consequences11. Thus, an enduring aim of research in the psychological and brain sciences is to establish nutritional supplements to enhance physical fitness and cognitive performance in active-duty military populations.
Accumulating evidence suggests that nutrients found in the Mediterranean diet promote brain and cognitive health. The Mediterranean diet includes olive oil as a source of monounsaturated fatty acids and polyphenols, fish and salmon that deliver phospholipids, omega-3 polyunsaturated fatty acids, lutein and vitamin D, and fruits and vegetables that provide antioxidants and vitamins B, C and E, carotenoids including lutein, folate, and polyphenols12–16. Elements of the Mediterranean diet are known to promote healthy vasculature by way of reducing total cholesterol and low-density lipoproteins (i.e., “bad cholesterol”)17, and improving endothelial function18. Broadly, these actions lower the risk of vascular comorbidities, dyslipidemia, hypertension, and coronary artery disease19. Within the brain, these actions are linked to a reduction in white matter lesions and promotion of white matter microstructure19. Furthermore, improvements in white matter brain structure are known to enhance cognitive performance (e.g., on measures of executive function and working memory20). Thus, combinations of these nutrients in the form of a novel nutritional supplement have the potential to improve the protective vascular, metabolic, antioxidant, and anti-inflammatory mechanisms promoted by individual nutrients and therefore to enhance brain function and cognition13.
Physical activity interventions improve cognition and the brain in multiple animal models, including rodents, dogs and monkeys21. Specifically, these models have demonstrated that exercise interventions result in (1) hippocampal neurogenesis; (2) increased synaptic activity in the brain; (3) growth of new blood vessels; (4) increased concentrations of brain derived neurotrophic factor (BDNF); (5) reduction of neurodegeneration; and (6) enhanced learning and memory21. The results from these animal models provide the basis for human studies of physical activity, fitness, and exercise. A meta-analysis of exercise interventions in humans revealed a moderate effect size for exercise leading to better cognition22,23. The meta-analysis showed that exercise improved a variety of cognitive tasks; but the largest effects were observed in tasks that engage the central executive network, including planning, problem solving, and working memory. Finally, the meta-analysis demonstrated that the combined effects of aerobic exercise, power and flexibility training had a greater cognitive benefit compared to only aerobic exercise training.
Motivated by these parallel lines of research, the present study examined the efficacy of a multi-modal fitness and nutritional intervention to enhance cognitive performance and physical fitness in United States Air Force Airmen. Specifically, we investigated: (1) whether a unimodal exercise training protocol enhanced fitness and cognition and (2) whether a multimodal fitness plus nutritional intervention resulted in fitness and cognitive gains beyond those conferred by the unimodal intervention.
Methods
Population
Study participants were active-duty Air Force Airmen recruited from Wright Patterson Air Force Base in Dayton, Ohio from January of 2016 through May of 2018. Study eligibility required participants to: (1) have active-duty status; (2) commit to study participation for 14 consecutive weeks; (3) be at least 18 but no older than 45 to minimize the risk of physical injury or cardiovascular occurrence due to the study’s required fitness activity; (4) not have a Department of Defense medical profile for mental and/or physical function limitation nor have a pregnancy profile; (5) not currently be taking prescription blood pressure medication; (6) cease taking herbal dietary supplements, performance supplements or any other substance that contained ingredients that might affect cardiovascular response with exercise one week before study participation began; (7) not have musculoskeletal injury that would limit their ability to engage in heavy resistance training and aerobic exercise; (8) not have cardiovascular or respiratory disease that would limit their ability to engage in heavy resistance training and aerobic exercise. 217 participants enrolled in the study; 205 began the intervention; and 148 completed the study. Only the data for those who completed the study were analyzed in this study and their demographics are presented in Table 1. Reasons for participants to drop (n = 70) include: (1) duty re-assignment or deployment; (2) muscle strain; and (3) attendance less than 80%; (4) not enough time.
Table 1.
Demographics (n = 148).
| Demographic | Supplement | Placebo | Total sample |
|---|---|---|---|
| Female | 12% | 16% | 28% |
| Average age | 30 | 30 | 30 |
| Age SD | 5.2 | 5.4 | 5.3 |
| Age range | 20–42 | 19–44 | 19–44 |
| Education | |||
| < 12 years | 3% | 3% | 6% |
| 12–16 years | 32% | 34% | 66% |
| > 16 years | 11% | 16% | 28% |
| Rank | |||
| Airman First Class | 6% | 3% | 9% |
| Staff Sergeant | 11% | 10% | 21% |
| Technical Sergeant | 8% | 7% | 15% |
| Major Sergeant | 1% | 4% | 5% |
| Lieutenant | 8% | 11% | 20% |
| Captain | 9% | 13% | 22% |
| Major | 2% | 4% | 6% |
| Colonel | 1% | 0% | 21% |
Percentages computed from total sample (n = 148).
SD standard deviation.
Standard protocol approval, participant consent and recruitment
All experimental protocols were approved by the 711th Human Performance Wing Institutional Review Board (IRB). The 711th Human Performance Wing is part of the United States Air Force and comprises three units: the Airman Systems Directorate, US Air Force School of Aerospace Medicine and the Human Systems Integration Directorate24. Informed consent was obtained from all participants in this study. All methods were carried out in accordance with Air Force Research Lab regulations. Participants were recruited at Wright Patterson Air Force Base through IRB approved flyers and emails.
Study design
Participants were randomly assigned to either the exercise plus nutritional supplement group (n = 70) or the exercise plus placebo group (n = 78). The sample size was based on a two-arm power analysis that specified an effect size of 0.20 and power of 0.90 and an attrition rate of 20%. Intervention assignment was counterbalanced for the 7 cohorts (about 25 participants per cohort) such that odd numbered cohorts received the supplement and even numbered cohorts received the placebo. Both experimenters and participants were blinded to group assignment. The exercise training intervention was identical for both groups. A battery of cognitive and physical fitness assessments was administered at pre- and post-intervention. This clinical trial was registered with the ISRCTN registry, number ISRCTN96342002, and was approved on July 28, 2020. This clinical trial was assigned a United States Air Force clearance of CLEARED on 28 Jul 2020 with case number MSC/PA-2020-0172.
Exercise intervention
The exercise intervention was designed to enhance mission relevant strength, cardiovascular health and fitness. The training consisted of a total body resistance circuit designed to improve upper- and lower-body strength (Monday and Wednesday); a moderate cardiovascular workout designed to facilitate active recovery, flexibility and core strength (Tuesday and Thursday); and a cardiovascular endurance training and high intensity cardiovascular workout (Friday). Fitness training sessions were scheduled five days a week (Monday–Friday) for 12 weeks and session each lasted 45 minutes. Participants were guided on proper form for each exercise. Air Force Research Lab research staff, consisting of exercise physiologists, athletic trainers and strength coaches, designed and supervised the exercise intervention. All exercise sessions were supervised by at least one member of the research staff. Average attendance rate for the fitness training sessions was 90%. Supplementary Fig. 1, Supplementary Tables 1–5 and Supplementary Note 1 provide more details of the exercise training.
Nutritional supplement and placebo intervention
The nutritional supplement beverage was designed to support both muscle and cognitive performance whereas the non-isocaloric standard of care placebo was designed to not promote physical or cognitive health. Both the supplement and placebo were ready-to-drink 8 fluid ounce liquid beverages, peach flavored and were packaged in identical plastic bottles to maintain blinding (Abbott Nutrition, Columbus, OH). The nutritional composition of each beverage is listed in Table 2. The nutritional supplement beverage is copyrighted by the University of Illinois Board of Trustees, 2020, and licensed under CC BY-NC 4.0 https://creativecommons.org/licenses/by-nc/4.0/.
