Abstract
E-cigarettes are popular among adolescents. Given that flavors enhance e-cigarette appeal, this study examined the influence of flavors on nicotine in e-cigarettes. Youth e-cigarette users (average 26.2 days [SD = 3.6] in past 28 days) were randomized to use e-cigarettes containing 6 or 12 mg/mL of freebase nicotine and completed 4 test sessions. During the first 3 test sessions, participants completed 3 fixed puffing bouts (1 puffing bout = 10 puffs, 3 s each, 30-s interval), using menthol, green-apple, and unflavored e-liquids (50 propylene glycol [PG]/50 vegetable glycerin [VG]) with their assigned nicotine concentration in a random order using a ~5.5-W V2 e-cigarette device. After each puffing bout, participants assessed subjective effects of nicotine and flavor. In the 4th test session, participants used any of the e-liquids they had tried in the earlier sessions, ad libitum for 60 min and the amount of e-liquid used for each flavor and the number of puffs was assessed. Participants (n = 49; 6 mg/mL [n = 24]; 12 mg/mL [n = 25]) were 63.3% male, 65.3% non-Hispanic White with an average age of 18.7 (SD = 0.9). Mixed models analysis revealed that green apple and 6 mg/mL of nicotine independently increased liking of e-cigarette taste. In addition, green apple produced higher ratings of fruitiness, sourness, sweetness, and menthol produced higher ratings of coolness. We did not observe any interactions between nicotine and flavor. Youth liked the taste of e-liquids containing green-apple flavor or low nicotine concentration which highlights the appeal of fruit flavors in e-cigarettes to adolescents.
Keywords: e-cigarette, youth, menthol, fruit, flavors
Adolescent e-cigarette use is considered a public health concern with more than 5 million high school and middle school students reporting current e-cigarette use in 2019 (Cullen et al., 2019). E-cigarette use in adolescents is alarming given the known detrimental effects of nicotine exposure to the developing brain (Abreu-Villaça et al., 2003; Yuan, Cross, Loughlin, Leslie, & Yuan, 2015) and recent studies suggest that e-cigarette use by adolescents may be a risk factor for future use of cigarettes (Barrington-Trimis et al., 2018; Bold et al., 2018; Soneji et al., 2017). Further, while some evidence suggests that e-cigarettes appear to appeal to youth who might otherwise not smoke cigarettes (Dutra & Glantz, 2014; Fulton, Gokal, Griffiths, & Wild, 2018), it has also been suggested that the relationship between e-cigarette and cigarette use is related to shared risk factors (Kim & Selya, 2019).
The appeal of e-cigarettes for youth has been linked to the availability of diverse e-liquid flavors. E-cigarettes are available in thousands of flavors without any regulations (Zhu et al., 2014), unlike cigarettes where all flavor additives except for menthol are banned (Food and Drug Administration, 2009). The 2009 flavor ban for cigarettes was prompted by research suggesting that flavored cigarettes encourage cigarette use in young people (Lewis & Wackowski, 2006). Indeed, most youth report their first tobacco product (including cigarettes, e-cigarettes, hookah etc.) was flavored (Ambrose et al., 2015; Harrell et al., 2017; Villanti et al., 2017) and youth rate appealing flavors as one of their top reasons for experimenting with e-cigarettes (Kong, Morean, Cavallo, Camenga, & Krishnan-Sarin, 2015; Zare, Nemati, & Zheng, 2018). Adolescents find candy-, fruit-, and menthol-flavored e-liquids more appealing than tobacco and alcohol flavored (Pepper, Ribisl, & Brewer, 2016) and trying more flavors is associated with more frequent use of e-cigarettes among youth (Morean et al., 2018).
Flavors in e-cigarettes may also be attractive to youth because they may alter the e-cigarette experience and reward. Menthol flavor has been found to improve the taste of e-liquid aerosol even at very low menthol concentrations and also to enhance wanting for high concentrations of nicotine (Krishnan-Sarin et al., 2017). The effects of menthol could be attributed to its cooling and analgesic properties via the transient receptor potential channels (Hatem, Attal, Willer, & Bouhassira, 2006; Wasner, Schattschneider, Binder, & Baron, 2004), which may reduce irritation from tobacco smoke and nicotine (Ha et al., 2015; Rosbrook & Green, 2016).
Given the evidence that both menthol and sweet flavors are liked by youth, the current study sought to compare the subjective effects of commercially available menthol, green apple and unflavored e-liquids with two nicotine concentrations (6 mg/mL, 12 mg/mL) among adolescent e-cigarette users. We conducted this study using an e-cigarette exposure paradigm that was used in our previous work and has been shown to produce reliable increases in nicotine levels following e-cigarette exposure (Krishnan-Sarin et al., 2017). We used green apple as the fruit flavor because it does not contain menthol, is a popular sweet flavor that has been shown to improve subjective reward from e-cigarettes containing nicotine (Audrain-McGovern, Strasser, & Wileyto, 2016) and increases the appeal of nicotine-free e-liquids (Devito et al., 2019). Based on our earlier evidence, we hypothesized that menthol flavor would enhance liking of e-cigarette taste and liking/wanting for e-cigarette drug effects at the high nicotine concentration because menthol masks the potential aversiveness of the high concentration of nicotine via menthol’s cooling flavor and analgesic effects. However, because green-apple flavor is unlikely to have analgesic effects, we hypothesized that green apple would increase liking of e-cigarette taste and liking/wanting for e-cigarette drug effects only at the low nicotine concentration because the low nicotine concentration would be less aversive. We also explored flavor sensations (sourness, coolness, fruitiness, sweetness, irritation/harshness), nicotine-specific subjective effects (craving, withdrawal, stimulation), and ad libitum use of the e-liquid flavors.
Method
Participant Recruitment and Screening
All the experimental procedures were approved by the Yale School of Medicine Human Investigations Committee (protocol number: 1307012312; title: “Flavors and E-Cigarette Effects in Adolescent Smokers”), registered on ClinicalTrials.gov (identifier: NCT03168191) and followed National Advisory Council on Drug Abuse (2012) guidelines for substance use research in children and adolescents and administration of drugs for research purposes. Participants were recruited from local high schools and through online advertisements. Participants had to (a) be between 16 and 20 years of age, (b) currently use e-cigarettes with nicotine (at least 10 days in the past month), (c) have baseline urinary cotinine levels of ≥150 ng/mL, (d) not currently trying to quit smoking or e-cigarette use, and (e) report having tried menthol flavored and green-apple-flavored e-liquids and/or were neutral or liked the menthol- and green-apple-flavored e-liquids and/or flavors.
Participants who were ≥18 years old provided consent and those <18 years old provided assent and parental permission. Participants received a physical examination (by an Advanced Practice Registered Nurse) and a clinical evaluation (by a licensed clinical psychologist) to rule out concerning physical or psychological conditions and substance use disorders (other than tobacco use disorder). Eligible youth participated in laboratory sessions at The John B. Pierce Laboratory (New Haven, CT) in a temperature controlled and ventilated room (with air exchange 11 times per hour). Participants were asked to abstain from cigarettes (confirmed by breath CO levels <10 ppm; Micro Direct, Inc., Lewiston, ME) and e-cigarettes for at least 12 h before each test session. At the end of the study, participants met with a licensed clinical psychologist who educated them on the risks on using tobacco products, provided them with health information regarding all tobacco products and encouraged them to rethink their tobacco use and explore quitting options. Participants were compensated $40 for experimental Sessions 1–3, $50 for experimental Session 4 (longer session), and $40 for completing the study.
Stimuli
The second-generation e-cigarette V2 Cigs (VMR Products LLC, Miami, Florida; ~5.5 W) with refillable tanks were used. All e-liquids were purchased from AmericaneLiquidStore (Wauwatosa, WI) and contained 50 propylene glycol (PG)/50 vegetable glycerin (VG) with the required combinations of freebase nicotine (6 mg/mL and 12 mg/mL), menthol and green apple. On the day of each session, the e-cigarette tanks were filled with 750 μl of e-liquid. The nicotine and characterizing flavor ingredients of menthol, green apple and unflavored e-liquids were characterized using gas chromatography (GC) coupled with mass spectrometry (Perkin Elmer Clarus SQ8S, Waltham, MA) using a Perkin Elmer Elite-5 MS column (length 60 m, internal diameter 0.25 mm, 0.25-μm film) and the quantification of selected compounds was carried out by GC-flame ionization detection (Shimadzu GC-2010 Plus, Kyoto, Japan) using an Agilent J&W DB-5 column (Santa Clara, CA; length 60 m, internal diameter 0.25 mm, 0.25-μm film; Erythropel et al., 2019).
