Abstract
As the incidence of cutaneous malignancies continues to rise and their treatment with immunotherapy expands, dermatologists and their patients are more likely to encounter these agents. While blockade of immune checkpoint target proteins (CTLA-4, PD-1, PD-L1) generates an antitumor response in a substantial fraction of patients, there is a critical need for reliable predictive biomarkers, as well as approaches to address refractory disease. This article reviews the indications, efficacy, safety profile and evidence supporting checkpoint inhibition as therapeutics for metastatic melanoma, cutaneous squamous cell carcinoma, and Merkel cell carcinoma. Pivotal studies resulting in the approval of ipilimumab, pembrolizumab, nivolumab, cemiplimab and avelumab by regulatory agencies for various cutaneous malignancies, as well as ongoing clinical research trials, are discussed.
INTRODUCTION
Immunotherapy has become a cornerstone of advanced tumor management. Via inhibition of the cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), programmed cell death-1 (PD-1), and programmed cell death ligand-1 (PD-L1), tumor cells are targeted and indirectly destroyed by activated T cells that infiltrate the tumor microenvironment. The first of the immune checkpoint inhibitors (CPIs) approved was ipilimumab [Yervoy®]; an additional four (nivolumab [Opdivo®], pembrolizumab [Keytruda®], cemiplimab [Libtayo®], and avelumab [Bavencio®] are approved by regulatory agencies for cutaneous malignancies. In addition to melanoma, CPIs are indicated for cutaneous squamous cell carcinoma (cSCC) and Merkel cell carcinoma (MCC). There are currently no CPIs approved for basal cell carcinoma (BCC), cutaneous lymphomas, cutaneous sarcomas, or cutaneous adnexal carcinomas (CACs).
Mechanism of action of immune checkpoint inhibitors
Ipilimumab works by blocking the negative regulator CTLA-4, resulting in increased T helper cells and decreased regulatory T cell (Treg) immunosuppressive activity.1 Pembrolizumab and nivolumab selectively block PD-1 receptors and suppress their expression by activated T cells, B cells, monocytes, and natural killer cells.2 Atezolizumab, avelumab, and durvalumab inhibit binding of PD-L1 to PD-1 receptors on T cells, thereby resulting in downregulation of T cell quiescence and reinvigoration of the antitumor immune response3 (Fig. 1).
Figure 1.
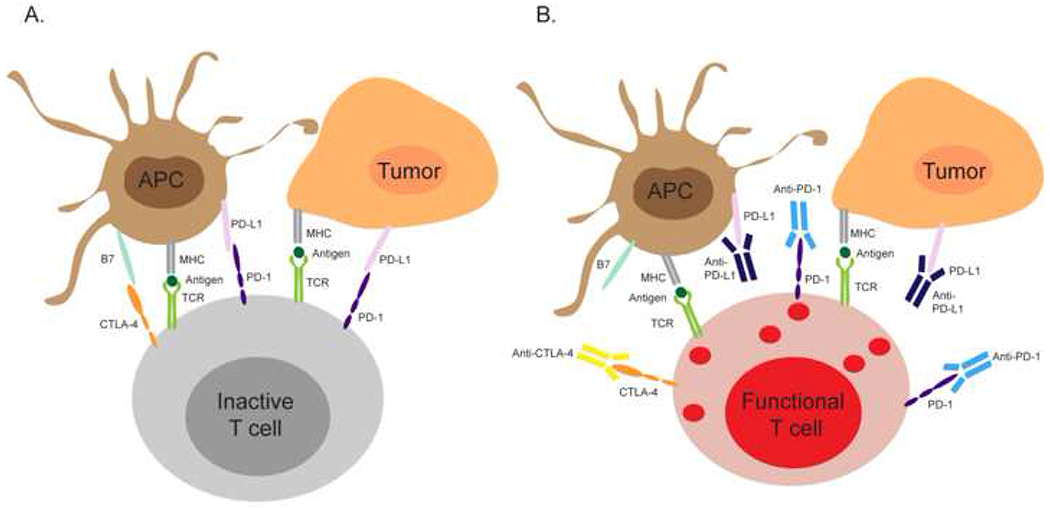
Immune checkpoint inhibitors reinvigorate antitumor immune responses.(A) Cytotoxic T cells in the tumor microenvironments express high level of inhibitory receptors such as CTLA-4 and PD-1. In the absence of immune checkpoint inhibitors, ligation of CTLA-4 and PD-1 by B7 or PD-L1 expressed by antigen presenting cells or tumor cells dampens the cytotoxic functions of T cells and inhibits their antitumor activity. (B) Anti-CTLA-4, anti-PD-1, and anti-PD-L1 can bind CTLA-4, PD-1, and PD-L1 and prevent the PD-1/PD-L1 and CTLA-4/B7 interactions, which restore the antitumor functions of cytotoxic T cells. Abbreviations: APC, antigen presenting cell; MHC, major histocompatibility complex; TCR, T-cell receptor; CTLA-4, cytotoxic T-lymphocyte-associated protein-4; PD-1, programmed cell death-1; PD-L1, programmed cell death ligand-1; B7, B7 protein.
