Abstract
Background:
The alcohol cue reactivity paradigm is increasingly used to screen medications for the treatment of alcohol use disorder (AUD) and other substance use disorders. Yet, its prospective association with craving and naturalistic drinking outcomes in clinical trials remains unknown. This study embedded repeated human laboratory assessments of alcohol cue reactivity within the context of a randomized controlled trial to examine the effects of varenicline tartrate (Chantix®), a partial agonist of α4β2 nicotinic acetylcholine receptors, on alcohol craving among treatment-seeking heavy drinkers with AUD. Our main objectives were to test whether varenicline, as compared to placebo, blunts alcohol-cue elicited craving and test whether alcohol cue reactivity observed in the human laboratory predicts subsequent alcohol craving and use during the remainder of the trial.
Design and Methods:
This double-blind, randomized, two-site study compared the effects of varenicline (up to 2 mg/day) and placebo on responses to in vivo alcohol cue and affective picture cue exposure in the human laboratory. Forty-seven volunteers (18 females, 29 males), ages 23 to 67 years (M = 43.7, SD = 11.5), were recruited from the community via advertisements to participate in a clinical trial designed to study the effects of varenicline on alcohol use. Participants were randomized to either varenicline or placebo for 6 weeks.
Results:
Varenicline did not attenuate cue-induced alcohol craving relative to placebo, but craving captured during the cue reactivity paradigm significantly predicted subsequent alcohol use in real-world settings during the clinical trial. Higher craving predicted heavier alcohol use.
Conclusions:
Our results are among the first to show alcohol cue-induced craving captured during a human laboratory paradigm predicts drinking outcomes in the context of a clinical trial.
Keywords: Varenicline, alcohol, cue reactivity, human laboratory
Introduction
Alcohol use disorder (AUD) affects nearly one-third of adults in the United States during their lifetime (Grant et al., 2015). Excessive alcohol use causes myriad acute and long-term medical, psychological, and social problems (Litten et al., 2012) and carries an economic burden of $249 billion each year (Sacks et al., 2015). Although advances in pharmacotherapy have improved treatment options for individuals who struggle to reduce their alcohol use, many people do not benefit from existing medications. Thus, efforts are needed to evaluate new medications and better understand how existing treatments work (Chung et al., 2016, Litten et al., 2015).
Human laboratory studies of acute responses to alcohol, alcohol cues, or other experimental manipulations have progressed in important ways and hold potential to advance our understanding of how medications exert beneficial effects on drinking outcomes (Plebani et al., 2012). Alcohol cue reactivity, which involves systematic exposure to in vivo alcohol cues and simulates high-risk situations for relapse, is one of the most widely studied laboratory paradigms in the context of AUD (Reynolds and Monti, 2013, Niaura et al., 1988). Exposure to alcohol cues reliably elicits responses presumed to relate to the motivational processes that underlie drinking (Monti et al., 2000), and it is sensitive to medication effects among treatment seeking and non-treatment seeking adults and adolescents. Alcohol cue-induced craving is blunted by naltrexone (Monti et al., 1999, Miranda et al., 2014, Davidson et al., 1999, McCaul et al., 2000, Ooteman et al., 2007, Lukas et al., 2013), gabapentin (Fox et al., 2012), mifepristone (MacKillop et al., 2015), prazosin (Hutchison et al., 2001), d-cycloserine (MacKillop et al., 2015) and olanzapine (Hutchison et al., 2001) as well as combinations of these medications (Myrick et al., 2008).
Although alcohol cue reactivity is increasingly used to screen medications for the treatment of AUD and other substance use disorders, its prospective association with craving and naturalistic drinking outcomes in clinical trials remains unknown. Medications that blunt alcohol cue-elicited craving in laboratory studies also typically decrease weekly self-reported craving in separate randomized clinical trials (RCTs) (Rosner et al., 2010, Vendruscolo et al., 2015, Mason et al., 2014, Volpicelli et al., 1992, O’Malley et al., 1992, Jonas et al., 2014, Anton et al., 2006).
Although there is evidence that neuroimaging phenotypes can predict drinking outcomes and related medication effects (Bach et al., 2020, Schacht et al., 2013, Schacht et al., 2017, Mann et al., 2014), to our knowledge, no study has directly tested whether cue reactivity, as measured by subjective craving in the human laboratory, predicts medication response (i.e., craving and naturalistic drinking) in the context of a single RCT. Inasmuch as modeling medication effects in the laboratory is meant to provide a time- and cost-efficient strategy for screening new drugs, it is essential that we understand whether these paradigms prospectively predict outcomes.
The present study leveraged the alcohol cue reactivity paradigm, in the context of a RCT, to examine the effects of varenicline tartrate (Chantix®), a partial agonist of α4β2 nicotinic acetylcholine receptors, on alcohol craving among treatment-seeking heavy drinkers with AUD. Varenicline was approved in the United States for smoking cession in 2006 (Gonzales et al., 2006). Four subsequent RCTs tested its efficacy for treating alcohol use among problem drinkers with mixed results (de Bejczy et al., 2015, Litten et al., 2013, O’Malley et al., 2018, Fucito et al., 2011). On the whole, there is insufficient evidence to conclude whether or how varenicline is an effective treatment for AUD. One human laboratory study tested varenicline’s effects on reactivity to in vivo alcohol cues and found that among nontreatment-seeking social drinkers, it attenuated alcohol cue-elicited craving relative to placebo among heavy drinkers, and this effect did not differ between smokers and nonsmokers (Roberts et al., 2017). In addition, a neuroimaging study tested the effects of varenicline administered for two-weeks on alcohol cue-elicited activation of reward-related brain areas among non-treatment-seeking individuals with alcohol dependence (Schacht et al., 2014). Results showed that varenicline, as compared to placebo, decreased alcohol cue-elicited activation of the bilateral orbitofrontal cortex but it did not affect other regions previously shown to be impacted by medications for AUD (e.g., ventral striatum, medial prefrontal cortex). Varenicline did not affect drinking outcomes, and there were no significant relationships between alcohol cue-related brain activation and alcohol use or craving during this brief two-week trial.
The present multisite study embedded repeated assessments of alcohol cue reactivity within the context of a short-duration RCT. Treatment-seeking heavy drinkers with moderate to severe AUD were randomized to varenicline (2 mg/daily) or placebo for six weeks. Our primary goal was to test varenicline’s effects on alcohol-cue elicited craving assessed after three and six weeks of medication or a matched placebo. Based on prior research that found varenicline blunted alcohol cue reactivity after only 1 week of varenicline treatment (Roberts et al. 2017), we examined medication effects on alcohol cue reactivity during Week 3. By studying effects during Week 3 we exceeded this timeframe and allowed for a 1-week titration and 2 weeks of target dosing. Our rationale for the Week 6 cue reactivity session was driven by results of Litten et al (2013), which found maximal treatment effects of varenicline on naturalistic outcomes starting at Week 6. Thus, we aimed to test whether the hypothesized cue reactivity treatment effect might be further enhanced with longer time on varenicline.
We used an established cue reactivity paradigm that pairs mood-induction procedures with in vivo alcohol cue exposure (Mason et al., 2008, Koob and Mason, 2016, Rubonis et al., 1994). This paradigm has been used to test medication effects with promising results (i.e., Mason et al., 2009). We hypothesized that varenicline would reduce alcohol cue-induced craving during both laboratory sessions. Our second objective was to directly test whether cue-elicited craving captured in the laboratory predicted subsequent naturalistic craving and drinking outcomes in the clinical trial. Specifically, we hypothesized that cue reactivity effects observed during first laboratory session (i.e., study week 3) would predict craving and alcohol use during the remainder of the trial. Finally, we evaluated the effects of varenicline compared with placebo on reduction of alcohol consumption, craving, cigarette smoking (among smokers) and nicotine use (among nicotine users), mood, sleep, study retention, and safety and tolerability throughout the last four weeks of the maintenance phase of the study.
Methods and Materials
Participants
Forty-seven volunteers (18 females, 29 males) who were seeking treatment for AUD were recruited from the community for a clinical trial studying the effects of a medication on alcohol use (NCT03035708). Key inclusion criteria were ≥ 21 years old; moderate to severe AUD according to DSM-5; consumed 28 or ≥ 35 drinks per week during the 28 days prior to consent for women and men, respectively; and had at least 1 HDD (≥ 4 for women, ≥ 5 for men) during the 7 days before randomization. Participants were required to demonstrate alcohol cue reactivity, defined as ≥ 3 points higher alcohol craving to alcohol cues compared to water cues on a 20-point visual analogue scale (VAS), during a brief cue reactivity assessment prior to randomization. This pre-randomization paradigm did not include the mood induction.
Key exclusion criteria included current substance use disorders, other than alcohol or nicotine; major psychiatric disorders; positive urine drug screen (except tetrahydrocannabinol); history of suicide attempts or actively suicidal; prior varenicline treatment; and contraindicated medical conditions or medications. Contraindicated medical conditions included serious or unstable medical illness or any potentially life-threatening or progressive medical condition other than addiction that might compromise safety. In addition, individuals with a history of gastric bypass surgery, atherosclerotic cardiovascular disease, or clinically significant electrocardiogram (ECG) results indicative of cardiovascular disease were excluded; stable hypertension was not exclusionary. Exclusionary medications included any pharmacotherapy for AUD (past 6 months), buprenorphine or methadone (past 30 days), and anticonvulsants, hypnotics, barbiturates, antipsychotics, psychomotor stimulants (e.g., methylphenidate), or benzodiazepines within 5 half-lives prior to randomization.