GC conditions were as follows: injection volume 1 μl, split ratio 300, injector temperature 250 °C, oven program: 30 °C for 7min, heated to 50 °C at 10 °C/min and held for 20 min, heated to 310 °C at 10 °C/min and held for 7 min. Helium (Airgas, Radnor, PA) was used as carrier gas, and the flame ionization detection was operated at 325 °C. E-liquid samples were weighed (Mettler-Toledo AB204-S, Columbus, OH) and diluted with methanol (high-performance liquid chromatography grade, Fisher Scientific, Hampton, NH) containing 1,4-dioxane as internal standard (99.8%, Sigma-Aldrich, St. Louis, MO). Quantification was achieved using calibration curves generated with commercially available standards: nicotine (>99%), hexyl-acetate (99%, both Sigma-Aldrich), DL-menthol (99%), and ethyl-2-methyl-butyrate (99%, both Acros Organics). GC analysis revealed menthol as the principal flavor component in “menthol” e-liquid, and hexyl-acetate and ethyl-2-methyl-butyrate in “green apple.” No menthol was found in the green apple e-liquid. Nicotine levels varied slightly from the label information, but variations did not exceed ~10% (see Table 1).
Table 1.
Components of Unflavored, Menthol, and Green Apple E-Liquids
| E-liquid | Label nicotine concentration | Nicotine | Menthol | Hexyl-acetate | Ethyl-2-methyl-butyrate |
|---|---|---|---|---|---|
| Green apple | 6 | 6.8 ± 0.0 μg/mg | n.d. | 0.7 ± 0.1 μg/mg | 3.3 ± 0.3 μg/mg |
| 12 | 12 ± 0.6 μg/mg | n.d. | 0.5 ± 0.1 μg/mg | 1.6 ± 0.1 μg/mg | |
| Unflavored | 6 | 6.0 ± 0.1 μg/mg | n.d. | n.d. | n.d. |
| 12 | 12 ± 0.3 μg/mg | n.d. | n.d. | n.d. | |
| Menthol | 6 | 6.5 ± 0.1 μg/mg | 29 ± 0.3 μg/mg | n.d. | n.d. |
| 2 | 13 ± 0.3 μg/mg | 30 ± 0.7 μg/mg | n.d. | n.d. |
Note. n.d. = not detected. Concentration was determined by gas chromatography (M ± 95% confidence interval, n = 3); 0.6% (6mg/mL) and 1.2% (12mg/mL) nicotine strength.
Experimental Design
Forty-nine participants (18 females, 31 males; see Table 2 for demographics) who reported e-cigarette use were included in the study and participated in four test sessions separated by at least 48 hr. Using randomization in blocks of size two stratified by gender, we first assigned each participant to one of two nicotine concentrations (6 mg/mL or 12 mg/mL) that represent the most common concentrations used by youth (Morean, Kong, Cavallo, Camenga, & Krishnan-Sarin, 2016). In this study we did not use a placebo control group (0 mg/mL nicotine) because the premise of this study was to understand the impact of menthol in the presence of nicotine in comparison to green-apple flavor. We have previously investigated menthol with 0 mg/mL nicotine and found that in the 0 mg/mL nicotine group, menthol was liked more compared to the no menthol condition and that the coolness of menthol is concentration dependent (Krishnan-Sarin et al., 2017). Therefore, we did not include a 0 mg/mL nicotine group in this study. Combined with their assigned nicotine concentration, participants were randomized in blocks of sizes 6 and 12 to the order in which they received the three flavors (unflavored, menthol, or green apple e-liquids) during the first three laboratory sessions (one flavor per session). The research assistants and the participants were blind to condition. To ensure blinding of the research assistants conducting the experiments, an independent research assistant who did not conduct experiments did the following: filled e-cigarette tanks with e-liquids, wrapped a white sticker around the tank to hide the color of the e-liquid and placed each e-liquid flavor in a sealed plastic bag to prevent detection of the odor of the e-liquids. Conducting the experiments in a ventilated chamber designed to remove odors also reduced the likelihood that the experimenter would detect the odor of the exhaled vapor.
Table 2.
Demographics of Participants
| Variable | Total sample (N = 49) | 6 mg (n= 24) | 12 mg (n = 25) |
|---|---|---|---|
| Age, M (SD) | 18.7 (0.9) | 18.8 (1.0) | 18.6 (0.8) |
| Gender | |||
| Male | 31 (63.3%) | 14 (58.3%) | 17 (68.0%) |
| Sex | |||
| Male | 30 (61.2%) | 14 (58.3%) | 16 (64.0%) |
| Transgender | 1 (2.0%) | 0 (0.0%) | 1 (4.0%) |
| Race/ethnicity | |||
| Non-Hispanic White | 32 (65.3%) | 18 (75.0%) | 14 (56.0%) |
| Non-Hispanic Asian | 4 (8.2%) | 0 (0.0%) | 4 (16.0%) |
| Non-Hispanic Black | 1 (2.0%) | 0 (0.0%) | 1 (4.0%) |
| Biracial/other | 2 (4.1%) | 0 (0.0%) | 2 (8.0%) |
| Hispanic | 10 (20.4%) | 6 (25%.0) | 4 (16.0%) |
| Cigarette use | |||
| Ever users | 42 (85.7%) | 21 (87.5%) | 21 (84.0%) |
| Age first tried cigarette, M (SD) | 15.8 (1.8) | 15.5 (2.0) | 16.1 (1.6) |
| Used cigarette in past 28 days | 22 (45.8%) | 13 (54.2%) | 9 (37.5%) |
| Missing | 1 | 0 | 1 |
| Days in past 28 days, M (SD) | 4.7 (9.3) | 5.5 (10.4) | 3.9 (8.2) |
| Missing | 1 | 0 | 1 |
| Average number of cigarettes per day among those who used cigarette in past 28 days, M (SD) | 2.8 (4.6) | 2.9 (4.5) | 2.7 (4.9) |
| Smoked menthol cigarettes in past month | 9 (18.4%) | 6 (25.0%) | 3 (12.0%) |
| Smoked 100 cigs in lifetime | 18 (36.7%) | 11 (45.8%) | 7 (28.0%) |
| E-cigarette use | |||
| Ever users | 49 (100.0%) | 24 (100.0%) | 25 (100.0%) |
| Age first tried e-cigarette, M (SD) | 15.3 (1.5) | 15.3 (1.7) | 15.2 (1.2) |
| Days in past 28 days, M (SD) | 26.2 (3.6) | 26.6 (2.6) | 25.8 (4.4) |
| Vaped with nicotine in past month | 49 (100.0%) | 24 (100.0%) | 25 (100.0%) |
| Nicotine concentration typically used, M (SD) | 25.9 (21.3) | 23.6 (20.3) | 28.1 (22.3) |
| Missing | 1 | 0 | 1 |
| Number of flavors used in past month, M (SD) | 3.5 (1.4) | 3.5 (1.4) | 3.4 (1.5) |
| E-cigarette device type use in past 30 days | |||
| Disposable, Cig-a-Like, or E-hookah | 9 (18.4%) | 7 (29.2%) | 2 (8.3%) |
| Hookah pen, vape pen, or EGO | 19 (38.8%) | 11 (45.8%) | 8 (32.0%) |
| JUUL | 38 (77.6%) | 17 (70.8%) | 21 (84.0%) |
| Mods/advanced personal vaporizers | 36 (73.5%) | 20(83.3%) | 16 (64.0%) |
| Preferred e-cigarette flavors | |||
| Fruit | 44 (89.8%) | 23 (95.8%) | 21 (84.0%) |
| Mint | 39 (79.6%) | 19 (79.2%) | 20 (80.0%) |
| Candy/dessert | 27 (55.1%) | 13 (54.2%) | 14 (56.0%) |
| Menthol | 25 (51.0%) | 12 (50.0%) | 13 (52.0%) |
| Vanilla | 13 (26.5%) | 8 (33.3%) | 5 (20.0%) |
| Alcohol | 9 (18.4%) | 5 (20.8%) | 4 (16.0%) |
| Coffee | 7 (14.3%) | 3 (12.5%) | 4 (16.0%) |
| Spice | 7 (14.3%) | 4 (16.7%) | 3 (12.0%) |
| Other | 7 (14.3%) | 5 (20.8%) | 2 (8.0%) |
| Tobacco | 7 (14.3%) | 2 (8.3%) | 5 (20.0%) |
| Don’t know | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Intake cotinine levels (ng/mL), M (SE) | 1,031.8 (103.4) | 933.0 (130.3) | 1,126.6 (159.8) |
Note. Data are presented as N (%) unless otherwise indicated.