Predictive biomarkers of response to immunotherapy
Markers of tumor response to immunotherapy have been investigated;4 and while some have been associated with increased overall survival (OS) in patients with melanoma, none have been validated. In accordance with the National Comprehensive Cancer Network (NCCN) Guidelines®, PD-L1 has potential utility in identifying melanoma patients who are more likely to respond to CPIs;5,6 however, the routine use of PD-L1 expression is not recommended for treatment decisions.5,7 Several additional immunotherapy biomarkers are under development for melanoma, including relative eosinophils, relative basophils, absolute monocytes, lactate dehydrogenase, and neutrophil-to-lymphocyte ratio.8–10 The occurrence of immune-related adverse events (irAEs) has also been implicated as potentially useful in tumor response to CPIs.11 In addition, a decrease in regulatory T-cells and an increase in activated CD8 positive cells have been cited.12–14 In advanced cSCC, although PD-L1 appears to be increased in high risk cSCC compared to normal skin specimens, its levels do not appear to correlate with the antitumor activity of PD-1 blockade.15–17 However, a higher tumor mutational burden is more commonly observed in immunocompromised cSCC patients.18–20 No predictors of response of MCC to CPIs are available yet.
MELANOMA
Key points
Ipilimumab, pembrolizumab, and nivolumab are approved for advanced melanoma.
In melanoma, combination therapy with nivolumab and ipilimumab results in higher OS compared to ipilimumab alone.
Nivolumab and pembrolizumab have each shown superior OS, with a better safety profile than ipilimumab.
Melanoma of the skin, despite its lower prevalence compared to other cutaneous malignancies, is one of the most aggressive forms of cancer. Non-invasive melanoma (melanoma in situ) has a good surgical prognosis; however, advanced melanoma lacks curative treatment options. Three CPIs are currently available to treat advanced melanoma: ipilimumab, nivolumab, and pembrolizumab.
Ipilimumab: anti-CTLA-4 therapy for advanced melanoma
Based on the improved OS results of the MDX010-20 phase 3 trial (Table I), ipilimumab (anti-CTLA-4) was approved in 2011, becoming the first CPI to be indicated for the treatment of nonresectable or metastatic melanoma (Fig. 2).21 Ipilimumab was found to elicit a dose-dependent effect on efficacy and safety measures, lending support to further studies at a dose of 10 mg/kg.22 However, while the 10 mg/kg dosing regimen of ipilimumab does result in significantly longer OS than does ipilimumab 3 mg/kg, it also leads to an increased frequency of treatment-related adverse events (TRAEs).23 In 2015, as significantly improved recurrence-free survival (RFS) for patients with completely resected high-risk stage III melanoma was observed in the EORTC 18071 phase 3 trial, ipilimumab was approved for this indication (Fig. 2). Significantly higher rates of RFS, OS, and distant metastasis-free survival (DMFS) compared to placebo were observed; 24–26 and the frequency of irAEs (Table I) was consistent with that observed in advanced melanoma.21,26 However, the adverse event (AE) profile was worse in the EORTC trial than in the MDX010-20 trial, in particular for endocrinopathies.
