Abstract
Short-term (3-day) consumption of a high fat diet (HFD) rich in saturated fats is associated with a neuroinflammatory response and subsequent cognitive impairment in aged, but not young adult, male rats. This exaggerated effect in aged rats could be due to a “primed” microglial phenotype observed in the normal aging process in rodents in which aged microglia display a potentiated response to immune challenge. Here, we investigated the impact of HFD on microglial priming and lipid composition in the hippocampus and amygdala of young and aged rats. Furthermore, we investigated the microglial response to palmitate, the main saturated fatty acid (SFA) found in HFD that is proinflammatory. Our results indicate that HFD increased gene expression of microglial markers of activation indicative of microglial priming, including CD11b, MHCII, CX3CR1, and NLRP3, as well as the pro-inflammatory marker IL-1β in both hippocampus and amygdala-derived microglia. Furthermore, HFD increased the concentration of SFAs and decreased the concentration of polyunsaturated fatty acids (PUFAs) in the hippocampus. We also observed a specific decrease in the anti-inflammatory PUFA docosahexaenoic acid (DHA) in the hippocampus and amygdala of aged rats. In a separate cohort of young and aged animals, isolated microglia from the hippocampus and amygdala exposed to palmitate in vitro induced an inflammatory gene expression profile mimicking the effects of HFD in vivo. These data suggest that palmitate may be a critical nutritional signal from the HFD that is directly involved in hippocampal and amygdalar inflammation. Interestingly, microglial activation markers were increased in response to HFD or palmitate in an age-independent manner, suggesting that HFD sensitivity of microglia, under these experimental conditions, is not the sole mediator of the exaggerated inflammatory response observed in whole tissue extracts from aged HFD-fed rats.
1. Introduction
Currently, over two-thirds of the U.S. population is either overweight or obese, leading to enormous strain on healthcare and a growing economic burden (Popkin and Doak, 2009). This rise in obesity is likely attributed to environmental factors including the overconsumption of unhealthy diets such as those high in saturated fats or sugars (Lake and Townshend, 2006). Over the last 15 years, consumption of a high saturated fat diet (HFD) has been shown to elicit a neuroinflammatory response in rodents, non-human primates, and humans (Grayson et al., 2010; Miller and Spencer, 2014). While initially constrained to the hypothalamus and hindbrain, chronic HFD-induced inflammation can eventually impact other regions such as the hippocampus and amygdala where it can have a detrimental impact on learning and memory and serve as a major risk factor for type 2 diabetes and neurodegenerative diseases (Guillemot-Legris and Muccioli, 2017; Miller and Spencer, 2014).
Normal aging is also associated with a neuroinflammatory response, particularly within the hippocampus and amygdala, and significant cognitive impairments in both rodents and humans (Barrientos et al., 2015; Godbout and Johnson, 2006). Heightened baseline inflammation in aged rodents can lead to exaggerated responses to immune challenges such as surgery, bacterial infection, or unhealthy diets (Barrientos et al., 2012, 2009; Skvarc et al., 2018; Spencer et al., 2017). We have previously shown that even just short-term HFD consumption (3-day) produces a rapid inflammatory response in the hippocampus and amygdala and leads to impairment of hippocampal- and amygdalar-dependent memories in aged rats but does not produce the same overt effects in young adults (Spencer et al., 2017). The potentiated effects of HFD in aged rats could be due to the “primed” microglial phenotype observed in aged animals, characterized by an increase in cell-surface receptors such as cluster of differentiation molecule 11 b (CD11b) and major histocompatibility complex class II (MHC II), which are indicators of microglial activation (Frank et al., 2010, 2006a; Perry et al., 1993; Rozovsky et al., 1998). This hyperactivity at baseline can lead to increased sensitivity to immune challenges such as the endotoxin lipopolysaccharide (LPS) (Frank et al., 2010). While previous work has shown HFD sensitizes young adult rats to a secondary LPS challenge and alters microglial morphology in aged animals, the effects of HFD on microglial priming have not been fully characterized (Sobesky et al., 2016, 2014; Spencer et al., 2019).
While the mechanism of how HFD evokes a neuroimmune response is not fully understood, the contribution of individual fatty acids has received a lot of attention. Briefly, consumption of a HFD increases circulating free fatty acids (FFAs) (Giles et al., 2016; Karmi et al., 2010) which can enter the brain via passive diffusion or transport protein-mediated mechanisms (Tracey et al., 2018). In the brain parenchyma, fatty acids or their metabolites can directly influence inflammation. Specifically, palmitic acid, the main saturated fatty acid (SFA) found in HFD can induce a proinflammatory response via toll-like receptor 4 (TLR4) activation or metabolism-dependent mechanisms and has been linked to several neuropathological conditions including obesity and Alzheimer’s disease (Fatima et al., 2019; Listenberger et al., 2001a; Sergi et al., 2018; Tse and Belsham, 2018; Wang et al., 2012). Furthermore, microglia can respond directly to fatty acids as palmitic acid treatment activates microglia in vitro and increases the release of proinflammatory cytokines (Duffy et al., 2015a; Wang et al., 2012; Yanguas-Casás et al., 2018). As such, changes in fatty acid concentrations in the hippocampus or amygdala and increased fatty acid sensitivity of aged microglia may be a potential mechanism for HFD-induced inflammation and cognitive deficits in aged rats.
Thus, the goal of the current study was to investigate the impact of short-term HFD consumption on indicators of microglial priming and inflammation in isolated microglia from the hippocampus and amygdala of adult and aged rats. We also investigated how short-term HFD consumption and age alters the lipid composition of the hippocampus and the amygdala and if treatment with palmitate induces an increased inflammatory response in aged microglia. We hypothesized that microglia from aged rats fed a HFD would have increased levels of microglial priming and proinflammatory markers compared to microglia from aged rats fed a standard low-fat chow diet and young adult rats fed a standard chow or HFD. We further hypothesized that short-term HFD consumption would increase the concentration of SFAs, specifically palmitic acid in the hippocampus and amygdala and that this increase would be greatest in aged rats. Finally, we hypothesized that microglia isolated from aged rats would have a greater sensitivity to palmitate treatment than microglia isolated from adult rats.
2. Materials and methods
2.1. Subjects
3 and 24 month old male F344×BN F1 rats (N=67) were utilized. Animals were obtained from the National Institute on Aging (Bethesda, MD). The animal colony was maintained at 22 °C on a 12-h light/dark cycle (lights on at 07:00 h). Animals were allowed free access to food and water and were given 1 week to acclimate to colony conditions before experimentation began. All experiments were conducted in accordance with protocols approved by the Ohio State University Institutional Animal Care and Use Committee.
2.2. Diet
A total of 67 animals were used in this study. At study onset, young and aged animals were randomly assigned to either continue consuming their regular chow (Teklad Diets, TD.8640; energy density of 3.0 kcal/g; 29% calories from protein, 54% from carbohydrates [no sweetener added], and 17% from fat [0.9% saturated, 1.2% monounsaturated, 2.7% polyunsaturated]), or an adjusted calorie 60% HFD (TD.06414, Envigo, energy density of 5.1 kcal/g; 18.4% calories from protein, 21.3% from carbohydrates [90 g/kg sucrose, 160 g/kg maltodextrin], and 60.3% from fat [37% saturated, 47% monounsaturated, 16% polyunsaturated]). Animals consumed HFD ad libitum for 3 days.
2.3. Tissue collection
Animals were given a lethal dose of sodium pentobarbital and transcardially perfused with ice-cold saline (0.9%) for 3 min to remove peripheral immune leukocytes from the CNS vasculature. Brains were rapidly extracted and placed on ice and hippocampus and amygdala were dissected for microglia isolations or frozen in liquid nitrogen and stored at −80°C until processing for lipid composition analysis.
2.4. Effects of 3-day HFD on isolated hippocampal and amygdalar microglia from young and aged animals.
This experiment had 4 conditions: young-chow (n=6), young-HFD (n=6), aged-chow (n=6), and aged-HFD (n=6). Hippocampal and amygdalar microglia were isolated using a Percoll density gradient. This well-validated method for rapidly isolating microglia (Cardona et al., 2006; Doorn et al., 2015; Frank et al., 2006b) yields a highly pure microglial population as we have previously determined by robust microglia-specific CD11b and MHCII amplification, but little to no amplification of astrocyte-specific GFAP or perivascular/meningeal macrophage marker CD163 using RT-PCR (Barrientos et al., 2015; Fonken et al., 2016b, 2016a, 2015; Frank et al., 2012, 2010). In the present experiment, we again observed robust CD11b and MHCII amplification. Due to limited volumes of cDNA we measured GFAP in a semi-randomized subset of our samples and confirmed no amplification across all groups (data not shown).
Microglia were suspended in Dulbecco’s modified Eagle’s medium (DMEM) + 10% fetal bovine serum (FBS) and microglia concentration determined by trypan blue exclusion. Cell number did not significantly differ between 3 and 24mo animals. Microglia concentration was adjusted to a density of 10,000/100μl and 90μl were added to individual wells of a 96-well v-bottom plate for a total of ~9,000 cells per well. After allowing the cells to incubate for 2 h at 37°C, 5% CO2, the plate was centrifuged at 1000×g for 10 min at 4 °C to pellet cells and cells were washed with ice-cold 1x PBS and centrifuged again at 1000×g for 10 min at 4 °C. Cell lysis/homogenization, DNase treatment, and cDNA synthesis were performed using the SuperScript III CellsDirect cDNA Synthesis System according to kit instructions (Invitrogen, Carlsbad, CA).
2.5. Effects of age and 3-day HFD on lipid composition in the hippocampus and amygdala.
In a separate cohort, rats were assigned to the following groups: young-chow (n =8), young-HFD (n = 8), aged-chow (n = 7), and aged-HFD (n = 8). Rats were euthanized and perfused as described above. Total lipids were extracted from all brain samples with 2:1 chloroform:methanol according to Folch (Folch et al., 1957). Phospholipids and neutral lipids were separated from hippocampal samples using solid phase extraction (Hamilton and Comai, 1988). Phospholipids and neutral lipids were not separated from amygdalar samples due to smaller tissue size and less total lipid yield. Fatty acids were methylated using 5% hydrochloric acid in methanol at 76°C (Stoffel et al., 1959). Fatty acid methyl esters were analyzed by gas chromatography using a 30-m Omegawax TM 320 fused silica capillary column (Supelco, Bellefonte, PA). Fatty acids are reported as a percent of total identified (Belury et al., 2016). For analysis, concentrations of individual fatty acids were summed to generate a percentage of total SFAs, polyunsaturated fatty acids (PUFAs), and monounsaturated fatty acids (MUFAs) for each animal for each brain. Palmitic acid, docosahexaenoic acid (DHA), and oleic acid were individually analyzed and reported for the hippocampus and amygdala due to their abundance and biological relevance. All fatty acid species of notable abundance are reported in tables 1–3.
Table 1.