Study Design and Procedures
This double-blind, randomized, parallel group, two-site study compared the effects of varenicline and placebo on responses to in vivo alcohol cue and affective picture cue exposure in the laboratory. Participants were randomized to varenicline or placebo for 6 weeks as outpatients. The protocol, consent, and all study-related materials were approved by the Institutional Review Board at each site (Brown University and Yale School of Medicine), the Food and Drug Administration (FDA), and the Data and Safety Monitoring Board. The study was conducted under International Conference on Harmonization (ICH) Good Clinical Practice (GCP) guidelines and all applicable regulatory requirements. The period of recruitment and follow-up was from May 1, 2017 through July 7, 2019. All participants were informed in writing that they would be exposed to alcohol cues during parts of the study.
Volunteers responded to advertisements and completed a telephone screening. Potentially eligible volunteers underwent additional in-person screening after providing informed consent. Eligible participants completed a baseline visit and were randomized to 6 weeks of either varenicline or matched placebo in a 1:1 ratio using a permuted block randomized procedure stratified by clinical site and nicotine use (past-week). The allocation sequence was computer generated by an independent statistician and the study medical provider assigned eligible participants to the next sequence number by nicotine status. Patients and staff other than the pharmacist staff were blind to treatment condition. During weeks 3 and 6, participants underwent identical alcohol cue reactivity assessments. A final follow-up telephone interview occurred 2 weeks after the last study visit.
Investigational Product
Varenicline or matched placebo was dispensed in identical blister cards at weekly in-clinic visits. Varenicline was titrated over the first week to the maintenance dose of 2 mg/day taken orally BID (i.e., 1 mg each morning and evening) for an additional 5 weeks. Specifically, the daily dose was titrated from 1 capsule (0.5 mg or placebo) on days 1 through 3, to 2 capsules (1.0 mg or placebo) on days 4 through 7, to 4 capsules (2.0 mg or placebo) for the remaining 5 weeks.
Medication compliance was calculated as the total number of capsules taken divided by the total number of capsules prescribed during the 6-week trial. If the blister card was not returned, we relied on self-reports of medication use.
Behavioral Platform
At each weekly visit, participants viewed Take Control modules (Devine et al., 2016), a computerized bibliotherapy platform derived from the National Institute on Alcohol Abuse and Alcoholism’s self-help approach, Rethinking Drinking (NIAAA, 2016), and used in prior pharmacotherapy trials (Litten et al., 2013, Falk et al., 2019).
Human Laboratory Paradigm
The cue exposure paradigm mirrored a published protocol (Mason et al., 2009) and occurred in the late afternoon whenever possible. Table 1 provides an overview of the paradigm. All participants tested negative for breath alcohol before the session and rated their alcohol craving prior to cue-reactivity trials using the Alcohol Craving Questionnaire – Short Form (ACQ-SF-R; Singleton et al., 1994). Cigarette smokers were given the opportunity but not required to smoke their last cigarette approximately 1 hour prior to cue exposure. Participants were escorted to a comfortable chair in a lighting-controlled, sound-attenuated room for mood induction and alcohol exposure.
Table 1.
Chronology of cue reactivity paradigm
| Time (min) | Cue reactivity trials & assessments |
|---|---|
| – 55 | Participant – arrived and rated their alcohol craving prior to cue-reactivity trials; smokers asked (but not required) to have their last cigarette before the cue-reactivity paradigm; participants were asked (but not required) to drink 4 oz of water; clinical and laboratory assessments were completed,a and participants were offered a bathroom break |
| – 5 | Participant was escorted to a comfortable chair located in a windowless, sound-attenuated testing room separated from a control room by a large one-way mirror. Participants were given instructions for the cue reactivity session and presented with a practice trial. |
| 0 | Step 1 — Image exposure: Participants were exposed to 12 affective images (pleasant, unpleasant, or neutral), 10 sec each, 4 sec between each image |
| 5 | Step 2 — In vivo beverage exposure: Alcohol or water beverage were placed in front of the participant for 90 sec while they recalled image-induced mood |
| 10 | Step 3 — Complete ratings: Participants completed VAS craving and in vivo cue manipulation check in presence of beverage, beverage removed from area after ratings completed; research staff collected rating materials and prepared for next trial |
| 60 | Repeated Steps 1–4 for remaining counterbalanced affect-beverage trial combinations; there was a total of six trials. |
Note. VAS = visual analogue scale;
clinical and laboratory assessments included vital signs, a urine toxicology screen, pregnancy/birth control (if female), adverse events, alcohol withdrawal, alcohol timeline follow back interview since the last session, medication compliance and accountability, prior and concomitant medication, smoking/nicotine quantity/frequency, suicidality, and mood/behavior/thinking questions.
As in work by Mason and colleagues (2008, 2009), a 3 Affective Image (positive, neutral, negative) × 2 Beverage (alcohol, water) within-subjects, block factorial design (6 repeated measures) was employed for the cue reactivity manipulation. All six mood-beverage combinations were presented to each participant in counterbalanced order. Positive (e.g. adventure sports, intimate kissing), neutral (e.g. household objects, mushrooms), and negative pictures (e.g., traumatic physical injuries, dangerous weapons) were selected from the International Affective Picture System. Although some images depicted sports events (e.g., surfing, volleyball, fishing, etc.), neither alcohol nor drugs were included in any of the images. In addition, the pairing of beverage cues with specific affective images was counterbalanced. Two sets of 12 images were selected for each affective category to reduce habituation across the 2 beverage conditions. Prior work verified the picture sets are associated with the expected affective category (Mason et al., 2009, Mason et al., 2008).
Photographs were displayed on a large screen positioned directly in front of the participant. For each trial, participants were exposed to 12 pictures within the relevant affective condition, with each picture presented for 10 seconds and a 4-second interval between them. Participants were instructed to view each picture for the entire presentation time and remember the mood evoked by the photographs. Immediately following the picture sequence, participants were presented with either their preferred alcoholic beverage or bottled water accompanied by its commercially labeled bottle for visual reference. Alcohol or water beverages were presented in each participant’s preferred mode of consumption (e.g., small tumbler for vodka, Pilsner glass for beer), including choices of mixers.
To standardize beverage cue exposure, audio recordings instructed participants to sniff the glass when high-pitched tones signaled and stop sniffing when low-pitched tones signaled. Each beverage cue exposure period lasted 90 seconds during which 13 5-second olfactory exposures occurred in variable intervals. Participants were instructed to “focus on the sensation you have while smelling the alcohol or water beverage and continue to feel the mood stirred up in your imagination by the pictures you have just viewed.” Following the beverage exposure period, the beverage was removed, and participants completed craving ratings. The procedure was repeated for the remaining mood-beverage combinations. Upon completion of all 6 trials, participants were debriefed and completed the ACQ-SF-R to verify a return to baseline levels. Before leaving the session, participants with any residual urge received counseling focused on coping skills from a licensed clinical psychologist or psychiatrist. Participants remained in the laboratory until their craving returned to baseline levels.
Manipulation Check
To provide a manipulation check on the in vivo beverage exposure, after each in vivo beverage exposure participants completed a 20-point VAS rating for the question ‘How much did you like the beverage just given to you?’ Response options ranged from ‘strongly disliked’ (0) to ‘strongly liked’ (20). In addition, to evaluate whether the positive and negative imagery evoked the expected emotional response, participants viewed each image again at the end of the week 6 cue-reactivity session and completed the Self-Assessment Manikin (SAM) — a cartoon figure used to assess the valence of each image on a 9-point scale (Bradley and Lang, 1994). Ratings could range from ‘unhappy’ (1) to ‘happy’ (9).
Primary Endpoint
Alcohol craving in response to each affect-beverage condition was assessed using four VAS items, with endpoints marked with a 0 indicating no craving and a 20 indicating severe craving. These items were adapted from the ACQ-SF-R (Singleton et al., 1994). Items represent expectancy for positive reinforcement (“Having a drink would make things just perfect”), strength of craving (“How strong is your craving to drink alcohol?”), intent (“If I could drink alcohol now, I would drink it”), and lack of control (“It would be hard to turn down a drink right now”). These items were averaged to create a single composite variable of craving (α = .91).
Exploratory Endpoints
Alcohol consumption during the 28-day baseline period prior to randomization and throughout the 4-week medication maintenance phase was captured using the Timeline Follow-Back interview (Sobell & Sobell, 1992; Miller, 1996). Drinks were converted into standard drink units (1 standard drink = 0.6 oz of pure alcohol) for all analyses. A priori, exploratory alcohol consumption endpoints included: percent HDD (≥ 4 for women, ≥ 5 for men), drinks per day, drinks per drinking day, percent days abstinent, percent very HDD (≥ 8 for women, ≥ 10 for men), percent subjects abstinent, percent subjects with no HDD and percent subjects with a reduction of at least 1 or 2 levels in the World Health Organization (WHO) drinking risk categories (Hasin et al., 2017).
Alcohol craving outside of the laboratory was assessed at baseline, Weeks 4–6, and at the end of study visit with the Penn Alcohol Craving Scale (Flannery et al., 1999). The Pittsburg Sleep Quality Index (PSQI) was administered at baseline and the end of study visit to assess sleep quality throughout the trial (Buysse et al., 1989), the Profile of Mood State (POMS) captured dimensions of affect or mood at baseline, Week 4, and at the end of study visit (McNair and Heuchert, 2005), and the Self-Reported Habit Index (SRHI) was administered at baseline and the end of study visit to assess the extent to which drinking was habitual (Morean et al., 2018). Finally, given varenicline’s well-documented effects on cigarette use, a smoking quantity-frequency interview was administered each week to capture the number of cigarettes smoked per week among smokers.