Table I.
Major studies investigating ipilimumab [Yervoy®] (anti-CTLA-4 immunotherapy) to treat melanoma
| Enrollment period | Trial phase/Identifier(s) | Patients | Randomization / Dosing regimen(s) | Primary endpoint(s) / Results | Median follow-up duration | Common severe (grade 3-5) irAEs: |
|---|---|---|---|---|---|---|
| 2004-2008 | Phase 3, MDX-010, NCT00094653 | Previously treated, unresectable stage III or IV melanoma patients, n=676 | Ipilimumab 3 mg/kg + gp100 every 3 weeks, for 4 treatments, n=403 Ipilimumab 3 mg/kg alone every 3 weeks for 4 treatments, n=137 gp100 alone every 3 weeks for 4 treatments, n=136 |
OS: Ipilimumab alone, 10.1 mo. Ipilimumab + gp100, 10 mo. gp100 alone, 6.4 mo. |
Ipilimumab alone: 27.8 mo. Ipilimumab + gp100: 21 mo. gp100 alone: 17.2 mo. |
Ipilimumab (+/− gp100): 10-15% gp100 alone: 3% |
| 2008-2011 | Phase 3, EORTC 18071, NCT00636168 | Previously untreated resected stage III cutaneous melanoma patients, n=951 | Ipilimumab, 10 mg/kg every 3 weeks for 4 doses; then every 3 months for up to 3 years, n=475 Placebo every 3 weeks for 4 doses; then every 3 months for up to 3 years, n=476 |
RFS: Ipilimumab: 26.1 mo. Placebo: 17.1 mo. 3-year RFS: Ipilimumab: 46.5% Placebo: 34.8% |
2.74 years | Ipilimumab vs. placebo: GI: 16% vs. <1% Hepatic: 11% vs. <1% Endocrine: 8% vs. 0% |
Abbreviations: glycoprotein 100 peptide vaccine (gp100); Overall survival (OS); Recurrence free survival (RFS)
Figure 2.
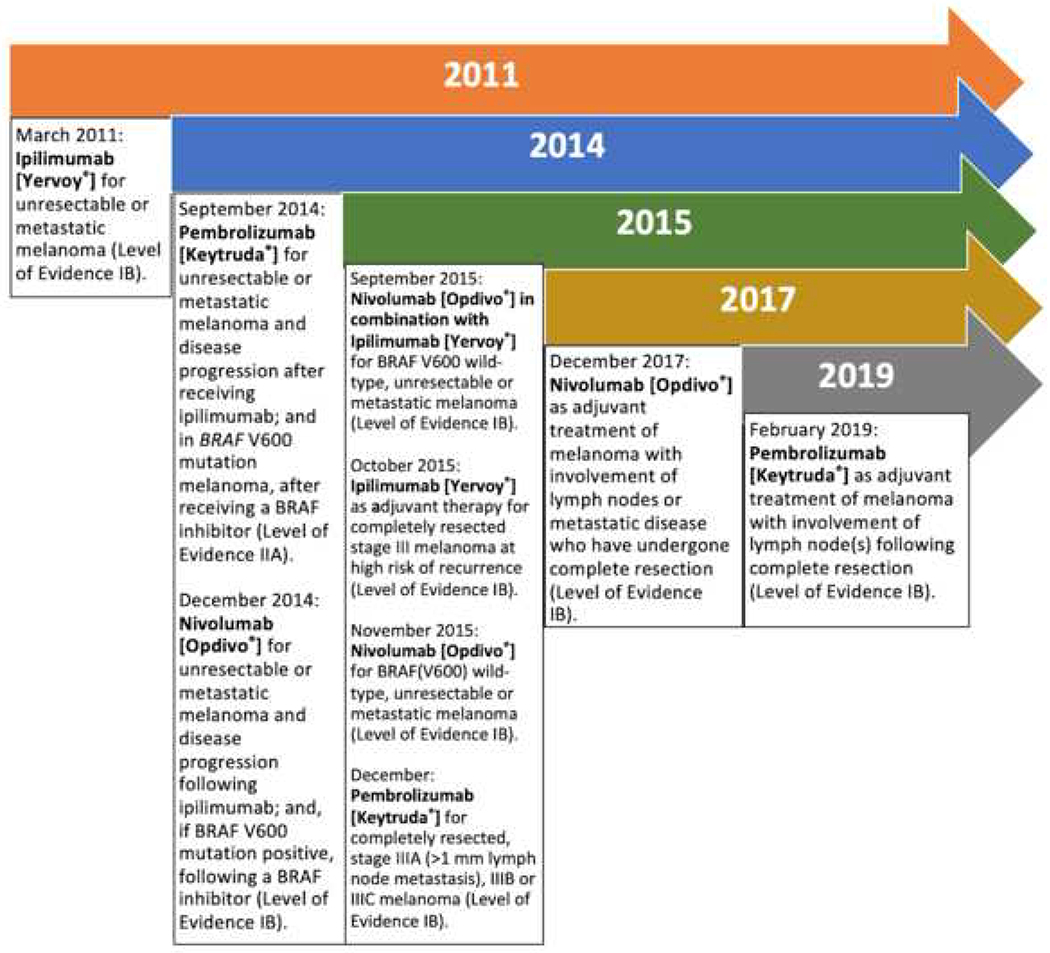
Timeline history of approved immune-checkpoint inhibitors to treat melanoma
Level IA evidence includes evidence from meta-analysis of randomized controlled trials.
