Abstract
Circadian clocks are cell-autonomous self-sustaining oscillators that allow organisms to anticipate environmental changes throughout the solar day and persist in nearly every cell examined. Environmental or genetic disruption of circadian rhythms increases the risk of several types of cancer, but the underlying mechanisms are not well understood. Here, we discuss evidence connecting circadian rhythms—with emphasis on the cryptochrome proteins (CRY1/2)—to cancer through in vivo models, mechanisms involving known tumor suppressors and oncogenes, chemotherapeutic efficacy, and human cancer risk.
Keywords: cell cycle, circadian clocks, circadian rhythm, cryptochromes, DNA repair, oncogenes carcinogenesis
1 |. INTRODUCTION
Circadian rhythms synchronize physiological processes and behaviors to zeitgebers (external timing cues) such as light and food availability. The ability to maintain an internal sense of time via a protein-based molecular oscillator is widespread throughout phylogeny. The suprachiasmatic nucleus (SCN) located in the anterior hypothalamus is the master clock that drives rhythms in hormone production, locomotor activity, and feeding behavior, which in turn entrain peripheral clocks located throughout the body.1 Keeping time throughout the body is important for overall health and well-being.
At the molecular level, mammalian circadian clocks are based on a transcription-translation feedback loop (TTFL) that regulates gene expression and contributes to oscillations of protein abundance. A heterodimer composed of circadian locomotor output cycles kaput (CLOCK) and brain and muscle ARNT-like protein 1 (BMAL1) drives expression of the CLOCK:BMAL1 repressors periods (PER 1–3) and cryptochromes (CRY 1–2) and other clock-controlled genes. An additional feedback loop in which the nuclear hormone receptors (NRs) RORα/γ and REV-ERBα/β drive rhythmic expression of the Bmal1 transcript is also required to sustain rhythms.2 This negative feedback produces approximately 24-hour oscillations in CLOCK:BMAL1 transcriptional activity, leading to oscillating expression of a significant fraction of the transcriptome and of the proteome in all mammalian organs that have been examined.3 Indeed, ~50% of mammalian genes are rhythmically expressed.4,5 These molecular rhythms modulate physiological processes such as metabolism, DNA repair, and inflammation—functions important for cancer growth.6 Deletion, mutation, or dysregulation of some core clock proteins has been implicated in cancer.7,8
The first evidence that environmental disruption of circadian rhythms influences breast cancer incidence was observed in the 1960s.9 Subsequently, through numerous epidemiological studies, the World Health Organization (WHO) designated circadian rhythm disruption a probable carcinogen.10 Circadian disruption can occur through a wide variety of human behaviors, most commonly shift work or trans-meridian travel.11 This disruption can be modeled experimentally in mice by altering their light schedules. In genetically engineered mouse models of breast, lung, and liver cancer,12–14 as well as xenografts of osteosarcoma and pancreatic adenocarcinoma,15 exposure to altered lighting conditions that mimic rotating shift work or chronic jet lag increased tumor formation and progression.
Cryptochromes evolved from bacterial light-activated DNA repair enzymes.16 In insects, the evolution of CRYs’ transcriptional repressor function seems to have co-occurred with loss of direct light sensing.17 Mammalian CRYs lack both innate light sensitivity and catalytic DNA repair activity but are sensitive to metabolic cues18 and may be regulated by magnetic fields.19 Their evolutionary relationship to DNA repair enzymes has motivated investigation of their potential role in protecting the genome by other means. In this review article, we discuss evidence connecting cryptochromes and cancer through in vivo mouse models of cancer and mechanistic or correlative studies involving tumor suppressors, oncogenes, and chemotherapy.
2 |. DOES MELATONIN PROVIDE PROTECTION AGAINST CANCER?
Because the SCN drives daily oscillations in the production and secretion of hormones, including predominantly glucocorticoids20 and melatonin,21 their levels in the circulation are altered by circadian disruption. Melatonin is synthesized by the pineal gland and its production is controlled by both light and circadian rhythms. Circadian regulation of melatonin drives its synthesis and secretion during the dark phase; exposure to light at night directly suppresses melatonin production; and insufficient exposure to daytime light results in delayed and/or reduced melatonin accumulation,21 which impacts sleep and likely other physiological systems as well. Melatonin modulates glucose homeostasis22–24 and has been reported to suppress cell and tumor growth.25 Many tumors exhibit reduced circadian rhythmicity, and studies have identified mechanisms that may underlie this phenomenon in specific contexts.26–28 Melatonin can resynchronize some circadian rhythm genes in prostate cancer cells.29 Research addressing the role of melatonin in tumor development in vivo is sparse, largely because the most commonly used inbred mouse strains do not synthesize melatonin due to genetic alterations that likely accumulated over generations of breeding in unnatural lighting conditions.30 If melatonin plays a major role in suppressing tumor development as suggested by some studies,31 the impact of circadian disruption on tumor development may in fact be greatly underestimated in studies that rely on inbred mouse models.
3 |. CRYPTOCHROMES ALTER DISEASE OUTCOMES IN MOUSE MODELS OF CANCER
Mouse models provide insight into the genetic determinants of cancer progression, and mice harboring mutations in core circadian clock components have been used to evaluate the potential for genetic disruption of circadian rhythms to alter tumor development.
A handful of studies have examined the impact of Cry1 and Cry2 deletion on tumor formation and survival in response to environmental exposures. Two groups measured overall survival in wildtype (WT) and Cry1−/−;Cry2−/− mice following exposure to 4 Gy ionizing radiation delivered at zeitgeber time (ZT, hours after lights on) 10 to mice at either six or eight weeks of age,32,33 respectively. One study reported increased tumor formation and decreased survival of Cry1−/−; Cry2−/− mice compared to WT littermates following irradiation.33 The other study32 did not observe a significant difference in response to radiation in Cry1−/−; Cry2−/− mice compared to WT mice of the same age and genetic background. It is unclear whether the different outcomes reflect the use of nonlittermate mice as controls in the 2005 study,32 exposure to irradiation at different ages, or some other detail of the methodology or housing conditions. More recently, it was demonstrated that Cry1−/−; Cry2−/− mice develop an increased tumor burden in response to diethylnitrosamine (DEN)-induced liver carcinogenesis.34 Together, these reports support the hypothesis that cryptochromes reduce tumor formation in response to at least some environmental exposures. Consistent with these observations, Cry1−/−;Cry2−/− mice exhibit decreased survival and form fewer but larger spontaneous hepatocellular carcinomas compared to WT mice.13
Several genetically engineered mouse models have been developed to mimic different types of cancer in humans. For example, TP53 is among the most widely inactivated human tumor suppressor genes and its deletion is used to accelerate the development of many tumor types in mice. Surprisingly, Tp53−/−; Cry1−/−; Cry2−/− mice have decreased tumor burden and increased overall survival compared to Tp53−/− mice35 possibly due to differential transcriptome level changes in nuclear factor kappa B (NF-κB) regulation.36 Thus, the effect of deleting Cry1 and Cry2 appears to impact tumor burden differently in different oncogenic settings. Specifically, this finding suggests that the impact of Cry1 and/or Cry2 deletion depends on the functional status of TP53.
Most studies that have investigated the role of cryptochromes in cancer have deleted Cry1 and Cry2 simultaneously (ie, Cry1−/−; Cry2−/− mice), presuming their functions to be redundant. However, while either CRY1 or CRY2 can support circadian rhythmicity37,38 subsequent studies have demonstrated that their functions are divergent both within37,39 and especially outside of40–43 the circadian clock mechanism. Therefore, it is important to study their potential roles in tumor formation separately. Cry2−/− mice and mice deficient for any three of the four Cry1 and Cry2 alleles in a Tp53−/− background exhibited increased survival compared to Tp53−/− mice35 while deletion of Cry2 increased tumor burden and decreased overall survival in a MYC-driven lymphoma mouse model.42
Chronic inflammation can promote tumor formation at affected sites.6 Altering circadian rhythms has been shown to impact immune responses.44 Cry1−/−;Cry2−/− mice display constitutive elevation of proinflammatory cytokines45–47 and an autoimmune phenotype.45,48 The proinflammatory phenotype of Cry1−/−; Cry2−/− mice may contribute to increased cancer formation upon irradiation.33
Together, these findings indicate that inactivation of cryptochromes impacts the development of a variety of cancers in mice. Furthermore, the effect of deleting Cry1 or Cry2 depends on the tissue of origin and on the underlying genetic drivers of tumorigenesis. Finally, CRY1 and CRY2 have unique functions in regulating cancer development and must be studied independently.