Table 2.
Nutritional composition of one 8-ounce placebo or nutritional supplement beverage.
| Composition | Unit | Supplement | Placebo |
|---|---|---|---|
| Energy | Kcal | 267 | 100 |
| Protein | g | 13 | 2 |
| Carbohydrates | g | 38 | 20 |
| Fat | g | 7 | 2 |
| Ca-HMB | g | 1.5 | – |
| Choline | mg | 100 | – |
| DHA | mg | 125 | – |
| Folic acid | µg | 100 | – |
| Lutein | mg | 6 | – |
| Magnesium | g | 0.1 | – |
| Phospholipid | g | 0.5 | – |
| Selenium | µg | 21 | – |
| Vitamin B1 | mg | 0.4 | – |
| Vitamin B2 | mg | 0.4 | – |
| Vitamin B3 | mg | 5.0 | – |
| Vitamin B5 | mg | 3.0 | – |
| Vitamin B6 | mg | 0.8 | – |
| Vitamin B12 | µg | 2 | – |
| Vitamin C | mg | 36 | – |
| Vitamin D | IU | 480 | – |
| Vitamin E | mg | 6.0 | – |
| Zinc | mg | 25 | – |
Participants were instructed to drink two 8-ounce servings a day for all 12 weeks of the intervention, for a total of 168 beverages throughout the study. Participants were advised to maintain their routine diet throughout the study and to drink one serving of the beverage 30 minutes before the fitness intervention and one serving 1 hour after the fitness intervention on Monday through Friday. On Saturday and Sunday, participants could consume the beverages at any time. The compliance rate for consuming the beverage was high. 47% of the participants consumed all 168 beverages; 51% of the participants consumed between 151 and 168 of the beverages; and 2% of the participants consumed between 141 and 151 beverages.
Physical fitness assessment
A fitness battery was administered at pre- and post-intervention to assess the efficacy of the exercise training and nutritional supplement. Measurements were taken for 6 domains of physical health and fitness: (1) power (2) strength and endurance (3) mobility and stability (4) blood pressure (5) heart rate (6) lean muscle mass. Table 3 lists the fitness tests included in each domain along with a brief description of how they were measured.
Table 3.
Tests administered for the physical fitness battery.
| Fitness domain | Brief description |
|---|---|
| Power | |
| Abdominal circumference (inches) | Measure hip circumference using a tape measure |
| Sled push and pull R/L (seconds) | Push (15 yards) and pull (15 yards) a 140 pound sled twice |
| Rotation smash ball R/L (inches) | Distance thrown of a heavy medicine ball |
| Weight (pounds) | Measure body weight with scale |
| Wingate upper body (Watts/kg) | Arm crank with fixed resistance for 30 seconds |
| Strength and endurance | |
| Body fat (% body weight) | DEXA overall and segmented body composition |
| Modified Illinois agility (seconds) | Quickly complete a weaving running course |
| Pull ups | 1 minute to complete as many as possible |
| Push ups | 1 minute to complete as many as possible |
| Sit ups | 1 minute to complete as many as possible |
| Standing long jump (inches) | Best of 3 jumps from a 2-foot static position |
| VO2 max (mL/kg/min) | Measure oxygen consumption on treadmill |
| Wingate lower body (Watts/kg) | Bicycle pedal with fixed resistance for 30 seconds |
| Mobility and stability | |
| Lateral bridge R/L (seconds) | Isometric hold until exhuastion |
| Lower Y balance test R/L | Multi-planar movement to test lower body balance |
| Supine bridge R/L (seconds) | Isometric hold until exhaustion |
| Upper Y balance test R/L | Multi-planar movement to test upper body balance |
| Blood pressure | |
| Diastolic blood pressure (mm Hg) | Average of 3 readings taken at rest |
| Systolic blood pressure (mm Hg) | Average of 3 readings taken at rest |
| Heart rate | |
| Maximum heart rate (beats/minute) | Number of beats/minute using electronic monitor |
| Resting heart rate (beats/minute) | Number of beats/minute using electronic monitor |
| Anthropometrics | |
| Height (inches) | Total body height |
| Leg length (inches) | Length of legs |
| Lean muscle mass (pounds) | DEXA overall and segmented body composition |
‘R/L’ is an average of the right and left sides of the body.
Cognitive assessment
A cognitive battery was administered at pre- and post-intervention to assess the efficacy of the exercise training and nutritional supplement. Measurements were taken for 6 domains of cognitive function: (1) short term memory (2) episodic memory (3) fluid intelligence (4) working memory (5) executive function and (6) processing efficiency and reaction time. Table 4 lists the cognitive tests included in each domain along with a brief description of how they were measured. Supplementary Note 2 provides further detail for each cognitive test.
Table 4.
Tests administered for the cognitive battery.
| Cognitive domain | Brief description |
|---|---|
| Short term memory | |
| Immediate free recall (IFR) words | Recall as many previously seen words as possible |
| Immediate free recall (IFR) pictures | Recall as many previously seen pictures as possible |
| Keep track | Recall the last instance of a given category from a previously seen set of words |
| Episodic memory | |
| Paired associates (PA) immediate recall | Given one word from a pair of words previously seen, recall the other word immediately |
| Paired associates (PA) delayed recall | Given one word from a pair of words previously seen, recall the other word after a delay |
| Fluid Intelligence | |
| Number series accuracy (Acc) | Identify the pattern of numbers |
| Letter series accuracy (Acc) | Identify the pattern of letters |
| Working memory | |
| Symmetry span | Hold items in memory while checking symmetry in a matrix |
| Rotation span | Hold items in memory while rotating letters |
| Executive function | |
| Stroop congruent accuracy (Acc) | Determine if the font color and word color match |
| Stroop incongruent accuracy (Acc) | Determine if the font color and word color mismatch |
| Processing efficiency | |
| Symbol digit modalities | Use a lookup table to translate symbols into numbers |
| Reaction time (RT) | |
| Number series, letter series, stroop congruent and incongruent | The amount of time to respond to each item |
Biomarker assessment
Blood plasma samples were collected at pre- and post-intervention and analyzed for vitamin B1225, folate26, triglycerides27, high-density lipoprotein (HDL), ferritin28, cortisol29 and several fatty acids (trans, saturated, mono-unsaturated, omega-3 and omega-6 polyunsaturated)30. Lutein was measured in the eye with Macular Pigment Optical Density (MPOD).
Statistical analysis
Statistical software
All data analyses were conducted using the R Studio interface (Version 1.0.143), which runs on the base R installation (Version 3.5.131,32). The R packages psych, effsize, compute.es, lsr and BayesFactor were used for the analyses in this manuscript33–37.
Statistical models
Principal component analysis, or PCA, was used to identify common structure in the physical fitness and cognitive test batteries. A component was retained if its eigenvalue was greater than 1.0. The number of components retained accounted for approximately 75% of the variance. A variable was included in a component if the loading had a magnitude with an absolute value of 0.50 or greater. Once the components were identified, composite scores were formed by averaging together variables which comprised each component from the PCA. The composite scores were computed separately for pre- and post-intervention. Composite scores reduce the number of analyses and reduce the likelihood of committing a Type 1 error.