level IB evidence includes evidence from at least one randomized controlled trial.
Level IIA evidence includes evidence from at least one controlled study without randomization.
Level IIB evidence includes evidence from at least one other type of experimental study.
Level III evidence includes evidence from nonexperimental descriptive studies (i.e. comparative, correlation & case-control).
Level IV evidence includes evidence from expert committee reports or opinions or clinical experience of respected authorities, or both.
Pembrolizumab: anti-PD-1 therapy for advanced melanoma
In September 2014, pembrolizumab was the first PD-1 inhibitor approved for patients with unresectable or ipilimumab-refractory advanced melanoma following treatment with a BRAF inhibitor if positive for the BRAF V600 mutation (Fig. 2).27 The phase 1 trial demonstrated that pembrolizumab was safe and efficacious at both doses of 2 mg/kg and 10 mg/kg every 3 weeks (Table II).28 In December 2015, based on the results of the phase 3 KEYNOTE-006 trial, which showed a substantial prolonged OS, progression-free survival (PFS), and less high-grade toxicity than did ipilimumab (Table II),29 the United States Food and Drug Administration (FDA) expanded the approval to include frontline treatment of patients with advanced melanoma with pembrolizumab regardless of BRAF status (Fig. 2). In February 2019, as per impactful results from the EORTC1325 / KEYNOTE-054 phase 3 trial showing improved RFS of pembrolizumab over placebo (Table II),30 pembrolizumab was approved for the adjuvant treatment of high-risk stage III melanoma patients with resected lymph nodes (Fig. 2).
Table II.