Safety Assessments
Safety was assessed each week using a combination of biological tests and subjective reports. Biological tests included vital signs, blood chemistry tests, urine tests for illicit drug use, ECG results, and blood alcohol concentrations, as measured by breathalyzer. Subjective assessments included self-reported adverse events assessed in the clinic and during telephone interviews using the open-ended question: “How have you been feeling since your last visit?” In addition, participants reported concomitant medication use, symptoms of alcohol withdrawal, measured by the Clinical Institute Withdrawal Assessment for Alcohol-revised (Sullivan et al., 1989), and suicidal ideation, measured by the Columbia Suicide Severity Rating Scale (Posner et al., 2011). Neuropsychiatric symptoms related to suicidality, mood, and behavior/thinking were assessed every week. The mood and behavior/thinking questions were adapted from the Brief Psychiatric Rating Scale (Overall & Gorham, 1962). Adverse events were coded using the Medical Dictionary of Regulatory Activities (MedDRA) preferred terms and grouped by system, organ, and class (SOC) designation. Each adverse event (based on preferred terminology) was counted once only for a given participant. If the same event occurred on multiple occasions, the highest severity was assumed.
Statistical Analyses
Analyses were conducted in SAS 9.4 (SAS Institute, Inc., Cary, North Carolina). Primary analyses focused on repeated assessments of alcohol cue-induced craving from each participant during the two human laboratory sessions conducted 3 and 6 weeks post-randomization. Mixed-effects models examined varenicline-placebo differences in craving in response to beverage exposure. Separate models were performed for each laboratory session. Treatment (varenicline = 1, placebo = 0) was treated as a fixed, between-subjects variable; beverage presentation (i.e., Cue type: alcohol = 1, water = 0) and affective stimuli were treated as fixed, within-subjects repeated measures; and subjects was treated as a random effect. Affect condition was coded as three exclusive binary variables (positive, neutral, or negative), with the neutral condition serving as the reference category. Site (Yale = 0; Brown = 1) and nicotine use status prior to randomization (nonuser = 0; user = 1) were stratification variables and were included as covariates. Other putative covariates, counterbalance order for beverage and affective picture presentation and gender, were explored based on their theoretical associations with craving, but were not substantively correlated with craving and were not included. Interactive effects of Affect condition with Cue type and Treatment were tested, did not alter the pattern of focal findings, were not consistently significant, and were removed for model parsimony. Final models included baseline alcohol cue reactivity (centered at the grand mean), Site, Nicotine use status at baseline, Affect condition, Treatment, Cue type, and Treatment × Cue type. The interactive effects of Treatment and Cue type constituted the focal test of varenicline’s effect on subjective alcohol cue reactivity in the human laboratory. Given the sample size, models were fit with restricted maximum likelihood estimation to avoid bias in variance components. An unstructured variance/covariance matrix did not impose any assumptions on the relations of variance components, and the between/within method of calculating degrees of freedom was used.
Although our main focus centered on human laboratory data, we examined the effects of varenicline on a number of exploratory secondary outcomes during the last three weeks of medication administration. Continuous secondary endpoints (percent HDDs, percent days abstinent, drinks per day, drinks per drinking day, number of cigarettes smoked per week, PACS, and POMS) were analyzed using a repeated measures mixed-model. A compound symmetry covariance structure was selected for all outcomes (except cigarettes smoked per week which used an autoregressive structure) because it had the smallest Bayesian Information Criteria from among three possible covariance structures tested: unstructured, compound symmetry, and autoregressive. Skewed outcomes were transformed as follows: untransformed (percent days abstinent, PACS, SHRI), square root (percent HDDs, drinks per drinking day, POMS, cigarettes per week, PSQI), and log (drinks per day). General Linear Models (ANCOVAs) were used to analyze the PSQI and SRHI at the end of treatment. All models controlled for site, time, nicotine use in the week before randomization, and their corresponding baseline measure. Models also included time by treatment group interaction term. Analysis of the dichotomous secondary endpoints (percentage subjects with no HDDs, percentage of subjects with very HDDs, percentage subjects abstinent from alcohol, percentage of subjects achieving at least a one and two-level shift in WHO alcohol consumption, and no nicotine use, and no cigarette smoking during the last 3-weeks of the maintenance period among those who used nicotine in the week prior to randomization) were conducted via logistic regression using the same covariates as models of continuous outcomes (without time and the time by treatment group interaction). For binary measures, we conducted sensitivity analysis by coding missing drinking data as failed to achieve the outcome.
For all statistical tests, p < 0.05 (2-tailed) was considered statistically significant. No adjustment was made for multiple comparisons across laboratory sessions. An estimated sample size of 40 participants (20 per arm) was expected to yield 83% power to detect a medication effect (d = 0.95) comparable to those observed in prior work (McKee et al., 2009). This effect size is consistent with those derived from other human laboratory studies that used a similar experimental paradigm as the present study but evaluated different medications: mefipristone (d = 1.25) (Vendruscolo et al., 2015) and gabapentin (d = 1.00) (Mason et al., 2009).
Results
Sample Characteristics
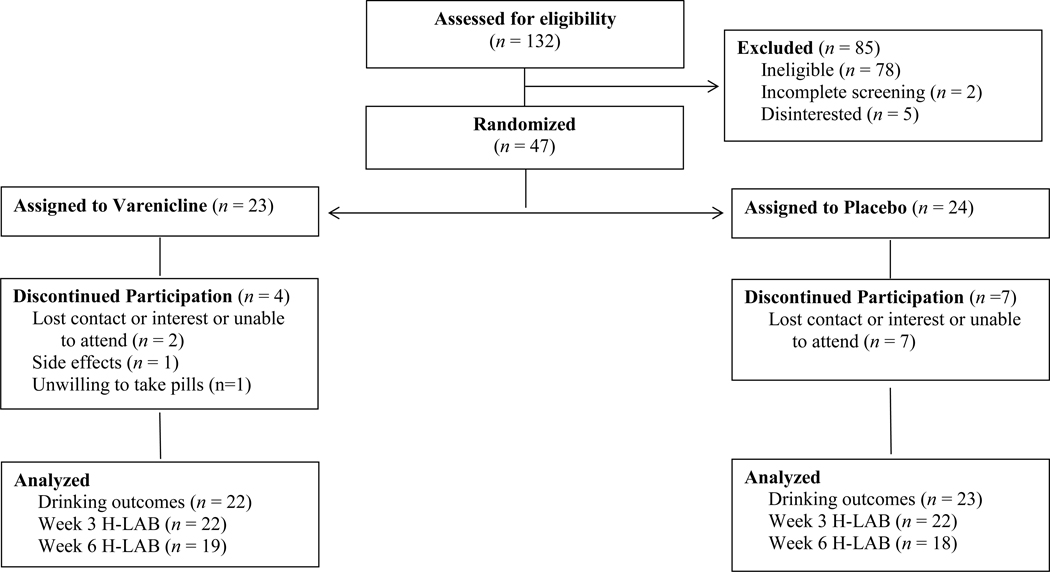
Of the 132 individuals assessed for eligibility, 47 were randomized to either varenicline (n = 23) or placebo (n = 24; see Figure 1). As in a prior varenicline trial (Litten et al., 2013), more participants withdrew in the placebo group (n = 7) than medication group (n = 4).
Figure 1.
Participant flow through the randomized double-blind study.
As shown in Table 2, participants were primarily White (76.6%), employed full time (63.8%), unmarried (74.5%), and male (61.7%), with an average age of 43.7, SD =11.5. The majority had “severe” AUD (76.6%), consumed on average 8.9 drinks per drinking day in the 28 days prior to intake (SD = 4.7), and met heavy drinking criteria on 69.1% of those days (SD = 25.3). Participants also drank frequently, reporting abstinence from alcohol on only 13.4% of the past 28 days (SD = 16.3). Nearly half (42.6%) smoked cigarettes in the past week, smoking an average of 73.7 cigarettes (SD = 50.8) over the week. The treatment groups were generally comparable across baseline characteristics.
Table 2.