4 |. CONNECTING CRYPTOCHROMES WITH TUMOR SUPPRESSORS
To sustain proliferation, tumor cells must overcome growth-inhibitory signals such as cell cycle inhibition via tumor suppressor genes. Recent reviews have described several molecular connections between cell cycle inhibition and circadian proteins.49,50 In this section, we focus on evidence suggesting that cryptochromes can interact with or influence widely known tumor suppressor pathways.
4.1 |. TP53
Tumor suppressor protein 53 (TP53) is the most frequently inactivated tumor suppressor in human cancer.6 In normal cells, it is activated by intracellular stresses, such as DNA damage and reactive oxygen species, leading to cell cycle arrest and/or apoptosis.6 Thus, its inactivation allows damaged cells to survive and expand. TP53 expression and/or TP53 phosphorylation exhibit daily oscillations in human oral mucosal cells51 and mouse thymus.33 Furthermore, TP53 protein exhibits a circadian rhythm in fibroblast-like cells either implanted in mice or subject to circadian synchronization in culture, while Tp53 mRNA was expressed at a constant level, which was attributed to rhythmic ATF4-driven repression of p19 interfering with MDM2-dependent TP53 destabilization.52 This suggests that TP53 protein levels are subject to posttranscriptional regulation by circadian clocks. Several studies have suggested that clock proteins can modulate the function of TP53.
TP53 stability is regulated by the E3 ubiquitin ligases mouse double minute 2 homolog (MDM2)53–55 and constitutive photomorphogenic 1 (COP1)56 and the ubiquitin-specific protease herpesvirus-associated ubiquitin-specific protease (HAUSP) that removes polyubiquitin chains from TP5357,58 (Figure 1). Upon DNA damage, HAUSP can deubiquitinate CRY1 to promote CRY1 stabilization.40 Whether CRY1 has any effect on TP53 function or stability mediated by its interaction with HAUSP remains unclear. DNA damage was also shown to decrease CRY2 stability by increasing the interaction between CRY2 and the Skp-Cullin-Fbox (SCF) E3 ubiquitin ligase substrate receptor F-box and leucine-rich repeat protein 3 (FBXL3).59–61 Both CRY1 and CRY2 can interact with De-etiolated1 (DET1), thus inhibiting recruitment of the substrate receptor COP1 to the Cullin 4 (CUL4)-based E3 ubiquitin ligase complex.62 This could result in CRY1/2-dependent TP53 stabilization as COP1 is a negative regulator of TP5356 (Figure 1). Consistent with this possibility, depletion of CRY1 in osteosarcoma cells decreased TP53 and P21 levels and increased proliferation and migration.63 Conversely, Cry1-deficient primary fibroblasts exhibit prolonged activation of P21 transcription in response to DNA damage, while loss of CRY2 suppressed P21 expression in the same context.40 Additionally, shRNA-mediated reduction of Tp53 results in increased cell proliferation and growth in low paracrine signaling conditions in Cry2−/− mouse embryonic fibroblasts (MEFs) compared to WT cells, while Cry1−/− MEFs grow more slowly than WT cells in response to depletion of TP53.42
FIGURE 1.
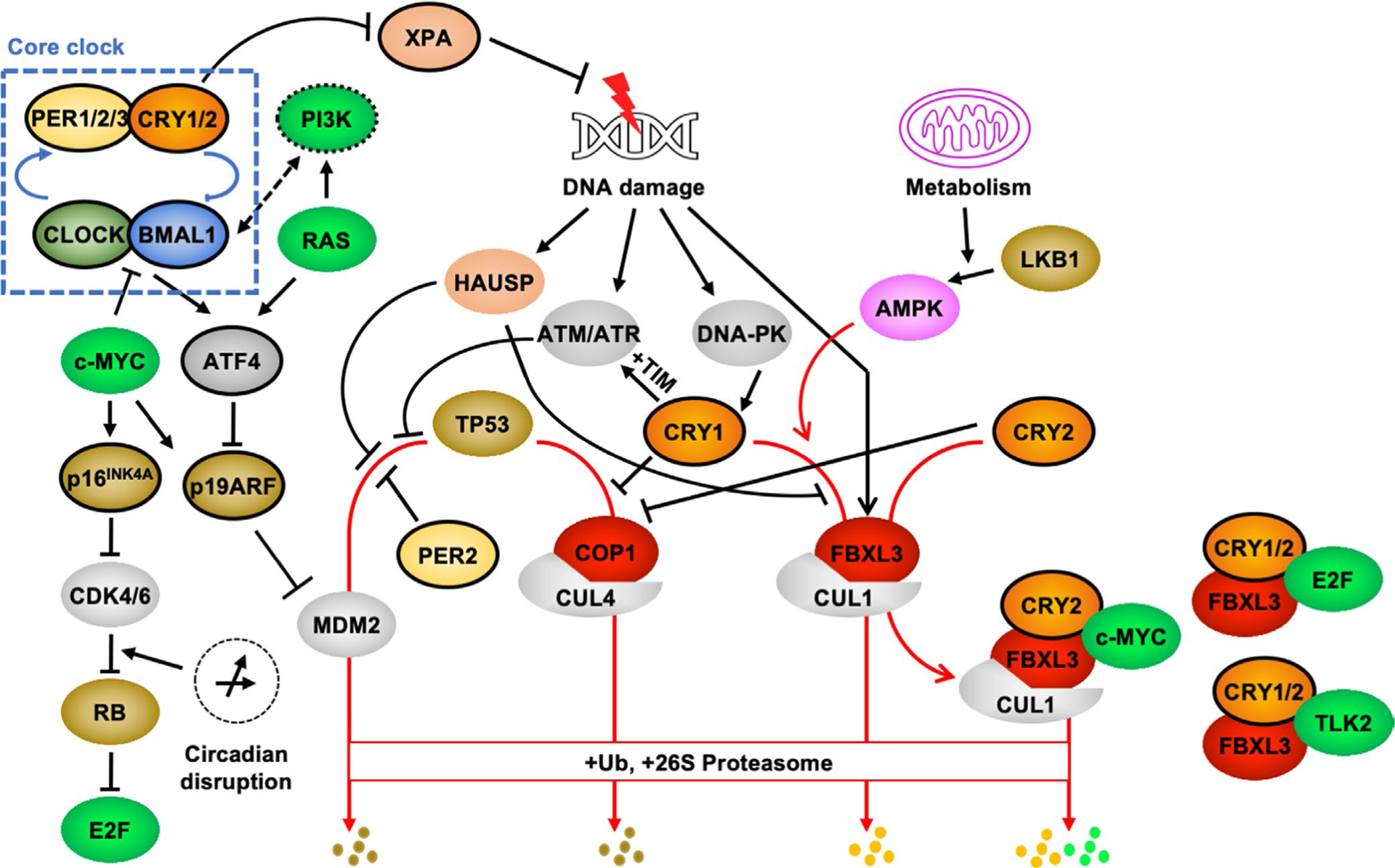
Molecular connections between CRY1, CRY2, and cancer-related pathways. The core clock mechanism involves a transcription-translation feedback loop (TTFL) in which the bHLH-PAS transcription factors CLOCK and BMAL1 drive expression of their own repressors, PERs and CRYs (for a more complete description of the molecular clock, see ref.1). In the face of growing interest in understanding the mechanistic underpinnings for enhanced cancer risk in people exposed to chronic circadian disruption, several molecular connections between clocks and proteins with established roles in regulating cell growth, DNA repair, and the cellular response to DNA damage have been described. See text for details. Proteins outlined in black exhibit widespread circadian expression at the mRNA level. Brown shading denotes tumor suppressors; bright green shading denotes oncogenes. Substrate receptors for multisubunit E3 ligases are shown in red. Red arrows indicate pathways that lead to ubiquitination and 26S proteasome-mediated degradation. Ub, ubiquitin
PERs and CRYs function as heterodimers to repress CLOCK- and BMAL1-driven transcription in the TTFL. PER2 can bind the C-terminal region of TP53 to prevent MDM2-mediated degradation, thereby promoting TP53 stabilization and increasing TP53-mediated target gene expression.64–66 Cryptochromes might influence these actions given their robust interactions with PER2. The impacts of cell type, stimulus context, and other factors in the role of cryptochromes in TP53 regulation remain to be determined.