Prior to the first session, participants were trained on puffing behavior by using tanks filled with only 50 PG/50 VG. In each puffing bout, participants were asked to take ten 3-s puffs with a 30-s interpuff interval as described previously (Krishnan-Sarin et al., 2017). Puffing behavior and interpuff interval were monitored by a timer during the fixed-exposure period. Participants were also trained on how to use the Labeled Magnitude Scale (LMS) and the Labeled Hedonic Scale (LHS) by rating the intensity and liking/disliking of 15 imagined or remembered sensations (i.e., the taste of plain bread, the bitter taste of black coffee, etc.). Sessions 1–3 consisted of a half hour fixed-exposure period that included three fixed puffing bouts, each separated by 5 min. During the 5-min rest period, participants completed subjective ratings and assessments (described below) in the following order: LMS (assessed intensity of coolness, craving, harshness/irritation, fruitiness, sweetness and sourness), LHS (assessed liking/disliking of taste), and the Drug Effect Questionnaire (DEQ; assessed liking and wanting of drug effects).
Following completion of the first three sessions, subjects participated in a fourth session in which they could use any of the e-liquids they had tried in the earlier three sessions ad libitum. To remind participants of the flavors, they were first instructed to take one puff of each of the three e-liquid flavors received during the test Sessions 1–3 (unflavored, menthol, and green apple) separated by 5 min. During the 5-min period, they completed the same assessments from Sessions 1–3. Following this, participants were left alone in the room and allowed to freely use all three flavors for 1 hr. The e-cigarette tanks were weighed before and after the session to determine the amount of each e-liquid used. All sessions were video-taped. A research assistant coded the video-tapes to determine number of puffs taken.
Subjective Assessments
Liking of e-cigarette taste (primary outcome).
Following each fixed-dose bout, we assessed how much participants liked/disliked the flavor of the e-cigarette using the LHS (Lim, Wood, & Green, 2009), a bipolar category-ratio scale that ranges from −100 (most disliked) to 100 (most liked), with neither liked nor disliked at the midpoint.
Liking/wanting of e-cigarette drug effects (primary outcome).
A modified version of the DEQ (Morean et al., 2013; Soria et al., 1996) was used in which participants rated acute responses to the e-cigarette on a scale from 0 mm (not at all) to 100-mm (extremely). Following each fixed concentration bout, we assessed e-cigarette liking/wanting (the average of “I feel good e-cigarette effects,” “I want more of that e-cigarette I received,” “I feel the e-cigarette strength,” and “I like the e-cigarette effect”).
Flavor intensity.
Immediately following each bout of vaping, participants rated the sensory effects they experienced (sourness, coolness, sweetness, fruitiness, and harshness/irritation) “right now” on the general version of the Labeled Magnitude Scale (gLMS; Bartoshuk et al., 2004; B. Green et al., 1996; B. G. Green, Shaffer, & Gilmore, 1993). The gLMS is a category ratio scale with seven semantic labels: “no sensation,” “barely detectable,” “weak,” “moderate,” “strong,” “very strong,” and “strongest imaginable,” positioned quasi-logarithmically per their empirically determined semantic magnitudes, with responses coded on a 0–100 scale.
Nicotine-specific subjective effects: Craving, withdrawal, stimulation.
Participants rated craving “right now” immediately following each bout of vaping on the general version of the gLMS (Bartoshuk et al., 2004; B. Green et al., 1996; B. G. Green et al., 1993) as mentioned previously. Following each fixed concentration bout, the DEQ (Morean et al., 2013; Soria et al., 1996) was used to assess stimulant effects (the average of “I feel energized” and “I feel high”) and withdrawal (the average of “ I feel sleepy,” “I feel angry,” “I feel irritable,” “I am having difficulty concentrating,” “I feel restless,” and “I feel hungry”).
Biochemical Analyses
Saliva nicotine samples were obtained at baseline and after each fixed dose bout for Sessions 1–3. For salivary nicotine samples, participants rinsed their mouths with water, chewed on a sterile dental cotton roll (Salivette; Sarstedt AG and Co, Nümbrecht Germany) for 30 s and deposited it into a plastic tube. These tubes were centrifuged to extract the saliva and nicotine levels were determined by Liquid Chromatography with tandem mass spectrometry employing a deuterated internal standard (Sofuoglu, Herman, Nadim, & Jatlow, 2012) and pH was also determined.
Urine samples for determination of menthol glucuronide levels and creatinine were obtained at baseline and at the end of Sessions 1–3. Urine menthol glucuronide was determined by Liquid Chromatography with tandem mass spectrometry employing a deuterated internal standard (Benowitz et al., 2010) and urine creatinine was determined using the Jaffe reaction to correct for urine dilution.
Statistical Analysis
We used a separate linear mixed model for each outcome. We log-transformed (after adding 1) or square-root-transformed non-normal outcomes. We started with models including fixed effects for nicotine level (i.e., 6 mg/mL, 12 mg/mL), flavor (i.e., unflavored, menthol, green apple), lab session (i.e., Session 1, 2, 3), time within session (Puffing Bout 1, 2, 3), all two-way interactions of these four variables, the three-way interactions Flavor × Nicotine × Session and Flavor × Nicotine × Time, and the stratification variable (gender). We used a subject random effect to model within-subject correlations and a repeated lab-within-subject effect to additionally model within-subject correlations during a lab session. We used Bayesian information criterion to select the latter correlation structure from unstructured, autoregressive (1), compound symmetry, and Toeplitz. After selecting the correlation, we performed backward elimination to remove nonstatistically significant interactions while maintaining hierarchically well-formulated models (we kept in the Nicotine × Flavor interaction to test the primary hypothesis). We used Bonferroni-adjusted α = .025 (0.05/2) for the two primary outcomes (liking/wanting of drug effects; liking of taste), α = .01 (0.05/6) for the six secondary outcomes (sourness, coolness, sweetness, fruitiness, irritation/harshness, craving) for which we had a priori hypotheses and unadjusted α = .05 for exploratory outcomes for which we did not have a priori hypotheses. Results are reported as adjusted least-squares means and their standard error. Models were fit in SAS 9.4. Following the CONSORT recommendations, we did not perform any test for baseline differences between the two groups (6 mg/mL and 12 mg/mL nicotine) because these tests are not needed in randomized trials (Moher et al., 2010).
Results
Primary Outcomes
Liking/disliking of e-cigarette taste.
There was no significant Nicotine × Flavor interaction for liking/disliking (Figure 1), F(2, 89.9) = 0.05, p = .95, for overall interaction, and t(89.9) = −0.25, p = .81, for the primary hypothesis. However, there was an overall main effect of flavor, F(2, 89.9) = 10.86, p < .0001, and nicotine concentration, F(1, 43.9) = 6.40, p = .02. Overall (and at each nicotine concentration), participants liked the taste of green-apple flavor (overall M = 29.29 [SE = 5.65]) more than either menthol (M = −1.37 [SE = 5.61]) or the unflavored e-liquids (M = −3.52 [SE = 5.63]; both p < .001; Cohen’s d = 0.52 for green apple vs. menthol and d = 0.88 for green apple vs. unflavored comparison). Further, participants liked the taste of 6 mg/mL nicotine more than the 12 mg/mL (M = 16.46 [SE = 4.68] vs. M = −0.19 [SE = 4.72], p = .02, d = 0.64).
Figure 1.