Summary of baseline characteristics by medication condition
| Variable | Placebo (n = 24) |
Varenicline (n = 23) |
||||
|---|---|---|---|---|---|---|
| n | Mean or % | SD | n | Mean or % | SD | |
| Demographics | ||||||
| Age | 43.1 | 12.1 | 44.4 | 11.0 | ||
| Gender (male) | 15 | 62.5 | 14 | 60.9 | ||
| Employed full time | 15 | 62.5 | 15 | 65.2 | ||
| Married | 8 | 33.3 | 4 | 17.4 | ||
| Race/ethnicity | ||||||
| White | 19 | 79.2 | 16 | 69.6 | ||
| Black | 2 | 8.3 | 6 | 26.1 | ||
| Hispanic | 2 | 8.3 | 1 | 4.4 | ||
| Other | 1 | 4.2 | 0 | 0.0 | ||
| Alcohol usea | ||||||
| Drinks per day | 7.6 | 2.7 | 6.9 | 2.4 | ||
| Drinking per drinking day | 9.3 | 5.2 | 8.5 | 4.1 | ||
| % days abstinent | 11.8 | 15.9 | 15.1 | 16.9 | ||
| % heavy drinking days | 71.9 | 24.3 | 66.3 | 26.5 | ||
| % very heavy drinking daysb | 33.8 | 28.2 | 32.6 | 30.3 | ||
| WHO very high-risk drinking | 15 | 62.5 | 11 | 47.8 | ||
| WHO high-risk drinking | 9 | 37.5 | 12 | 52.2 | ||
| Alcohol use disorder severity | ||||||
| Moderate (4 or 5 symptoms) | 4 | 16.7 | 7 | 30.4 | ||
| Severe (6+ symptoms) | 20 | 83.3 | 16 | 69.6 | ||
| Cue reactivity VAS craving for alcohol | ||||||
| Alcohol cue | 14.0 | 5.1 | 12.1 | 5.6 | ||
| Water cue | 8.6 | 5.8 | 7.7 | 5.6 | ||
| Alcohol – water cue | 5.3 | 3.7 | 4.5 | 3.9 | ||
| Motivation to achieve treatment goal | 8.8 | 1.5 | 8.0 | 2.0 | ||
| Confidence to achieve treatment goal | 6.3 | 2.4 | 6.3 | 2.6 | ||
| CIWA-AR | 1.2 | 1.6 | 1.2 | 2.0 | ||
| Penn Alcohol Craving Scale score | 17.7 | 3.8 | 18.0 | 4.5 | ||
| Pittsburg Sleep Quality Index score | 6.3 | 3.3 | 5.9 | 2.3 | ||
| Profile of Mood States: Total mood disturbance score | 9.0 | 23.7 | 5.4 | 18.9 | ||
| Self-Reported Habit Index score | 32.2 | 15.3 | 33.5 | 12.5 | ||
| Nicotine use (past week) | 11 | 45.8 | 11 | 47.8 | ||
| Current smoker (past week) | 11 | 45.8 | 9 | 39.1 | ||
| Cigarettes per week (past week, among smokers) | 82.1 | 54.5 | 63.4 | 47.0 | ||
| FTND (among smokers) | 3.9 | 3.1 | 2.8 | 2.4 | ||
Note.
Derived from the 28-day timeline follow-back interview administered at baseline;
Defined as 8+/10+ drinks per day for women/men; FTND = Fagerstrom Test for Nicotine Dependence; VAS = Visual Analog Scale; CIWA-AR = Clinical Institute Withdrawal Assessment for Alcohol-Revised
All participants were seeking treatment for AUD. A range of treatment goals were endorsed by the sample, including abstinence (28%) and controlled or occasional use (i.e., cut down but not stop completely; 72%). Participants also rated their motivation to achieve their treatment goal (‘how motivated are you to reach this goal’) as well as their confidence (‘how confident are you that you will be able to reach this goal’). Both items were rated on 10-point scales that ranged from ‘not motivated/confident’ (1) to ‘extremely motivated/confident’ (10). Treatment groups were comparable in their treatment goals, and across both conditions participants had strong motivation and confidence to achieve their goals (see Table 2).
Medication Compliance and Tolerability
Participants were compliant with the medication regimen. The percentage of prescribed pills taken was similar for the varenicline (M = 91.8%, SD = 17.2) and placebo (M = 87.1%, SD = 18.7) conditions (p = .374).
There was one serious adverse event (SAE) of a seizure and overnight hospitalization leading to withdrawal in the varenicline group and was considered “possibly related” to varenicline. Table 3 summarizes other adverse effects reported by 10% or more participants in either arm of the study. Consistent with its product label, compared to placebo, varenicline was associated with higher rates of nausea (43.5% vs 16.7%) and abnormal dreams (30.4% vs 20.8%), though these differences were not statistically significant.
Table 3.
Summary of adverse events reported by at least 10% of participants in either arm of the study
| MedDRA SOC/Preferred term | Placebo (n = 24) | Varenicline (n = 23) | p |
|---|---|---|---|
| Nausea | 4 (16.7%) | 10 (43.5%) | 0.060 |
| Abnormal dreams | 5 (20.8%) | 7 (30.4%) | 0.517 |
| Nasopharyngitis | 6 (25.0%) | 1 (4.3%) | 0.097 |
| Bright urine | 1 (4.2%) | 3 (13.0%) | 0.348 |
Note. Multiple occurrences of a specific adverse event for a participant were counted once in the frequency for the adverse event.
Effects of Varenicline on Alcohol Cue Reactivity
Validity of beverage and affective cues were investigated in several ways. First, we examined their effects on ratings of beverage liking and valence of the affective images. We found significant effects of beverage cue on beverage liking for both lab sessions, with alcohol preferred to water (Study Week 3: b = 4.18, SE = 0.53, p < .001; Study Week 6: b = 3.19, SE = 0.51, p < .001). Least squares means and tests of differences of least squares means for a mixed model predicting emotional valence (unhappy to happy) from affect condition, accounting for Site and Nicotine use status at baseline, showed expected directionality, i.e., positive > neutral > negative. There were significant differences in least squares means for positive and negative image exposure relative to neutral images on emotional valence (Study Week 3: b positive = 0.44, SE = 0.19, p = .025; b negative = −1.58, SE = 0.19, p < .001; Study Week 6: b positive = 0.49, SE = 0.19, p = .012; b negative = −1.22, SE = 0.19, p < .001). The least squares means for positive, neutral, and negative affect conditions in Week 3 were 6.17, 5.73, and 4.15, respectively, and in Week 6 were 6.41, 5.92, 4.70, respectively.
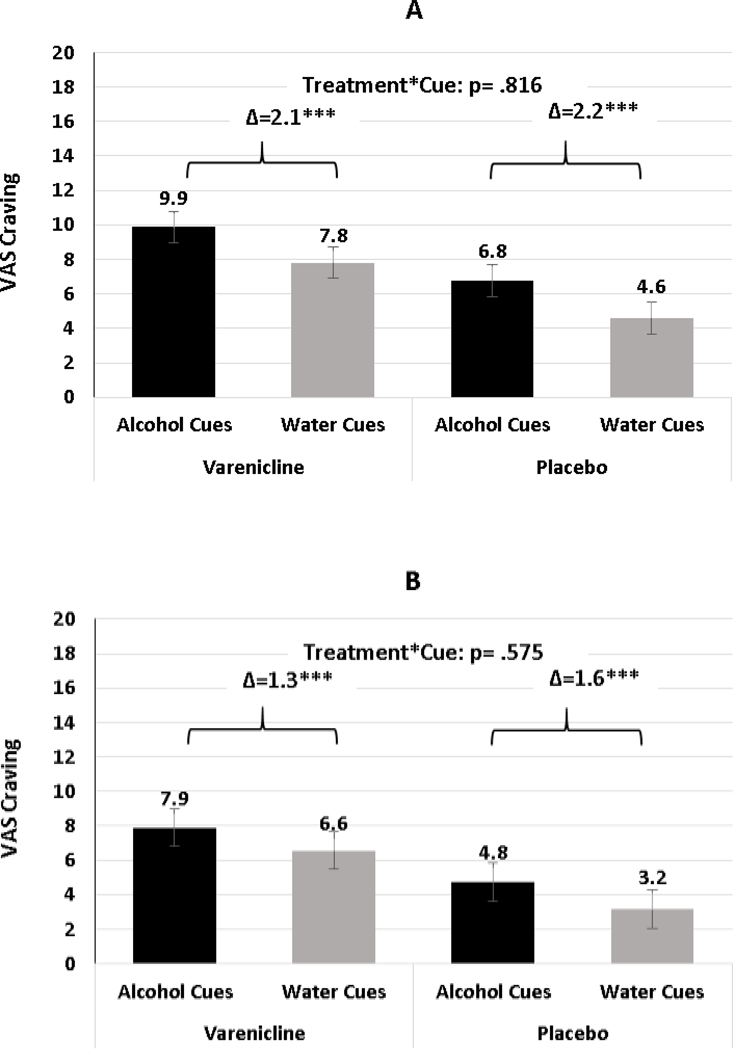
Primary outcomes were the differences in alcohol craving following presentation of alcohol vs. water cues between the varenicline and placebo groups at Study Weeks 3 and 6 (Table 4). Alcohol cues were associated with greater craving relative to water cues, ps < .001, indicating that alcohol cues elicited the desired cue reactivity effect expected from this paradigm. An unexpected main effect of varenicline on craving emerged, such that varenicline increased ratings of craving over placebo, irrespective of cue type, for Study Weeks 3 and 6 (Week 3: b = 3.22, SE = 1.30, p = .018; Week 6: b = 3.41, SE = 1.56, p = .036). Contrary to hypotheses, the focal interactive effect of varenicline (v. placebo) and alcohol cues (v. water), was not significant at either Week (Week 3: b = −0.12, SE = 0.50, p = .816; Week 6: b = −0.26, SE = 0.47, p = .575). As illustrated in Figure 2, the adjusted mean difference in craving after being presented with alcohol v. water beverages were nearly identical for varenicline and placebo (2.1 vs 2.2, respectively [Week 3, Panel A]; 1.3 vs 1.6, respectively [Week 6, Panel B]). Results were similar when each the 4 individual VAS craving outcome items was evaluated separately (vs. the average of the 4 items) (data not shown).
Table 4.