4.2 |. RB
Inactivation of RB causes childhood retinoblastoma. RB suppresses cell growth by preventing adenoviral early region 2 binding factor (E2F) from activating the expression of cell cycle promoting genes.67 Phosphorylation of RB by cyclin-dependent kinases 4 and 6 (CDK4/6) prevents its interaction with E2Fs, and CDK4/6 inhibitors are effective in treating cancers with intact RB function.67 CDK4/6 protein and RB phosphorylation were enhanced by treatments that mimic chronic circadian disruption in human osteosarcoma cells (U2OS).68 This phenomenon was blocked by genetic depletion of either Bmal1 or of both Cry1 and Cry2. We recently discovered an interaction of both CRY1 and CRY2 with several E2F family transcription factors. While only CRY2 stimulates the ubiquitination and turnover of c-MYC by recruiting it to SCFFBXL3,42 we found that both CRY1 and CRY2 can interact with E2F family members and stimulate their interaction with FBXL369 (Figure 1). Steady-state levels of E2F4 and E2F8 are robustly affected by modulating Cry1 and/or Cry2 expression, but E2F1 protein levels are less susceptible to modulation by cryptochromes. CRY1 and CRY2 may influence E2F-dependent transcription through direct repression, but this requires further investigation.
4.3 |. LKB1
Peutz-Jeghers syndrome is an inherited cancer susceptibility caused by inactivating mutations in liver kinase B1 (LKB1).70 LKB1 phosphorylates and thereby activates a family of fourteen related kinases that includes adenosine monophosphate–activated protein kinase (AMPK).71 Recent work demonstrated that the tumor suppressor function of LKB1 depends on activation of a subfamily of four AMPK-related kinases, the microtubule affinity regulating kinases (MARK1–4).72 AMPK phosphorylates CRY1 on two serines (S71 and S280) (Figure 1), and thereby disrupts its interaction with PER273 and promotes CRY1 association with FBXL3, leading to CRY1 ubiquitination and degradation18 (Figure 1). All of the LKB1-activated kinases are expected to phosphorylate substrates containing similar amino acid sequences as those phosphorylated by AMPK, but it is unclear whether MARKs can phosphorylate CRY1 or CRY2.
AMPK is a master regulator of metabolic function and is a target of the widely used diabetes drug, metformin,71 which is currently being evaluated for therapeutic efficacy in cancer.74 A recent study examined the relationship between expression of clock genes and sensitivity to a variety of established or proposed chemotherapeutic agents. The drugs tested include two that activate AMPK (AICAR and phenformin). Intriguingly, among the fourteen core clock genes examined, only the expression of CRY1 is positively correlated and of PER2 is negatively correlated with sensitivity to both drugs,8 suggesting that AMPK-dependent disruption of CRY1-PER2 interaction may influence cell viability. Although neither the transport and metabolism of metformin, nor the expression of AMPK catalytic subunits are affected by the time of day, activation of AMPK in the liver by equivalent doses of metformin is much greater during the active phase in mice,75 suggesting that timing of metformin delivery may influence its efficacy in diabetes and/or cancer treatment.
4.4 |. DNA damage–responsive kinases
Ataxia telangiectasia mutated (ATM) and ataxia telangiectasia and Rad3-related (ATR) are the central kinases that initiate the DNA damage response in cells. ATM is activated by double-strand breaks, while ATR responds to stalled DNA replication forks.76 TIMELESS (TIM) is a large intrinsically disordered protein that is required for circadian rhythms in Drosophila melanogaster.77 The role of mammalian TIM in circadian rhythms has been difficult to study due to its essential function in embryonic survival.78 Human TIM mutants cause advanced sleep phase,79 suggesting that TIM also influences the mammalian clock,80,81 an idea that is also supported by protein-protein interactions between mammalian TIM, CRY1,81,82 and CRY2.83 TIM has been shown to facilitate the ATR replication checkpoint,84 and CRY1 can participate in the ATR-mediated DNA damage checkpoint via interaction with TIM82 (Figure 1).
Following DNA damage, DNA-dependent protein kinase (DNA-PK) can phosphorylate the C-terminal tail of CRY1 at Serine 58840,85 (Figure 1). It is unclear whether or how this phosphorylation impacts the interaction of CRY1 with TIM and/or ATR-mediated signaling. Furthermore, TIM has a role in genome integrity through facilitating sister chromatid cohesion.86 Consequently, alterations in CRY1 or CRY2 interaction could result in aberrant mitosis and disrupted genome integrity.
Though less well studied than the glutamine-directed protein kinases that are activated by DNA damage (ATM, ATR, and DNA-PK), the mammalian tousled-like kinases, TLK1 and TLK2, are maximally active in dividing cells at the time of peak DNA replication and are further activated in response to treatment with a variety of DNA-damaging agents during S phase.87 TLK2 is frequently amplified in estrogen receptor–positive breast tumors and its overexpression enhanced aggressive growth of breast cancer–derived cell lines.88 CRY1 and CRY2 can recruit TLK2 to FBXL3, and genetic deletion of Cry1 and Cry2 increased the steady-state protein level of inducibly expressed TLK2 in primary fibroblasts.89
5 |. CRY-ING OVER ONCOGENES
To become malignant, cells must replicate indefinitely.6 This can be achieved through overactivation of pro-growth pathways or deregulation of the cell cycle. The circadian and cell cycles influence each other,90–94 and several molecular connections between the two have been described. Cryptochromes impact both expression and posttranslational regulation of established cell cycle regulators. Deletion of Cry1 and/or Cry2 alters the expression of several genes involved in cell cycle regulation, including Cyclin D1, Wee1, Cyclin A2, and Cyclin B1,90 and affects the steady-state protein levels of c-MYC42 and of several members of the E2F family of transcription factors (E2Fs).69 A recent study developed a method to approximate chronic circadian disruption in cultured cells and demonstrated that it increases proliferation. The enhanced proliferation caused by circadian disruption was prevented by depletion of Cry1 and Cry2,68 suggesting that cryptochromes may play an important role in linking circadian disruption to enhanced cancer risk.
5.1 |. MYC
c-MYC is among the most highly amplified oncogenes in human cancer.95 c-MYC is an important regulator of cell proliferation, transcription, differentiation, apoptosis, and cell migration.96 In primary MEFs, c-MYC stable overexpression leads to increased proliferation and growth in low paracrine signaling conditions; these effects were greatly enhanced in Cry2−/− compared to WT or Cry1−/− MEFs.42 Similar to the well-established regulation of c-MYC by FBXW7,97 CRY2 cooperates with FBXL3 to interact with c-MYC and promote its ubiquitination and degradation.42 Intriguingly, not only does DNA damage enhance the interaction between FBXL3 and CRY2,40 but FBXL3 was one of only four F-box substrate adaptors that exhibited significantly enhanced recruitment to CUL1 in response to DNA damage in a recent study.98 In line with this finding, knockdown of CRY2 in osteosarcoma cells increased c-MYC and decreased TP53 protein levels.99 Fbxw7 deletion in either T cells or hematopoietic stem cells can cause thymic lymphoma100 or acute lymphoblastic leukemia,101 respectively. These effects of Fbxw7 deletion are accelerated in Tp53−/−, Pten−/−, or NOTCH activation mouse models.102 Germline deletion of Cry2 in the Eμ-Myc lymphoma mouse model increased tumor burden and decreased overall survival.42 Additional studies are needed to understand whether Cry2 deletion has a cell-autonomous effect on tumor development and to elucidate the context-dependent roles of Cry2 deletion in tumor development in vivo. Conversely, MYC opposes BMAL1 expression and/or activity and thus dampens circadian rhythms.26,103,104 Reciprocal regulation of circadian rhythms and MYC further highlights the dynamic interaction between circadian and cell cycle oscillations.
5.2 |. RAS
In the early days of oncogene discovery, several tumor-inducing retroviruses that cause the formation of sarcomas in rats were found to promote tumorigenesis by way of a common viral gene. This so-called rat sarcoma (ras) viral oncogene has several mammalian orthologs—encoded by the Kirsten (K), Harvey (H), and neuroblastoma (N) ras oncogenes. The protein products of Kras, Hras, and Nras, collectively referred to as RAS, are activated by growth factor receptors and hydrolyze guanosine triphosphate (GTP) to promote the activation of downstream signaling pathways, including phosphatidylinositol 3-kinases (PI3Ks) and mitogen-activated protein kinases (MAPKs). RAS proteins are the most frequent targets of mutation in cancer, with mutations that prevent their association with GTPase-activating proteins (GAPs; eg, HRasG12V a.k.a HRasV12) having the most powerful influence on cellular transformation.