Fatty acid profile of neutral lipids extracted from the hippocampus. Values are expressed as percent of total fatty acids.
| Young-Chow | Aged-Chow | Young-HFD | Aged-HFD | |
|---|---|---|---|---|
| Palmitic Acid | 38.12 ± 1.32 | 36.48 ± 1.38 | 39.25 ± 1.30 | 39.53 ± 0.74 |
| Stearic Acid | 34.40 ± 0.52 | 33.70 ± 0.86 | 35.09 ± 0.39 | 34.50 ± 0.32 |
| Arachidonic acid | 11.06 ± 0.86 | 12.21 ± 1.10 | 10.67 ± 0.76 | 10.31 ± 0.60 |
| Oleic Acid | 6.01 ± 0.39 | 7.59 ± 0.69 | 5.81 ± 0.22 | 6.41 ± 0.32 |
| Docosahexaenoic Acid | 2.41 ± 0.26 | 2.29 ± 0.15 | 2.17 ± 0.19 | 2.12 ± 0.09 |
| Vaccenic Acid | 2.14 ± 0.09 | 2.38 ± 0.23 | 2.00 ± 0.08 | 2.24 ± 0.09 |
| Linoleic Acid | 0.57 ± 0.06 | 0.81 ± 0.09 | 0.53 ± 0.07 | 0.77 ± 0.10 |
| Adrenic Acid | 0.63 ± 0.08 | 0.55 ± 0.04 | 0.55 ± 0.04 | 0.50 ± 0.03 |
| Gondoic Acid | 0.64 ± 0.12 | 0.53 ± 0.06 | 0.72 ± 0.10 | 0.73 ± 0.09 |
Table 3.
Fatty acid profile of total lipids extracted from the amygdala. Values are expressed as percent of total fatty acids.
| Young-Chow | Aged-Chow | Young-HFD | Aged-HFD | |
|---|---|---|---|---|
| Palmitic Acid | 22.41 ± 0.34 | 22.80 ± 0.45 | 22.95 ± 0.32 | 22.53 ± 0.26 |
| Stearic Acid | 22.65 ± 0.35 | 22.81 ± 0.26 | 23.21 ± 0.20 | 22.80 ± 0.13 |
| Arachidonic acid | 11.95 ± 0.37 | 12.35 ± 0.27 | 12.10 ± 0.22 | 12.18 ± 0.29 |
| Oleic Acid | 15.18 ± 0.37 | 15.69 ± 0.49 | 14.56 ± 0.37 | 15.90 ± 0.36 |
| Docosahexaenoic Acid | 15.18 ± 0.19 | 13.87 ± 0.22 | 15.33 ± 0.19 | 14.02 ± 0.17 |
| Vaccenic Acid | 2.89 ± 0.10 | 2.82 ± 0.11 | 2.84 ± 0.07 | 2.83 ± 0.07 |
| Linoleic Acid | 0.55 ± 0.02 | 0.47 ± 0.03 | 0.59 ± 0.01 | 0.45 ± 0.02 |
| Adrenic Acid | 3.71 ± 0.12 | 3.92 ± 0.09 | 3.81 ± 0.08 | 3.93 ± 0.07 |
| Gondoic Acid | 0.98 ± 0.10 | 1.28 ± 0.13 | 0.91 ± 0.09 | 1.31 ± 0.10 |
2.6. Ex vivo immune stimulation of hippocampal and amygdalar microglia with palmitate
In a separate cohort, young (n=6) and aged (n=6) chow-fed male rats were euthanized and hippocampal and amygdalar microglia were isolated and plated as described above. A 100x concentration of sodium palmitate (Sigma, P9767) was reconstituted in molecular biology grade water and heated to 70°C for ~ 5 min before being diluted 1:10 in DMEM + 10% FBS heated to 37 °C. Palmitate was further diluted 1:10 to the working concentration upon delivery to the wells. Microglia were incubated with palmitate (50, 100, and 500μM) or vehicle for 2 h at 37 °C, 5% CO2. These doses were chosen in accordance with previous studies that treated microglia, astrocytes, or neurons with palmitate (Duffy et al., 2015b; Frago et al., 2017; Hidalgo-Lanussa et al., 2017; Listenberger et al., 2001b; Sergi et al., 2018; Tse and Belsham, 2018; Wang et al., 2012; Yanguas-Casás et al., 2018) and are consistent with a physiological range of plasma palmitic acid levels (Abdelmagid et al., 2015). Following treatment, the plate was centrifuged at 1000×g for 10 min at 4 °C to pellet cells and cells washed 1× in ice-cold PBS and centrifuged at 1000×g for 10 min at 4 °C. Cell lysis/homogenization, DNase treatment, and cDNA synthesis were performed as described above.
2.7. Real time PCR (RT-PCR) measurement of gene expression
A detailed description of the PCR amplification protocol has been published previously (Frank et al., 2006). cDNA sequences were obtained from Genbank at the National Center for Biotechnology Information (NCBI;www.ncbi.nlm.nih.gov). Primer sequences were designed using the Qiagen Oligo Analysis & Plotting Tool (oligos.qiagen.com/oligos/toolkit.php?) and tested for sequence specificity using the Basic Local Alignment Search Tool at NCBI (Altschul et al.,1997). Primers were obtained from Invitrogen. Primer specificity was verified by melt curve analysis. All primers were designed to exclude amplification of genomic DNA. Primer sequences are as follows: β-Actin, F-TTCCTTCCTGGGTATGGAAT, R-GAGGAGCAATGATCTTGATC; CD11b, F-CTGGTACATCGAGACTTCTC, R-TTGGTCTCTGTCTGAGCCTT; interleukin 1-β (IL-1β), F-CCTTGTGCAAGTGTCTGAAG, R-GGGCTTGGAAGCAATCCTTA; MHC II, F-AGCACTGGGAGTTTGAA-GAG, R-AAGCCATCACCTCCTGGTAT; nod-like receptor protein 3 (NLRP3), F-AGAAGCTGGGGTTGGTGAATT, R-GTTGTCTAACTCCAGCATCTG; high mobility group box 1 (HMGB1), F-GAGGTGGAAGACCATGTCTG, R-AAGAAGAAGGCCGAAGGAGG; CX3C receptor 1 (CX3CR1), F-TCAGGACCTCACCATGCCTA, R-CGAACGTGAAGACAAGGGAG. PCR amplification of cDNA was performed using the Quantitect SYBR Green PCR Kit (Qiagen, Valencia, CA). Formation of PCR product was monitored in real time using the QuantStudio 3 Real-Time PCR System (Applied Biosystems, Waltham, MA). Relative gene expression was determined by the ΔΔCT method of qPCR analysis normalized to β-Actin. The mean ΔΔCT of the young-chow group or the young-vehicle group was used as the calibrator to calculate LOG fold-changes (control group set to 0) in mRNA concentrations.
2.8. Data analysis
All data are presented as means + SEM. Statistical analyses were computed using GraphPad Prism version 7. Unequal sample sizes both within and across experiments are related to the exclusion of subjects due to low cell yields, particularly from amygdala dissections. In addition, due to animal ordering limitations by the National Institute on Aging, the desired number of animals is not always available, which also contributes to unequal sample sizes. Two-way ANOVAs were run for the experiments that had a 2 × 2 factorial design. In the case of significant interactions, Tukey’s multiple comparisons post hoc tests were run. The threshold for significance was set as α = 0.05. Only significant F-values were reported in the results section.
3. Results
3.1. HFD consumption increases inflammatory mRNA concentration in young and aged microglia from the hippocampus and amygdala
We investigated the impact of age and 3-day consumption of HFD on a variety of proinflammatory genes in microglia isolated from the hippocampus and amygdala. Results indicate a main effect of diet, where HFD increased mRNA concentration of the microglial activation marker CD11b relative to chow (F (1, 19) = 7.757, p < 0.05; Fig. 1A) in hippocampal, but not amygdalar microglia (Fig. 2A). There was also an increase in aged hippocampal and amygdalar microglia but this was not statistically significant. There was, however, a main effect of age for another microglial activation marker, MHCII, (F (1, 19) = 12.84, p < 0.01; Fig. 1B) in microglia isolated from the hippocampus but not from the amygdala (Fig. 2B). However, HFD increased MHCII in amygdalar microglia (F (1, 16) = 6.158, p < 0.05; Fig. 2B). In hippocampal microglia there was also a main effect of HFD for the fractalkine receptor CX3CR1, the inflammasome component NLRP3, and the proinflammatory cytokine IL-1β (CX3CR1: F (1, 20) = 5.955, p < 0.05; Fig. 1D; NLRP3: F (1, 20) = 6.542, p < 0.05; Fig. 1E; IL-1β: F (1, 19) = 5.086; Fig. 1F). There was also a main effect of age for CX3CR1 (F (1, 20) = 7.118, p < 0.05; Fig. 1D). In microglia isolated from the amygdala, there was a main effect of HFD for CX3CR1 and NLRP3 (CX3CR1: F (1, 16) = 4.666, p < 0.05; Fig. 2D; NLRP3: F (1, 16) = 8.942, p < 0.01; Fig. 2E). Age increased these markers as well but was not statistically significant. However, there was no significant effect of age or HFD on IL-1β in the amygdala, though both groups were increased (Fig. 2F). There was no significant effect of age or diet on the endogenous danger signal HMGB1 in hippocampal (Fig. 1C) or amygdalar (Fig. 2C) microglia, although it was increased in the amygdala. There were no significant diet × age interactions.
Fig. 1.