One-way paired t-tests assessed differences between pre- and post-intervention measures within intervention groups. The ANOVA model was used to test for difference at pre-intervention between groups. The ANCOVA model tests for post-intervention differences across groups, while controlling for baseline performance.
Model evidence
p values for the statistical models provide evidence for an improvement from pre- to post-intervention if they are less than the threshold of 0.05. Cohen’s d measures the magnitude of the effect size from pre- to post-intervention. A Cohen’s d value of 0.0 ≤ d < 0.01 is a very small effect size, 0.01 ≤ d < 0.20 is a small effect size, 0.20 ≤ d < 0.50 is a medium effect size, 0.50 ≤ d < 0.80 is a large effect size, 0.80 ≤ d < 1.20 is a very large effect size and d ≥ 1.20 is huge38,39. Bayes factors, Bf, quantify the likelihood of a change from pre- to post-intervention. A Bf < 1 means there is no change from pre- to post-intervention; 1 ≤ Bf < 3 is some evidence for a change from pre- to post-intervention; 3 ≤ Bf < 20 is positive evidence of a change from pre- to post-intervention; 20 ≤ Bf < 150 is strong evidence of a change from pre- to post-intervention; and Bf > 150 is decisive evidence for a change from pre- to post-intervention40.
Results
Checking for random assignment
Because participants were randomly assigned to intervention groups, differences in demographics, fitness or cognitive test measures at pre-intervention were not expected. Table 1 presents the demographic composition of the participants, including Air Force Rank. The sample was 28% female; had an average age of 30; and had an educational composition with 6% not completing high school, 66% completing some college and 28% completing graduate or professional schooling. Demographics did not differ between the exercise plus supplement and exercise plus placebo groups (see Supplementary Note 3 for statistical results). Supplementary Tables 6 and 7 present the means and standard deviations of all physical fitness measures and cognitive tests included in the pre-intervention test battery. Pre-intervention means did not differ between the exercise plus supplement and exercise plus placebo intervention for 41 out of 42 fitness and cognitive measures (see Supplementary Note 3 for statistical results). The similarity between the exercise plus supplement and exercise plus placebo groups for both demographic composition and pre-intervention fitness and cognitive scores provides strong evidence that participants were randomly assigned into groups.
Principal component analysis
Principal Component Analysis (PCA) was used to identify the underlying structure of the fitness and cognitive batteries. For fitness, 21 variables from Table 3 were included in PCA and five components accounted for 77% of the variance. The component names, also listed in Table 3 are Power, Strength and Endurance, Mobility and Stability, Blood Pressure and Heart Rate. Supplementary Table 8 presents the loadings for each component. For cognition, 11 variables from Table 4 were included in PCA and five components accounted for 72% of the variance. The component names, also listed in Table 4 are Short Term Memory, Episodic Memory, Fluid Intelligence, Working Memory and Executive Function. Supplementary Table 9 presents the loadings for each component. Supplementary Note 4 presents further details of the PCA.
Efficacy of the exercise training plus placebo intervention
Table 5 reports the statistical results providing evidence for changes in physical fitness, cognition and biomarkers associated with the exercise training plus placebo intervention. Overall, 61% (14 out of 23) of the assessment metrics changed. Five of the six physical fitness measures improved from pre- to post-intervention: power, strength and endurance, mobility and stability, heart rate and lean muscle mass. Blood pressure did not improve. Four of the eight cognitive measures improved from pre- to post-intervention in the exercise training plus placebo intervention: episodic memory, fluid intelligence accuracy, executive function reaction time and processing efficiency. Cognitive measures that did not improve include short term memory, executive function accuracy, fluid intelligence reaction time and working memory. Five of the biomarkers decreased in concentration (cortisol, ferritin, folate, vitamin B12 and low-density lipoprotein) while nine biomarkers did not change (lutein density in the fovea or parafovea, HDL, triglyceride levels and all five fatty acids). Supplementary Tables 10–12 report the pre- and post-intervention means and standard deviations for the physical fitness and cognitive batteries and biomarkers for the exercise training plus placebo group.
Table 5.
Statistical results for the efficacy of the exercise plus placebo intervention.
| t-stat | df | p value | d | Bf | |
|---|---|---|---|---|---|
| Physical fitness | |||||
| Power* | 3.22 | 73 | 0.001 | 0.37 | 28.1 |
| Strength and endurance* | 12.3 | 65 | 10−16 | 1.52 | 1016 |
| Mobility and stability* | 5.53 | 74 | 10−7 | 0.64 | 106 |
| Blood pressure | − 0.87 | 76 | 0.19 | 0.10 | 0.29 |
| Heart rate* | − 2.16 | 75 | 0.018 | 0.25 | 2.2 |
| Lean muscle mass* | 3.42 | 76 | 0.001 | 0.39 | 48.9 |
| Cognition | |||||
| Short term memory | − 2.4 | 72 | 0.99 | 0.29 | 0.04 |
| Episodic memory* | 2.4 | 55 | 0.01 | 0.32 | 3.65 |
| Fluid intelligence acc* | 5.5 | 73 | 10−7 | 0.64 | 105 |
| Fluid intelligence RT | 0.55 | 72 | 0.71 | 0.06 | 0.09 |
| Working memory | − 1.64 | 73 | 0.95 | 0.19 | 0.05 |
| Executive function acc | − 1.14 | 73 | 0.87 | 0.13 | 0.06 |
| Executive function RT* | − 3.2 | 73 | 0.0011 | 0.37 | 23.2 |
| Processing efficiency* | 2.6 | 73 | 0.005 | 0.23 | 6.4 |
| Biomarkers | |||||
| Cortisol (µg/dL)* | − 1.60 | 77 | 0.056 | 0.18 | 0.80 |
| Ferritin (ng/mL)* | − 6.58 | 76 | 10−9 | 0.75 | 106 |
| Folate (ng/mL)* | − 1.65 | 76 | 0.051 | 0.19 | 0.87 |
| High density lipoprotein (mg/dL) | 0.77 | 77 | 0.22 | 0.09 | 0.26 |
| Low density lipoprotein (mg/dL)* | − 2.33 | 77 | 0.01 | 0.26 | 3.14 |
| Lutein density in fovea | − 0.13 | 30 | 0.55 | 0.02 | 0.17 |
| Lutein density in parafovea | − 0.43 | 30 | 0.66 | 0.08 | 0.14 |
| Triglycerides (mg/dL) | − 0.19 | 76 | 0.58 | 0.02 | 0.11 |
| Vitamin B12 (pg/mL)* | − 3.36 | 76 | 0.001 | 0.38 | 40.6 |
| Saturated fatty acids (mol%) | 0.30 | 77 | 0.38 | 0.04 | 0.16 |
| Monounsaturated fatty acids (mol%) | 1.25 | 77 | 0.11 | 0.14 | 0.47 |
| Omega-3 PUFAs (mol%) | − 0.43 | 77 | 0.67 | 0.05 | 0.18 |
| Omega-6 PUFAs (mol%) | − 0.51 | 77 | 0.69 | 0.06 | 0.09 |
| Trans fatty acids (mol%) | − 1.53 | 77 | 0.06 | 0.17 | 0.71 |
t-stat is the value of the 1-sided t-statistic (where negative values represent decreases from pre- to post-intervention); df is the degrees of freedom of the t-test; p value is the frequentist p value of the t-test; d is Cohen’s d; and Bf is the Bayes factor for the t-test. An * in the row header indicates the exercise plus placebo intervention modified that indicator. PUFA stands for polyunsaturated fatty acid.