HRasV12 overexpression lengthens circadian period and decreases Cry1 expression in MEFs.27,105 MEFs generated from Bmal1−/− or Clock mutant mice are resistant to oncogenic transformation by combined overexpression of H-RasV12 and SV40 large T antigen (SV40LT). This is likely due to their inability to activate transcription of Atf4 and thereby repress the tumor suppressor p19Arf, such that p19ARF maintains its suppression of MDM2-mediated TP53 degradation.106 In the absence of SV40LT co-expression, depletion of Bmal1 had no impact on proliferation or senescence in MEFs overexpressing H-RasV12.105 MEFs deficient in period or cryptochrome repressors expressed normal levels of ATF4 and were transformed by HRasV12 to a similar extent as WT cells. This difference in transformation could reflect reduced expression of genes directly controlled by CLOCK and BMAL1 in Bmal1 or Clock mutants, while loss of Per2, or Cry1 and Cry2, may result in elevated expression of those genes either constitutively or with an enhanced amplitude of rhythmicity. Finally, Cry2−/− MEFs were more susceptible to H-RasV12 transformation than WT or Cry1−/− MEFs.42 These studies suggest a possible CRY2-specific effect on RAS-dependent signaling; it is unclear whether this is mediated by effects on ATF4, c-MYC, core clock function, or some other mechanism. Like c-MYC, RAS has been shown to reciprocally affect the circadian clock.27
6 |. EXPRESSION PATTERNS AND POLYMORPHISMS OF CRY1 AND CRY2 IN HUMAN CANCERS
Many studies have demonstrated that long-term shift work increases the relative risk of several types of cancer.107–114 Even the longitudinal position within a time zone at which a person resides significantly impacts cancer risk.115,116 Together, these findings suggest that chronic alterations in circadian environmental exposures alter tumor development. Association studies have identified single nucleotide polymorphisms (SNPs) in CRYs that associate with risk or mortality for various cancers, including prostate cancer,117–119 non-Hodgkin’s lymphoma,120 and breast cancer.121 Further research is needed to determine whether these SNPs are reproducibly associated with tumor development in additional populations. Although amplification, deletion, and missense mutation of core clock genes are rare in cancer, several studies have measured significantly altered expression of CRY1 or CRY2 in a variety of cancer types (Table 1). Generally, there seems to be a strong tendency toward decreased expression of CRY1 and especially of CRY2 in a variety of human cancers. Furthermore, CRY2 expression has a much greater tendency than that of CRY1 to be associated with altered activity of established oncogenic or tumor suppressive pathways.8 This suggests that CRY2 could have a more dominant role than CRY1 in altering cancer-relevant signaling pathways. Notably, these analyses used exome sequencing data compiled in The Cancer Genome Atlas (TCGA)122; the recently released Pan-cancer analysis of whole genomes (PCAWG)123 will enable investigation of noncoding variants.
TABLE 1.
Correlation of CRY1 and/ or CRY2 expression with human tumor phenotype (vs normal control tissue) and with survival outcome
| Cancer type | CRY1 | CRY2 | References |
|---|---|---|---|
| Gastric | Increased; associated with advanced stage NC | NC Decreased |
125 8 |
| Thyroid | NC Decreased |
Decreased Decreased |
126 8 |
| Glioma | NC | Increased Associated with poor outcome |
127 8 |
| Skin | Decreased NC |
Decreased |
128 42 |
| Ovarian | Increased; associated with improved survival |
Decreased | 129 |
| Pancreas (PDAC) | Decreased | Decreased | 130 |
| Colorectal | NC Decreased NC |
Decreased Decreased Decreased |
131 132 8 |
| Lung | Decreased (LUSC and LUAD) Decreased |
Decreased (LUSC and LUAD) Decreased |
8 42 |
| Kidney | Decreased in KICH | Increased (KICH), Decreased (KIRP); low expression associated with poor outcomes (KIRP, KIRC) | 8 |
| Breast | NC NC |
Decreased; alters subtype Decreased |
8 42 |
| Liver | NC | NC; low expression associated with worse outcome | 8 |
| Head and Neck | NC | Decreased | 8 |
| Bladder | NC | Decreased | 8 |
| Esophagus | Decreased | Decreased | 8 |
| Prostate | NC | NC | 8 |
| Bone | NC | Decreased | 42 |
Abbreviations: KICH, kidney chromophobe; KIRC, kidney renal clear cell carcinoma; KIRP, kidney renal papillary carcinoma; LUAD, lung adenocarcinoma; LUSC, lung squamous cell carcinoma; NC, no change; PDAC, pancreatic ductal adenocarcinoma.
Tumors tend to have reduced or absent circadian rhythmicity either due to individual oncogenes,26,27 proteins up-regulated by oncogenic transformation,28 or lack of access to peripheral synchronizing factors.124 It is unclear whether the loss of rhythmicity could confound measurements of gene expression. Furthermore, most of these studies only examined CRY1/2 expression and did not investigate whether these tumors maintained circadian rhythmicity. Typically, tumor resection was performed between 9:00 AM and 5:00 PM, but few studies precisely record the time of resection. In addition, lighting conditions are usually not well described. These factors could influence CRY1/2 expression, and the lack of such information complicates the interpretation of data from samples for which they are not recorded.
7 |. CRYPTOCHROMES MAY IMPACT DRUG EFFICACY
The idea of chronotherapy considers the time of day when the therapy is dosed to improve efficacy or to avert negative side effects or toxicity of the therapy.133 This idea suggests that the circadian clock and its core components most likely play a role in modulating therapy outcomes.
Cry1−/−; Cry2−/− mice showed less toxic side effects and were more resistant toward the anticancer drug, cyclophosphamide, compared to their WT littermates. This effect was not due to changes in cyclophosphamide metabolism and/ or detoxification rates,134 although cryptochromes have been shown to suppress other drug metabolism pathways.135 Whether chemoresistance to cyclophosphamide in Cry1−/−; Cry2−/− mice compared to WT littermates applies in a cancer mouse model or xenograft model will be an interesting area for further study.
Platinum-based chemotherapeutic agents, including cisplatin and oxaliplatin, drive cytotoxicity in dividing cells by forming adducts with nuclear DNA, and thus causing DNA damage and interfering with replication and transcription. Cryptochromes are evolutionarily related to bacterial DNA repair enzymes known as photolyases.16 While mammalian CRY1 and CRY2 lack DNA repair activity, daily oscillations in the transcript and protein levels136 of the nucleotide excision repair enzyme xeroderma pigmentosum group A (XPA) have been documented in livers extracted from mice. Furthermore, XPA and nucleotide excision repair activity are elevated in Cry1−/−;Cry2−/− livers.136 Thus, cryptochromes could suppress DNA repair in response to platinum-based chemotherapeutic treatments; if so, the timing of treatment delivery could impact its effectiveness and/ or toxicity.
In addition to modulating DNA repair activity through XPA expression, CRY1 and CRY2 can influence the cellular response to DNA damage. The apoptotic effects of oxaliplatin in RAS-transformed, Tp53−/− cells are increased by deletion of Cry1 and Cry2 due to increased expression of Egr1 and p73, but the apoptotic effects of doxorubicin are not impacted by Cry mutation.137 Consistent with the indication that CRY1 and/or CRY2 oppose the cytotoxic effect of oxaliplatin, chemoresistant colon cancer samples were found to have high expression of CRY2 and depletion of CRY2 rendered them sensitive to oxaliplatin.138 Furthermore, Tp53−/−; Cry1−/−; Cry2−/− xenografts were reportedly more sensitive to oxaliplatin treatment than Tp53−/− xenografts, due to increased expression of p73 leading to increased apoptosis in response to oxaliplatin.139 Together, these findings suggest that cryptochromes impact the efficacy of platinum-based chemotherapeutic agents. Additional investigations will be required to understand why their impact on the response to other DNA-damaging agents like doxorubicin seems to be different.