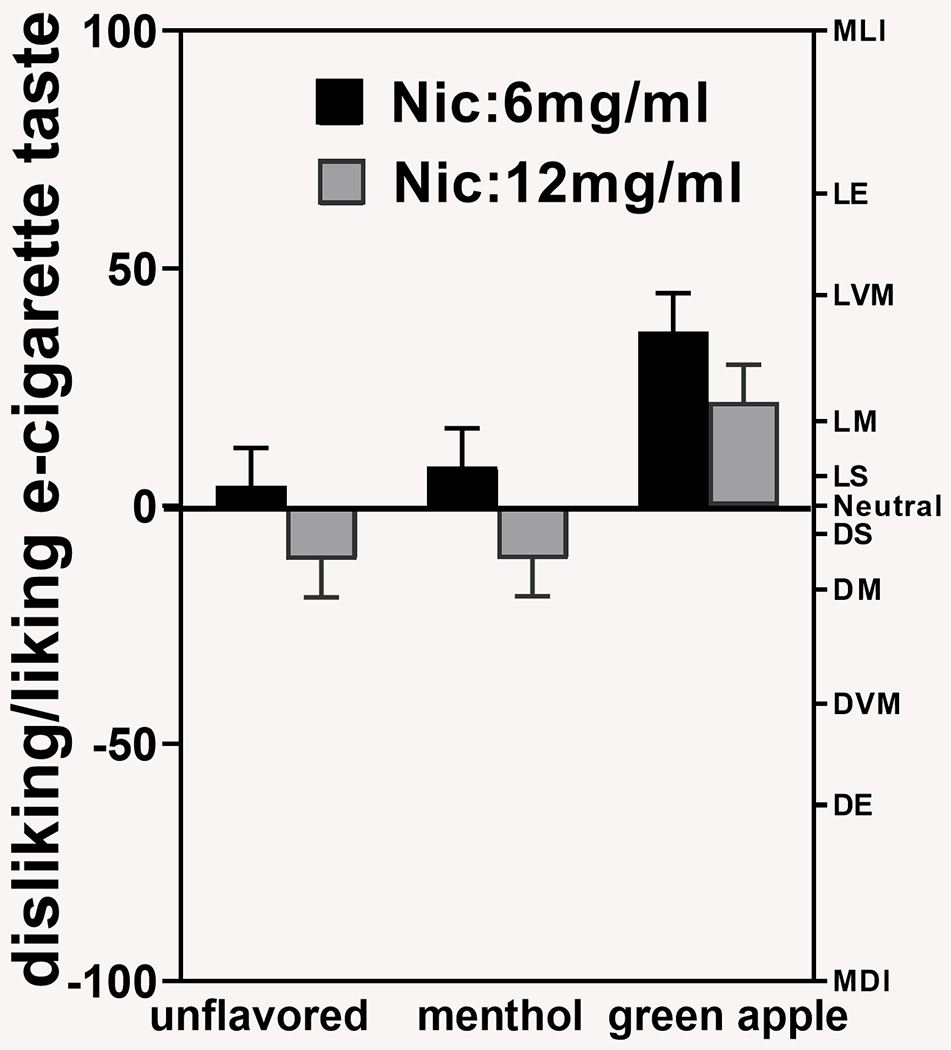
Liking of e-cigarette taste (Labeled Hedonic Scale [LHS]) during fixed-puffing bout sessions. The taste of green-apple-flavored e-liquid was liked more than menthol and unflavored e-liquids. Six mg/mL of nicotine enhanced the liking of e-cigarette taste significantly more than 12 mg/mL of nicotine. Error bars represent the standard errors of the means. Vertical bars represent least squares means. Nic = nicotine. The letters on the right y-axis represent labels on the LHS: MDI = most dislike imaginable; DE = dislike extremely; DVM = dislike very much; DM = dislike moderately; DS = dislike slightly; N = neutral; LS = like slightly; LM = like moderately; LVM = like very much; LE = like extremely; MLI = most like imaginable.
Liking/wanting of e-cigarette drug effects.
There were no statistically significant interactions with respect to Nicotine × Flavor, F(2, 92) = 0.06, p = .94, and no main effect of nicotine concentration, F(1, 46.9) = 0.77, p = .38, or flavor type, F(2, 92) = 0.83, p = .44. Means were as follows: unflavored 6 mg/mL of nicotine, overall M = 44.93 (SE = 4.36); unflavored 12 mg/mL of nicotine, M = 41.86 (SE = 4.37); menthol 6 mg/mL of nicotine, M = 46.71 (SEM = 4.38); menthol 12 mg/mL of nicotine, M = 41.29 (SE = 4.35); green apple 6 mg/mL of nicotine, M = 49.43 (SE = 4.38); green apple 12 mg/mL of nicotine, M = 45.15 (SE = 4.34). Post hoc analysis with the four items in the drug effect questionnaire liking/wanting construct separately revealed that for 3 out of the 4 items had no evidence of a significant interaction between nicotine and flavor or a main effect for nicotine or flavor. For the “I want more of the e-cigarette I received” item, the main effect of flavor was significant (p = .02), with green-apple flavor having a higher mean score than both menthol (p = .01) and no flavor (p = .02).
Secondary and Exploratory Outcomes
There were no significant interactions with respect to Nicotine × Flavor for any secondary and exploratory outcome. Significant main effects are described below.
Sourness.
Flavor was the only significant predictor (Figure 2A), F(2, 92) = 27.55, p < .0001, of sourness ratings. Participants reported greater sourness with green apple compared to both menthol and unflavored e-liquids (p < .0001).
Figure 2.
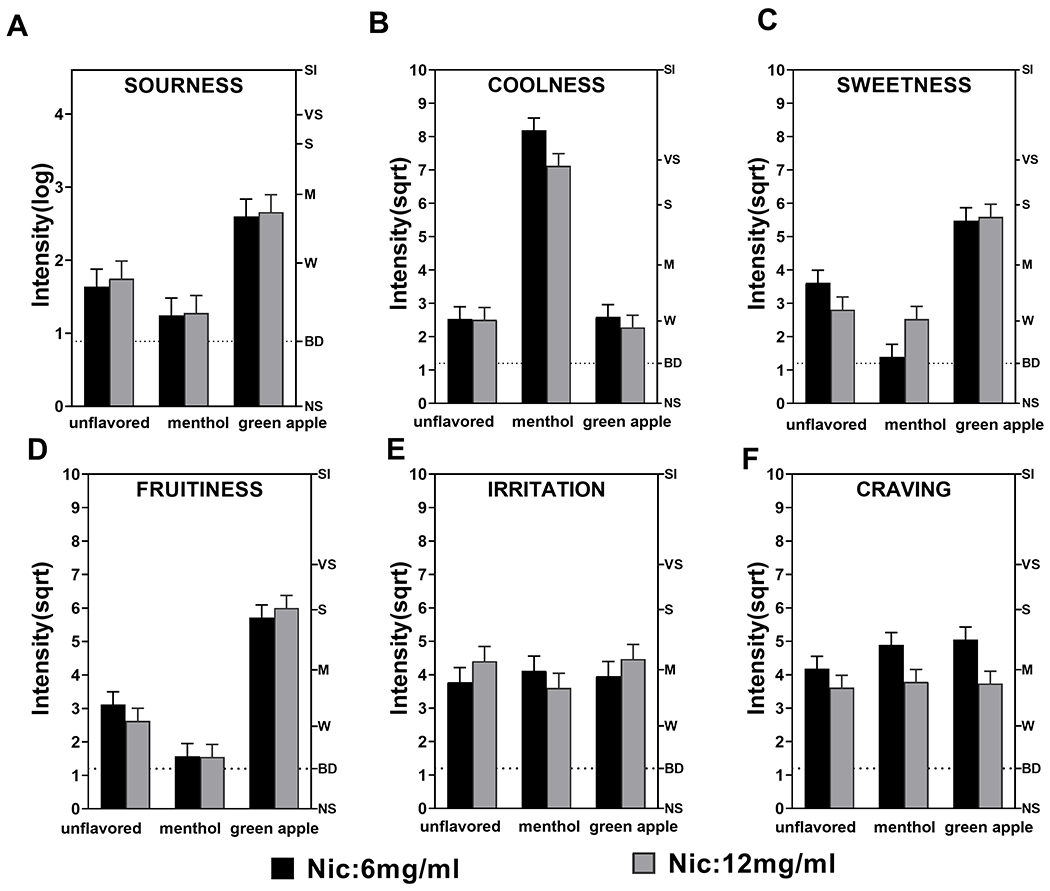
Mean “coolness,” “sourness,” “sweetness,” “fruitiness,” “irritation/harshness,” and “craving” rating (general version of the Labeled Magnitude Scale) during fixed-puffing bout sessions. (A) Green-apple-flavored e-liquid had a higher sourness rating in comparison to unflavored and menthol e-liquids. (B) Menthol-flavored e-liquid had a higher coolness rating versus unflavored and green-apple e-liquids. (C) Green-apple-flavored e-liquid had the highest sweetness rating versus menthol and unflavored e-liquids. Unflavored e-liquid had a higher sweetness rating than menthol. (D) Green-apple-flavored e-liquid had the highest fruitiness rating versus menthol and unflavored e-liquids. Unflavored e-liquid had a higher fruitiness rating than menthol. (E) There were no significant differences in irritation/harshness. (F) 6 mg/mL of nicotine had higher craving ratings than 12 mg/mL of nicotine. Sourness was log transformed and all other data was square-root transformed. Vertical bars represent least squares means. Error bars represent the standard errors of the means. Nic = nicotine. Letters on the right y-axis denote semantic labels of sensation intensity on the general Labeled Magnitude Scale: NS = no sensation; BD = barely detectable; W = weak; M = moderate; S = strong; VS = very strong; SI = strongest imaginable.