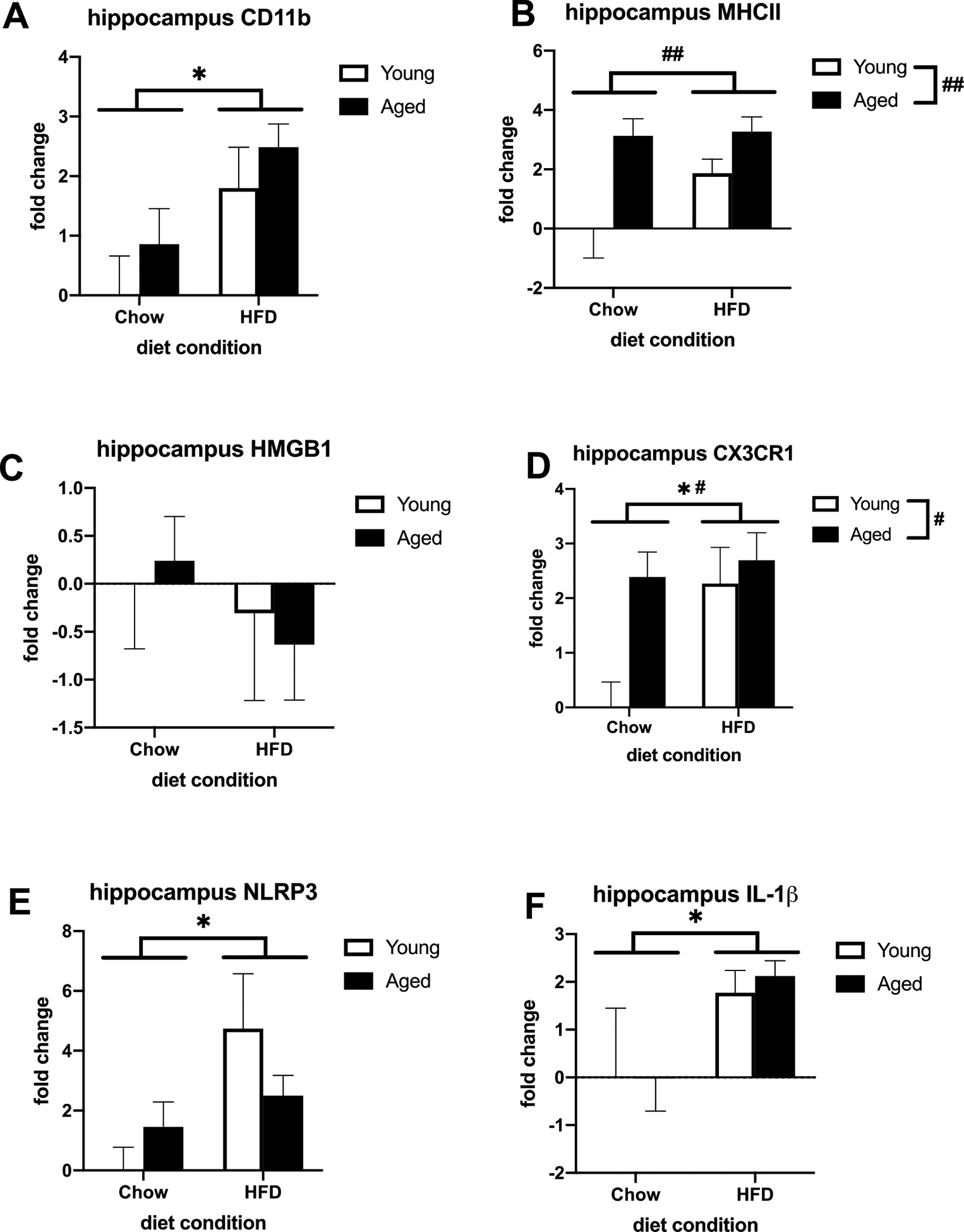
Priming and proinflammatory markers in hippocampal microglia isolated from adult and aged animals fed a standard chow or a HFD. (A) Fold change in CD11b mRNA (B) Fold change in MHC II mRNA (C) Fold change in HMGB1 mRNA (D) Fold change in CX3CR1 mRNA (E) Fold change in NLRP3 mRNA (F) Fold change in IL-1β mRNA. *p < 0.05 (main effect of diet), # p < 0.05 (main effect of age), ## p < 0.01.
Fig. 2.
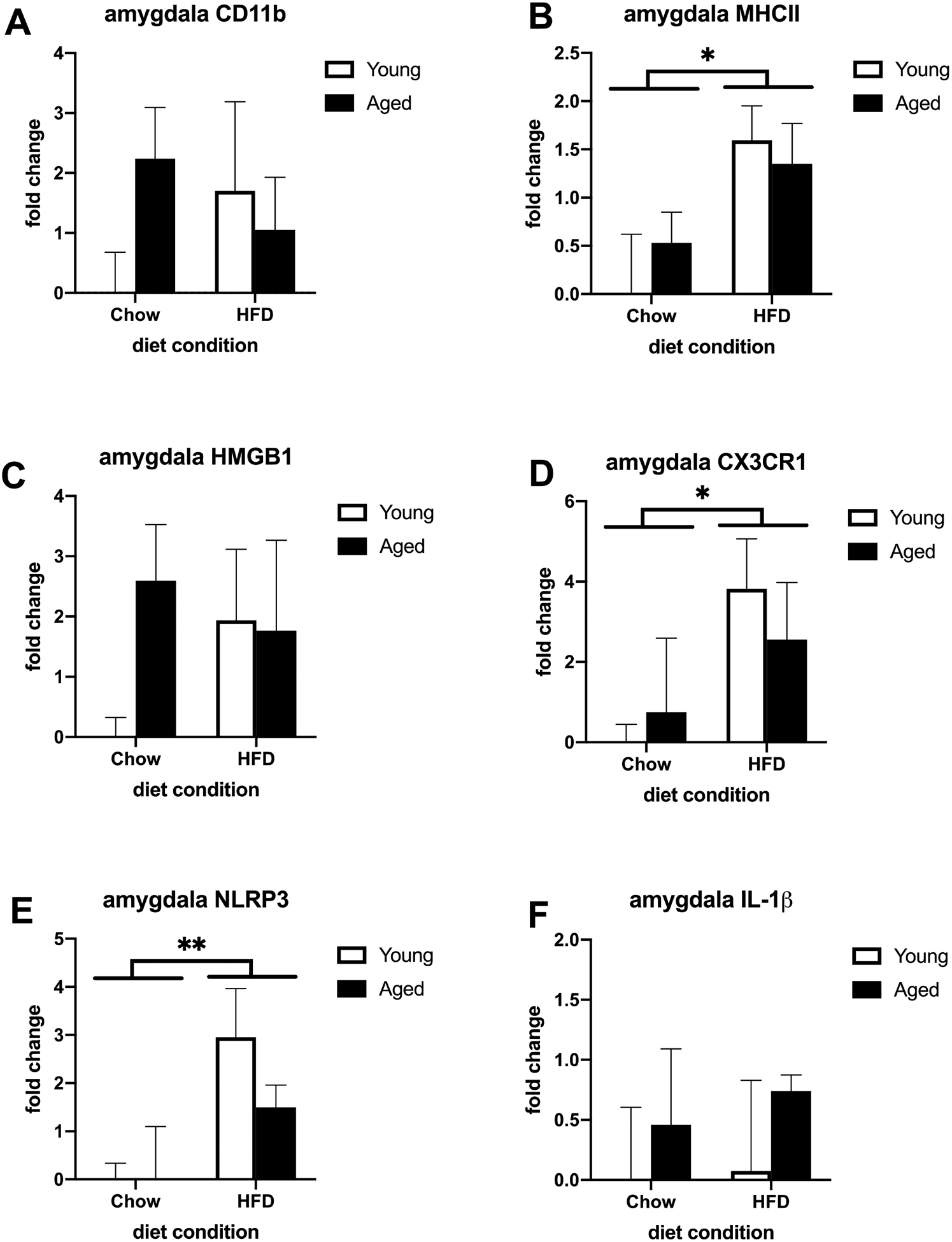
Priming and proinflammatory markers in amydalar microglia isolated from adult and aged animals fed a standard chow or a HFD. (A) Fold change in CD11b mRNA (B) Fold change in MHC II mRNA (C) Fold change in HMGB1 mRNA (D) Fold change in CX3CR1 mRNA (E) Fold change in NLRP3 mRNA (F) Fold change in IL-1β mRNA. *p < 0.05 (main effect of diet), **p < 0.01 (main effect of diet).
3.2. Age and diet alters the lipid composition in the hippocampus and amygdala
To investigate how age and HFD might be impacting the concentration of various fatty acids in brain regions associated with learning and memory, we measured the lipid composition of the hippocampus and amygdala. For hippocampal neutral lipids, HFD increased total SFA concentration (F (1, 25) = 8.332, p < 0.01; Fig. 3A) and decreased total PUFA concentration (F (1, 26) = 4.942, p < 0.05; Fig. 3C). Additionally, age increased the concentration of total MUFAs (F (1, 27) = 7.165, p < 0.05; Fig. 3E). Of the SFAs, there was a trend for an increase in palmitic acid (F (1, 27) = 3.020, p = 0.093; Fig. 3B). There was an increase in the MUFA oleic acid in aged animals (F (1, 27) = 6.740, p < 0.05; Fig. 3F). While diet appeared to decrease DHA, a prominent PUFA in the brain, this did not reach statistical significance (Fig. 3D). Within extracted hippocampal phospholipids, age decreased total SFAs, total PUFAs, and DHA (SFAs: F (1, 27) = 14.59, p < 0.01; Fig. 4A; PUFAs: F (1, 27) = 25.74, p < 0.01; Fig. 4C; DHA: F (1, 27) = 17.38, p < 0.01; Fig. 4D) and increased total MUFAs and oleic acid (MUFAs: F (1, 27) = 46.67, p < 0.01; Fig. 4E; oleic acid: F (1, 27) = 36.69, p < 0.01; Fig. 4F). There was no effect of diet or age on palmitic acid in hippocampal phospholipids (Fig. 4B). For total lipids extracted from the amygdala, aging decreased the concentration of DHA (F (1, 26) = 46.32, p < 0.01; Fig. 5D) and increased total MUFAs and oleic acid (MUFAs: F (1, 27) = 5.778, p < 0.05; Fig. 5E; oleic acid: F (1, 27) = 5.538, p < 0.05; Fig. 5F). There were no effects of diet or age on total SFAs (Fig. 5A), PUFAs (Fig. 5C), or palmitic acid in the amygdala (Fig. 5B). Overall, the only effects of diet on lipid composition were in the hippocampal neutral lipids as there were no main effects of diet on hippocampal phospholipids or amygdalar total lipids. There were no significant diet × age interactions in any brain region.
Fig. 3.
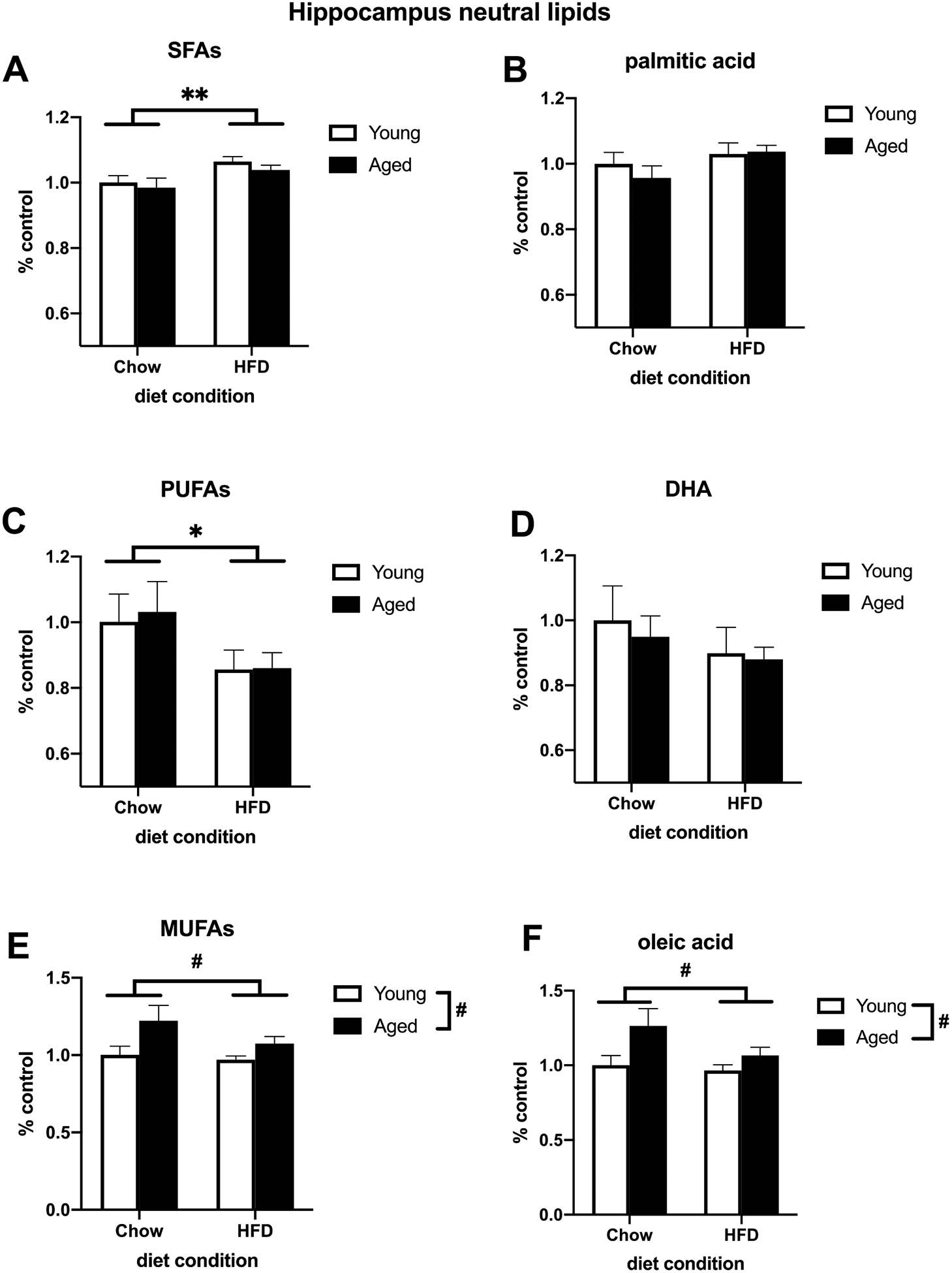
Relative concentration of neutral lipids extracted from the hippocampus of adult and aged rats fed either a standard chow or a HFD. (A) Total SFAs (B) Palmitic acid (C) Total PUFAs (D) DHA (E) Total MUFAs (F) Oleic acid. *p < 0.05 (main effect of diet), **p < 0.01 (main effect of diet), # p < 0.05 (main effect of age).