The xenobiotic receptors pregnane X receptor (PXR) and constitutive androstane receptor (CAR) are members of the nuclear hormone receptor (NR) superfamily of transcription factors.140 PXR and CAR are activated by a wide variety of lipophilic ligands including many drugs and environmental toxins. Cryptochromes interact with many NRs, including PXR and CAR, and suppress NR-driven transcriptional activation.46,135,141 Because PXR and CAR activate transcriptional networks that stimulate drug transport, activation, and metabolism, this suggests that circadian modulation of their activity by cryptochromes could alter the effectiveness and/or toxicity of clinically useful compounds in a time-of-day-dependent manner. In addition, several cancers are influenced by NR actions. Additional studies are needed to reveal whether and how cryptochromes and NRs function together to influence outcomes in hormone-dependent cancers, like breast and prostate cancers, in which estrogen, progesterone, and androgen receptors’ expression and/or mutation play an important role.142–144
8 |. CHEMICAL COMPOUNDS THAT DIRECTLY TARGET CRYPTOCHROMES
In addition to the potential for cryptochromes to alter the pharmacokinetics or efficacy of other drugs, selective modulation of cryptochromes themselves may be therapeutic. A handful of compounds that stabilize or inhibit cryptochromes have been described. The first such small molecule identified (KL001) stabilizes both CRY1 and CRY2145 by inhibiting their interaction with FBXL3.146 KL001 suppresses gluconeogenesis in hepatocytes, suggesting that pharmacological targeting of cryptochromes could be useful in metabolic disease.145,147 Many CRY-stabilizing derivatives have been made,148–150 and it was recently demonstrated that both KL001 and the derivative SHP656 selectively kill glioblastoma stem cells in vitro and in vivo.150
KS15 is a small molecule that inhibits CRY1- and CRY2-dependent repression of CLOCK and BMAL1 without affecting CRY1/2 stability.151Treatment of breast cancer cells in vitro with high concentrations of KS15 reduced their proliferation and increased their sensitivity to doxorubicin.152 It is unclear whether these reported effects of KS15 require its regulation of CRY1 and/or CRY2.
9 |. CONCLUSIONS AND OUTLOOK
Cryptochromes participate in a wide variety of cellular functions that may influence cancer growth in addition to generating circadian rhythms. While we focused on cryptochromes in this review, there are likely multiple mechanisms by which circadian clocks influence the cell cycle and cancer. Alteration in the expression of Cry or any other core clock gene undoubtedly will affect expression of other circadian proteins and their downstream targets, making it difficult to delineate the direct and indirect impacts of any perturbation of the clock. CRY1 and CRY2 are often thought to have similar functions, but it is imperative that they are investigated as separate entities because they clearly have several differentiated roles. Also, cryptochromes can function differently in different cellular pathways depending on context. Further development of cryptochromes as therapeutic targets in cancer or other diseases will require additional clinical and structure-function relationship studies. Circadian rhythms are pervasive and influence many of the widely accepted cancer hallmarks.6,11,153 Incorporating a better understanding of the role of circadian rhythms and time of day in basic and clinical cancer research will improve evidence-based recommendations for public health and clinical practice.
ACKNOWLEDGEMENTS
We thank Dr Marie Pariollaud for helpful discussions and apologize for the omission of literature that we were unable to cite due to space constraints.
Funding information
NIH grants CA211187 and ES031000 to KAL.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
REFERENCES
- 1.Partch CL, Green CB, Takahashi JS. Molecular architecture of the mammalian circadian clock. Trends Cell Biol. 2014;24(2):90–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Takahashi JS. Transcriptional architecture of the mammalian circadian clock. Nat Rev Genet. 2017;18(3):164–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ruben MD, Wu G, Smith DF, et al. A database of tissue-specific rhythmically expressed human genes has potential applications in circadian medicine. Sci Transl Med. 2018;10(458):eaat8806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang R, Lahens NF, Ballance HI, Hughes ME, Hogenesch JB. A circadian gene expression atlas in mammals: implications for biology and medicine. Proc Natl Acad Sci USA. 2014;111(45):16219–16224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mure LS, Le HD, Benegiamo G, et al. Diurnal transcriptome atlas of a primate across major neural and peripheral tissues. Science. 2018;359(6381):eaao0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. [DOI] [PubMed] [Google Scholar]
- 7.Lamia KA. Ticking time bombs: connections between circadian clocks and cancer. F1000Research. 2017;6:1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ye Y, Xiang YU, Ozguc FM, et al. The genomic landscape and pharmacogenomic interactions of clock genes in cancer chronotherapy. Cell Syst. 2018;6(3):314–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamilton T Influence of environmental light and melatonin upon mammary tumour induction. Br J Surg. 1969;56(10):764–766. [DOI] [PubMed] [Google Scholar]
- 10.Ward EM, Germolec D, Kogevinas M, et al. Carcinogenicity of night shift work. Lancet Oncol. 2019;20(8):1058–1059. [DOI] [PubMed] [Google Scholar]
- 11.Sulli G, Manoogian ENC, Taub PR, Panda S. Training the circadian clock, clocking the drugs, and drugging the clock to prevent, manage, and treat chronic diseases. Trends Pharmacol Sci. 2018;39(9):812–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Papagiannakopoulos T, Bauer M, Davidson S, et al. Circadian rhythm disruption promotes lung tumorigenesis. Cell Metab. 2016;24(2):324–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kettner NM, Voicu H, Finegold MJ, et al. Circadian homeostasis of liver metabolism suppresses hepatocarcinogenesis. Cancer Cell. 2016;30(6):909–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Dycke K, Rodenburg W, van Oostrom C, et al. Chronically alternating light cycles increase breast cancer risk in mice. Curr Biol. 2015;25(14):1932–1937. [DOI] [PubMed] [Google Scholar]
- 15.Filipski E, Levi F. Circadian disruption in experimental cancer processes. Integr Cancer Ther. 2009;8(4):298–302. [DOI] [PubMed] [Google Scholar]
- 16.Partch CL, Sancar A. Photochemistry and photobiology of cryptochrome blue-light photopigments: the search for a photocycle. Photochem Photobiol. 2005;81(6):1291–1304. [DOI] [PubMed] [Google Scholar]
- 17.Yuan Q, Metterville D, Briscoe AD, Reppert SM. Insect cryptochromes: gene duplication and loss define diverse ways to construct insect circadian clocks. Mol Biol Evol. 2007;24(4):948–955. [DOI] [PubMed] [Google Scholar]
- 18.Lamia KA, Sachdeva UM, DiTacchio L, et al. AMPK regulates the circadian clock by cryptochrome phosphorylation and degradation. Science. 2009;326(5951):437–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Foley LE, Emery P Drosophila cryptochrome: variations in blue. J Biol Rhythms. 2020;35(1):16–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oster H, Challet E, Ott V, et al. The functional and clinical significance of the 24-hour rhythm of circulating glucocorticoids. Endocr Rev. 2017;38(1):3–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gorman MR. Temporal organization of pineal melatonin signaling in mammals. Mol Cell Endocrinol. 2019;503:110687. [DOI] [PubMed] [Google Scholar]
- 22.Owino S, Buonfiglio DDC, Tchio C, Tosini G. Melatonin signaling a key regulator of glucose homeostasis and energy metabolism. Front Endocrinol. 2019;10:488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Owino S, Contreras-Alcantara S, Baba K, Tosini G. Melatonin signaling controls the daily rhythm in blood glucose levels independent of peripheral clocks. PLoS ONE. 2016;11(1):e0148214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Contreras-Alcantara S, Baba K, Tosini G. Removal of melatonin receptor type 1 induces insulin resistance in the mouse. Obesity (Silver Spring). 2010;18(9):1861–1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blask DE, Sauer LA, Dauchy RT. Melatonin as a chronobiotic/anticancer agent: cellular, biochemical, and molecular mechanisms of action and their implications for circadian-based cancer therapy. Curr Top Med Chem. 2002;2(2):113–132. [DOI] [PubMed] [Google Scholar]