Efficacy of the exercise training plus supplement intervention
Table 6 reports the statistical results providing evidence for changes in physical fitness, cognition and biomarkers associated with the exercise training plus supplement. Overall, 83% (19 out of 23) of the assessment metrics changed. All six physical fitness measures improved from pre- to post-intervention: power, strength and endurance, mobility and stability, heart rate, blood pressure and lean muscle mass. Six of the eight cognitive measures improved in the exercise training plus nutritional supplement intervention: episodic memory, fluid intelligence accuracy and reaction time, working memory, executive function reaction time and processing efficiency. Accuracy for short term memory and executive function did not improve. Eight of the biomarkers changed (cortisol, ferritin, omega-6 polyunsaturated fats and low-density lipoprotein decreased; folate, vitamin B12, omega-3 polyunsaturated fats and lutein density in the fovea all increased). Follow-up analyses of the omega-3 fatty acids revealed that serum concentrations of both docosahexaenoic (DHA) and eicosapentaenoic (EPA) increased but docosapentaenoic (DPA) decreased. For omega-6 fatty acids, follow-up analyses revealed concentrations of arachadonic, adrenic, and docosapentaenoic acid all decreased. Six biomarkers did not change (HDL, triglycerides, saturated fatty acids, trans fatty acids, mono-unsaturated fatty acids and lutein density in the parafovea). Supplementary Tables 10–12 report the pre- and post-intervention means and standard deviations for the physical fitness and cognitive batteries and biomarkers for the exercise training plus supplement group.
Table 6.
Statistical results for the efficacy of the exercise plus supplement intervention.
| t-stat | df | p value | d | Bf | |
|---|---|---|---|---|---|
| Physical fitness | |||||
| Power* | 4.97 | 63 | 10−6 | 0.62 | 6324 |
| Strength and endurance* | 10.9 | 50 | 10−15 | 1.52 | 1012 |
| Mobility and stability* | 6.1 | 69 | 10−8 | 0.73 | 106 |
| Blood pressure* | − 2.7 | 69 | 0.004 | 0.33 | 7.9 |
| Heart rate* | − 6.2 | 68 | 10−8 | 0.75 | 106 |
| Lean muscle mass* | 9.2 | 69 | 10−14 | 1.10 | 1010 |
| Cognition | |||||
| Short term memory | − 2.2 | 63 | 0.98 | 0.27 | 0.05 |
| Episodic memory* | 2.28 | 58 | 0.013 | 0.30 | 3.05 |
| Fluid intelligence acc* | 5.3 | 65 | 10−7 | 0.65 | 104 |
| Fluid intelligence RT* | − 1.85 | 65 | 0.034 | 0.23 | 1.30 |
| Working memory* | 1.81 | 67 | 0.04 | 0.22 | 1.20 |
| Executive function acc | − 4.3 | 66 | 0.001 | 0.52 | 0.03 |
| Executive function RT* | − 5.6 | 66 | 10−7 | 0.68 | 104 |
| Processing efficiency* | 5.4 | 69 | 10−7 | 0.47 | 104 |
| Biomarkers | |||||
| Cortisol (µg/dL)* | − 1.61 | 54 | 0.057 | 0.22 | 0.92 |
| Ferritin (ng/mL)* | − 5.96 | 54 | 10−8 | 0.80 | 105 |
| Folate (ng/mL)* | 1.74 | 54 | 0.043 | 0.24 | 1.15 |
| High density lipoprotein (mg/dL) | 1.04 | 54 | 0.15 | 0.14 | 0.41 |
| Low density lipoprotein (mg/dL)* | − 2.54 | 54 | 0.007 | 0.35 | 5.45 |
| Lutein density in fovea* | 1.62 | 13 | 0.06 | 0.43 | 1.42 |
| Lutein density in parafovea | 1.07 | 13 | 0.15 | 0.29 | 0.73 |
| Triglycerides (mg/dL) | 1.25 | 54 | 0.11 | 0.17 | 0.54 |
| Vitamin B12 (pg/mL)* | 2.02 | 54 | 0.024 | 0.27 | 1.89 |
| Saturated fatty acids (mol%) | 0.24 | 55 | 0.41 | 0.04 | 0.18 |
| Monounsaturated fatty acids (mol%) | − 0.15 | 55 | 0.56 | 0.02 | 0.13 |
| Omega-3 PUFAs (mol%)* | 6.72 | 55 | 10−9 | 0.90 | 106 |
| Omega-6 PUFAs (mol%)* | − 3.52 | 55 | 0.00043 | 0.47 | 62.2 |
| Trans fatty acids (mol%) | − 0.86 | 55 | 0.20 | 0.11 | 0.33 |
t-stat is the value of the 1-sided t-statistic (where negative values represent decreases from pre- to post-intervention); df is the degrees of freedom of the t-test; p value is the frequentist p value of the t-test; d is Cohen’s d; and Bf is the Bayes factor for the t-test. An * in the row header indicates the exercise plus placebo intervention modified that indicator. PUFA stands for polyunsaturated fatty acid.
Efficacy of the unimodal versus the multimodal intervention
The unimodal exercise plus placebo intervention was compared to the multimodal exercise plus supplement using an ANCOVA model to determine if the addition of the nutritional supplement conferred incremental physical and cognitive benefits. Measures were only tested in the ANCOVA if there was a change from pre- to post-intervention in Table 5 or 6. The multimodal exercise plus supplement resulted in further improvement beyond those observed for the exercise plus placebo intervention for heart rate, lean muscle mass, fluid intelligence reaction time, working memory, processing efficiency, folate, vitamin B12, omega-3 and omega-6 polyunsaturated fatty acids (Table 7). Figure 1 plots the average maximum heart rate for the multimodal versus the unimodal intervention group and demonstrates a steeper rate of decline in maximum heart rate for the nutritional supplement. For the remaining measures in Table 7 (power, strength and endurance, mobility and stability, blood pressure, episodic memory, fluid intelligence accuracy, executive function reaction time, cortisol and ferritin levels and lutein density in the fovea) the multimodal exercise plus supplement did not confer incremental benefits beyond the unimodal exercise plus placebo. Supplementary Table 13 provides the mean values underlying the statistical tests in Table 7. Supplementary Table 14 presents Bayes factor ratios comparing the two interventions to provide a quantitative measure of their relative efficacy, which provides further evidence of the benefit of the nutritional supplement. Supplementary Note 5 provides further discussion of Supplementary Table 14.
Table 7.