Fig. 4.
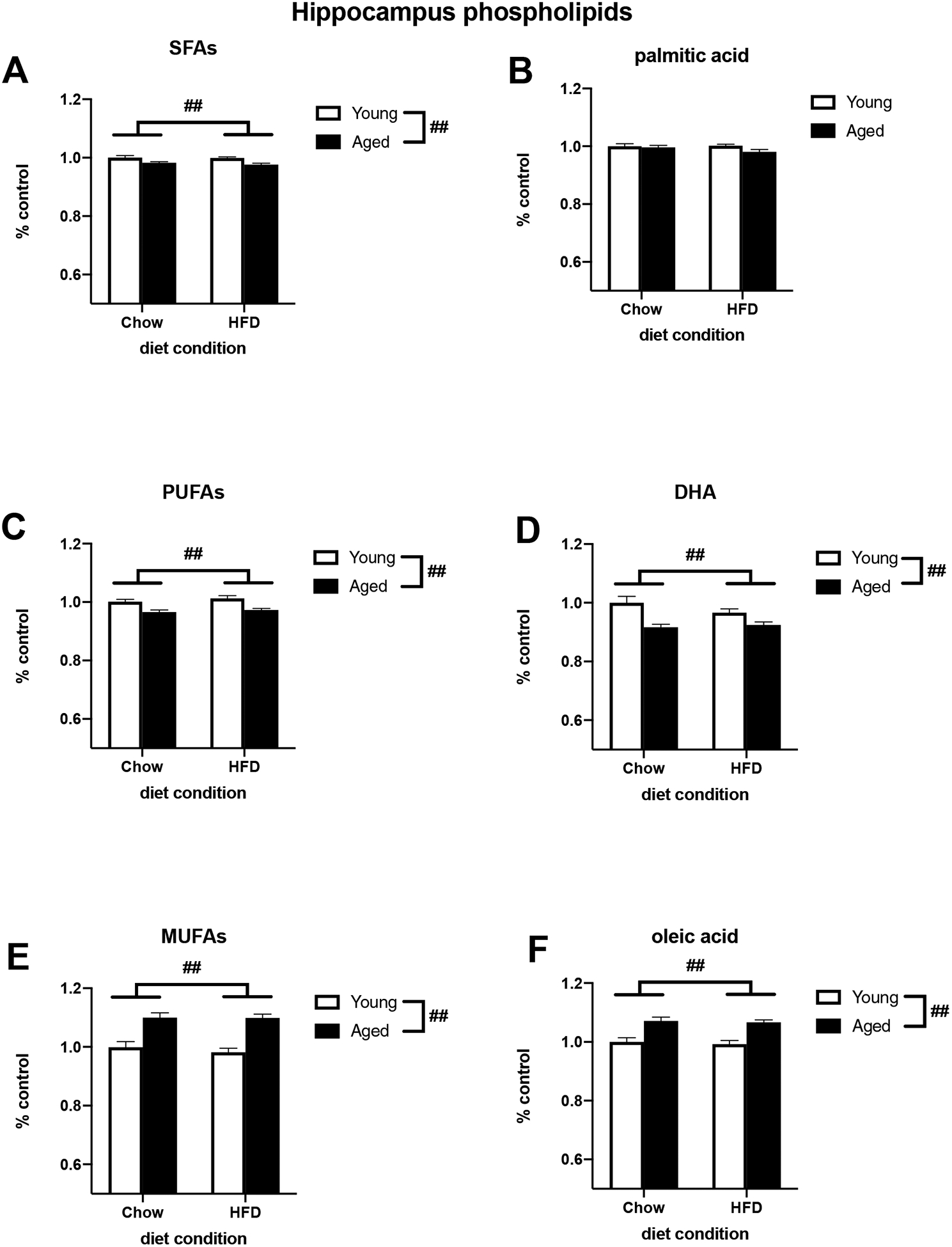
Relative concentration of phospholipids extracted from the hippocampus of adult and aged rats fed either a standard chow or a HFD. (A) Total SFAs (B) Palmitic acid (C) Total PUFAs (D) DHA (E) Total MUFAs (F) Oleic acid. ## p < 0.01 (main effect of age).
Fig. 5.
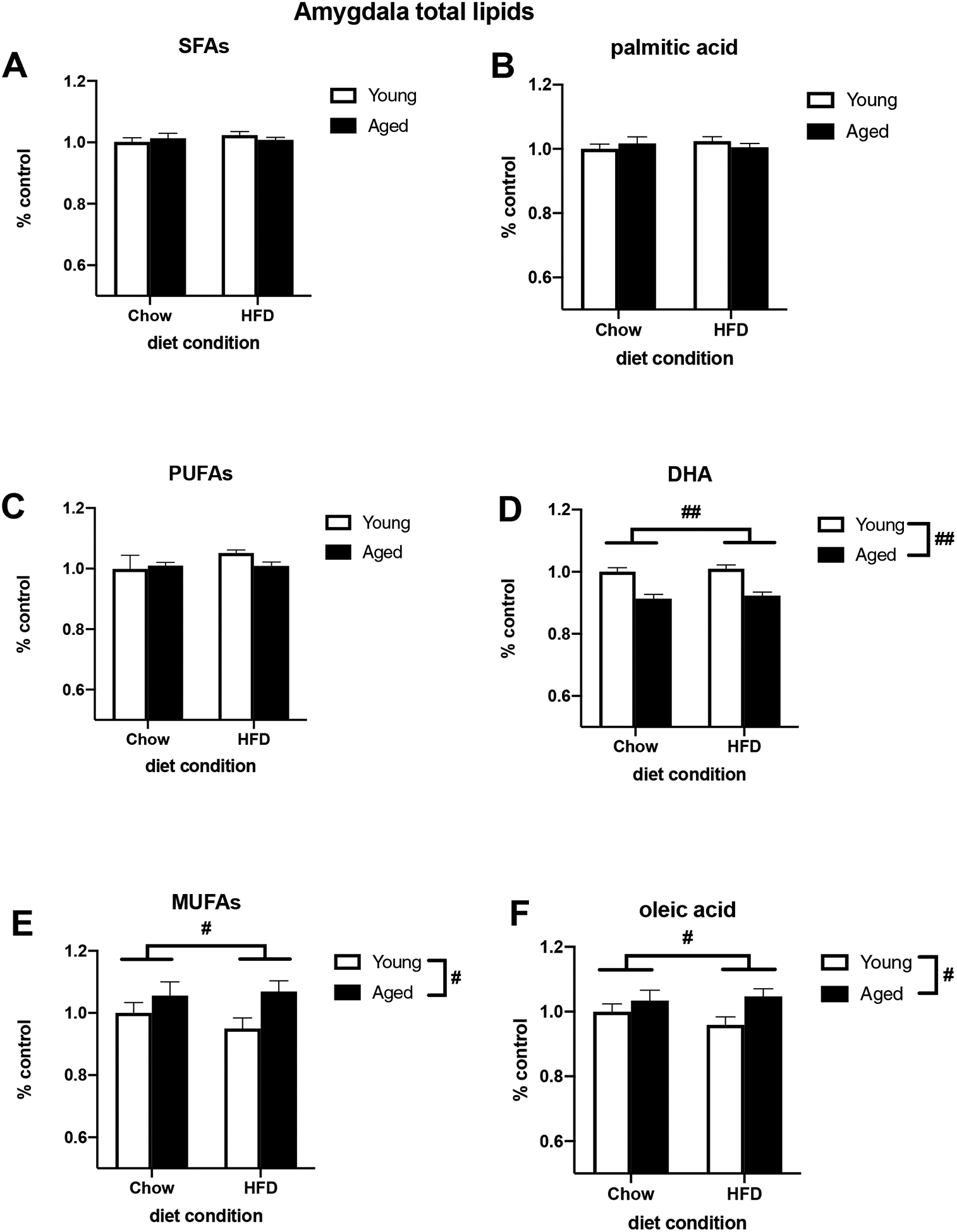
Relative concentration of total lipids extracted from the amygdala of adult and aged rats fed either a standard chow or a HFD. (A) Total SFAs (B) Palmitic acid (C) Total PUFAs (D) DHA (E) Total MUFAs (F) Oleic acid. # p < 0.05 (main effect of age), ## p < 0.01 (main effect of age).
3.3. Palmitate treatment increases inflammatory mRNA concentration in young and aged microglia from the hippocampus and amygdala in a dose-dependent manner
To investigate the impact of the most abundant SFA found in HFD, we treated isolated microglia with palmitate and investigated the same inflammatory genes as in the first experiment. We saw a main effect of palmitate treatment on CD11b and MHCII in both hippocampal (CD11b: F (3, 40) = 5.432, p < 0.01; Fig. 6A; MHCII: F (3, 40) = 9.499, p < 0.01; Fig. 6B) and amygdalar (CD11b: F (3, 39) = 4.602, p < 0.01; Fig. 7A; MHCII: F (3, 40) = 8.311, p < 0.0005; Fig. 7B) microglia. There was also an increase in these markers in aged microglia from both regions but this was not statistically significant. Palmitate treatment also significantly increased proinflammatory genes CX3CR1, NLRP3, and IL-1β in microglia isolated from both the hippocampus (CX3CR1: F (3, 40) = 12.52, p < 0.01; Fig. 6D; NLRP3: F (3, 40) = 7.570, p < 0.01; Fig. 6E; IL-1β: F (3, 40) = 7.852, p < 0.01; Fig. 6F) and amygdala (CX3CR1: F (3, 40) = 4.578, p < 0.01; Fig. 7D; NLRP3: F (3, 38) = 6.387, p < 0.05; Fig. 7E; IL-1β: F (3, 37) = 9.845, p < 0.01; Fig. 7F). Palmitate treatment increased HMGB1 in amygdalar microglia (F (3, 40) = 4.344, p < 0.01; Fig. 7C) but not in hippocampal microglia (Fig. 6C). There were no significant diet × age interactions.