Major studies investigating pembrolizumab [Keytruda®] (anti-PD-1 immunotherapy) to treat meanoma
| Enrollment period | Trial phase/Identifier | Patients | Randomization / Dosing regimen(s) | Primary endpoint / Results | Median follow-up duration | Common severe (grade 3-5) irAEs: |
|---|---|---|---|---|---|---|
| 2012-2013 | Phase 1, KEYNOTE-001, NCT01295827 | Previously treated, ipilimuma-brefractory advanced melanoma, n=173 | Pembrolizumab 2 mg/kg every 3 weeks, n=89 Pembrolizumab 10 mg/kg every 3 weeks, n=84 |
ORR: Pembrolizumab 2 mg/kg: 26% Pembrolizumab 10 mg/kg: 26% |
8 mo. | Pembrolizumab 2 mg/kg: 3% Pembrolizumab 10 mg/kg: 0% |
| 2013-2014 | Phase 3, KEYNOTE-006, NCT01866319 | Previously treated and untreated (65.8%) advanced melanoma patients, n=834 | Pembrolizumab 10 mg/kg every 2 weeks, n=279 Pembrolizumab 10 mg/kg every 3 weeks, n=277 Ipilimumab 3 mg/kg (4 doses) every 3 weeks, n=278 |
6 mo-PFS, 12-mo OS, RR: Pembrolizumab 10 mg/kg every 2 weeks: 47.3%, 74.1%, 33.7% Pembrolizumab 10 mg/kg every 3 weeks: 46.4%, 68.4%, 32.9% Ipilimumab 3 mg/kg (4 doses) every 3 weeks: 26.5%, 58.2%, 11.9% |
7.9 mo. | Pembrolizumab 10 mg/kg every 2 weeks: 13.3% Pembrolizumab 10 mg/kg every 3 weeks: 10.1% Ipilimumab 3 mg/kg (4 doses) every 3 weeks: 19.9% |
| 2015-2016 | Phase 3, EORTC132, KEYNOTE-054, NCT02362594 | Previously treated, completely resected stage III melanoma patients, n=1019 PD-L1 positive subgroup, n=853 |
Pembrolizumab 200 mg every 3 weeks for a total of 18 doses (~1 year), n=514 Placebo every 3 weeks for a total of 18 doses (~1 year), n=505 |
RFS in overall intention to treat group: Pembrolizumab: 75.4% Placebo: 61.0% 1-year rate of RFS in PD-L1 positive subgroup: Pembrolizumab: 77.1% Placebo: 62.6% |
15 mo. | Pembrolizumab: Pembrolizumab: 14.7% Placebo: 3.4% |
Abbreviations: Overall survival (OS); Recurrence free survival (RFS); Objective response rate (ORR); Progression free survival (PFS); Response rate (RR)
Nivolumab: anti-PD-1 therapy for advanced melanoma
Following the results of the CHECKMATE-037 phase 3 trial31 (Table III), in which nivolumab led to a greater proportion of confirmed objective responses and fewer toxic effects compared to chemotherapy in patients with ipilimumab- and BRAF inhibitor-refractory melanoma, the FDA granted accelerated approval in December 201432 (Fig. 2). The following year, after a favorable benefit-risk profile associated with significant improvements in OS and PFS (as compared with dacarbazine) was demonstrated by the phase 3 trial33 (Table III), nivolumab received additional FDA approval as first-line single agent treatment of patients with BRAF(V600) wild-type, unresectable or metastatic melanoma34 (Fig. 2).
Table III.
Major studies investigating nivolumab [Opdivo®] (anti-PD-1 immunotherapy) to treat melanoma
| Enrollment period | Trial phase/Identifier | Patients | Randomization / Dosing regimen(s) | Primary endpoint / Results | Median follow-up | Common severe (grade 3-5) irAEs: |
|---|---|---|---|---|---|---|
| 2012-2014 | Phase 3, CheckMate 037, NCT01721746 | Previously treated, unresectable or metastatic ipilimumab-refractory melanoma; or (if BRAF V600 mutation-positive) ipilimumab + BRAF inhibitor-refractory melanoma, n=631 | Nivolumab 3 mg/kg every 2 weeks, n=272 Chemotherapy (dacarbazine 1000 mg/m2 every 3 weeks or paclitaxel 175 mg/m2 combined with carboplatin area under the curve 6 every 3 weeks), n=133 |
OR: Nivolumab (n=120): 37.1% Chemotherapy (n=47): 10.6% |
8.4 mo. | Nivolumab: 5% Chemotherapy: 9% |
| 2013-2014 | Checkmate 066, NCT01721772 | Previously untreated melanoma without BRAF mutation, n=418 | Nivolumab 3 mg/kg every 2 weeks and dacarbazinematched placebo every 3 weeks, n=210 Dacarbazine 1000 mg/m^2 BSA every 3 weeks and nivolumabmatched placebo every 2 weeks, n=208 |