Coolness.
There was a significant effect of flavor (Figure 2B), F(2, 96.4) = 195.86, p < .0001, with menthol rated as “cooler” than the green apple and unflavored e-liquids (p < .0001).
Sweetness.
There was a significant main effect of flavor (Figure 2C), F(2, 92) = 61.89, p < .0001. Participants rated green apple as “sweeter” than both menthol and unflavored (p < .0001). The unflavored e-liquid was rated as “sweeter” than menthol (p = .0002).
Fruitiness.
The effect of flavor was significant (Figure 2D), F(2, 92) = 88.85, p < .0001. Participants reported higher fruitiness scores for the green-apple flavor compared to menthol and unflavored e-liquids (p < .0001). Ratings of fruitiness of the unflavored e-liquid were higher than for menthol (p = .0001).
Irritation/harshness.
There was no significant main effect of flavor (Figure 2E), F(2, 91.6) = 0.43, p = .65, or nicotine concentration, F(1, 45.6) = 0.25, p = .62. Females reported higher irritation/harshness than males (M = 4.54 [SE = 0.35] vs. M = 3.58 [SE = 0.26] on the square-root transformed scale), though the difference was not significant at the 0.01 level (p = .03).
Biochemical Analyses
Urine menthol glucuronide (ng/mg).
There was a significant Flavor × Time interaction (Figure 3A), F(2, 131) = 68.1, p < .0001. As expected, the urine menthol glucuronide values increased (from baseline to end of lab) when subjects used menthol-flavored e-liquids (from M = 8.11 [SE = 0.19] to M = 9.96 [SE = 0.19] on the log-transformed scale, p < .0001). There was no evidence of a change in urine menthol for fruit (p = .21) or unflavored e-liquids (p = .24).
Figure 3.
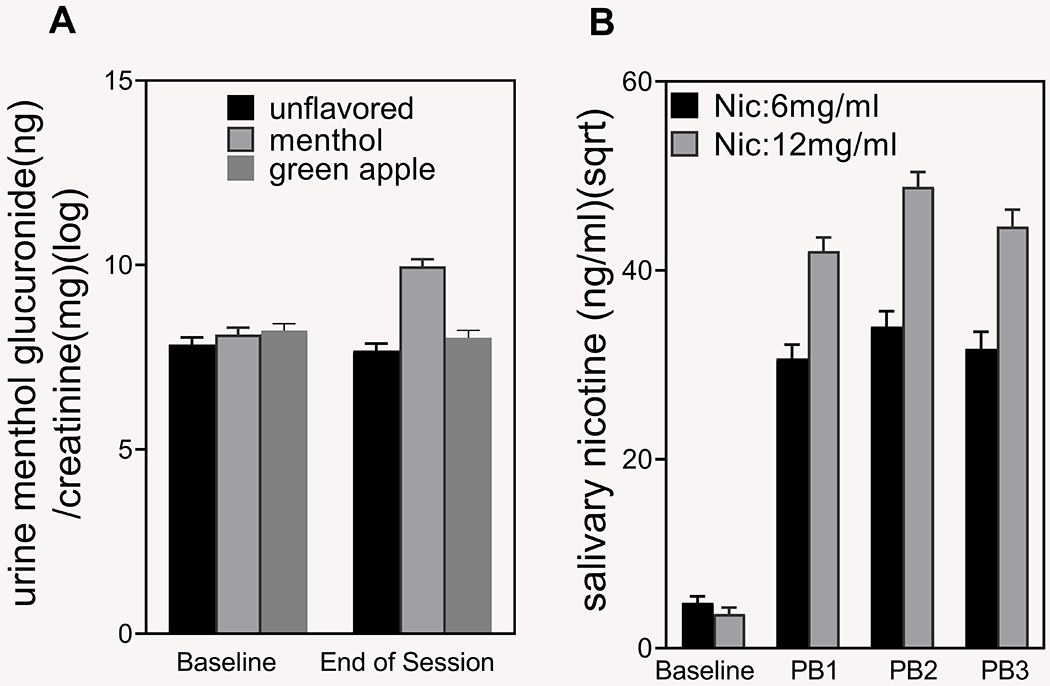
Urine menthol glucuronide and salivary nicotine levels during fixed-puffing bout sessions. (A) Urine menthol glucuronide levels were enhanced at the end of the menthol exposure session only. (B) Salivary nicotine was enhanced with both e-liquid nicotine concentrations (6 mg/mL and 12 mg/mL) after each puffing bout compared to baseline. Twelve mg/mL of nicotine produced higher salivary nicotine levels than 6 mg/mL of nicotine. Data for (A) were log transformed and for (B) were square-root transformed. Vertical bars represent least squares (means. Error bars represent the standard errors of the means. PB1 = post-Bout 1; PB2 = post-Bout 2; PB3 = post-Bout 3). Nic = nicotine.
Saliva nicotine (ng/ml).
The Nicotene × Time interaction was significant (Figure 3B), F(3, 142) = 16.78, p < .0001. At baseline (prebouts), there was no significant difference in salivary nicotine levels in the two nicotine groups (3.61 at 12 mg vs. 4.82 at 6 mg on the square-root transformed scale, p = .21). Salivary nicotine levels increased in both groups after baseline, with higher levels in the 12 mg group than in the 6 mg group after each puff bout (p < .0001; M = 44.67 [SE = 1.78] vs. M = 31.69 [SE = 1.81] after third puffing bout).
Nicotine-Specific Subjective Effects: Craving, Nicotine Withdrawal, and Stimulation
Craving.
The main effect of flavor, F(2, 90.8) = 1.47, p = .24, was not significant, but there was an effect of nicotine, F(1, 44.8) = 7.45, p = .01, with participants reporting more craving for the 6 mg/mL nicotine versus 12 mg/mL (M = 4.71 [SE = 0.26] vs. M = 3.71 [SE = 0.26] on the square-root transformed scale; Figure 2F).
Nicotine withdrawal.
Withdrawal was associated with time within session, F(3, 144) = 15.14, p < .0001. Within a session, withdrawal improved from baseline to each one of the three subsequent postpuffing bout timepoints (all p < .0001). The means for the square-root transformed data were as follows: baseline (overall M = 4.30 [SE = 0.26]), after Puffing Bout 1 (M = 3.64 [SE = 0.25]), after Puffing Bout 2 (M = 3.58 [SE = 0.25]), and after Puffing Bout 3 (M = 3.58 [SE = 0.26]).
Stimulation.
Stimulation was associated with time within session, F(3, 144) = 3.36, p = .02. Stimulant effects after first puffing bout were higher than stimulant effects after the second (p = .04) and third (p = .008) puffing bouts in a lab session. The means for square-root transformed data were as follows: baseline (overall M = 5.22 [SE = 0.18]), after Puffing Bout 1 (M = 5.37 [SE = 0.18]), after Puffing Bout 2 (M = 5.22 [SEM = 0.19]) and after Puffing Bout 3 (M = 5.10 [SE = 0.20]).
Ad Libitum Session
Number of puffs during ad lib session.
The effect of flavor was significant (Figure 4A), F(2, 88) = 11.05, p < .0001, for the number of puffs. Participants took more puffs from the green-apple flavor e-liquid (Mdn = 28, interquartile range [IQR] = 11–54) than both menthol (Mdn =13, IQR = 2–36) and unflavored (Mdn =5, IQR = 2–15), p = .002, p < .0001, respectively. There was no statistically significant difference in number of puffs between menthol and no flavor (p = .17). We observed similar results when analyzing puff duration (data not shown).
Figure 4.