Statistical results comparing the efficacy of the unimodal exercise plus placebo intervention to the multimodal exercise plus supplement intervention.
| F-stat | df | p value | d | Bf | |
|---|---|---|---|---|---|
| Physical fitness | |||||
| Power | 1.46 | (2,135) | 0.23 | 0.20 | 0.35 |
| Strength and endurance | 1.80 | (2,114) | 0.18 | 0.22 | 0.48 |
| Mobility and stability | 0.05 | (2,142) | 0.82 | 0.04 | 0.18 |
| Blood pressure | 0.75 | (2,144) | 0.39 | 0.14 | 0.48 |
| Heart rate* | 7.42 | (2,142) | 0.007 | 0.45 | 7.03 |
| Lean muscle mass* | 13.9 | (2,144) | 0.0003 | 0.61 | 63.4 |
| Cognition | |||||
| Episodic memory | 1.77 | (2,115) | 0.19 | 0.22 | 0.14 |
| Fluid intelligence acc | 0.27 | (2,140) | 0.60 | 0.09 | 0.20 |
| Fluid intelligence RT* | 2.28 | (2,139) | 0.13 | 0.25 | 2.09 |
| Working memory* | 6.81 | (2,142) | 0.01 | 0.43 | 8.27 |
| Executive function RT | 0.99 | (2,141) | 0.32 | 0.16 | 0.53 |
| Processing efficiency* | 3.12 | (2,141) | 0.08 | 0.29 | 1.07 |
| Biomarkers | |||||
| Cortisol (µg/dL) | 0.04 | (2,129) | 0.84 | 0.03 | 0.21 |
| Low density lipoprotein (mg/dL) | 0.13 | (2,129) | 0.72 | 0.06 | 0.21 |
| Ferritin (ng/mL) | 0.30 | (2,129) | 0.59 | 0.09 | 0.27 |
| Folate (ng/mL)* | 14.6 | (2,129) | 0.0002 | 0.66 | 2.65 |
| Lutein density in fovea | 0.48 | (2,42) | 0.49 | 0.12 | 0.49 |
| Vitamin B12 (pg/mL)* | 16.5 | (2,129) | 10−5 | 0.70 | 79.9 |
| Omega-3 PUFAs (mol%)* | 46.1 | (2,131) | 10−16 | 1.18 | 107 |
| Omega-6 PUFAs (mol%)* | 6.7 | (2,131) | 0.011 | 0.45 | 5.93 |
F-stat is the value of the ANCOVA model which included group as a factor and pre-intervention performance as a covariate; df is the degrees of freedom of the ANCOVA model; p value is the frequentist p value of the ANCOVA model; d is Cohen’s d; and Bf is the Bayes factor for the ANCOVA. An * in the row header means the exercise plus supplement intervention improved that indicator more than the exercise plus placebo intervention. To minimize the number of analyses conducted—and the resulting Type 1 error—Table 7 only includes rows from Table 5 or 6 that had a significant result. PUFA stands for polyunsaturated fatty acid. Supplementary Table 13 lists the means underlying these statistical tests.
Figure 1.
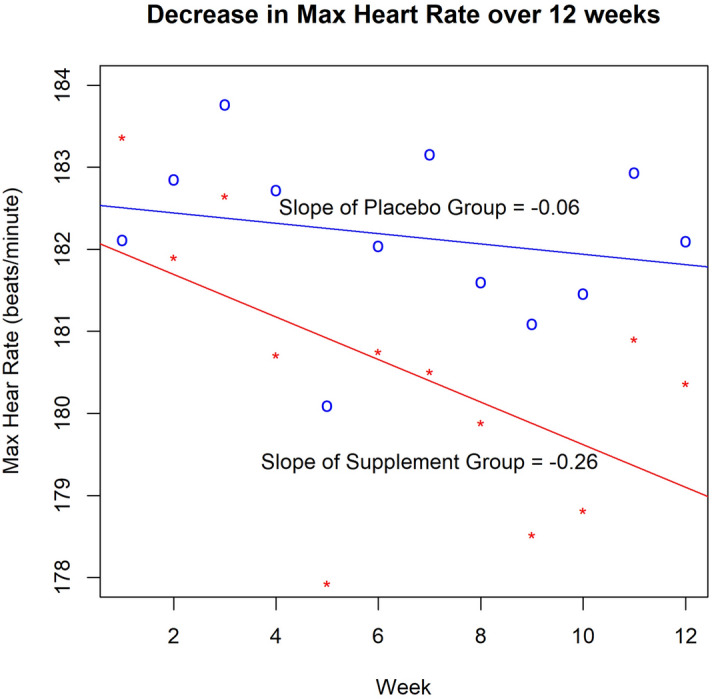
Maximum heart rate for the exercise plus supplement (red line) and exercise plus placebo (blue line) interventions. The x-axis is the week of the study and the y-axis is the average maximum heart rate. Each point on the plot represents the average maximum heart rate across all participants in that intervention condition for that week.
Discussion
We conducted an RCT in healthy, fit and active duty Air Force servicemen and women (n = 148) to investigate the efficacy of an exercise training intervention and a novel nutritional supplement for improving physical fitness and cognition. Exercise training interventions are known to improve overall physical health and cognition and we also observed these improvements in the current study41. Our study extends this body of work by demonstrating that the combination of a nutritional supplement and an exercise training intervention provided physical health and cognitive gains beyond those from exercise alone. The large sample size of the present study and the large number of assessments across physical fitness, cognition and biomarkers permit five primary conclusions.
First, both interventions improved measures of physical fitness. The exercise training plus placebo intervention improved 21 individual measures of physical fitness and the exercise training plus nutritional supplement intervention improved 23 individual measures of physical fitness. Given the huge Cohen’s d effect sizes in some cases, and the decisive Bf results, there is clear and compelling evidence that the exercise training intervention was effective and that it improved physical fitness.
Second, both interventions improved measures of cognition. The exercise training plus placebo intervention improved 8 individual measures of cognition. The exercise training plus nutritional supplement intervention effectively improved 11 individual measures of cognition. These results suggest that the combination of a nutritional supplement with the exercise training intervention selectively extended the benefits of the exercise training plus placebo intervention for some of the cognitive outcomes. Consistent with previous research41, the exercise training plus placebo intervention drove improvements in cognitive function.
The third primary conclusion from the study is that the multimodal exercise training plus nutritional supplement intervention conferred advantages on physical fitness and cognition beyond the effects of the unimodal exercise training plus placebo intervention. Heart rate, lean muscle mass, fluid intelligence reaction time, working memory and processing efficiency all improved more for the multimodal exercise training plus nutritional supplement. Broadly, this is consistent with recent studies demonstrating advantages for multimodal interventions, relative to unimodal interventions, for improving physical fitness or cognition42,43.
Fourth, changes in biomarkers provide further evidence to support the efficacy of the interventions. One inflammatory marker, ferritin, decreased for both intervention groups; but the nutritional supplement did not reduce ferritin levels more than the exercise training intervention. Increasingly, ferritin is recognized as an important marker of inflammation and cell damage; higher levels are associated with worse cognitive functioning44. In the current study, the exercise intervention decreased inflammation, as measured by ferritin. Vitamin B12, folate, the omega-3 polyunsaturated fatty acid docosahexaenoic acid (DHA) and lutein were consumed daily as part of the nutritional supplement intervention. Increased serum concentrations of these four nutrients were observed in the exercise training and nutritional supplement intervention only. Concentrations of vitamin B12 and folate decreased in the exercise training plus placebo intervention. Concentrations of omega-6 polyunsaturated fatty-acids, which were not part of the nutritional supplement, decreased only for the exercise plus nutritional supplement intervention. Importantly, concentrations of trans, saturated and monounsaturated fatty acids did not change from pre- to post-intervention for either intervention group. Because neither the nutritional formula nor the placebo contained any of these biomarkers, we observe selectivity of biological uptake for the biomarkers that were included in the nutritional formula.