Effects of Varenicline on Subjective Alcohol Cue Reactivity
| 95% CI |
|||||
|---|---|---|---|---|---|
| Model and predictor variables | b | SE | LL | UL | p |
| Study Week 3 (n = 44) | |||||
| Intercept | 4.07 | 1.30 | 1.44 | 6.70 | .003 |
| Alcohol cue reactivity (baseline) | 0.51 | 0.13 | 0.24 | 0.77 | < .001 |
| Study site | 2.23 | 1.35 | − 0.51 | 4.96 | .108 |
| Nicotine use (baseline) | − 1.56 | 1.29 | − 4.15 | 1.04 | .234 |
| Varenicline (v. placebo) | 3.22 | 1.30 | 0.59 | 5.85 | .018 |
| Alcohol cue (v. water) | 2.17 | 0.35 | 1.46 | 2.89 | < .001 |
| Negative affect (v. neutral) | 0.27 | 0.31 | − 0.34 | 0.88 | .386 |
| Positive affect (v. neutral) | 0.48 | 0.31 | − 0.13 | 1.09 | .123 |
| Varenicline × Alcohol cue | − 0.12 | 0.50 | − 1.13 | 0.89 | .816 |
| Study Week 6 (n = 37) | |||||
| Intercept | 1.70 | 1.48 | − 1.31 | 4.72 | .259 |
| Alcohol cue reactivity (baseline) | 0.31 | 0.16 | − 0.02 | 0.64 | .068 |
| Study site | 3.48 | 1.68 | 0.05 | 6.90 | .047 |
| Nicotine use (baseline) | − 0.62 | 1.55 | − 3.78 | 2.54 | .692 |
| Varenicline (v. placebo) | 3.41 | 1.56 | 0.23 | 6.58 | .036 |
| Alcohol cue (v. water) | 1.58 | 0.33 | 0.91 | 2.26 | < .001 |
| Negative affect (v. neutral) | 0.04 | 0.28 | − 0.53 | 0.61 | .897 |
| Positive affect (v. neutral) | − 0.04 | 0.28 | − 0.61 | 0.53 | .887 |
| Varenicline × Alcohol cue | − 0.26 | 0.47 | − 1.21 | 0.68 | .575 |
Note. b = unstandardized effect; CI = confidence interval; LL = lower limit; UL = upper limit. Interactive effects reflect the influence of varenicline treatment on subjective alcohol cue reactivity.
Figure 2.
Least squared mean differences in reactivity to alcohol and water cues for vareniclineand placebo treatment groups at Study Weeks 3 (Panel A) and 6 (Panel B). *** p < .001.
Given prior reports regarding varenicline’s effects on alcohol cue reactivity and drinking among smokers and men, we explored whether nicotine use status and sex moderated varenicline effects. Baseline smoking status did not moderate the interaction of medication and cue type on craving, evidenced by nonsignificant three-way interactions, i.e., Smoking Status × Cue × Treatment (b = 0.30, SE = 1.01, p = .767 in Week 3; and b = −1.29, SE= .93, p = .174 in Week 6; Supplementary Figure 1 of the online supplemental materials). Sex did not moderate the interaction of medication and cue type on craving, evidenced by nonsignificant three-way interactions, i.e., Sex × Cue × Treatment (b = −0.78, SE = 1.02, p = .448 in Week 3; and b = −0.83, SE= .94, p = .381 in Week 6; Supplementary Figure 2 of the online supplemental materials).
Exploratory Effects of Varenicline on Naturalistic Drinking and Non-Drinking Outcomes
Table 5 presents the results for other exploratory outcomes. The percentage of participants with very heavy drinking was the only drinking related outcome to differ significantly between varenicline and placebo, with the percentage being higher for those on varenicline. Paralleling this finding, drinks per drinking day was higher, although not significantly for the active treatment condition. There were no differences on drinks per day of percent of days abstinent. On the FDA accepted measures of response, the percentage of participants with no HDDs (varenicline = 27.3%, placebo = 30.4%) and the percentage of participants who were abstinent (varenicline = 9.1%; placebo = 17.4%) were also statistically similar between groups (results were similar when missing data were imputed as failure, data not shown). There were no significant differences between groups on the Penn Alcohol Craving scale, SRHI, the PSQI, POMS, or cigarettes smoked per week. No participants in either condition were abstinent from nicotine or cigarettes during the treatment period.
Table 5.
Treatment Outcomes: Differences between Placebo and Varenicline during the Last Month of Treatment (Weeks 3– 6)
| Placebo (n = 23) |
Varenicline ( n = 22) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| LSMEAN | SE | 95% CI | LSMEAN | SE | 95% CI | LSMEAN Δ | SE | d | p | |
| Drinking Outcomes | ||||||||||
| % HDD | 21.0 | 4.9 | 11.1 – 30.9 | 30.7 | 5.0 | 20.7 – 40.7 | 9.7 | 7.0 | 0.09 | 0.262 |
| % days abstinent | 54.2 | 6.4 | 41.4 – 67.1 | 48.6 | 6.4 | 35.6 – 61.6 | −5.6 | 9.1 | −0.01 | 0.543 |
| Drinks per day | 2.5 | 0.5 | 1.4 – 3.5 | 3.7 | 0.5 | 2.6 – 4.7 | 1.2 | 0.8 | 0.49 | 0.205 |
| Drinks per drinking day | 4.0 | 0.8 | 2.4 – 5.6 | 6.8 | 0.8 | 5.2 – 8.3 | 2.8 | 1.1 | 0.48 | 0.061 |
| % | n | denom | % | n | denom | % Δ | aOR (95% CI) | p | ||
| % subjects with no HDD | 30.4 | 7 | 23 | 27.3 | 6 | 22 | −3.1 | 0.83 (0.22–3.17) | 0.790 | |
| % subjects abstinent | 17.4 | 4 | 23 | 9.1 | 2 | 22 | −8.3 | 0.45 (0.07–2.95) | 0.405 | |
| WHO 1-shift reduction | 82.6 | 19 | 23 | 63.6 | 14 | 22 | −19.0 | 0.35 (0.08–1.43) | 0.148 | |
| WHO 2-shift reduction | 60.9 | 14 | 23 | 40.9 | 9 | 22 | −20.0 | 0.44 (0.13–1.47) | 0.182 | |
| % subjects with very HDD | 34.8 | 8 | 23 | 63.6 | 14 | 22 | 28.8 | 4.32(1.13–16.51) | 0.032 | |
| Non-Drinking Outcomes | ||||||||||
| % abstinent from nicotinea | 0.0 | 0 | 0 | 0.0 | 0 | 0 | 0.0 | N/A | 1.000 | |
| % abstinent from smokingb | 0.0 | 0 | 0 | 0.0 | 0 | 0 | 0.0 | N/A | 1.000 | |
| LSMEAN | SE | 95% CI | LSMEAN | SE | 95% CI | LSMEAN Δ | SE | d | p | |
| Cigarettes per weekb | 64.0 | 7.6 | 48.0 – 80.1 | 47.0 | 7.7 | 30.6 – 63.4 | −17.0 | 10.9 | −0.25 | 0.198 |
| Penn Alcohol Craving | 10.9 | 1.1 | 8.7 – 13.0 | 10.8 | 1.1 | 8.7 – 13.0 | 0.0 | 1.5 | 0.00 | 0.997 |
| Self-report Habit Index | 44.4 | 3.3 | 37.8 – 51.0 | 48.7 | 3.3 | 42.1 – 55.4 | 4.3 | 4.6 | 0.15 | 0.352 |
| Pittsburg Sleep Quality Inventory | 6.0 | 0.4 | 5.1 – 6.9 | 5.3 | 0.4 | 4.4 – 6.2 | −0.6 | 0.6 | −0.18 | 0.557 |
| POMS | 7.3 | 3.9 | −0.6 – 15.2 | 2.9 | 3.9 | −5.0 – 10.8 | −4.5 | 5.5 | −0.15 | 0.325 |
Note. HDD = heavy drinking days, LSMEANS = least squared means, SE = standard error, CI = confidence interval, Δ = varenicline - placebo difference, denom = denominator, d= Cohen’s d (varenicline-placebo), aOR = adjusted odds ratio, POMS = Profile of Mood Scale (POMS) - Total Mood Disturbance score Models were based on a mITT population that included subjects who received at least one dose of medication. No imputation was used for missing data. For continuous outcomes, LSMEANS were estimated from fully adjusted models on untransformed outcomes (for interpretive purposes); corresponding Cohen’s d and p-values were based on the same model but with the appropriately transformed outcome.
% subjects; model for any nicotine outcome included only 21 participants who were nicotine users at baseline and had non-missing data (varenicline n=11, placebo n=10).
% subjects; models for smoking outcomes included only 20 participants who were smokers at baseline and had non-missing data (varenicline n=9, placebo n=11
There were no significant time by treatment group interaction effects for any outcome, except for one. Although the main effect was not statistically significant, for the subset of participants who smoked cigarettes at baseline (n = 20), there was a significant treatment by time interaction for cigarettes smoked per week (F (3,43.9 = 3.37, p = .027) such that, compared to placebo, varenicline reduced the mean number of cigarettes smoked per week during weeks 5 and 6 (p = .057 and p = .059, respectively; Supplementary Figure 3 of online supplemental material).