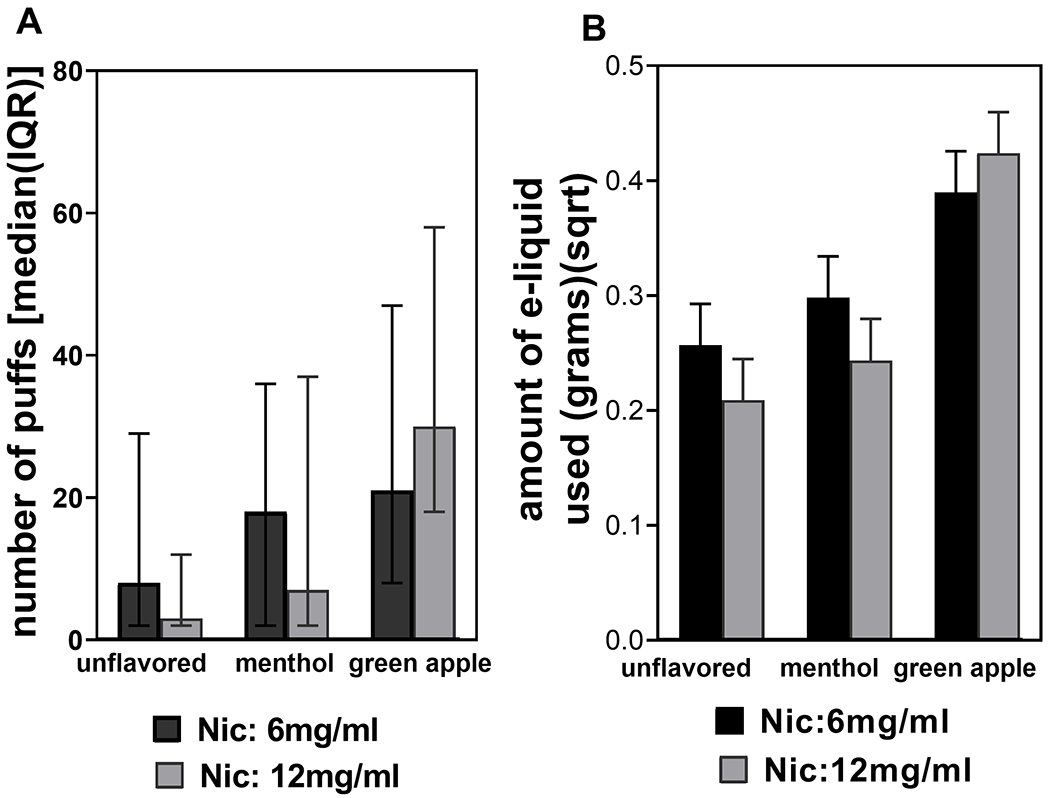
Ad libitum session-number of puffs and amount of e-liquid used. (A) E-cigarettes containing green-apple-flavored e-liquids had a higher number of puffs versus menthol and unflavored e-liquids. (B) Green-apple flavor had the highest amount of e-liquid used compared to menthol and unflavored e-liquids. Vertical bars in (A) represent the median and the error bars represent the interquartile range. Data in (B) were square-root transformed. The error bars represent the standard errors of the means. The vertical bar represents least squares means. Nic = nicotine.
Amount of e-liquid used during ad lib session (grams).
Only the effect of flavor was significant (Figure 4B), F(2, 93.9) = 13.66, p < .0001. Participants used more green-apple flavor e-liquid (M = 0.41 square-root grams [SE = 0.03]) than both menthol (M = 0.27 [SE = 0.03]) and unflavored e-liquids (M = 0.23 [SE = 0.03]; p < .0001, p = .0002, respectively). There was no statistically significant difference between the amount of menthol and unflavored e-liquids used (p = .28).
Discussion
This study sought to compare the effects of flavors (menthol, unflavored, and green apple) on nicotine concentration (6 mg/mL, 12 mg/mL) in commercially available e-liquids using an e-cigarette exposure paradigm in adolescent e-cigarette users. We primarily hypothesized that menthol flavor would interact with 12 mg/mL nicotine to enhance e-cigarette taste and liking/wanting for e-cigarette drug effects and that green-apple flavor would improve e-cigarette taste and liking/wanting for e-cigarette drug effects at 6 mg/mL nicotine. Contrary to our hypothesis, we did not find an interaction between nicotine concentration and flavor on liking e-cigarette taste or on liking/wanting of e-cigarette drug effects. However, we did observe that green-apple flavor and 6 mg/mL of nicotine independently increased liking of e-cigarette taste (see Figure 1). In addition, green-apple flavor produced higher ratings of fruitiness, sourness, and sweetness, and menthol produced higher ratings of coolness (see Figure 2).
Interestingly, while we observed that green-apple flavor enhanced the liking of e-cigarette taste, we found no evidence of a similar effect of green-apple flavor on liking/wanting of e-cigarette exposure study in young adult cigarette smokers also did not observe significant effects of a fruit-flavored e-liquid on e-cigarette drug effects (Cobb et al., 2019). Our finding that youth liked the taste of green apple (fruit) flavor e-cigarettes is consistent with other studies in young adults (Audrain-McGovern et al., 2016; Goldenson et al., 2016; Leventhal, Goldenson, Barrington-Trimis, Pang, & Kirkpatrick, 2019) and a recent preclinical study that demonstrated that a green-apple flavor constituent, farnesol, caused reward-related behaviors in male mice (Avelar et al., 2019). The sweetness of the green-apple flavor (vs. menthol and unflavored e-liquids; Figure 2C) may explain the greater liking for this flavor in our study (Kim et al., 2016). In the ad libitum session (see Figure 4) participants also took more puffs on the e-cigarette containing green-apple flavor more frequently and consumed more of the green-apple-flavored e-liquid compared to the menthol and unflavored e-liquids when they could puff on any of the 3 flavors. The e-liquid flavors contained nicotine (6 mg/mL or 12 mg/mL as assigned); therefore, the greater use of the green-apple flavor very likely resulted in higher nicotine exposure. Although we did not measure saliva nicotine levels during the ad libitum session, a previous ad libitum study found that higher use of a fruit-flavored e-liquid produced higher plasma nicotine levels in comparison to a tobacco-flavored e-liquid (St. Helen, Dempsey, Havel, Jacob, & Benowitz, 2017).
We also observed a main effect of nicotine concentration on liking of e-cigarette taste. Specifically, participants liked the taste of the 6 mg/mL of nicotine more than the 12 mg/mL nicotine concentration, While it could be hypothesized that the taste of the 6 mg/mL concentration was liked more because it was less irritating than the 12 mg/mL concentration, we did not find any statistically significant differences in harshness/irritation between 6 mg/mL and 12 mg/mL of nicotine (Figure 2E), and biochemical analysis demonstrated that there were higher saliva nicotine levels in the 12 mg/mL group versus the 6 mg/mL group (Figure 3B) which is consistent with our previous study (Krishnan-Sarin et al., 2017). The lack of harshness in the current study is in contrast with our previous study in which 12 mg/mL had higher ratings of harshness/irritation than 6 mg/mL (Rosbrook & Green, 2016). This may also explain why we did not observe an interactive effect of menthol on e-cigarette taste liking or drug effects based on nicotine concentration as we observed previously (Krishnan-Sarin et al., 2017). In that study, menthol enhanced the liking of drug effects at 12 mg/mL of nicotine but not at 6 mg/mL of nicotine. Our previous studies used menthol e-liquids with a 70 PG/30 VG ratio (Krishnan-Sarin et al., 2017; Rosbrook & Green, 2016), while the current study used commercial e-liquids with a 50 PG/50 VG ratio. Differential PG/VG ratios in e-liquids have been shown to alter nicotine delivery (Spindle et al., 2018) and they may alter sweetness ratings as well. Even though sweetness was not measured in our previous studies, in the current study the unflavored e-liquid was rated sweeter than the menthol-containing e-liquid (Figure 2C). The chemical analysis of e-liquids ruled out the presence of a noncharacterizing but sweet chemical agent (e.g., vanillin) in the unflavored e-liquid. The sweetness of 50 PG/50 VG ratio may have affected the perception of harshness of the nicotine concentrations and in turn altering the impact of menthol flavor. In addition, these discrepant findings could also be related to the fact that our earlier study enlisted more menthol cigarette smokers (30 out of 60) compared to the current study (nine out of 49), suggesting that prior menthol exposure may be an important factor in the effects of menthol containing e-liquids. These differences highlight the importance of controlling for other PG/VG levels constituents and subject characteristics when conducting flavor studies with e-cigarettes. Future studies need to examine the sensory responses to e-liquid flavors in the presence of different PG/VG levels.