- 26.Altman B, Hsieh A, Sengupta A, et al. MYC disrupts the circadian clock and metabolism in cancer cells. Cell Metab. 2015;22(6):1009–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Relógio A, Thomas P, Medina-Pérez P, et al. Ras-mediated deregulation of the circadian clock in cancer. PLoS Genet. 2014;10(5):e1004338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Michael A, Harvey S, Sammons P, et al. Cancer/Testis antigen PASD1 silences the circadian clock. Mol Cell. 2015;58(5):743–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jung-Hynes B, Huang W, Reiter RJ, Ahmad N. Melatonin resynchronizes dysregulated circadian rhythm circuitry in human prostate cancer cells. J Pineal Res. 2010;49(1):60–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kasahara T, Abe K, Mekada K, Yoshiki A, Kato T. Genetic variation of melatonin productivity in laboratory mice under domestication. Proc Natl Acad Sci USA. 2010;107(14):6412–6417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Favero G, Moretti E, Bonomini F, Reiter RJ, Rodella LF, Rezzani R. Promising antineoplastic actions of melatonin. Front Pharmacol. 2018;9:1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gauger MA, Sancar A. Cryptochrome, circadian cycle, cell cycle checkpoints, and cancer. Cancer Res. 2005;65(15):6828–6834. [DOI] [PubMed] [Google Scholar]
- 33.Lee S, Donehower LA, Herron AJ, Moore DD, Fu L. Disrupting circadian homeostasis of sympathetic signaling promotes tumor development in mice. PLoS ONE. 2010;5(6):e10995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mteyrek A, Filipski E, Guettier C, et al. Critical cholangiocarcinogenesis control by cryptochrome clock genes. Int J Cancer. 2017;140(11):2473–2483. [DOI] [PubMed] [Google Scholar]
- 35.Ozturk N, Lee JH, Gaddameedhi S, Sancar A. Loss of cryptochrome reduces cancer risk in p53 mutant mice. Proc Natl Acad Sci USA. 2009;106(8):2841–2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cavga AD, Tardu M, Korkmaz T, et al. Cryptochrome deletion in p53 mutant mice enhances apoptotic and anti-tumorigenic responses to UV damage at the transcriptome level. Funct Integr Genomics. 2019;19(5):729–742. [DOI] [PubMed] [Google Scholar]
- 37.Horst GTJVD, Muijtjens M, Kobayashi K, et al. Mammalian Cry1 and Cry2 are essential for maintenance of circadian rhythms. Nature. 1999;398(6728):627–630. [DOI] [PubMed] [Google Scholar]
- 38.Vitaterna MH, Selby CP, Todo T, et al. Differential regulation of mammalian Period genes and circadian rhythmicity by cryptochromes 1 and 2. Proc Natl Acad of Sciences. 1999;96(21):12114–12119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rosensweig C, Reynolds KA, Gao P, et al. An evolutionary hotspot defines functional differences between CRYPTOCHROMES. Nat Commun. 2018;9(1):1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Papp SJ, Huber AL, Jordan SD, et al. DNA damage shifts circadian clock time via Hausp-dependent Cry1 stabilization. eLife. 2015;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Griebel G, Ravinet-Trillou C, Beeske S, Avenet P, Pichat P. Mice deficient in cryptochrome 1 (cry1 (−/−)) exhibit resistance to obesity induced by a high-fat diet. Front Endocrinol. 2014;5:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huber A-L, Papp SJ, Chan AB, et al. CRY2 and FBXL3 cooperatively degrade c-MYC. Mol Cell. 2016;64(4):774–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Michael AK, Fribourgh JL, Van Gelder RN, Partch CL. Animal cryptochromes: divergent roles in light perception, circadian timekeeping and beyond. Photochem Photobiol. 2017;93(1):128–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scheiermann C, Gibbs J, Ince L, Loudon A. Clocking in to immunity. Nat Rev Immunol. 2018;18(7):423–437. [DOI] [PubMed] [Google Scholar]
- 45.Narasimamurthy R, Hatori M, Nayak SK, Liu F, Panda S, Verma IM. Circadian clock protein cryptochrome regulates the expression of proinflammatory cytokines. Proc Natl Acad Sci USA. 2012;109(31):12662–12667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lamia KA, Papp SJ, Yu RT, et al. Cryptochromes mediate rhythmic repression of the glucocorticoid receptor. Nature. 2011;480(7378):552–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hand LE, Hopwood TW, Dickson SH, et al. The circadian clock regulates inflammatory arthritis. FASEB journal. 2016;30(11):3759–3770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cao QI, Zhao X, Bai J, et al. Circadian clock cryptochrome proteins regulate autoimmunity. Proc Natl Acad Sci USA. 2017;114(47):12548–12553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Circadian SA. Circadian clock, cell division, and cancer: from molecules to organism. Int J Mol Sci. 2017;18(4):873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Altman BJ. Cancer clocks out for lunch: disruption of circadian rhythm and metabolic oscillation in cancer. Front Cell Dev Biol. 2016;4:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bjarnason GA, Jordan RC, Sothern RB. Circadian variation in the expression of cell-cycle proteins in human oral epithelium. Am J Pathol. 1999;154(2):613–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Horiguchi M, Koyanagi S, Hamdan AM, et al. Rhythmic control of the ARF-MDM2 pathway by ATF4 underlies circadian accumulation of p53 in malignant cells. Cancer Res. 2013;73(8):2639–2649. [DOI] [PubMed] [Google Scholar]
- 53.Honda R, Tanaka H, Yasuda H. Oncoprotein MDM2 is a ubiquitin ligase E3 for tumor suppressor p53. FEBS Lett. 1997;420(1):25–27. [DOI] [PubMed] [Google Scholar]
- 54.Haupt Y, Maya R, Kazaz A, Oren M. Mdm2 promotes the rapid degradation of p53. Nature. 1997;387(6630):296–299. [DOI] [PubMed] [Google Scholar]
- 55.Kubbutat MH, Jones SN, Vousden KH. Regulation of p53 stability by Mdm2. Nature. 1997;387(6630):299–303. [DOI] [PubMed] [Google Scholar]
- 56.Dornan D, Wertz I, Shimizu H, et al. The ubiquitin ligase COP1 is a critical negative regulator of p53. Nature. 2004;429(6987):86–92. [DOI] [PubMed] [Google Scholar]
- 57.Li M, Chen D, Shiloh A, et al. Deubiquitination of p53 by HAUSP is an important pathway for p53 stabilization. Nature. 2002;416(6881):648–653. [DOI] [PubMed] [Google Scholar]
- 58.Li M, Brooks CL, Kon N, Gu W. A dynamic role of HAUSP in the p53-Mdm2 pathway. Mol Cell. 2004;13(6):879–886. [DOI] [PubMed] [Google Scholar]
- 59.Siepka SM, Yoo S-H, Park J, et al. Circadian mutant Overtime reveals F-box protein FBXL3 regulation of cryptochrome and period gene expression. Cell. 2007;129(5):1011–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Busino L, Bassermann F, Maiolica A, et al. SCFFbxl3 controls the oscillation of the circadian clock by directing the degradation of cryptochrome proteins. Science. 2007;316(5826):900–904. [DOI] [PubMed] [Google Scholar]
- 61.Godinho SIH, Maywood ES, Shaw L, et al. The after-hours mutant reveals a role for Fbxl3 in determining mammalian circadian period. Science. 2007;316(5826):897–900. [DOI] [PubMed] [Google Scholar]
- 62.Rizzini L, Levine DC, Perelis M, Bass J, Peek CB, Pagano M. Cryptochromes-mediated inhibition of the CRL4(Cop1)-complex assembly defines an evolutionary conserved signaling mechanism. Curr Biol. 2019;29(12):1954–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhou L, Yu Y, Sun S, Zhang T, Wang M. Cry 1 regulates the clock gene network and promotes proliferation and migration via the Akt/P53/P21 pathway in human osteosarcoma cells. J Cancer. 2018;9(14):2480–2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gotoh T, Vila-Caballer M, Santos CS, Liu J, Yang J, Finkielstein CV. The circadian factor Period 2 modulates p53 stability and transcriptional activity in unstressed cells. Mol Biol Cell. 2014;25(19):3081–3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gotoh T, Vila-Caballer M, Liu J, Schiffhauer S, Finkielstein CV. Association of the circadian factor Period 2 to p53 influences p53’s function in DNA-damage signaling. Mol Biol Cell. 2015;26(2):359–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gotoh T, Kim JK, Liu J, et al. Model-driven experimental approach reveals the complex regulatory distribution of p53 by the circadian factor Period 2. Proc Natl Acad Sci USA. 2016;113(47):13516–13521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kent LN, Leone G. The broken cycle: E2F dysfunction in cancer. Nat Rev Cancer. 2019;19(6):326–338. [DOI] [PubMed] [Google Scholar]
- 68.Lee Y, Lahens NF, Zhang S, Bedont J, Field JM, Sehgal A. G1/S cell cycle regulators mediate effects of circadian dysregulation on tumor growth and provide targets for timed anticancer treatment. PLoS Biol. 2019;17(4):e3000228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chan AB, Huber AL, Lamia KA. Cryptochromes modulate E2F family transcription factors. Sci Rep. 2020;10(1):4077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Boudeau J, Sapkota G, Alessi DR. LKB1, a protein kinase regulating cell proliferation and polarity. FEBS Lett. 2003;546(1):159–165. [DOI] [PubMed] [Google Scholar]