Fig. 6.
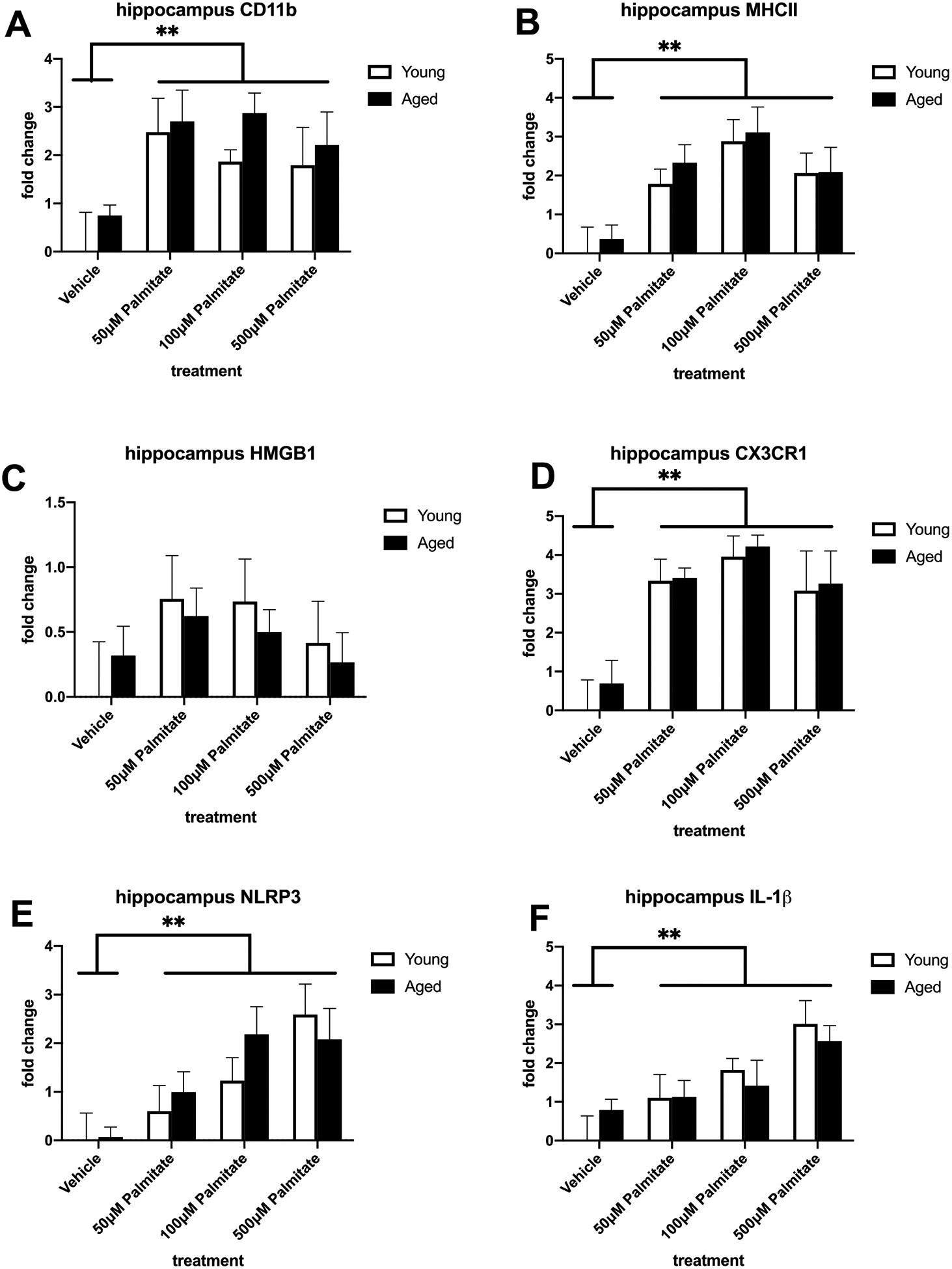
Priming and proinflammatory markers in hippocampal microglia isolated from adult and aged animals and treated with vehicle or palmitate. (A) Fold change in CD11b mRNA (B) Fold change in MHC II mRNA (C) Fold change in HMGB1 mRNA (D) Fold change in CX3CR1 mRNA (E) Fold change in NLRP3 mRNA (F) Fold change in IL-1β mRNA. **p < 0.01 (main effect of treatment)
Fig. 7.
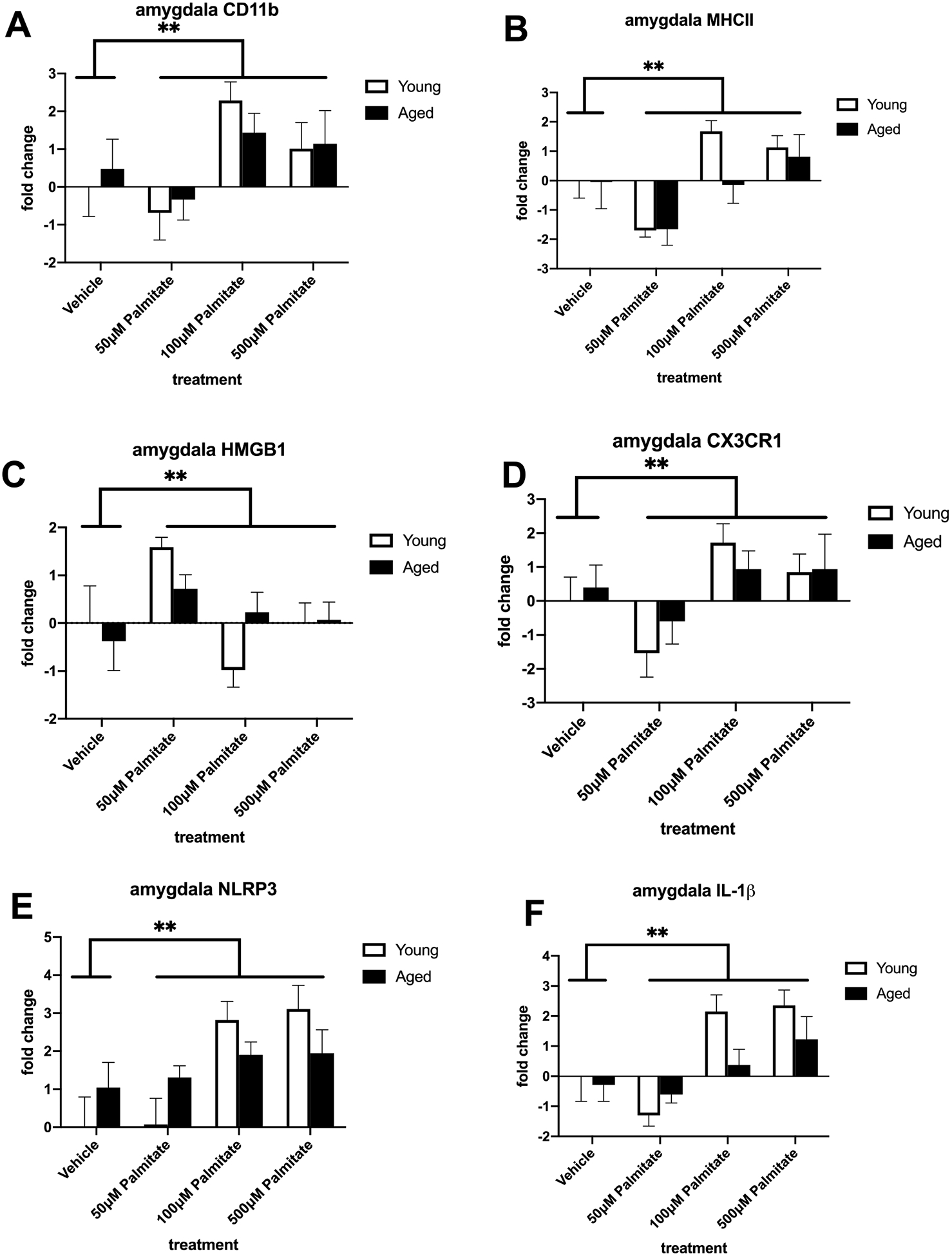
Priming and proinflammatory markers in amygdalar microglia isolated from adult and aged animals and treated with vehicle or palmitate. (A) Fold change in CD11b mRNA (B) Fold change in MHC II mRNA (C) Fold change in HMGB1 mRNA (D) Fold change in CX3CR1 mRNA (E) Fold change in NLRP3 mRNA (F) Fold change in IL-1β mRNA. **p < 0.01 (main effect of treatment).
4. Discussion
Together, our data show that HFD consumption and aging alter microglial priming and the lipid composition of memory-associated brain regions in male rats. We report that 3-day HFD consumption increased basal levels of microglial priming markers in rapidly isolated microglia from the adult and aged hippocampus and amygdala. HFD also increased the concentration of proinflammatory SFAs and decreased the concentration of anti-inflammatory PUFAs in lipids isolated from the hippocampus. Furthermore, there was an age-dependent decrease in the anti-inflammatory PUFA, DHA, in both the hippocampus and amygdala. Lastly, we showed that treatment of rapidly isolated microglia with the most abundant SFA, palmitate, resulted in a similar proinflammatory profile to HFD consumption in an age-independent manner.
Previous studies have shown an interaction between age and diet, such that 3-day HFD consumption increased the expression of IL-1β in the hippocampus and amygdala in aged rats, with little to no effect in young adult rats (Spencer et al., 2017). This same study showed an age and diet interaction for the neuroinflammatory priming marker, MHC II. Importantly, this study looked at whole hippocampal and amygdalar tissue extracts, so the relative contributions of specific cellular phenotypes were not examined. Here, we extend these data by investigating this phenomenon in rapidly isolated microglia from the hippocampus and the amygdala. We demonstrated that HFD increased the cell surface receptor CD11b in hippocampal microglia and MHCII in amygdalar microglia, regardless of age.
We also saw a HFD-induced increase in the fractalkine receptor CX3CR1 and the inflammasome component NLRP3. Functionally, NLRP3 regulates the cleavage and release of IL-1β (Khare et al., 2010; Schroder and Tschopp, 2010), which was also increased in both adult and aged microglia in animals fed a HFD. Interestingly, we saw no effect of diet on the endogenous danger signal HMGB1 in hippocampal microglia, which is in line with a previous study that showed no effect of age or diet on HMGB1 in hippocampal tissue (Spencer et al., 2017). We did see, however, a trend for age and diet to increase HMGB1 in amygdalar microglia, which extends a previous study showing an effect of age on HMGB1 mRNA in amygdala tissue (Spencer et al., 2017).