Exploratory Effects on Drinking and Naturalistic Craving
Next, general linear models were used to model drinking, craving in daily life, and smoking outcomes during Study Weeks 4 through 6 from subjective alcohol craving after exposure to in vivo alcohol cues in the human laboratory at Study Week 3. Baseline equivalents of outcomes were included as covariates, as well as study site, nicotine use status, gender, and treatment. Higher levels of cue-elicited alcohol craving predicted more drinks per day, b = .02, SE = .01, p = .022, partial η2 = .15, and percent HDDs, b = 0.23, SE = .09, p = .018, partial η2 = .16 (Supplementary Figure 4 of the online supplemental materials). Laboratory craving did not significantly predict drinks per drinking day, p = .084, partial η2 = .09, percent days abstinent, p = .101, partial η2 = .08, weekly craving, p = .129, partial η2 = .07, or the average number of cigarettes smoked per day, p = .587, partial η2 = .02. Greater differences in craving for alcohol in response to alcohol cues relative to water were not significantly predictive of drinks per day, p = .394, partial η2 = .02, drinks per drinking day, p = .472, partial η2 = .02, percent days abstinent, p = .516, partial η2 = .01, percent HDDs, p = .835, partial η2 = .001, weekly craving, p = .534, partial η2 = .01, or average number of cigarettes smoked per day, p = .117, partial η2 = .17.
Sensitivity Analysis
Cue reactivity studies have shown the potential for carryover effects when alcohol cue exposure is followed by exposure to water cues. In the present study, however, water- and alcohol-cue presentation was counterbalanced. Presenting the alcohol cue first may have elicited alcohol craving that persisted through the presentation of the water cue. To address this possibility, sensitivity analyses were conducted on the subset of data where participants were exposed to water cues before alcohol cues. Results of these sensitivity analyses for Weeks 3 and 6 showed that participants reported significantly greater alcohol craving following alcohol cues compared to water cues (Week 3: b = 4.24, SE = 1.09, p = .001; Week 6: b = 2.89, SE = 1.17, p = .025), and varenicline did not significantly alter this effect (Week 3: b = 0.19, SE = 1.39, p = .893; Week 6: b = 0.40, SE = 1.45, p = .784).
Discussion
This multisite study tested whether varenicline blunts the strength of alcohol craving during an alcohol cue reactivity paradigm administered in the laboratory among treatment-seeking heavy drinking adults with moderate to severe AUD. A previous single-session human laboratory study tested the effects of varenicline on alcohol-cue-induced craving after a brief 10-day medication trial (Roberts et al., 2017), but no one had evaluated its effects during repeated laboratory sessions embedded within the context of a longer RCT. Moreover, no one had examined the effects of varenicline during an alcohol cue exposure paradigm paired with a mood induction despite growing interest in its influence on affect and motivational processes, especially in the context of alcohol and nicotine use (Childs et al., 2012, Fedota et al., 2015). We advanced prior work by testing the effects of varenicline on emotion-enhanced alcohol cue reactivity at two points, separated by 3 weeks, in a 6-week clinical trial. In addition, we investigated naturalistic drinking, craving, smoking and other outcomes during a 4-week follow-up period and examined whether alcohol cue reactivity effects observed in the human laboratory predicted these subsequent outcomes. Incorporating a laboratory paradigm within a clinical trial allowed us to test not only whether varenicline altered cue-induced craving but also the degree to which laboratory-based findings predict real-world outcomes.
Contrary to our hypothesis, varenicline did not attenuate alcohol cue-induced craving relative to placebo. In fact, individuals randomized to varenicline reported higher craving for alcohol in general, for both alcohol and water cues, than those on placebo. This effect was surprising given prior research found varenicline blunted craving in response to alcohol cues in the laboratory (Roberts et al., 2017) and it reduced weekly alcohol craving in all RCTs in which it was assessed (Litten et al., 2013, Fucito et al., 2011, de Bejczy et al., 2015).
There are several potential explanations for our lack of effects. A major difference between the present investigation and that by Roberts et al. (2017) is that we required that individuals remain abstinent prior to the cue exposure sessions, whereas this was not required by Roberts et al. (2017). Another key difference was that Roberts et al. (2017) did not use a mood induction procedure and reduced the likelihood of possible carryover effects of alcohol cue exposure by presenting water cues first for all participants. Integrating a mood induction with this paradigm may have added complexities that obscured our ability to detect medication effects. There is mounting evidence that varenicline affects mood and motivational processes associated with alcohol and drug use. Due to our small sample size, however, we lacked statistical power to test this possibility. It should be noted that using this same paradigm in which abstinence was required prior to cue exposure, Mason and colleagues (2008) found that gabapentin significantly attenuated craving in response to alcohol cues and blunted subjective arousal to both alcohol cues and positive and negative affective stimuli.
It is also possible that differences in participant selection influenced findings. Our sample was composed of individuals seeking treatment for AUD whereas Roberts et al. (2017) studied non-treatment seeking heavy drinkers. In addition, we selected people who demonstrated alcohol cue reactivity effects during the screening session (i.e., a 3-point minimum increase in alcohol craving to alcohol cues compared to water cues). Roberts et al (2017) did not use this selection criterion. Recent research also suggests that men experience greater benefit from varenicline for the treatment of AUD than women (O’Malley et al., 2018). Our small sample size, however, precluded our ability to test whether sex moderated outcomes. Finally, it is possible that varenicline blunts cue-induced craving early in treatment but this effect abates over time. Roberts et al. (2017) tested varenicline’s effects on alcohol cue-elicited craving after a 1-week medication period, whereas we tested its effects after two and five weeks of a stabilized dose following 3 days of required abstinence. This potential explanation is questionable, however, given RCTs found varenicline treatment reduced weekly reports of craving over the target dose period.
Laboratory results paralleled the weekly self-reported alcohol craving and drinking outcomes in the naturalistic clinical trial. Compared to placebo, varenicline had no beneficial effect on weekly alcohol craving or alcohol consumption outcomes. These results are consistent with some prior studies and inconsistent with others, which adds to the growing body of mixed findings regarding the efficacy of varenicline for the treatment of AUD. In addition, a higher percentage of participants in the varenicline condition reported very HDD during the trial compared to placebo. Our study adds to a growing number of mixed findings from RCTs that tested the efficacy of varenicline for treating alcohol use among problem drinkers. A preliminary RCT with 30 heavy drinking smokers found that varenicline (2 mg/day) reduced alcohol craving and heavy drinking days (HDDs) compared to placebo (Fucito et al., 2011). Similar findings were observed in a larger 13-week multisite RCT of 200 smokers and nonsmokers with alcohol dependence (Litten et al., 2013). Compared to placebo, varenicline lowered weekly percent HDDs, drinks per day, drinks per drinking day, and alcohol craving. In contrast, a 12-week RCT of 160 alcohol dependent patients found no benefit of varenicline on self-reported drinking outcomes as compared to placebo; however, compared to placebo, varenicline significantly reduced craving and lowered levels of the alcohol marker phosphatidylethanol (de Bejczy et al., 2015). Recently, a 16-week RCT of 131 alcohol dependent smokers found no overall varenicline-placebo difference in heavy drinking outcomes; however, relative to placebo, varenicline reduced HDDs and increased the percentage of participants with no HDDs in men but not women (O’Malley et al., 2018).
Findings provide useful information about the utility of the alcohol cue reactivity paradigm for screening novel medications. Although medication effects were not detected using the paradigm, craving in response to alcohol cues captured during cue reactivity predicted subsequent alcohol use in real-world settings during the clinical trial. Higher alcohol craving in the laboratory prospectively predicted more drinks per day and heavier alcohol use the remaining weeks of the clinical trial. Interestingly, the difference in alcohol craving in response to alcohol and water cues did not significantly predict naturalistic outcomes, which calls for additional research with larger samples to further test these associations.
Although medications (e.g., gabapentin, naltrexone) that decrease cue-elicited craving in the laboratory studies typically also reduce craving and drinking in separately conducted clinical trials (Mason et al., 2014, Vendruscolo et al., 2015, Tidey et al., 2008, Miranda et al., 2014), this is the first study to examine whether alcohol craving captured during the cue reactivity paradigm in the laboratory predicts drinking outcomes in the same pharmacotherapy trial. One early trial examined the effects of cue-exposure treatment combined with coping skills training on alcohol cue-elicited craving and drinking outcomes (Monti et al., 1993). Only pre-treatment cue reactivity predicted drinking outcomes; cue reactivity assessed during treatment was not associated with outcomes. A study of adolescent problem drinkers paired the alcohol cue reactivity paradigm with data collected in the natural environment using ecological momentary assessment methods (Ramirez and Miranda, 2014). Alcohol cues elicited craving in the laboratory (compared to water cues), and this effect generalized to the natural environment such that adolescents experienced greater alcohol craving in nondrinking moments when alcohol cues were present in real-world settings. These effects were stronger for adolescents with more alcohol problems, and higher craving predicted subsequent greater drinking levels. Other naturalistic studies have found that subjective (i.e., craving) and physiological reactivity to alcohol cues predicted time to relapse following inpatient alcohol detoxification (Sinha et al., 2011, Cooney et al., 1997). On the whole, there is mounting support for alcohol craving captured during cue reactivity as a clinically relevant endophenotype predictive of alcohol use outcomes, and thus may be a useful tool for understanding how interventions work and identifying promising new treatment options.