This study only examined two e-liquid flavors of the thousands of e-liquid flavors available. An additional caveat is that inclusion of flavors that are both sweet and cool (i.e., “vanilla chill”) and other categories of flavors may have yielded different results. While the tested nicotine concentrations (6 mg/mL and 12 mg/mL) are used among youth (Morean et al., 2016), there was a lack of significant differences in irritation between the nicotine concentrations. Use of higher nicotine concentrations may have resulted in significant differences in irritation especially considering that our participants reported typically using high nicotine concentrations in their e-liquids (25.9 mg/mL [SD = 21.3]; Table 2) and may therefore have not found the lower nicotine concentrations used in our study (6 and 12 mg/mL) to be irritating. Also, it is worth noting that our “high” nicotine condition, was much lower than the high nicotine concentrations (e.g., > 60 mg/mL) used in some e-liquids. If there were significant differences in irritation between the nicotine concentrations for the current study, we may have observed an effect of menthol flavor. Another limitation to consider is the use of second generation V2 Cigs e-cigarette device which may have low levels of use among youth, but evidence from 2014–2017 suggests that adolescents and young adults are currently using second-generation e-cigarettes such as vape pens (Barrington-Trimis et al., 2018; Krishnan-Sarin et al., 2019). Lastly, there were no attempts to bio verify e-cigarette abstinence or the use of a “bogus pipeline” in this study. Therefore, we cannot confirm e-cigarette abstinence before the test sessions in our sample.
In conclusion, this study highlights the significant role fruit flavors play in adolescent e-cigarette use. Green-apple flavor, a fruit flavor, appears to significantly improve the taste of e-liquids regardless of the nicotine concentration, suggesting a means by which fruit flavors may enhance the appeal of e-liquids for youth. Regulatory efforts to reduce/prevent youth access to fruit-flavored e-liquids may reduce the appeal of e-cigarettes to youth.
Public Health Significance.
This study demonstrated that green-apple flavor and low nicotine concentration was appealing to youth past-month e-cigarette users. Regulatory efforts to reduce/prevent youth access to fruit-flavored e-liquids may reduce the appeal of e-cigarettes to youth.
Acknowledgments
The findings in this study were presented as an oral presentation at the 2019 Society for Research on Nicotine and Tobacco Conference in San Francisco, California. This research reported in this publication was supported by National Institutes of Health Grants P50DA036151 and U54 DA036151-07 (Yale Tobacco Center of Regulatory Science) and the Food and Drug Administration Center for Tobacco Products. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Food and Drug Administration. The funding source had no other role other than financial support. Asti Jackson assisted with the design and conduct of the study, created the initial draft of the manuscript, revised, created and approved the final draft. Suchitra Krishnan-Sarin, Stephanie S. O’Malley, and Barry Green conceptualized and designed the study, helped with drafting the initial manuscript, and approved the final manuscript as submitted. Grace Kong and Dana A. Cavallo assisted with the design and conduct of the study, reviewed and revised the manuscript, and approved the final manuscript as submitted. Hanno C. Erythropel and Tore Eid assisted with the analyses of the e-liquids, nicotine, and menthol glucuronide levels and approved final manuscript as submitted. Eugenia Buta and Ralitza Gueorguieva carried out the initial analyses, reviewed and revised the manuscript, and approved the final manuscript as submitted. The authors have no financial relationships relevant to this article to disclose. The authors have no conflicts of interest relevant to this article to disclose.
Contributor Information
Asti Jackson, Department of Psychiatry, Yale School of Medicine.
Barry Green, The John B. Pierce Laboratory and Department of Surgery, Yale School of Medicine.
Hanno C. Erythropel, Department of Chemical and Environmental Engineering, Yale University.
Grace Kong, Department of Psychiatry, Yale School of Medicine.
Dana A. Cavallo, Department of Psychiatry, Yale School of Medicine
Tore Eid, Department of Laboratory Medicine, Yale School of Medicine.
Ralitza Gueorguieva, Department of Biostatistics, Yale School of Public Health.
Eugenia Buta, Department of Biostatistics, Yale School of Public Health.
Stephanie S. O’Malley, Department of Psychiatry, Yale School of Medicine
Suchitra Krishnan-Sarin, Department of Psychiatry, Yale School of Medicine.
References
- Abreu-Villaça Y, Seidler FJ, Qiao D, Tate CA, Cousins MM, Thillai I, & Slotkin TA (2003). Short-term adolescent nicotine exposure has immediate and persistent effects on cholinergic systems: Critical periods, patterns of exposure, dose thresholds. Neuropsychopharmacology, 28, 1935–1949. 10.1038/sj.npp.1300221 [DOI] [PubMed] [Google Scholar]
- Ambrose BK, Day HR, Rostron B, Conway KP, Borek N, Hyland A, & Villanti AC (2015). Flavored tobacco product use among U.S. youth aged 12–17 years, 2013–2014. Journal of the American Medical Association, 314, 1871–1873. 10.1001/jama.2015.13802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audrain-McGovern J, Strasser AA, & Wileyto EP (2016). The impact of flavoring on the rewarding and reinforcing value of e-cigarettes with nicotine among young adult smokers. Drug and Alcohol Dependence, 166, 263–267. 10.1016/j.drugalcdep.2016.06.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avelar AJ, Akers AT, Baumgard ZJ, Cooper SY, Casinelli GP, & Henderson BJ (2019). Why flavored vape products may be attractive: Green apple tobacco flavor elicits reward-related behavior, upregulates nAChRs on VTA dopamine neurons, and alters midbrain dopamine and GABA neuron function. Neuropharmacology, 158, 107729. 10.1016/j.neuropharm.2019.107729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrington-Trimis JL, Gibson LA, Halpern-Felsher B, Harrell MB, Kong G, Krishnan-Sarin S, … Weaver SR (2018). Type of e-cigarette device used among adolescents and young adults: Findings from a pooled analysis of 8 studies of 2,166 vapers. Nicotine & Tobacco Research, 20, 271–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrington-Trimis JL, Kong G, Leventhal AM, Liu F, Mayer M, Boley Cruz T, … Mcconnell R (2018). E-cigarette use and subsequent smoking frequency among adolescents. Pediatric Research, 142, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartoshuk LM, Duffy VB, Green BG, Hoffman HJ, Ko CW, Lucchina LA, … Weiffenbach JM (2004). Valid across-group comparisons with labeled scales: The gLMS versus magnitude matching. Physiology & Behavior, 82, 109–114. 10.1016/j.physbeh.2004.02.033 [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Dains KM, Dempsey D, Havel C, Wilson M, & Jacob P III. (2010). Urine menthol as a biomarker of mentholated cigarette smoking. Cancer Epidemiology, Biomarkers & Prevention, 19, 3013–3019. 10.1158/1055-9965.EPI-10-0706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bold KW, Kong G, Camenga DR, Simon P, Cavallo DA, Morean ME, & Krishnan-Sarin S (2018). Trajectories of e-cigarette and conventional cigarette use among youth. Pediatrics, 141, e20171832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb CO, Lopez AA, Soule EK, Yen MS, Rumsey H, Lester Scholtes R, … Eissenberg T (2019). Influence of electronic cigarette liquid flavors and nicotine concentration on subjective measures of abuse liability in young adult cigarette smokers. Drug and Alcohol Dependence, 203, 27–34. 10.1016/j.drugalcdep.2019.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen KA, Gentzke AS, Sawdey MD, Chang JT, Anic GM, Wang TW, … King BA (2019). e-Cigarette Use Among Youth in the United States, 2019. Journal of the American Medical Association, 322, 2095–2103. 10.1001/jama.2019.18387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVito EE, Jensen KP, O’Malley SS, Gueorguieva R, Krishnan-Sarin S, Valentine G, … Sofuoglu M (2019). Modulation of “protective” nicotine perception and use profile by flavorants: Preliminary findings in e-cigarettes. Nicotine & Tobacco Research: Official Journal of the Society for Research on Nicotine and Tobacco. Advance online publication. 10.1093/ntr/ntz057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutra LM, & Glantz SA (2014). High international electronic cigarette use among never smoker adolescents. The Journal of Adolescent Health, 55, 595–597. 10.1016/j.jadohealth.2014.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erythropel HC, Jabba SV, DeWinter TM, Mendizabal M, Anastas PT, Jordt SE, & Zimmerman JB (2019). Formation of flavorant-propylene glycol adducts with novel toxicological properties in chemically unstable e-cigarette liquids. Nicotine & Tobacco Research, 21, 1248–1258. 10.1093/ntr/nty192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Food and Drug Administration. (2009). Family Smoking Prevention and Tobacco Control Act. P.L 111, 1–84. [Google Scholar]