We observed a trend for age to increase all markers measured, but only MHCII and CX3CR1 in hippocampal microglia were significantly increased by age. It is important to note that several markers, including CX3CR1 and NLRP3, are not necessarily specific to microglia as there are several studies showing that astrocytes express these same markers (Albalawi et al., 2017; Dorf et al., 2000). MHC II, traditionally thought of as an indicator of microglial activation, is also expressed on astrocytes under certain inflammatory conditions (Dong and Benveniste, 2001). While a less robust age effect on pro-inflammatory markers is inconsistent with previous work in whole-tissue extracts, the discrepancy is likely related to the fact that these data were collected from isolated microglia. It is possible that removal of microglia from their endogenous microenvironment could have dampened neuronal or astrocytic signals that contribute to microglia-mediated inflammation (Barbierato et al., 2013; Szepesi et al., 2018). It is also possible that the stress of microglial isolation procedures may have elevated basal levels of microglial activity, making it more challenging to detect subtle interactions between diet and aging. However, these explanations are unlikely as previous work has demonstrated primary microglia retain their in vivo phenotype using multiple methods of isolation (Floden and Combs, 2007; Nikodemova and Watters, 2012). Taken together, these data indicate that HFD affects microglial priming in an age-independent manner, suggesting microglia may not be the main driver of the age and diet interaction in inflammatory signaling that has been well-characterized in previous reports. Of course, it is important to bear in mind that this study only examined short-term consumption of HFD and it is possible that longer durations would yield different outcomes. For example, a study in adult mice found that while short-term HFD consumption led to potentiated neuroinflammatory responses, prolonged consumption evoked an increase in anti-inflammatory responses, explaining the lack of a sensitized response to an LPS challenge in hypothalamic microglia (Baufeld et al., 2016). Whether similar protective mechanisms would be intact in the aging brain, and specifically in the hippocampus or amygdala, remains to be examined. Given the known impairments in microglial regulation in aging rodents (Frank et al., 2006b) it is possible that prolonged consumption would in fact exacerbate the neuroinflammatory response compared to what was observed here. It should be noted however, that examining prolonged consumption of HFD cannot be done without also causing obesity and invoking multiple systems (e.g. metabolic, hormonal, cardiovascular, etc…) that would interfere with interpretations about the specific contribution of diet on microglial responses.
Given the vast literature investigating the impact of dietary fatty acids on the inflammatory milieu in the brain, we investigated the lipid composition of the hippocampus and amygdala in response to aging and 3-day HFD consumption. HFD consumption increased SFAs and decreased PUFAs in neutral lipids extracted from the hippocampus. Because neutral lipids serve as the reservoir to provide energy for the cell, levels of neutral lipids can typically accumulate from excessive energy intake (MacDonald et al., 1996). In support of this notion, neutral lipids were the only lipids to be altered by our short-term diet manipulation in the current study. Previous work has shown that fatty acids delivered to the gut via enteric gavage are rapidly incorporated in brain regions critical for food intake and energy homeostasis (Valdearcos et al., 2014). Labeled fatty acids have also been shown to be rapidly transported into various regions of the brain (Mitchell and Hatch, 2011). However, to the best of our knowledge, this is the first evidence that a short-term dietary manipulation can alter the lipid composition of the hippocampus.
An increase in SFAs and a decrease in PUFAs in brain tissue is consistent with a proinflammatory microenvironment as the majority of studies suggest that SFAs are generally proinflammatory and PUFAs are anti-inflammatory (Layé, 2010; Wang et al., 2012). Specifically, we reported a trend for a HFD-induced increase in palmitic acid in the hippocampus, and palmitic acid was the most abundant fatty acid in the neutral lipid extracts. This is noteworthy because palmitic acid has previously been implicated in proinflammatory gene expression in a variety of brain pathologies, including diet-induced inflammation, diabetes, and Alzheimer’s disease (Fatima et al., 2019). We also demonstrated a diet-induced reduction in total PUFAs and a trend for HFD to decrease DHA in the hippocampus. Moreover, in hippocampal phospholipid and amygdalar total lipid extracts, we showed that aging decreased PUFAs, specifically DHA, regardless of diet condition. This is consistent with previous research in both rodents and humans showing that aging is associated with a decrease in DHA (Horrocks and Yeo, 1999; Little et al., 2007). DHA exerts anti-inflammatory effects in the brain and decreases proinflammatory gene expression (Layé et al., 2018). Conversely, decreased DHA levels have previously been linked to a proinflammatory state and cognitive impairment in both rodents and humans (Horrocks and Yeo, 1999; Labrousse et al., 2012). Briefly, DHA is cleaved from the phospholipid membrane and metabolized to produce active metabolites that resolve inflammation (Lacombe et al., 2018; Layé et al., 2018). Thus, decreased DHA levels in aged animals could be linked to exaggerated neuroimmune responses.
We also reported an increase in MUFAs, particularly oleic acid, in aged animals in the hippocampus and amygdala, which is consistent with a previous study that measured cortical MUFA concentration in aging mice (Albouery et al., 2020). While some studies suggest MUFAs, and oleic acid in particular, can decrease proinflammatory gene expression (Tse and Belsham, 2018), this conclusion is still controversial as other findings report both SFAs and MUFAs to increase proinflammatory gene expression (Button et al., 2014). A limitation of this study was our inability to separate neutral and phospholipids in the amygdala due to tissue size. Thus, this could have contributed to not detecting diet-induced changes in fatty acid composition in the amygdala as neutral lipids were the only lipids to be altered by diet in the hippocampus. Regardless, a decrease in PUFAs in the amygdala due to age could still be linked to HFD-induced amygdalar inflammation and amygdala-dependent cognitive deficits.
While SFAs are likely not the only contributor to diet-induced inflammation, they do have direct proinflammatory effects in the brain (Rogero and Calder, 2018). Likewise, due to the abundance of palmitic acid in HFD and the brain, as well as its previous implications in neuroinflammatory signaling (Fatima et al., 2019; Listenberger et al., 2001a; Sergi et al., 2018; Tse and Belsham, 2018), we treated microglia isolated from the hippocampus and the amygdala with various doses of palmitate, a commonly used technique to model HFD in vitro (Lacombe et al., 2018). Interestingly, palmitate appeared to mimic the results of our first experiment (Fig. 1 and 2) in which animals fed a HFD showed increased levels of pro-inflammatory mRNA and microglial priming. Likewise, palmitate increased mRNA of CD11b, MHC II, CX3CR1, NLRP3, and IL-1β in hippocampal and amygdalar microglia. Furthermore, there was no effect of palmitate on HMGB1 mRNA in hippocampal microglia but there was an effect of the low dose of palmitate on HMGB1 in amygdalar microglia. This is consistent with the ex vivo work in the current study (Fig. 2) and with our previous in vivo work that showed an HMGB1 increase in the amygdala but not hippocampus in response to aging and HFD (Spencer et al., 2017). In general, it appeared that amygdalar microglia were less sensitive to palmitate than hippocampal microglia as evidenced by a weaker effect at lower concentrations. These data, coupled with the lipid composition changes by HFD, could suggest the hippocampus has increased susceptibility to HFD-induced inflammation compared to the amygdala. One possible explanation for this is that the hippocampus is proximal to a more permeable blood-brain barrier than the amygdala and, thus, exposed to higher concentrations of circulating signals.
Our findings are consistent with previous in vitro studies that demonstrated a palmitate-induced increase in proinflammatory cytokine response in primary (neonatal) and immortalized microglia. Briefly, these studies showed that treatment of cultured microglia with palmitate increased MHC II, proinflammatory cytokine, and inducible nitric oxide synthase (iNOS) gene expression (Duffy et al., 2015a; Wang et al., 2012; Yanguas-Casás et al., 2018). While these previous palmitate studies focused more heavily on proinflammatory cytokine expression and release, here we chose to investigate genes associated with microglial priming due to their well-documented implications in the aged microglial phenotype. To the best of our knowledge, this is the first study investigating the effects of palmitate in microglia isolated specifically from the hippocampus and the amygdala, and the first to compare fatty acid sensitivity between adult and aged microglia in the context of inflammation. This is a critical extension of the literature as there is evidence supporting brain-region-specific and aging-related responses of microglia to inflammatory stimuli, including HFD (Butler et al., 2020; Spencer et al., 2019, 2017). Interestingly, palmitate produced a similar increase in microglial priming and inflammation regardless of age. This is inconsistent with previous studies that showed aged microglia have greater sensitivity to immune challenges such as LPS (Frank et al., 2010). One explanation for this could be due to the differences in the mechanism of action between LPS and fatty acids as palmitate can induce inflammation through multiple mechanisms (Listenberger et al., 2001a; Tse and Belsham, 2018; Wang et al., 2012). So while it is true that TLR-dependent mechanisms may be sensitized in aging, other pathways may not be. Unfortunately, due to limitations in tissue samples we were unable to quantify the effects of palmitate or HFD on TLR4 gene expression. However, future studies will investigate the role of TLR4 in age-dependent microglial priming in this context.
Overall, our data suggest that microglia are not the sole mediator of the potentiated neuroinflammatory response to HFD in aged animals as HFD had a similar impact on microglial priming and inflammation in adult and aged microglia. Palmitate also had similar effects in adult and aged microglia suggesting there were no differences in fatty acid sensitivity in aged microglia. Given that palmitate mimicked the effect of HFD in microglia, it could be a critical nutritional signal from the HFD that mediates the inflammatory response. Moreover, decreased PUFAs, specifically the anti-inflammatory DHA, in aged animals may be a contributing factor to neuroimmune responses in aging. Similar to microglia, astrocytes also directly sense FFAs and astrogliosis has been reported following both short- and long-term consumption of HFD (Frago et al., 2017; Thaler et al., 2012). Furthermore, astrocytes have been shown to adapt a senescence-associated secretory phenotype associated with neuroinflammation in aged animals (Salminen et al., 2011), setting the stage for a potential diet-age interaction. Future studies could investigate the potential additive or synergistic roles of microglia and astrocytes in mediating potentiated HFD-induced neuroinflammation in aged rats.
Table 2.
Fatty acid profile of phospholipids extracted from the hippocampus. Values are expressed as percent of total fatty acids.
| Young-Chow | Aged-Chow | Young-HFD | Aged-HFD | |
|---|---|---|---|---|
| Palmitic Acid | 20.55 ± 0.19 | 20.47 ± 0.15 | 20.59 ± 0.11 | 20.16 ± 0.17 |
| Stearic Acid | 22.12 ± 0.14 | 21.54 ± 0.05 | 22.04 ± 0.09 | 21.45 ± 0.05 |
| Arachidonic acid | 12.52 ± 0.31 | 12.79 ± 0.12 | 13.24 ± 0.15 | 12.93 ± 0.08 |
| Oleic Acid | 16.59 ± 0.23 | 17.77 ± 0.21 | 16.45 ± 0.21 | 17.70 ± 0.13 |
| Docosahexaenoic Acid | 14.42 ± 0.32 | 13.22 ± 0.14 | 13.93 ± 0.19 | 12.93 ± 0.08 |
| Vaccenic Acid | 3.11 ± 0.04 | 3.04 ± 0.03 | 3.04 ± 0.03 | 3.02 ± 0.01 |
| Linoleic Acid | 0.52 ± 0.02 | 0.37 ± 0.01 | 0.47 ± 0.01 | 0.39 ± 0.00 |
| Adrenic Acid | 4.04 ± 0.06 | 4.00 ± 0.03 | 4.13 ± 0.01 | 4.04 ± 0.03 |
| Gondoic Acid | 1.16 ± 0.07 | 1.62 ± 0.06 | 1.11 ± 0.05 | 1.66 ± 0.05 |
Highlights.