These changes in serum concentrations of biomarkers in participants in the nutritional supplement intervention suggest possible mechanisms that might have driven improvements in cognition and physical fitness. B vitamins are critical for brain health45. Younger adult populations show stronger associations between B vitamin supplementation and cognitive improvement46. Therefore, it is reasonable to conclude that B vitamins from the nutritional supplement in the current study are partially responsible for some of the observed improvements in cognitive function in the exercise training plus nutritional supplement group. The nutritional supplement intervention group had increased concentrations of both docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA). Long chain polyunsaturated fatty acids, or PUFAs, like DHA and EPA, are found in high concentrations in the brain’s gray matter. PUFAs in the brain are known to promote neuronal growth and synapse formation and facilitate neural transmission47. Long chain omega-6 polyunsaturated fatty acids are important for brain and cognitive health; but this effect is most pronounced when they are in a balanced ratio with the omega-3 polyunsaturated fats48,49. Long chain omega-3 and omega-6 fatty acids compete for chemical conversion to various structures and molecules inside and outside cells. In the current study, for the nutritional intervention group, omega-6 concentrations for arachadonic, adrenic, and docosapentaenoic acids all decreased but the omega-3 concentrations of DHA and EPA increased. The increased availability of omega-3’s from the nutritional supplement resulted in a reduction in the ratio of omega-6/omega-3 from 10:1 at the start of the study to 8:1 by the end of the intervention. The ratio remained unchanged at 10:1 from pre- to post-intervention for the exercise plus placebo group. The observed reduction in concentration of several omega-6 fatty acids in the nutritional supplement group was most likely due to the increased availability of omega-3 fatty acids from the nutritional supplement, thereby driving the smaller omega-6/omega-3 ratio which, in turn, contributed to the improved cognitive outcomes for the group receiving the nutritional supplement.
Protein, HMB (β-hydroxy β-methylbutyrate) and vitamin D, all of which were included in the nutritional supplement, increase muscle protein synthesis and muscle growth and improve physical fitness. Protein supplementation increases muscle mass and muscle strength50. Furthermore, a dose-dependent relationship exists between the amount of dietary protein and uptake into the body for overall whole-body protein levels and incorporation into muscle fibers51. β-hydroxy β-methylbutyrate (HMB), which was included in the nutritional supplement, synergistically works with protein for increasing lean muscle mass and improves recovery in resistance exercise52,53. Vitamin D facilitates absorption of calcium in bones and supports muscle growth54. Enhancements to overall physical fitness and health emerged when protein, HMB, protein and vitamin D were taken together as part of an oral nutritional supplement55. And the effects of protein supplementation can be augmented by exercise. Interventions that combine exercise training with nutritional supplements that promote muscle recovery and growth provide the body with necessary building blocks for greater fitness training gains56–58.
Although the current study provides evidence that the nutritional supplement improved physical fitness and cognition when coupled with exercise training, more research is needed to refine these findings. First, the present study only considered the efficacy of the intervention at the group level. Having demonstrated group level changes to the interventions, follow-up studies should seek to understand the role of individual variability. There is a growing body of research demonstrating that different individuals may need a different number of intervention sessions to achieve the same gain59. And different individuals start interventions with different levels of physical fitness and cognitive ability. Accounting for these differential starting points, and possibly using baseline information as a predictor of the possible training gains, could allow further tailoring of intervention protocols and sensitivity of testing to achieve optimal performance gains for individuals. Second, it is not known if interventions like exercise training and a nutritional supplement beverage have upper limits to the physical fitness or cognitive gains achievable, whether at the group or individual level. Dose–response curves would help tease this out. Third, the current study used principal component analysis (PCA) to aggregate physical fitness, cognitive and biomarker measures according to common structure or function. Part of the motivation for using PCA was to amplify the underlying signal and reduce the number of statistical tests required; but there are other statistical approaches which could derive further insights about the efficacy of the interventions. Fourth, it was not possible to include a true control condition because active duty military populations necessarily undergo a certain amount of physical movement in their day-to-day activity. However, Supplementary Note 6 provides the results of an analysis including a passive control group from another study, where study participants matched the demographic and physiological profile of the current study, and who also completed the same cognitive battery as participants in the current study. These results are consistent with the results reported in the current study that did not include a true control group. Fifth, the mechanism of improvement for cognition from the addition of the nutritional supplement needs to be elucidated. There are at least 3 possibilities: (a) the supplement enhanced fitness, which in turn improved cognition more than the exercise training intervention alone, (b) the supplement directly enhanced cognition, (c) the supplement improved fitness, which improved cognition and the supplement also directly improved cognition. Functional and structural brain imaging analyses would facilitate an understanding of how the brain changes in an intervention, as would a study with intervention groups designed to isolate this mechanism. Sixth, more research needs to be conducted to further understand the mechanism of some biomarker results. Serum B vitamin concentrations increased for the nutritional supplement group but decreased for the placebo group. Hence, the nutritional formula restored the B vitamins depleted through exercise. Future research should examine whether increased B vitamin concentration is partially responsible for the gains in cognition observed in the nutritional supplement intervention. Finally, because the nutritional supplement is a liquid that needs to be refrigerated, its use would be limited to garrison or home settings. However, because the nutritional supplement appears to enhance the body and brain with continued usage, it should not be considered to aid immediate safety or performance, as might be required in a field environment.
The nutritional supplement was a user-friendly way to ensure each participant received equivalent amounts of scientifically proven daily allowances of both macro- and micro-nutrients that supported health benefits. A study using a whole food intervention might confer some advantages over a nutritional supplement, such as greater bioavailability and adsorption of key nutrients; but running a 3-month RCT with whole food intervention presents several challenges. First, the whole food intervention would need to select a variety of foods but still ensure equivalent dosage of key nutrients. Second, in the current study, the non-isocaloric placebo represented a normal diet since those participants had no dietary restrictions. In a whole food intervention, it would be harder to have a true placebo. Third, administering whole food meals is costly (both in terms of time and money) and challenging to track consumption. In the current study, it was easy to watch participants drink two 8-ounce servings of the liquid nutritional supplement in the lab every day; but tracking the consumption of whole food meals is much more challenging. Despite these challenges, future work should attempt to implement a nutritional intervention that includes an isocaloric control. Moreover, researchers should seek to develop nutritional supplements that more closely approximate whole food. To this end, some researchers have begun to elucidate how patterns of nutrient biomarkers, which presumably relate to eating a healthy, whole food diet, enhance cognitive and brain function60,61.
In conclusion, the current study supports the efficacy of a multimodal fitness and nutritional intervention to improve both physical fitness and cognition in Air Force Airmen. Moreover, these changes in the body and brain align with corresponding changes in blood-based biomarkers of nutrition. Our study also demonstrates a wider range of improvements and larger effect sizes due to multimodal training compared to unimodal fitness training alone. While the findings are based on a large sample of Air Force Airmen and demonstrate that physical fitness and cognitive enhancement is achievable, the multimodal lifestyle intervention documented in this study could easily be implemented in other real-world contexts to optimize human performance.
Supplementary information
Acknowledgements
Steven A. Culpepper provided valuable comments about Bayes factors analyses. This work was supported by a grant from Abbott Nutrition through the Center for Nutrition, Learning, and Memory at the University of Illinois (ANGC1402; PI: Barbey).
Author contributions
C.E.Z. conducted the study analysis, contributed to the interpretation of findings, and drafted the manuscript. A.S. and E.J. assisted with the study design; led data collection; contributed to the analysis, interpretation and write up of the physical fitness results; and assisted with drafting part of the methods. E.A. helped interpret and write up findings, organized data, and assisted with data analysis. J.J. assisted with data collection and the analysis and interpretation of biomarkers. T.D. and M.J.K. both contributed to the study design and drafting of the manuscript. A.K.B. conceptualized the study as part of a larger grant, led study procedures, and contributed to the interpretation of findings and drafting of the manuscript. All authors reviewed the manuscript.