- 71.Hardie DG, Alessi DR. LKB1 and AMPK and the cancer-metabolism link - ten years after. BMC Biol. 2013;11:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Goodwin JM, Svensson RU, Lou HJ, Winslow MM, Turk BE, Shaw RJ. An AMPK-independent signaling pathway downstream of the LKB1 tumor suppressor controls Snail1 and metastatic potential. Mol Cell. 2014;55(3):436–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xing W, Busino L, Hinds TR, et al. SCF(FBXL3) ubiquitin ligase targets cryptochromes at their cofactor pocket. Nature. 2013;496(7443):64–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Samuel SM, Varghese E, Kubatka P, Triggle CR, Metformin BD. The answer to cancer in a flower? Current knowledge and future prospects of metformin as an anti-cancer agent in breast, Cancer. Biomolecules 2019;9(12):846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Henriksson E, Huber A-L, Soto EK, et al. The liver circadian clock modulates biochemical and physiological responses to metformin. J Biol Rhythms. 2017;32(4):345–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Awasthi P, Foiani M, Kumar A. ATM and ATR signaling at a glance. J Cell Sci. 2015;128(23):4255–4262. [DOI] [PubMed] [Google Scholar]
- 77.Sehgal A, Price JL, Man B, Young MW. Loss of circadian behavioral rhythms and per RNA oscillations in the Drosophila mutant timeless. Science. 1994;263(5153):1603–1606. [DOI] [PubMed] [Google Scholar]
- 78.Gotter AL, Manganaro T, Weaver DR, et al. A time-less function for mouse timeless. Nat Neurosci. 2000;3(8):755–756. [DOI] [PubMed] [Google Scholar]
- 79.Kurien P, Hsu P-K, Leon J, et al. TIMELESS mutation alters phase responsiveness and causes advanced sleep phase. Proc Natl Acad Sci USA. 2019;116(24):12045–12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Barnes JW, Tischkau SA, Barnes JA, et al. Requirement of mammalian Timeless for circadian rhythmicity. Science. 2003;302(5644):439–442. [DOI] [PubMed] [Google Scholar]
- 81.Engelen E, Janssens RC, Yagita K, Smits VA, van der Horst GT, Tamanini F. Mammalian TIMELESS is involved in period determination and DNA damage-dependent phase advancing of the circadian clock. PLoS ONE. 2013;8(2):e56623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kang TH, Leem SH. Modulation of ATR-mediated DNA damage checkpoint response by cryptochrome 1. Nucleic Acids Res. 2014;42(7):4427–4434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Unsal-Kacmaz K, Mullen TE, Kaufmann WK, Sancar A. Coupling of human circadian and cell cycles by the timeless protein. Mol Cell Biol. 2005;25(8):3109–3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ünsal-Kaçmaz K, Chastain PD, Qu P-P, et al. The human Tim/ Tipin complex coordinates an Intra-S checkpoint response to UV that slows replication fork displacement. Mol Cell Biol. 2007;27(8):3131–3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gao P, Yoo S-H, Lee K-J, et al. Phosphorylation of the cryptochrome 1 C-terminal tail regulates circadian period length. J Biol Chem. 2013;288(49):35277–35286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Leman AR, Noguchi C, Lee CY, Noguchi E. Human timeless and tipin stabilize replication forks and facilitate sister-chromatid cohesion. J Cell Sci. 2010;123(Pt 5):660–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sillje HH, Takahashi K, Tanaka K, Van Houwe G, Nigg EA. Mammalian homologues of the plant Tousled gene code for cell-cycle-regulated kinases with maximal activities linked to ongoing DNA replication. EMBO J. 1999;18(20):5691–5702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kim J-A, Tan Y, Wang X, et al. Comprehensive functional analysis of the tousled-like kinase 2 frequently amplified in aggressive luminal breast cancers. Nat Commun. 2016;7:12991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Correia SP, Chan AB, Vaughan M, et al. The circadian E3 ligase complex SCF(FBXL3+CRY) targets TLK2. Sci Rep. 2019;9(1):198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Matsuo T, Yamaguchi S, Mitsui S, Emi A, Shimoda F, Okamura H. Control mechanism of the circadian clock for timing of cell division in vivo. Science. 2003;302(5643):255–259. [DOI] [PubMed] [Google Scholar]
- 91.Geyfman M, Kumar V, Liu Q, et al. Brain and muscle Arnt-like protein-1 (BMAL1) controls circadian cell proliferation and susceptibility to UVB-induced DNA damage in the epidermis. Proc Natl Acad Sci USA. 2012;109(29):11758–11763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kowalska E, Ripperger JA, Hoegger DC, et al. NONO couples the circadian clock to the cell cycle. Proc Natl Acad Sci USA. 2013;110(5):1592–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hong CI, Zamborszky J, Baek M, et al. Circadian rhythms synchronize mitosis in Neurospora crassa. Proc Natl Acad Sci USA. 2014;111(4):1397–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bieler J, Cannavo R, Gustafson K, Gobet C, Gatfield D, Naef F. Robust synchronization of coupled circadian and cell cycle oscillators in single mammalian cells. Mol Syst Biol. 2014;10:739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dang CV. MYC on the path to cancer. Cell. 2012;149(1):22–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bretones G, Delgado MD, Leon J. Myc and cell cycle control. Biochim Biophys Acta. 2015;1849(5):506–516. [DOI] [PubMed] [Google Scholar]
- 97.Crusio KM, King B, Reavie LB, Aifantis I. The ubiquitous nature of cancer: the role of the SCF(Fbw7) complex in development and transformation. Oncogene. 2010;29(35):4865–4873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Reitsma JM, Liu X, Reichermeier KM, et al. Composition and Regulation of the Cellular Repertoire of SCF Ubiquitin Ligases. Cell. 2017;171(6):1326–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yu Y, Li Y, Zhou L, Yang G, Wang M, Hong Y. Cryptochrome 2 (CRY2) suppresses proliferation and migration and regulates clock gene network in osteosarcoma cells. Med Sci Monit. 2018;24:3856–3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Onoyama I, Tsunematsu R, Matsumoto A, et al. Conditional inactivation of Fbxw7 impairs cell-cycle exit during T cell differentiation and results in lymphomatogenesis. J Exp Med. 2007;204(12):2875–2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Matsuoka S, Oike Y, Onoyama I, et al. Fbxw7 acts as a critical fail-safe against premature loss of hematopoietic stem cells and development of T-ALL. Genes Dev. 2008;22(8):986–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Davis RJ, Welcker M, Clurman BE. Tumor suppression by the Fbw7 ubiquitin ligase: mechanisms and opportunities. Cancer Cell. 2014;26(4):455–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shostak A, Ruppert B, Diernfellner A, Brunner M. Correspondence: Reply to ‘Oncogenic MYC persistently up-regulates the molecular clock component REV-ERBalpha’. Nat Commun. 2017;8:14918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Shostak A, Ruppert B, Ha N, et al. MYC/MIZ1-dependent gene repression inversely coordinates the circadian clock with cell cycle and proliferation. Nat Commun. 2016;7:11807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.El-Athman R, Genov NN, Mazuch J, et al. The Ink4a/Arf locus operates as a regulator of the circadian clock modulating RAS activity. PLoS Biol. 2017;15(12):e2002940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Katamune C, Koyanagi S, Shiromizu S, et al. Different roles of negative and positive components of the circadian clock in oncogene-induced neoplastic transformation. J Biol Chem. 2016;291(20):10541–10550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hansen J, Stevens RG. Case-control study of shift-work and breast cancer risk in Danish nurses: impact of shift systems. Eur J Cancer. 2012;48(11):1722–1729. [DOI] [PubMed] [Google Scholar]
- 108.Knutsson A, Hammar N, Karlsson B. Shift workers’ mortality scrutinized. Chronobiol Int. 2004;21(6):1049–1053. [DOI] [PubMed] [Google Scholar]
- 109.Karlsson B, Alfredsson L, Knutsson A, Andersson E, Toren K. Total mortality and cause-specific mortality of Swedish shift- and dayworkers in the pulp and paper industry in 1952–2001. Scand J Work Environ Health. 2005;31(1):30–35. [DOI] [PubMed] [Google Scholar]
- 110.Straif K, Baan R, Grosse Y, et al. Carcinogenicity of shift-work, painting, and fire-fighting. Lancet Oncol. 2007;8(12):1065–1066. [DOI] [PubMed] [Google Scholar]
- 111.Hansen J, Lassen CF. Nested case-control study of night shift work and breast cancer risk among women in the Danish military. Occup Environ Med. 2012;69(8):551–556. [DOI] [PubMed] [Google Scholar]