Strengths of this study include the rigorous evaluation of possible outcomes under two identical laboratory cue-exposure sessions and in real-world settings in the context of a larger clinical trial, the low rate of missing data, increased generalizability of findings by the inclusion of treatment-seeking individuals with AUD, use of a standardized behavioral platform, and high treatment retention. On balance, limitations included the lack of statistical power to detect possible moderator effects, such as sex differences. In addition, individuals with significant alcohol withdrawal and psychiatric comorbidities were excluded from the study, which may limit how well our findings generalize to the types of patients with AUD who are treated in typical clinical practice. In addition, the counterbalanced presentation of water and alcohol cues may have impacted our ability to detect medication effects given the known potential for carryover effects when alcohol cue exposure is followed by exposure to water cues. However, sensitivity analyses on the subset of data where participants were exposed to water cues before alcohol cues found the same results, which mitigates this concern. Finally, we cannot rule out the possibility that participants experienced fatigue or overload given the number of in vivo and image cue exposures presented, which might have dampened our ability to detect medication effects. This concern is mitigated, however, by the strong cue reactivity effects we and others observed with this paradigm (Mason et al., 2008), and other studies using the same paradigm did detect medication effects (Mason et al., 2009).
In summary, although a previous laboratory study found varenicline blunted alcohol cue-induced craving among adult heavy drinkers with DSM-IV alcohol abuse or dependence, this multisite investigation did not find this effect. It is possible that methodological differences between the present study and prior work or heterogeneity in the medication response accounted for our null findings. Additional studies are needed to test for possible heterogeneity in varenicline effects, namely potential sex differences, and to disentangle how mood impacts alcohol cue reactivity effects and how this might impact sensitivity to medication effects. Although our null efficacy results for drinking outcomes call to question the utility of varenicline for the treatment of AUD, it should be noted that large-scale RCTs found it reduced alcohol use and craving in daily life. Finally, our results are among the first to show alcohol cue-induced craving captured during a laboratory paradigm predicts drinking outcomes in the context of a clinical trial.
Supplementary Material
Acknowledgments
Supported by the following contract from the National Institute on Alcohol Abuse and Alcoholism, HHSN275201500007I, and in part by P50AA12870; K24AA026326; K23AA024808. ClinicalTrials.gov: NCT03035708.
Dr. O’Malley reports the following disclosures: member of the American Society of Clinical Psychopharmacology’s Alcohol Clinical Trials Initiative, supported by Alkermes, Amygdala Neurosciences, Arbor Pharmaceuticals, Ethypharm, Indivior, Lundbeck, Mitsubishi, and Otsuka; Consultant/advisory board member, Alkermes, Amygdala (without compensation), Opiant; Medication supplies, Astra Zeneca, Novartis; NIDA DSMB member, Emmes Corporation. None of the other authors have disclosures.
Footnotes
Disclaimer: The views and opinions expressed in this article are those of the authors and should not be construed to represent the views of any of the sponsoring organizations, agencies, or the US government.
References
- Anton RF, O’malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, Gastfriend DR, Hosking JD, Johnson BA, Locastro JS, Longabaugh R, Mason BJ, Mattson ME, Miller WR, Pettinati HM, Randall CL, Swift R, Weiss RD, Williams LD, Zweben A. & Group CSR 2006. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA, 295, 2003–17. [DOI] [PubMed] [Google Scholar]
- Bach P, Weil G, Pompili E, Hoffmann S, Hermann D, Vollstadt-Klein S, Mann K, Perez-Ramirez U, Moratal D, Canals S, Dursun SM, Greenshaw AJ, Kirsch P, Kiefer F. & Sommer WH 2020. Incubation of neural alcohol cue reactivity after withdrawal and its blockade by naltrexone. Addict Biol, 25, e12717. [DOI] [PubMed] [Google Scholar]
- Bradley MM & Lang PJ 1994. Measuring emotion: the Self-Assessment Manikin and the Semantic Differential. J Behav Ther Exp Psychiatry, 25, 49–59. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR & Kupfer DJ 1989. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res, 28, 193–213. [DOI] [PubMed] [Google Scholar]
- Childs E, Roche DJ, King AC & De Wit H. 2012. Varenicline potentiates alcohol-induced negative subjective responses and offsets impaired eye movements. Alcohol Clin Exp Res, 36, 906–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung T, Noronha A, Carroll KM, Potenza MN, Hutchison K, Calhoun VD, Gabrieli JD, Morgenstern J, Nixon SJ, Wexler BE, Brewer J, Ray L, Filbey F, Strauman TJ, Kober H. & Feldstein Ewing SW 2016. Brain mechanisms of Change in Addictions Treatment: Models, Methods, and Emerging Findings. Curr Addict Rep, 3, 332–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooney NL, Litt MD, Morse PA, Bauer LO & Gaupp L. 1997. Alcohol cue reactivity, negative-mood reactivity, and relapse in treated alcoholic men. J Abnorm Psychol, 106, 243–50. [DOI] [PubMed] [Google Scholar]
- Davidson D, Palfai T, Bird C. & Swift R. 1999. Effects of naltrexone on alcohol self-administration in heavy drinkers. Alcohol Clin Exp Res, 23, 195–203. [PubMed] [Google Scholar]
- De Bejczy A, Lof E, Walther L, Guterstam J, Hammarberg A, Asanovska G, Franck J, Isaksson A. & Soderpalm B. 2015. Varenicline for treatment of alcohol dependence: a randomized, placebo-controlled trial. Alcohol Clin Exp Res, 39, 2189–99. [DOI] [PubMed] [Google Scholar]
- Falk DE, Ryan ML, Fertig JB, Devine EG, Cruz R, Brown ES, Burns H, Salloum IM, Newport DJ, Mendelson J, Galloway G, Kampman K, Brooks C, Green AI, Brunette MF, Rosenthal RN, Dunn KE, Strain EC, Ray L, Shoptaw S, Ait-Daoud Tiouririne N, Gunderson EW, Ransom J, Scott C, Leggio L, Caras S, Mason BJ, Litten RZ, National Institute on Alcohol, A. & Alcoholism Clinical Investigations Group Study, G. 2019. Gabapentin Enacarbil Extended-Release for Alcohol Use Disorder: A Randomized, Double-Blind, Placebo-Controlled, Multisite Trial Assessing Efficacy and Safety. Alcohol Clin Exp Res, 43, 158–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedota JR, Sutherland MT, Salmeron BJ, Ross TJ, Hong LE & Stein EA 2015. Reward Anticipation Is Differentially Modulated by Varenicline and Nicotine in Smokers. Neuropsychopharmacology, 40, 2038–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flannery BA, Volpicelli JR & Pettinati HM 1999. Psychometric properties of the Penn Alcohol Craving Scale. Alcohol Clin Exp Res, 23, 1289–95. [PubMed] [Google Scholar]
- Fox HC, Anderson GM, Tuit K, Hansen J, Kimmerling A, Siedlarz KM, Morgan PT & Sinha R. 2012. Prazosin effects on stress- and cue-induced craving and stress response in alcohol-dependent individuals: preliminary findings. Alcohol Clin Exp Res, 36, 351–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fucito LM, Toll BA, Wu R, Romano DM, Tek E. & O’malley SS 2011. A preliminary investigation of varenicline for heavy drinking smokers. Psychopharmacology (Berl), 215, 655–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales D, Rennard SI, Nides M, Oncken C, Azoulay S, Billing CB, Watsky EJ, Gong J, Williams KE, Reeves KR & Varenicline Phase 3 Study, G. 2006. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. JAMA, 296, 47–55. [DOI] [PubMed] [Google Scholar]
- Grant BF, Goldstein RB, Saha TD, Chou SP, Jung J, Zhang H, Pickering RP, Ruan WJ, Smith SM, Huang B. & Hasin DS 2015. Epidemiology of DSM-5 Alcohol Use Disorder: Results From the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry, 72, 757–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin DS, Wall M, Witkiewitz K, Kranzler HR, Falk D, Litten R, Mann K, O’malley SS, Scodes J, Robinson RL, Anton R. & Alcohol Clinical Trials Initiative, W. 2017. Change in non-abstinent WHO drinking risk levels and alcohol dependence: a 3 year follow-up study in the US general population. Lancet Psychiatry, 4, 469–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison KE, Swift R, Rohsenow DJ, Monti PM, Davidson D. & Almeida A. 2001. Olanzapine reduces urge to drink after drinking cues and a priming dose of alcohol. Psychopharmacology (Berl), 155, 27–34. [DOI] [PubMed] [Google Scholar]
- Jonas DE, Amick HR, Feltner C, Bobashev G, Thomas K, Wines R, Kim MM, Shanahan E, Gass CE, Rowe CJ & Garbutt JC 2014. Pharmacotherapy for adults with alcohol use disorders in outpatient settings: a systematic review and meta-analysis. JAMA, 311, 1889–900. [DOI] [PubMed] [Google Scholar]
- Koob GF & Mason BJ 2016. Existing and Future Drugs for the Treatment of the Dark Side of Addiction. Annu Rev Pharmacol Toxicol, 56, 299–322. [DOI] [PubMed] [Google Scholar]