Data availability
The individual de-identified participant data and related study documents can be made available upon request.
Code availability
All analyses were conducted in R using standard packages and functions (see "Methods" section).
Competing interests
TD and MJK are research scientists at Abbott Nutrition. There are no other competing interests to declare for all other authors.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-74140-7.
References
- 1.Nindl BC, et al. Perspectives on resilience for military readiness and preparedness: report of an international military physiology roundtable. J. Sci. Med. Sport. 2018;21:1116–1124. doi: 10.1016/j.jsams.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 2.Lieberman HR. Nutrition, brain function, and cognitive performance. Appetite. 2003;40:245–254. doi: 10.1016/S0195-6663(03)00010-2. [DOI] [PubMed] [Google Scholar]
- 3.Special Operations Forces: Preservation of the Force and Family (accessed 15 June 2020); https://www.socom.mil/POTFF/Pages/About-POTFF.aspx (2020).
- 4.Taylor MK, et al. Physical fitness influences stress reactions to extreme military training. Mil. Med. 2008;173:738–742. doi: 10.7205/MILMED.173.8.738. [DOI] [PubMed] [Google Scholar]
- 5.Morgan CA, Doran A, Steffian G, Hazlett G, Southwick SM. Stress-induced deficits in working memory and visuo-constructive abilities in special operations soldiers. Biol. Psychiatry. 2006;60:722–729. doi: 10.1016/j.biopsych.2006.04.021. [DOI] [PubMed] [Google Scholar]
- 6.Bovill ME, Tharion WJ, Lieberman HR. Nutritional knowledge and supplement use among elite US Army soldiers. Mil. Med. 2003;168:997–1000. doi: 10.1093/milmed/168.12.997. [DOI] [PubMed] [Google Scholar]
- 7.Lieberman HR, et al. Use of dietary supplements among active-duty US Army soldiers. Am. J. Clin. Nutr. 2010;92:985–995. doi: 10.3945/ajcn.2010.29274. [DOI] [PubMed] [Google Scholar]
- 8.Deuster PA, Lindsey AT, Scott JM. Dietary supplements: regulatory challenges and issues in the department of defense. Mil. Med. 2018;183:53–55. doi: 10.1093/milmed/usx067. [DOI] [PubMed] [Google Scholar]
- 9.Crawford C, Saldanha RC, Deuster PA. Dietary supplements for musculoskeletal pain: science versus claims. J. Spec. Oper. Med. 2018;18:110–114. doi: 10.55460/8VTS-JFKO. [DOI] [PubMed] [Google Scholar]
- 10.Doan BK, Hickey PA, Lieberman HR, Fischer JR. Caffeinated tube food effect on pilot performance during a 9-hr simulated nighttime U-2 mission. Aviat. Space Environ. Med. 2006;77:1034–1040. [PubMed] [Google Scholar]
- 11.Cohen PA, Travis JC, Keizers PHJ, Deuster P, Venhuis BJ. Four experimental stimulants found in sports and weight loss supplements: 2-amino-6-methylheptane (octodrine), 1,4-dimethylamylamine (1,4-DMAA), 1,3-dimethylamylamine (1,3-DMAA) and 1,3-dimethylbutylamine (1,3-DMBA) Clin. Toxicol. 2018;56:421–426. doi: 10.1080/15563650.2017.1398328. [DOI] [PubMed] [Google Scholar]
- 12.Zamroziewicz M, Barbey AK. Role of the Mediterranean Diet in the Brain and Neurodegenerative Diseases. Amsterdam: Elsevier; 2017. [Google Scholar]
- 13.Sofi F, Macchi C, Casini A. Mediterranean diet and minimizing neurodegeneration. Curr. Nutr. Rep. 2013;2:75–80. doi: 10.1007/s13668-013-0041-7. [DOI] [Google Scholar]
- 14.Parletta N, Milte CM, Meyer BJ. Nutritional modulation of cognitive function and mental health. J. Nutr. Biochem. 2013;24:725–743. doi: 10.1016/j.jnutbio.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 15.Hammond BR, et al. Effects of lutein/zeaxanthin supplementation on the cognitive function of community dwelling older adults: a randomized, double-masked, placebo-controlled trial. Front. Aging Neurosci. 2017;9:254. doi: 10.3389/fnagi.2017.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Renzi-Hammond LM, et al. Effects of a lutein and zeaxanthin intervention on cognitive function: a randomized, double-masked, placebo-controlled trial of younger healthy adults. Nutrients. 2017;9:1246. doi: 10.3390/nu9111246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Féart C, et al. Adherence to a Mediterranean diet and plasma fatty acids: data from the Bordeaux sample of the Three-City study. Br. J. Nutr. 2011;106:149–158. doi: 10.1017/S0007114510005805. [DOI] [PubMed] [Google Scholar]
- 18.Rallidis LS, et al. Close adherence to a Mediterranean diet improves endothelial function in subjects with abdominal obesity. Am. J. Clin. Nutr. 2009;90:263–268. doi: 10.3945/ajcn.2008.27290. [DOI] [PubMed] [Google Scholar]
- 19.Frisardi V, et al. Nutraceutical properties of Mediterranean diet and cognitive decline. J. Alzheimer's Dis. 2010;22:715–740. doi: 10.3233/JAD-2010-100942. [DOI] [PubMed] [Google Scholar]
- 20.Witte AV, et al. Long-chain omega-3 fatty acids improve brain function and structure in older adults. Cereb. Cortex. 2014;24:3059–3068. doi: 10.1093/cercor/bht163. [DOI] [PubMed] [Google Scholar]
- 21.Hertzog C, Kramer AF, Wilson RS, Lindenberger U. Enrichment effects on adult cognitive development: can the functional capacity of older adults be preserved and enhanced? Psychol. Sci. Public Interest. 2009;9:1–65. doi: 10.1111/j.1539-6053.2009.01034.x. [DOI] [PubMed] [Google Scholar]
- 22.Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol. Sci. 2003;14:125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- 23.Colcombe SJ, et al. Cardiovascular fitness, cortical plasticity, and aging. Proc. Natl. Acad. Sci. U. S. A. 2004;101:3316–3321. doi: 10.1073/pnas.0400266101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wright Patterson Air Force Base. (accessed 15 June 2020); https://www.wpafb.af.mil/afrl/711hpw/ (2020).
- 25.Roche. B12 (accessed 15 June 2020); https://pim-eservices.roche.com/eLD_SF/us/en/Documents/GetDocument?documentId=3d2ed552-5b35-e911-a49d-00215a9b3428.
- 26.Roche. Folate (accessed 15 June 2020); https://pim-eservices.roche.com/eLD_SF/us/en/Documents/GetDocument?documentId=21a71400-a546-e911-939f-00215a9b3428.
- 27.Roche. Triglycerides (accessed 15 June 2020); https://pim-eservices.roche.com/eLD_SF/us/en/Documents/GetDocument?documentId=1aabd09a-50c4-e711-b48d-00215a9b3428.
- 28.Roche. Ferritin (accessed 15 June 2020); https://pim-eservices.roche.com/eLD_SF/us/en/Documents/GetDocument?documentId=544f48b4-c593-e811-828e-00215a9b3428.
- 29.Roche. Cortisol. (accessed 15 June 2020); https://pim-eservices.roche.com/eLD_SF/us/en/Documents/GetDocument?documentId=c2c16f20-20a4-e911-f08c-f4a30bc86a7b.