- 112.Papantoniou K, Castaño-Vinyals G, Espinosa A, et al. Night shift work, chronotype and prostate cancer risk in the MCC-Spain case-control study. Int J Cancer. 2015;137(5):1147–1157. [DOI] [PubMed] [Google Scholar]
- 113.Fenga C Occupational exposure and risk of breast cancer. Biomed Rep. 2016;4(3):282–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wegrzyn LR, Tamimi RM, Rosner BA, et al. Rotating night-shift work and the risk of breast cancer in the nurses’ health studies. Am J Epidemiol. 2017;186(5):532–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gu F, Xu S, Devesa SS, et al. Longitude Position in a Time Zone and Cancer Risk in the United States. Cancer Epidemiol Biomark Prev. 2017;26(8):1306–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Borisenkov MF. Latitude of residence and position in time zone are predictors of cancer incidence, cancer mortality, and life expectancy at birth. Chronobiol Int. 2011;28(2):155–162. [DOI] [PubMed] [Google Scholar]
- 117.Chu LW, Zhu Y, Yu K, et al. Variants in circadian genes and prostate cancer risk: a population-based study in China. Prostate Cancer Prostatic Dis. 2008;11(4):342–348. [DOI] [PubMed] [Google Scholar]
- 118.Zhu Y, Stevens RG, Hoffman AE, et al. Testing the circadian gene hypothesis in prostate cancer: a population-based case-control study. Cancer Res. 2009;69(24):9315–9322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lin DW, FitzGerald LM, Fu R, et al. Genetic variants in the LEPR, CRY1, RNASEL, IL4, and ARVCF genes are prognostic markers of prostate cancer-specific mortality. Cancer Epidemiol Biomarkers Prev. 2011;20(9):1928–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hoffman AE, Zheng T, Stevens RG, et al. Clock-cancer connection in non-Hodgkin’s lymphoma: a genetic association study and pathway analysis of the circadian gene cryptochrome 2. Cancer Res. 2009;69(8):3605–3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Dai H, Zhang L, Cao M, et al. The role of polymorphisms in circadian pathway genes in breast tumorigenesis. Breast Cancer Res Treat. 2011;127(2):531–540. [DOI] [PubMed] [Google Scholar]
- 122.Weinstein JN, Collisson EA, Mills GB, et al. The cancer genome atlas pan-cancer analysis project. Nat Genet. 2013;45(10):1113–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Consortium ITP-CAoWG. Pan-cancer analysis of whole genomes. Nature. 2020;578(7793):82–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Morgan MN, Dvuchbabny S, Martinez C-A, Kerr B, Cistulli PA, Cook KM. Ticking: links between circadian rhythms and cancer. Clocks Sleep. 2019;1(4):435–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hu M-L, Yeh K-T, Lin P-M, et al. Deregulated expression of circadian clock genes in gastric cancer. BMC Gastroenterol. 2014;14:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mannic T, Meyer P, Triponez F, et al. Circadian clock characteristics are altered in human thyroid malignant nodules. J Clin Endocrinol Metab. 2013;98(11):4446–4456. [DOI] [PubMed] [Google Scholar]
- 127.Luo Y, Wang F, Chen L-A, et al. Deregulated expression of cry1 and cry2 in human gliomas. Asian Pac J Cancer Prev. 2012;13(11):5725–5728. [DOI] [PubMed] [Google Scholar]
- 128.Lengyel Z, Lovig C, Kommedal S, et al. Altered expression patterns of clock gene mRNAs and clock proteins in human skin tumors. Tumour Biol. 2013;34(2):811–819. [DOI] [PubMed] [Google Scholar]
- 129.Tokunaga H, Takebayashi Y, Utsunomiya H, et al. Clinicopathological significance of circadian rhythm-related gene expression levels in patients with epithelial ovarian cancer. Acta Obstet Gynecol Scand. 2008;87(10):1060–1070. [DOI] [PubMed] [Google Scholar]
- 130.Relles D, Sendecki J, Chipitsyna G, Hyslop T, Yeo CJ, Arafat HA. Circadian gene expression and clinicopathologic correlates in pancreatic cancer. J Gastrointest Surg. 2013;17(3):443–450. [DOI] [PubMed] [Google Scholar]
- 131.Mazzoccoli G, Panza A, Valvano MR, et al. Clock gene expression levels and relationship with clinical and pathological features in colorectal cancer patients. Chronobiol Int. 2011;28(10):841–851. [DOI] [PubMed] [Google Scholar]
- 132.Mazzoccoli G, Colangelo T, Panza A, et al. Deregulated expression of cryptochrome genes in human colorectal cancer. Mol Cancer. 2016;15:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ballesta A, Innominato PF, Dallmann R, Rand DA, Levi FA. Systems Chronotherapeutics. Pharmacol Rev. 2017;69(2):161–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Gorbacheva VY, Kondratov RV, Zhang R, et al. Circadian sensitivity to the chemotherapeutic agent cyclophosphamide depends on the functional status of the CLOCK/BMAL1 transactivation complex. Proc Natl Acad Sci USA. 2005;102(9):3407–3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kriebs A, Jordan SD, Soto E, et al. Circadian repressors CRY1 and CRY2 broadly interact with nuclear receptors and modulate transcriptional activity. Proc Natl Acad Sci USA. 2017;114(33):8776–8781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kang TH, Lindsey-Boltz LA, Reardon JT, Sancar A. Circadian control of XPA and excision repair of cisplatin-DNA damage by cryptochrome and HERC2 ubiquitin ligase. Proc Natl Acad Sci USA. 2010;107(11):4890–4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lee JH, Gaddameedhi S, Ozturk N, Ye R, Sancar A. DNA damage-specific control of cell death by cryptochrome in p53-mutant ras-transformed cells. Can Res. 2013;73(2):785–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Fang L, Yang Z, Zhou J, et al. Circadian clock gene CRY2 degradation is involved in chemoresistance of colorectal cancer. Mol Cancer Ther. 2015;14(6):1476–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lee JH, Sancar A. Circadian clock disruption improves the efficacy of chemotherapy through p73-mediated apoptosis. Proc Natl Acad Sci USA. 2011;108(26):10668–10672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Evans RM, Mangelsdorf DJ. Nuclear Receptors, RXR, and the Big Bang. Cell. 2014;157(1):255–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Jordan SD, Kriebs A, Vaughan M, et al. CRY1/2 Selectively Repress PPARdelta and Limit Exercise Capacity. Cell Metab. 2017;26(1):pp. 243–255 e246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lesicka M, Jabłońska E, Wieczorek E, et al. Altered circadian genes expression in breast cancer tissue according to the clinical characteristics. PLoS ONE. 2018;13(6):e0199622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Doan TB, Graham JD, Clarke CL. Emerging functional roles of nuclear receptors in breast cancer. J Mol Endocrinol. 2017;58(3):R1 69–R190. [DOI] [PubMed] [Google Scholar]
- 144.Huang H, Tindall DJ. The role of the androgen receptor in prostate cancer. Crit Rev Eukaryot Gene Expr. 2002;12(3):193–207. [DOI] [PubMed] [Google Scholar]
- 145.Hirota T, Lee JW, St. John PC, et al. Identification of small molecule activators of cryptochrome. Science. 2012;337(6098):1094–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Nangle S, Xing W, Zheng N. Crystal structure of mammalian cryptochrome in complex with a small molecule competitor of its ubiquitin ligase. Cell Res. 2013;23(12):1417–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Humphries PS, Bersot R, Kincaid J, et al. Carbazole-containing amides and ureas: Discovery of cryptochrome modulators as antihyperglycemic agents. Bioorg Med Chem Lett. 2018;28(3):293–297. [DOI] [PubMed] [Google Scholar]
- 148.Oshima T, Yamanaka I, Kumar A, et al. C-H activation generates period-shortening molecules that target cryptochrome in the mammalian circadian clock. Angew Chem Int Ed Engl. 2015;54(24):7193–7197. [DOI] [PubMed] [Google Scholar]
- 149.Lee JW, Hirota T, Kumar A, Kim NJ, Irle S, Kay SA. Development of Small-Molecule Cryptochrome Stabilizer Derivatives as Modulators of the Circadian Clock. ChemMedChem. 2015;10(9):1489–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Dong Z, Zhang G, Qu M, et al. Targeting glioblastoma stem cells through disruption of the circadian clock. Cancer Discov. 2019;9(11):1556–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Chun SK, Jang J, Chung S, et al. Identification and validation of cryptochrome inhibitors that modulate the molecular circadian clock. ACS Chem Biol. 2014;9(3):703–710. [DOI] [PubMed] [Google Scholar]
- 152.Chun SK, Chung S, Kim H-D, et al. A synthetic cryptochrome inhibitor induces anti-proliferative effects and increases chemosensitivity in human breast cancer cells. Biochem Biophys Res Commun. 2015;467(2):441–446. [DOI] [PubMed] [Google Scholar]
- 153.Shafi AA, Knudsen KE. Cancer and the circadian clock. Cancer Res. 2019;79(15):3806–3814. [DOI] [PMC free article] [PubMed] [Google Scholar]