3-day HFD primes hippocampal and amygdalar microglia independent of age
HFD increases SFAs and decreases PUFAs in the hippocampus
Aging decreases DHA in the hippocampus and amygdala
Palmitate treatment primes rapidly isolated microglia independent of age
Microglia may not solely drive the exaggerated immune response to HFD in aging
Acknowledgements:
This work is supported in part by grants from the National Institute on Aging AG028271 and AG067061 (to R.M.B), and the Ohio Agriculture Research and Development Denter, OSU (to M.A.B.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdelmagid SA, Clarke SE, Nielsen DE, Badawi A, El-Sohemy A, Mutch DM, Ma DWL, 2015. Comprehensive profiling of plasma fatty acid concentrations in young healthy canadian adults. PLoS One 10. doi: 10.1371/journal.pone.0116195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albalawi F, Lu W, Beckel JM, Lim JC, McCaughey SA, Mitchell CH, 2017. The P2X7 Receptor Primes IL-1β and the NLRP3 Inflammasome in Astrocytes Exposed to Mechanical Strain. Front. Cell. Neurosci 11, 227. doi: 10.3389/fncel.2017.00227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albouery M, Buteau B, Grégoire S, Cherbuy C, Pais de Barros JP, Martine L, Chain F, Cabaret S, Berdeaux O, Bron AM, Acar N, Langella P, Bringer MA, 2020. Age-Related Changes in the Gut Microbiota Modify Brain Lipid Composition. Front. Cell. Infect. Microbiol 9, 444. doi: 10.3389/fcimb.2019.00444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbierato M, Facci L, Argentini C, Marinelli C, Skaper S, Giusti P, 2013. Astrocyte-Microglia Cooperation in the Expression of a Pro-Inflammatory Phenotype. CNS Neurol. Disord. - Drug Targets 12, 608–618. doi: 10.2174/18715273113129990064 [DOI] [PubMed] [Google Scholar]
- Barrientos RM, Hein AM, Frank MG, Watkins LR, Maier SF, 2012. Intracisternal interleukin-1 receptor antagonist prevents postoperative cognitive decline and neuro inflammatory response in aged rats. J. Neurosci 32, 14641–14648. doi: 10.1523/JNEUROSCI.2173-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos RM, Kitt MM, Watkins LR, Maier SF, 2015. Neuroinflammation in the normal aging hippocampus. Neuroscience. doi: 10.1016/j.neuroscience.2015.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos Ruth M., Thompson VM, Kitt MM, Amat J, Hale MW, Frank MG, Crysdale NY, Stamper CE, Hennessey PA, Watkins LR, Spencer RL, Lowry CA, Maier SF, 2015. Greater glucocorticoid receptor activation in hippocampus of aged rats sensitizes microglia. Neurobiol. Aging 36, 1483–1495. doi: 10.1016/j.neurobiolaging.2014.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos RM, Watkins LR, Rudy JW, Maier SF, 2009. Characterization of the sickness response in young and aging rats following E. coli infection. Brain. Behav. Immun 23, 450–454. doi: 10.1016/j.bbi.2009.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baufeld C, Osterloh A, Prokop S, Miller KR, Heppner FL, 2016. High-fat diet-induced brain region-specific phenotypic spectrum of CNS resident microglia. Acta Neuropathol. 132, 361–375. doi: 10.1007/s00401-016-1595-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belury MA, Cole RM, Bailey BE, Ke JY, Andridge RR, Kiecolt-Glaser JK, 2016. Erythrocyte linoleic acid, but not oleic acid, is associated with improvements in body composition in men and women. Mol. Nutr. Food Res 60, 1206–1212. doi: 10.1002/mnfr.201500744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler MJ, Perrini AA, Eckel LA, 2020. Estradiol treatment attenuates high fat diet-induced microgliosis in ovariectomized rats. Horm. Behav 120. doi: 10.1016/j.yhbeh.2020.104675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Button EB, Mitchell AS, Domingos MM, Chung JH-J, Bradley RM, Hashemi A, Marvyn PM, Patterson AC, Stark KD, Quadrilatero J, Duncan RE, 2014. Microglial Cell Activation Increases Saturated and Decreases Monounsaturated Fatty Acid Content, but Both Lipid Species are Proinflammatory. Lipids 49, 305–316. doi: 10.1007/s11745-014-3882-y [DOI] [PubMed] [Google Scholar]
- Cardona AE, Huang DR, Sasse ME, Ransohoff RM, 2006. Isolation of murine microglial cells for RNA analysis or flow cytometry. Nat. Protoc 1, 1947–1951. doi: 10.1038/nprot.2006.327 [DOI] [PubMed] [Google Scholar]
- Dong Y, Benveniste EN, 2001. Immune function of astrocytes. Glia 36, 180–190. doi: 10.1002/glia.1107 [DOI] [PubMed] [Google Scholar]
- Doorn KJ, Brevé JJP, Drukarch B, Boddeke HW, Huitinga I, Lucassen PJ, van Dam AM, 2015. Brain region-specific gene expression profiles in freshly isolated rat microglia. Front. Cell. Neurosci 9, 84. doi: 10.3389/fncel.2015.00084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorf ME, Berman MA, Tanabe S, Heesen M, Luo Y, 2000. Astrocytes express functional chemokine receptors. J. Neuroimmunol doi: 10.1016/S0165-5728(00)00371-4 [DOI] [PubMed] [Google Scholar]
- Duffy CM, Yuan C, Wisdorf LE, Billington CJ, Kotz CM, Nixon JP, Butterick TA, 2015a. Role of orexin A signaling in dietary palmitic acid-activated microglial cells. Neurosci. Lett 606, 140–144. doi: 10.1016/j.neulet.2015.08.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy CM, Yuan C, Wisdorf LE, Billington CJ, Kotz CM, Nixon JP, Butterick TA, 2015b. Role of orexin A signaling in dietary palmitic acid-activated microglial cells. Neurosci. Lett 606, 140–144. doi: 10.1016/j.neulet.2015.08.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatima S, Hu X, Gong RH, Huang C, Chen M, Wong HLX, Bian Z, Kwan HY, 2019. Palmitic acid is an intracellular signaling molecule involved in disease development. Cell. Mol. Life Sci doi: 10.1007/s00018-019-03092-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floden AM, Combs CK, 2007. Microglia repetitively isolated from in vitro mixed glial cultures retain their initial phenotype. J. Neurosci. Methods 164, 218–224. doi: 10.1016/j.jneumeth.2007.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folch J, Lees M, Sloane Stanely GH, 1957. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem 226, 497–509. doi: 10.3989/scimar.2005.69n187 [DOI] [PubMed] [Google Scholar]
- Fonken LK, Frank MG, Kitt MM, Barrientos RM, Watkins LR, Maier SF, 2015. Microglia inflammatory responses are controlled by an intrinsic circadian clock. Brain. Behav. Immun 45, 171–179. doi: 10.1016/j.bbi.2014.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonken LK, Frank MG, Kitt MM, D’Angelo HM, Norden DM, Weber MD, Barrientos RM, Godbout JP, Watkins LR, Maier SF, 2016a. The alarmin HMGB1 mediates age-induced neuroinflammatory priming. J. Neurosci 36, 7946–7956. doi: 10.1523/JNEUROSCI.1161-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonken LK, Kitt MM, Gaudet AD, Barrientos RM, Watkins LR, Maier SF, 2016b. Diminished circadian rhythms in hippocampal microglia may contribute to age-related neuroinflammatory sensitization. Neurobiol. Aging 47, 102–112. doi: 10.1016/j.neurobiolaging.2016.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frago LM, Canelles S, Freire-Regatillo A, Argente-Arizón P, Barrios V, Argente J, Garcia-Segura LM, Chowen JA, 2017. Estradiol Uses Different Mechanisms in Astrocytes from the Hippocampus of Male and Female Rats to Protect against Damage Induced by Palmitic Acid. Front. Mol. Neurosci 10, 330. doi: 10.3389/fnmol.2017.00330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Barrientos RM, Biedenkapp JC, Rudy JW, Watkins LR, Maier SF, 2006a. mRNA up-regulation of MHC II and pivotal pro-inflammatory genes in normal brain aging. Neurobiol. Aging 27, 717–722. doi: 10.1016/j.neurobiolaging.2005.03.013 [DOI] [PubMed] [Google Scholar]
- Frank MG, Barrientos RM, Thompson BM, Weber MD, Watkins LR, Maier SF, 2012. IL-1RA injected intra-cisterna magna confers extended prophylaxis against lipopolysaccharide-induced neuroinflammatory and sickness responses. J. Neuroimmunol 252, 33–39. doi: 10.1016/j.jneuroim.2012.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Barrientos RM, Watkins LR, Maier SF, 2010. Aging sensitizes rapidly isolated hippocampal microglia to LPS ex vivo. J. Neuroimmunol 226, 181–184. doi: 10.1016/j.jneuroim.2010.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Wieseler-Frank JL, Watkins LR, Maier SF, 2006b. Rapid isolation of highly enriched and quiescent microglia from adult rat hippocampus: Immunophenotypic and functional characteristics. J. Neurosci. Methods 151, 121–130. doi: 10.1016/j.jneumeth.2005.06.026 [DOI] [PubMed] [Google Scholar]
- Giles C, Takechi R, Mellett NA, Meikle PJ, Dhaliwal S, Mamo JC, 2016. The Effects of Long-Term Saturated Fat Enriched Diets on the Brain Lipidome. PLoS One 11, e0166964. doi: 10.1371/journal.pone.0166964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godbout JP, Johnson RW, 2006. Age and Neuroinflammation: A Lifetime of Psychoneuroimmune Consequences. Neurol. Clin doi: 10.1016/j.ncl.2006.03.010 [DOI] [PubMed] [Google Scholar]
- Grayson BE, Levasseur PR, Williams SM, Smith MS, Marks DL, Grove KL, 2010. Changes in Melanocortin Expression and Inflammatory Pathways in Fetal Offspring of Nonhuman Primates Fed a High-Fat Diet. Endocrinology 151, 1622–1632. doi: 10.1210/en.2009-1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillemot-Legris O, Muccioli GG, 2017. Obesity-Induced Neuroinflammation: Beyond the Hypothalamus. Trends Neurosci. doi: 10.1016/j.tins.2017.02.005 [DOI] [PubMed] [Google Scholar]