- 30.Matthan NR, Ip B, Resteghini N, Ausman LM, Lichtenstein AH. Plasma phospholipid fatty acid biomarkers of dietary fat quality and endogenous metabolism predict coronary heart disease risk: a nested case-control study within the Women's Health Initiative observational study. J. Lipid Res. 2010;51:2826–2832. doi: 10.1194/jlr.D007534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.RStudio . Integrated Development for R. Boston: RStudio, Inc.; 2015. [Google Scholar]
- 32.R Foundation for Statistical Computing . R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing; 2017. [Google Scholar]
- 33.psych: Procedures for Personality and Psychological Research v. R package version = 1.8.10 (Northwestern University, Evanston, Illinois, USA, 2018).
- 34.effsize: Efficient Effect Size Computation. v. R package version 0.7.4 (2018).
- 35.compute.es: Compute Effect Sizes. v. R package version 0.2-2 (2013).
- 36.Navarro, D. J. (University of Adelaide, Adelaide, Australia, 2015).
- 37.BayesFactor: Computation of Bayes Factors for common designs. v. R package version 0.9.12-4.2. (2018).
- 38.Sawilowsky S. New effect size rules of thumb. J. Mod. Appl. Stat. Methods. 2009;8:467–474. doi: 10.22237/jmasm/1257035100. [DOI] [Google Scholar]
- 39.Cohen J. A power primer. Psychol. Bull. 1992;112:155–159. doi: 10.1037/0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- 40.Kass RE, Raftery AE. Bayes factors. J. Am. Stat. Assoc. 1995;90:773–795. doi: 10.1080/01621459.1995.10476572. [DOI] [Google Scholar]
- 41.Northey JM, Cherbuin N, Pumpa KL, Smee DJ, Rattray B. Exercise interventions for cognitive function in adults older than 50: a systematic review with meta-analysis. Br. J. Sports Med. 2018;152:154–160. doi: 10.1136/bjsports-2016-096587. [DOI] [PubMed] [Google Scholar]
- 42.Daugherty AM, et al. Multi-modal fitness and cognitive training to enhance fluid intelligence. Intelligence. 2017;66:32–43. doi: 10.1016/j.intell.2017.11.001. [DOI] [Google Scholar]
- 43.Zwilling CE, et al. Enhanced decision-making through multimodal training. Sci. Learn. 2019 doi: 10.1038/s41539-019-0049-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kell DB, Pretorius E. Serum ferritin is an important inflammatory disease marker, as it is mainly a leakage product from damaged cells. Metallomics. 2014;6:748–773. doi: 10.1039/C3MT00347G. [DOI] [PubMed] [Google Scholar]
- 45.Kennedy DO. B vitamins and the brain: mechanisms, dose and efficacy—a review. Nutrients. 2016;8:68. doi: 10.3390/nu8020068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Qin B, et al. Intake of niacin, folate, vitamin B-6, and vitamin B-12 through young adulthood and cognitive function in midlife: the Coronary Artery Risk Development in Young Adults (CARDIA) study. Am. J. Clin. Nutr. 2017;106:1032–1040. doi: 10.3945/ajcn.117.157834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Das UN. Autism as a disorder of deficiency of brain-derived neurotrophic factor and altered metabolism of polyunsaturated fatty acids. Nutrition. 2013;29:1175–1185. doi: 10.1016/j.nut.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 48.Simopoulos AP. Evolutionary aspects of diet: the omega-6/omega-3 ratio and the brain. Mol. Neurobiol. 2011;44:203–215. doi: 10.1007/s12035-010-8162-0. [DOI] [PubMed] [Google Scholar]
- 49.Andruchow ND, Konishi K, Shatenstein B, Bohbot VD. A lower ratio of omega-6 to omega-3 fatty acids predicts better hippocampus-dependent spatial memory and cognitive status in older adults. Neuropsychology. 2017;31:724–734. doi: 10.1037/neu0000373. [DOI] [PubMed] [Google Scholar]
- 50.Hanach NI, McCullough F, Avery A. The impact of dairy protein intake on muscle mass, muscle strength, and physical performance in middle-aged to older adults with or without existing sarcopenia: a systematic review and meta-analysis. Adv. Nutr. 2019;10:59–69. doi: 10.1093/advances/nmy065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Holwerda AM, et al. Dose-dependent increases in whole-body net protein balance and dietary protein-derived amino acid incorporation into myofibrillar protein during recovery from resistance exercise in older men. J. Nutr. 2019;149:221–230. doi: 10.1093/jn/nxy263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Portal S, et al. The effect of HMB supplementation on body composition, fitness, hormonal and inflammatory mediators in elite adolescent volleyball players: a prospective randomized, double-blind, placebo-controlled study. Eur. J. Appl. Physiol. 2011;111:2261–2269. doi: 10.1007/s00421-011-1855-x. [DOI] [PubMed] [Google Scholar]
- 53.Durkalec-Michalski K, Jeszka J. The efficacy of a β-hydroxy-β-methylbutyrate supplementation on physical capacity, body composition and biochemical markers in elite rowers: a randomised, double-blind, placebo-controlled crossover study. J. Int. Soc. Sports Nutr. 2015;12:31. doi: 10.1186/s12970-015-0092-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Holick ME. Vitamin D deficiency. N. Engl. J. Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 55.Sanz-Paris A, et al. Role of oral nutritional supplements enriched with β-hydroxy-β-methylbutyrate in maintaining muscle function and improving clinical outcomes in various clinical settings. J. Nutr. Health Aging. 2018;22:664–675. doi: 10.1007/s12603-018-0995-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Eley HL, Russell ST, Tisdale MJ. Attenuation of depression of muscle protein synthesis induced by lipopolysaccharide, tumor necrosis factor, and angiotensin II by beta-hydroxy-beta-methylbutyrate. Am. J. Physiol. Endocrinol. Meab. 2008;295:E1409–E1416. doi: 10.1152/ajpendo.90530.2008. [DOI] [PubMed] [Google Scholar]
- 57.Girón MD, et al. β-Hydroxy-β-Methylbutyrate (HMB) normalizes dexamethasone-induced autophagy-lysosomal pathway in skeletal muscle. PLoS ONE. 2015;10:e0117520. doi: 10.1371/journal.pone.0117520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Alway SE, Pereira SL, Edens NK, Hao Y, Bennett BT. β-Hydroxy-β-methylbutyrate (HMB) enhances the proliferation of satellite cells in fast muscles of aged rats during recovery from disuse atrophy. Exp. Gerontol. 2013;48:973–984. doi: 10.1016/j.exger.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 59.Montero D, Lundby C. Refuting the myth of non-response to exercise training: “non-responders” do respond to higher dose of training. J. Physiol. 2017;595:3377–3387. doi: 10.1113/JP273480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zwilling CE, Talukdar T, Zamroziewicz MK, Barbey AK. Nutrient biomarker patterns, cognitive function, and fMRI measures of network efficiency in the aging brain. NeuroImage. 2018;188:239–251. doi: 10.1016/j.neuroimage.2018.12.007. [DOI] [PubMed] [Google Scholar]
- 61.Bowman GL, et al. Nutrient biomarker patterns, cognitive function, and MRI measures of brain aging. Neurology. 2012;78:241–249. doi: 10.1212/WNL.0b013e3182436598. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The individual de-identified participant data and related study documents can be made available upon request.
All analyses were conducted in R using standard packages and functions (see "Methods" section).