- Fulton E, Gokal K, Griffiths S, & Wild S (2018). More than half of adolescent e-cigarette users had never smoked a cigarette: Findings from a study of school children in the U.K. Public Health, 161, 33–35. 10.1016/j.puhe.2018.04.014 [DOI] [PubMed] [Google Scholar]
- Goldenson NI, Kirkpatrick MG, Barrington-Trimis JL, Pang RD, McBeth JF, Pentz MA, … Leventhal AM (2016). Effects of sweet flavorings and nicotine on the appeal and sensory properties of e-cigarettes among young adult vapers: Application of a novel methodology. Drug and Alcohol Dependence, 168, 176–180. 10.1016/j.drugalcdep.2016.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green BG, Dalton P, Cowart B, Shaffer G, Rankin K, & Higgins J (1996). Evaluating the “Labeled Magnitude Scale” for measuring sensations of taste and smell. Chemical Senses, 21, 323–334. 10.1093/chemse/21.3.323 [DOI] [PubMed] [Google Scholar]
- Green BG, Shaffer GS, & Gilmore MM (1993). Derivation and evaluation of a semantic scale of oral sensation magnitude with apparent ratio properties. Chemical Senses, 18, 683–702. 10.1093/chemse/18.6.683 [DOI] [Google Scholar]
- Ha MA, Smith GJ, Cichocki JA, Fan L, Liu YS, Caceres AI, … Morris JB (2015). Menthol attenuates respiratory irritation and elevates blood cotinine in cigarette smoke exposed mice. PLoS ONE, 10, e0117128. 10.1371/journal.pone.0117128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrell MB, Weaver SR, Loukas A, Creamer M, Marti CN, Jackson CD, … Eriksen MP (2017). Flavored e-cigarette use: Characterizing youth, young adult, and adult users. Preventive Medicine Reports, 5, 33–40. 10.1016/j.pmedr.2016.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatem S, Attal N, Willer J-C, & Bouhassira D (2006). Psychophysical study of the effects of topical application of menthol in healthy volunteers. Pain, 122, 190–196. 10.1016/j.pain.2006.01.026 [DOI] [PubMed] [Google Scholar]
- Kim H, Lim J, Buehler SS, Brinkman MC, Johnson NM, Wilson L, … Clark PI (2016). Role of sweet and other flavours in liking and disliking of electronic cigarettes. Tobacco Control, 25, ii55–ii61. 10.1136/tobaccocontrol-2016-053221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, & Selya AS (2019). The relationship between electronic cigarette use and conventional cigarette smoking is largely attributable to shared risk factors. Nicotine & Tobacco Research. Advance online publication. 10.1093/ntr/ntz157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong G, Morean ME, Cavallo DA, Camenga DR, & Krishnan-Sarin S (2015). Reasons for electronic cigarette experimentation and discontinuation among adolescents and young adults. Nicotine & Tobacco Research, 17, 847–854. 10.1093/ntr/ntu257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan-Sarin S, Green BG, Kong G, Cavallo DA, Jatlow P, Gueorguieva R, … O’Malley SS (2017). Studying the interactive effects of menthol and nicotine among youth: An examination using e-cigarettes. Drug and Alcohol Dependence, 180, 193–199. 10.1016/j.drugalcdep.2017.07.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan-Sarin S, Jackson A, Morean M, Kong G, Bold KW, Camenga DR, … Wu R (2019, January). E-cigarette devices used by high-school youth. Drug and Alcohol Dependence, 194, 395–400. 10.1016/j.drugalcdep.2018.10.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM, Goldenson NI, Barrington-Trimis JL, Pang RD, & Kirkpatrick MG (2019). Effects of non-tobacco flavors and nicotine on e-cigarette product appeal among young adult never, former, and current smokers. Drug and Alcohol Dependence, 203, 99–106. 10.1016/j.drugalcdep.2019.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis MJ, & Wackowski O (2006). Dealing with an innovative industry: A look at flavored cigarettes promoted by mainstream brands. American Journal of Public Health, 96, 244–251. 10.2105/AJPH.2004.061200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim J, Wood A, & Green BG (2009). Derivation and evaluation of a labeled hedonic scale. Chemical Senses, 34, 739–751. 10.1093/chemse/bjp054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D, Hopewell S, Schulz KF, Montori V, Gøtzsche PC, Devereaux PJ, … Altman DG (2010). CONSORT 2010 explanation and elaboration: Updated guidelines for reporting parallel group randomised trials. British Medical Journal, 340, c869. 10.1136/bmj.c869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morean ME, Butler ER, Bold KW, Kong G, Camenga DR, Cavallo DA, … Krishnan-Sarin S (2018). Preferring more e-cigarette flavors is associated with e-cigarette use frequency among adolescents but not adults. PLoS ONE, 13, e0189015. 10.1371/journal.pone.0189015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morean ME, de Wit H, King AC, Sofuoglu M, Rueger SY, & O’Malley SS (2013). The drug effects questionnaire: Psychometric support across three drug types. Psychopharmacology, 227, 177–192. 10.1007/s00213-012-2954-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morean ME, Kong G, Cavallo DA, Camenga DR, & Krishnan-Sarin S (2016). Nicotine concentration of e-cigarettes used by adolescents. Drug and Alcohol Dependence, 167, 224–227. 10.1016/j.drugalcdep.2016.06.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Advisory Council on Drug Abuse. (2012). Guidelines for substance abuse research involving children and adolescents. Retrieved from https://archives.drugabuse.gov/advisory-council-minutes/nacda-guidelines-substance-abuse-research-involving-children-adolescents
- Pepper JK, Ribisl KM, & Brewer NT (2016). Adolescents’ interest in trying flavoured e-cigarettes. Tobacco Control, 25, ii62–ii66. 10.1136/tobaccocontrol-2016-053174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosbrook K, & Green BG (2016). Sensory effects of menthol and nicotine in an e-cigarette. Nicotine & Tobacco Research, 18, 1588–1595. 10.1093/ntr/ntw019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofuoglu M, Herman AI, Nadim H, & Jatlow P (2012). Rapid nicotine clearance is associated with greater reward and heart rate increases from intravenous nicotine. Neuropsychopharmacology, 37, 1509–1516. 10.1038/npp.2011.336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soneji S, Barrington-Trimis JL, Wills TA, Leventhal AM, Unger JB, Gibson LA, … Sargent JD (2017). Association between initial use of e-cigarettes and subsequent cigarette smoking among adolescents and young adults: A systematic review and meta-analysis. Journal of the American Medical Association Pediatrics, 171, 788–797. 10.1001/jamapediatrics.2017.1488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soria R, Stapleton JM, Gilson SF, Sampson-Cone A, Henningfield JE, & London ED (1996). Subjective and cardiovascular effects of intravenous nicotine in smokers and non-smokers. Psychopharmacology, 128, 221–226. 10.1007/s002130050129 [DOI] [PubMed] [Google Scholar]
- Spindle TR, Talih S, Hiler MM, Karaoghlanian N, Halquist MS, Breland AB, … Eissenberg T (2018). Effects of electronic cigarette liquid solvents propylene glycol and vegetable glycerin on user nicotine delivery, heart rate, subjective effects, and puff topography. Drug and Alcohol Dependence, 188, 193–199. 10.1016/j.drugalcdep.2018.03.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- St. Helen G, Dempsey DA, Havel CM, Jacob P III, & Benowitz NL (2017). Impact of e-liquid flavors on nicotine intake and pharmacology of e-cigarettes. Drug and Alcohol Dependence, 178, 391–398. 10.1016/j.drugalcdep.2017.05.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villanti AC, Johnson AL, Ambrose BK, Cummings KM, Stanton CA, Rose SW, … Hyland A (2017). Flavored tobacco product use in youth and adults: Findings from the first wave of the PATH Study (2013–2014). American Journal of Preventive Medicine, 53, 139–151. 10.1016/j.amepre.2017.01.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasner G, Schattschneider J, Binder A, & Baron R (2004). Topical menthol—A human model for cold pain by activation and sensitization of C nociceptors. Brain: A Journal of Neurology, 127, 1159–1171. 10.1093/brain/awh134 [DOI] [PubMed] [Google Scholar]
- Yuan M, Cross SJ, Loughlin SE, Leslie FM, & Yuan M (2015). Nicotine and the adolescent brain. The Journal of Physiology, 593, 3397–3412. 10.1113/JP270492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zare S, Nemati M, & Zheng Y (2018). A systematic review of consumer preference for e-cigarette attributes: Flavor, nicotine strength, and type. PLoS ONE, 13, e0194145. 10.1371/journal.pone.0194145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S-H, Sun JY, Bonnevie E, Cummins SE, Gamst A, Yin L, & Lee M (2014). Four hundred and sixty brands of e-cigarettes and counting: Implications for product regulation. Tobacco Control, 23, iii3–iii9. 10.1136/tobaccocontrol-2014-051670 [DOI] [PMC free article] [PubMed] [Google Scholar]