- Hamilton JG, Comai K, 1988. Rapid separation of neutral lipids, free fatty acids and polar lipids using prepacked silica sep-Pak columns. Lipids 23, 1146–1149. doi: 10.1007/BF02535281 [DOI] [PubMed] [Google Scholar]
- Hidalgo-Lanussa O, Ávila-Rodriguez M, Baez-Jurado E, Zamudio J, Echeverria V, Garcia-Segura LM, Barreto GE, 2017. Tibolone Reduces Oxidative Damage and Inflammation in Microglia Stimulated with Palmitic Acid through Mechanisms Involving Estrogen Receptor Beta. Mol. Neurobiol doi: 10.1007/s12035-017-0777-y [DOI] [PubMed] [Google Scholar]
- Horrocks LA, Yeo YK, 1999. Health benefits of docosahexaenoic acid (DHA). Pharmacol. Res 40, 211–225. doi: 10.1006/phrs.1999.0495 [DOI] [PubMed] [Google Scholar]
- Karmi A, Iozzo P, Viljanen A, Hirvonen J, Fielding BA, Virtanen K, Oikonen V, Kemppainen J, Viljanen T, Guiducci L, Haaparanta-Solin M, Nagren K, Solin O, Nuutila P, 2010. Increased Brain Fatty Acid Uptake in Metabolic Syndrome. Diabetes 59, 2171–2177. doi: 10.2337/db09-0138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khare S, Luc N, Dorfleutner A, Stehlik C, 2010. Inflammasomes and their activation. Crit. Rev. Immunol doi: 10.1615/critrevimmunol.v30.i5.50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labrousse VF, Nadjar A, Joffre C, Costes L, Aubert A, Grégoire S, Bretillon L, Layé S, 2012. Short-term long chain Omega3 diet protects from neuroinflammatory processes and memory impairment in aged mice. PLoS One 7. doi: 10.1371/journal.pone.0036861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacombe RJS, Chouinard-Watkins R, Bazinet RP, 2018. Brain docosahexaenoic acid uptake and metabolism. Mol. Aspects Med doi: 10.1016/j.mam.2017.12.004 [DOI] [PubMed] [Google Scholar]
- Lake A, Townshend T, 2006. Obesogenic environments: exploring the built and food environments. J. R. Soc. Promot. Health 126, 262–7. [DOI] [PubMed] [Google Scholar]
- Layé S, 2010. Polyunsaturated fatty acids, neuroinflammation and well being. Prostaglandins Leukot. Essent. Fat. Acids 82, 295–303. doi: 10.1016/j.plefa.2010.02.006 [DOI] [PubMed] [Google Scholar]
- Layé S, Nadjar A, Joffre C, Bazinet RP, 2018. Anti-inflammatory effects of omega-3 fatty acids in the brain: Physiological mechanisms and relevance to pharmacology. Pharmacol. Rev 70, 12–38. doi: 10.1124/pr.117.014092 [DOI] [PubMed] [Google Scholar]
- Listenberger LL, Ory DS, Schaffer JE, 2001a. Palmitate-induced Apoptosis Can Occur through a Ceramide-independent Pathway. J. Biol. Chem 276, 14890–14895. doi: 10.1074/jbc.M010286200 [DOI] [PubMed] [Google Scholar]
- Listenberger LL, Ory DS, Schaffer JE, 2001b. Palmitate-induced Apoptosis Can Occur through a Ceramide-independent Pathway. J. Biol. Chem 276, 14890–14895. doi: 10.1074/jbc.M010286200 [DOI] [PubMed] [Google Scholar]
- Little SJ, Lynch MA, Manku M, Nicolaou A, 2007. Docosahexaenoic acid-induced changes in phospholipids in cortex of young and aged rats: A lipidomic analysis. Prostaglandins Leukot. Essent. Fat. Acids 77, 155–162. doi: 10.1016/j.plefa.2007.08.009 [DOI] [PubMed] [Google Scholar]
- MacDonald RS, Zhang W, Zhang J-P, Sun GY, 1996. Brain Neutral Lipids and Phospholipids Are Modified by Long-Term Feeding of Beef Tallow vs. Corn Oil Diets. J. Nutr 126, 1554–1562. doi: 10.1093/jn/126.6.1554 [DOI] [PubMed] [Google Scholar]
- Miller AA, Spencer SJ, 2014. Obesity and neuroinflammation: A pathway to cognitive impairment. Brain. Behav. Immun 42, 10–21. doi: 10.1016/j.bbi.2014.04.001 [DOI] [PubMed] [Google Scholar]
- Mitchell RW, Hatch GM, 2011. Fatty acid transport into the brain: Of fatty acid fables and lipid tails. Prostaglandins Leukot. Essent. Fat. Acids 85, 293–302. doi: 10.1016/j.plefa.2011.04.007 [DOI] [PubMed] [Google Scholar]
- Nikodemova M, Watters JJ, 2012. Efficient isolation of live microglia with preserved phenotypes from adult mouse brain. J. Neuroinflammation 9, 635. doi: 10.1186/1742-2094-9-147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry VH, Matyszak MK, Fearn S, 1993. Altered antigen expression of microglia in the aged rodent CNS. Glia 7, 60–67. doi: 10.1002/glia.440070111 [DOI] [PubMed] [Google Scholar]
- Popkin BM, Doak CM, 2009. The Obesity Epidemic Is a Worldwide Phenomenon. Nutr. Rev 56, 106–114. doi: 10.1111/j.1753-4887.1998.tb01722.x [DOI] [PubMed] [Google Scholar]
- Rogero MM, Calder PC, 2018. Obesity, inflammation, toll-like receptor 4 and fatty acids. Nutrients. doi: 10.3390/nu10040432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozovsky I, Finch CE, Morgan TE, 1998. Age-related activation of microglia and astrocytes: In vitro studies show persistent phenotypes of aging, increased proliferation, and resistance to down-regulation. Neurobiol. Aging 19, 97–103. doi: 10.1016/S0197-4580(97)00169-3 [DOI] [PubMed] [Google Scholar]
- Salminen A, Ojala J, Kaarniranta K, Haapasalo A, Hiltunen M, Soininen H, 2011. Astrocytes in the aging brain express characteristics of senescence-associated secretory phenotype. Eur. J. Neurosci doi: 10.1111/j.1460-9568.2011.07738.x [DOI] [PubMed] [Google Scholar]
- Schroder K, Tschopp J, 2010. The Inflammasomes. Cell. doi: 10.1016/j.cell.2010.01.040 [DOI] [PubMed] [Google Scholar]
- Sergi D, Morris AC, Kahn DE, McLean FH, Hay EA, Kubitz P, MacKenzie A, Martinoli MG, Drew JE, Williams LM, 2018. Palmitic acid triggers inflammatory responses in N42 cultured hypothalamic cells partially via ceramide synthesis but not via TLR4. Nutr. Neurosci doi: 10.1080/1028415X.2018.1501533 [DOI] [PubMed] [Google Scholar]
- Skvarc DR, Berk M, Byrne LK, Dean OM, Dodd S, Lewis M, Marriott A, Moore EM, Morris G, Page RS, Gray L, 2018. Post-Operative Cognitive Dysfunction: An exploration of the inflammatory hypothesis and novel therapies. Neurosci. Biobehav. Rev doi: 10.1016/j.neubiorev.2017.11.011 [DOI] [PubMed] [Google Scholar]
- Sobesky JL, Barrientos RM, De May HS, Thompson BM, Weber MD, Watkins LR, Maier SF, 2014. High-fat diet consumption disrupts memory and primes elevations in hippocampal IL-1β, an effect that can be prevented with dietary reversal or IL-1 receptor antagonism. Brain. Behav. Immun 42, 22–32. doi: 10.1016/j.bbi.2014.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobesky JL, D’Angelo HM, Weber MD, Anderson ND, Frank MG, Watkins LR, Maier SF, Barrientos RM, 2016. Glucocorticoids mediate short-term high-fat diet induction of neuroinflammatory priming, the NLRP3 inflammasome, and the danger signal HMGB1. eNeuro 3. doi: 10.1523/ENEURO.0113-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer SJ, Basri B, Sominsky L, Soch A, Ayala MT, Reineck P, Gibson BC, Barrientos RM, 2019. High-fat diet worsens the impact of aging on microglial function and morphology in a region-specific manner. Neurobiol. Aging 74, 121–134. doi: 10.1016/j.neurobiolaging.2018.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer SJ, D’Angelo H, Soch A, Watkins LR, Maier SF, Barrientos RM, 2017. High-fat diet and aging interact to produce neuroinflammation and impair hippocampal- and amygdalar-dependent memory. Neurobiol. Aging 58, 88–101. doi: 10.1016/j.neurobiolaging.2017.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoffel W, Chu F, Ahrens EH, 1959. Analysis of Long-Chain Fatty Acids by Gas-Liquid Chromatography: Micromethod for Preparation of Methyl Esters. Anal. Chem 31, 307–308. doi: 10.1021/ac60146a047 [DOI] [Google Scholar]
- Szepesi Z, Manouchehrian O, Bachiller S, Deierborg T, 2018. Bidirectional Microglia-Neuron Communication in Health and Disease. Front. Cell. Neurosci doi: 10.3389/fncel.2018.00323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaler JP, Yi CX, Schur EA, Guyenet SJ, Hwang BH, Dietrich MO, Zhao X, Sarruf DA, Izgur V, Maravilla KR, Nguyen HT, Fischer JD, Matsen ME, Wisse BE, Morton GJ, Horvath TL, Baskin DG, Tschöp MH, Schwartz MW, 2012. Obesity is associated with hypothalamic injury in rodents and humans. J. Clin. Invest 122, 153–162. doi: 10.1172/JCI59660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey TJ, Steyn FJ, Wolvetang EJ, Ngo ST, 2018. Neuronal Lipid Metabolism: Multiple Pathways Driving Functional Outcomes in Health and Disease. Front. Mol. Neurosci 11, 10. doi: 10.3389/fnmol.2018.00010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse EK, Belsham DD, 2018. Palmitate induces neuroinflammation, ER stress, and Pomc mRNA expression in hypothalamic mHypoA-POMC/GFP neurons through novel mechanisms that are prevented by oleate. Mol. Cell. Endocrinol 472, 40–49. doi: 10.1016/j.mce.2017.11.017 [DOI] [PubMed] [Google Scholar]
- Valdearcos M, Robblee MM, Benjamin DI, Nomura DK, Xu AW, Koliwad SK, 2014. Microglia Dictate the Impact of Saturated Fat Consumption on Hypothalamic Inflammation and Neuronal Function. Cell Rep. 9, 2124–2139. doi: 10.1016/j.celrep.2014.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Liu D, Wang F, Liu S, Zhao S, Ling EA, Hao A, 2012. Saturated fatty acids activate microglia via Toll-like receptor 4/NF-κB signalling. Br. J. Nutr 107, 229–241. doi: 10.1017/S0007114511002868 [DOI] [PubMed] [Google Scholar]
- Yanguas-Casás N, Crespo-Castrillo A, de Ceballos ML, Chowen JA, Azcoitia I, Arevalo MA, Garcia-Segura LM, 2018. Sex differences in the phagocytic and migratory activity of microglia and their impairment by palmitic acid. Glia 66, 522–537. doi: 10.1002/glia.23263 [DOI] [PubMed] [Google Scholar]