- Litten RZ, Egli M, Heilig M, Cui C, Fertig JB, Ryan ML, Falk DE, Moss H, Huebner R. & Noronha A. 2012. Medications development to treat alcohol dependence: a vision for the next decade. Addict Biol, 17, 513–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litten RZ, Ryan ML, Falk DE, Reilly M, Fertig JB & Koob GF 2015. Heterogeneity of alcohol use disorder: understanding mechanisms to advance personalized treatment. Alcohol Clin Exp Res, 39, 579–84. [DOI] [PubMed] [Google Scholar]
- Litten RZ, Ryan ML, Fertig JB, Falk DE, Johnson B, Dunn KE, Green AI, Pettinati HM, Ciraulo DA, Sarid-Segal O, Kampman K, Brunette MF, Strain EC, Tiouririne NA, Ransom J, Scott C, Stout R. & Group NS 2013. A double-blind, placebo-controlled trial assessing the efficacy of varenicline tartrate for alcohol dependence. J Addict Med, 7, 277–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukas SE, Lowen SB, Lindsey KP, Conn N, Tartarini W, Rodolico J, Mallya G, Palmer C. & Penetar DM 2013. Extended-release naltrexone (XR-NTX) attenuates brain responses to alcohol cues in alcohol-dependent volunteers: a bold FMRI study. Neuroimage, 78, 176–85. [DOI] [PubMed] [Google Scholar]
- Mackillop J, Few LR, Stojek MK, Murphy CM, Malutinok SF, Johnson FT, Hofmann SG, Mcgeary JE, Swift RM & Monti PM 2015. D-cycloserine to enhance extinction of cue-elicited craving for alcohol: a translational approach. Transl Psychiatry, 5, e544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann K, Vollstadt-Klein S, Reinhard I, Lemenager T, Fauth-Buhler M, Hermann D, Hoffmann S, Zimmermann US, Kiefer F, Heinz A. & Smolka MN 2014. Predicting naltrexone response in alcohol-dependent patients: the contribution of functional magnetic resonance imaging. Alcohol Clin Exp Res, 38, 2754–62. [DOI] [PubMed] [Google Scholar]
- Mason BJ, Light JM, Escher T. & Drobes DJ 2008. Effect of positive and negative affective stimuli and beverage cues on measures of craving in non treatment-seeking alcoholics. Psychopharmacology (Berl), 200, 141–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason BJ, Light JM, Williams LD & Drobes DJ 2009. Proof-of-concept human laboratory study for protracted abstinence in alcohol dependence: effects of gabapentin. Addict Biol, 14, 73–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason BJ, Quello S, Goodell V, Shadan F, Kyle M. & Begovic A. 2014. Gabapentin treatment for alcohol dependence: a randomized clinical trial. JAMA Intern Med, 174, 70–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaul ME, Wand GS, Eissenberg T, Rohde CA & Cheskin LJ 2000. Naltrexone alters subjective and psychomotor responses to alcohol in heavy drinking subjects. Neuropsychopharmacology, 22, 480–92. [DOI] [PubMed] [Google Scholar]
- Mcnair D. & Heuchert J. 2005. Profile of Mood States (POMS) Tonawanda, NY, Multi-Health Systems. [Google Scholar]
- Miranda R Jr., Ray L, Blanchard A, Reynolds E, Monti PM, Chun T, Justus A, Swift RM, Tidey J, Gwaltney CJ & Ramirez J. 2014. Effects of naltrexone on adolescent alcohol cue reactivity and sensitivity: An initial randomized trial. Addiction Biology, 19, 941–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monti PM, Rohsenow DJ & Hutchison KE 2000. Toward bridging the gap between biological, psychobiological and psychosocial models of alcohol craving. Addiction, 95 Suppl 2, S229–36. [DOI] [PubMed] [Google Scholar]
- Monti PM, Rohsenow DJ, Hutchison KE, Swift RM, Mueller TI, Colby SM, Brown RA, Gulliver SB, Gordon A. & Abrams DB 1999. Naltrexone’s effect on cue-elicited craving among alcoholics in treatment. Alcohol Clin Exp Res, 23, 1386–94. [PubMed] [Google Scholar]
- Monti PM, Rohsenow DJ, Rubonis AV, Niaura RS, Sirota AD, Colby SM, Goddard P. & Abrams DB 1993. Cue exposure with coping skills treatment for male alcoholics: a preliminary investigation. J Consult Clin Psychol, 61, 1011–9. [DOI] [PubMed] [Google Scholar]
- Morean ME, Demartini KS, Foster D, Patock-Peckham J, Garrison KA, Corlett PR, Krystal JH, Krishan-Sarin S. & O’malley SS 2018. The Self-Report Habit Index: Assessing habitual marijuana, alcohol, e-cigarette, and cigarette use. Drug Alcohol Depend, 186, 207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myrick H, Anton RF, Li X, Henderson S, Randall PK & Voronin K. 2008. Effect of naltrexone and ondansetron on alcohol cue-induced activation of the ventral striatum in alcohol-dependent people. Arch Gen Psychiatry, 65, 466–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niaura RS, Rohsenow DJ, Binkoff JA, Monti PM, Pedraza M. & Abrams DB 1988. Relevance of cue reactivity to understanding alcohol and smoking relapse. J Abnorm Psychol, 97, 133–52. [DOI] [PubMed] [Google Scholar]
- O’malley SS, Jaffe AJ, Chang G, Schottenfeld RS, Meyer RE & Rounsaville B. 1992. Naltrexone and coping skills therapy for alcohol dependence. A controlled study. Arch Gen Psychiatry, 49, 881–7. [DOI] [PubMed] [Google Scholar]
- O’malley SS, Zweben A, Fucito LM, Wu R, Piepmeier ME, Ockert DM, Bold KW, Petrakis I, Muvvala S, Jatlow P. & Gueorguieva R. 2018. Effect of Varenicline Combined With Medical Management on Alcohol Use Disorder With Comorbid Cigarette Smoking: A Randomized Clinical Trial. JAMA Psychiatry, 75, 129–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooteman W, Koeter MW, Verheul R, Schippers GM & Van Den Brink W. 2007. The effect of naltrexone and acamprosate on cue-induced craving, autonomic nervous system and neuroendocrine reactions to alcohol-related cues in alcoholics. Eur Neuropsychopharmacol, 17, 558–66. [DOI] [PubMed] [Google Scholar]
- Plebani JG, Ray LA, Morean ME, Corbin WR, Mackillop J, Amlung M. & King AC 2012. Human laboratory paradigms in alcohol research. Alcohol Clin Exp Res, 36, 972–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez J. & Miranda R Jr. 2014. Alcohol craving in adolescents: bridging the laboratory and natural environment. Psychopharmacology (Berl), 231, 1841–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds EK & Monti PM 2013. The cue reactivity paradigm in addiction research In: MACKILLOP J. & DE WIT H. (eds.) The Wiley-Blackwell Handbook of Addiction Psychopharmacology. West Sussex: UK: John Wiley & Sons Ltd. [Google Scholar]
- Roberts W, Harrison ELR & Mckee SA 2017. Effects of varenicline on alcohol cue reactivity in heavy drinkers. Psychopharmacology (Berl), 234, 2737–2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosner S, Hackl-Herrwerth A, Leucht S, Vecchi S, Srisurapanont M. & Soyka M. 2010. Opioid antagonists for alcohol dependence. Cochrane Database Syst Rev, CD001867. [DOI] [PubMed] [Google Scholar]
- Rubonis AV, Colby SM, Monti PM, Rohsenow DJ, Gulliver SB & Sirota AD 1994. Alcohol cue reactivity and mood induction in male and female alcoholics. J Stud Alcohol, 55, 487–94. [DOI] [PubMed] [Google Scholar]
- Sacks JJ, Gonzales KR, Bouchery EE, Tomedi LE & Brewer RD 2015. 2010 National and State Costs of Excessive Alcohol Consumption. Am J Prev Med, 49, e73–9. [DOI] [PubMed] [Google Scholar]
- Schacht JP, Anton RF, Randall PK, Li X, Henderson S. & Myrick H. 2013. Effects of a GABA-ergic medication combination and initial alcohol withdrawal severity on cue-elicited brain activation among treatment-seeking alcoholics. Psychopharmacology (Berl), 227, 627–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacht JP, Anton RF, Randall PK, Li X, Henderson S. & Myrick H. 2014. Varenicline effects on drinking, craving and neural reward processing among non-treatment-seeking alcohol-dependent individuals. Psychopharmacology (Berl), 231, 3799–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacht JP, Randall PK, Latham PK, Voronin KE, Book SW, Myrick H. & Anton RF 2017. Predictors of Naltrexone Response in a Randomized Trial: Reward-Related Brain Activation, OPRM1 Genotype, and Smoking Status. Neuropsychopharmacology, 42, 2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Fox HC, Hong KI, Hansen J, Tuit K. & Kreek MJ 2011. Effects of adrenal sensitivity, stress- and cue-induced craving, and anxiety on subsequent alcohol relapse and treatment outcomes. Arch Gen Psychiatry, 68, 942–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan JT, Sykora K, Schneiderman J, Naranjo CA & Sellers EM 1989. Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar). Br J Addict, 84, 1353–7. [DOI] [PubMed] [Google Scholar]
- Tidey JW, Monti PM, Rohsenow DJ, Gwaltney CJ, Miranda R Jr., Mcgeary JE, Mackillop J, Swift RM, Abrams DB, Shiffman S. & Paty JA 2008. Moderators of naltrexone’s effects on drinking, urge, and alcohol effects in non-treatment-seeking heavy drinkers in the natural environment. Alcohol Clin Exp Res, 32, 58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendruscolo LF, Estey D, Goodell V, Macshane LG, Logrip ML, Schlosburg JE, Mcginn MA, Zamora-Martinez ER, Belanoff JK, Hunt HJ, Sanna PP, George O, Koob GF, Edwards S. & Mason BJ 2015. Glucocorticoid receptor antagonism decreases alcohol seeking in alcohol-dependent individuals. J Clin Invest, 125, 3193–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpicelli JR, Alterman AI, Hayashida M. & O’brien CP 1992. Naltrexone in the treatment of alcohol dependence. Arch Gen Psychiatry, 49, 876–80. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.