1-year-OS: Nivolumab: 72.9% Dacarbazine: 42.1% |
Nivolumab: 8.9 mo. Dacarbazine: 6.8 mo |
Nivolumab: 11.7% Dacarbazine: 17.6% |
| 2015 | Phase 3, Checkmate 238, NCT02388906 | Completely resected, advanced (stage IIIb, IIIc or IV) melanoma patients, n=906 | Nivolumab 3 mg/kg every 2 weeks, n=453 Ipilimumab, 10 mg/kg every 3 weeks for 4 doses; then every 12 weeks, n=453 |
RFS in overall intention to treat group: Nivolumab: 70.5% Ipilimumab: 60.8% |
18 mo. | Nivolumab: 14.4% Ipilimumab: 45.9% |
Abbreviations: Investigator’s choice of chemotherapy (ICC); body surface area (BSA)
In December 2017, as further improvements in RFS and a lower rate of grade 3 or 4 AEs were seen in the CHECKMATE-238 phase 3 trial of 906 patients with resectable high risk and advanced melanoma35 (Table III), nivolumab was approved as adjuvant therapy (Fig. 2). Since then, long-term favorable efficacy and tolerability perseveres in patients with advanced or recurrent melanoma who were treated with nivolumab, irrespective of melanoma type,36 with or without BRAF mutations.37,38
Nivolumab plus ipilimumab: combination therapy for advanced melanoma
In 2015, the results of the CheckMate-069 phase 2 trial39 led to accelerated FDA approval of the first-ever immunotherapy combination of nivolumab plus ipilimumab for patients with BRAF V600 wild-type, unresectable or metastatic melanoma (Fig. 2). Among 109 patients, the combination had a response rate (RR) of 60% compared to 11% for ipilimumab alone, and an acceptable safety profile (Table IV).39 Afterward, based on longer PFS rates observed with combination immunotherapy as opposed to ipilimumab alone on the CheckMate-067 phase 3 trial, ipilimumab plus nivolumab was granted accelerated approval in January 2016 for patients with BRAF V600 mutation-positive unresectable or metastatic melanoma (Fig. 2).40
Table IV.
Major studies investigating combination of nivolumab [Opdivo®] plus ipilimumab [Yervoy®] (anti-PD-1 + anti-CTLA-4 immunotherapy) to treat melanoma
| Enrollment period | Trial phase/Identifier | Patients | Randomization / Dosing regimen(s) | Primary endpoint / Results | Median follow-up | Grade 3-4 irAEs |
|---|---|---|---|---|---|---|
| 2013-2014 | Phase 2, CheckMate-069, NCT01927419 | Untreated metastatic melanoma patients, n=142 | Ipilimumab 3 mg/kg + nivolumab 1 mg/kg (combination group) once every 3 weeks for four doses, followed by nivolumab 3 mg/kg every 3 weeks for four doses or placebo every 2 weeks, n=95 Ipilimumab 3 mg/kg + placebo, followed by nivolumab 3 mg/kg every 3 weeks for four doses or placebo every 2 weeks, n=47 |
OR among patients with BRAF V600 wild type tumors: Ipilimumab + nivolumab (n=72): 61% Ipilimumab + placebo (n=37): 11% |
11 mo. | Combination group: 54% Ipilimumab monotherapy: 24% |
| 2013-2014 | Phase 3, CheckMate-067, NCT01844505 | Untreated, unresectable stage III or IV melanoma patients, n=945 | Nivolumab alone, n=316 Nivolumab + ipilimumab, n=314 Ipilimumab alone, n=315 |
PFS Nivolumab + ipilimumab: 11.5 mo. Nivolumab alone: 6.9 mo. Ipilimumab alone: 2.9 mo. |
12.2-12.5 mo. | Nivolumab alone: 16.3% Nivolumab + ipilimumab: 55% Ipilimumab alone: 27.3% |
Among patients with advanced melanoma, therapy with nivolumab plus ipilimumab or nivolumab alone results in longer PFS and OS than with ipilimumab alone6,41 (Fig. 4); and according to the most recently published data, a sunstained long-term OS rate has been observed at 5 years in the nivolumab-plus-ipilimumab (52%) versus nivolumab (44%) versus ipilimumab group (26%).6 However, the nivolumab plus ipilimumab combination results in a high degree of side effects; and choosing which patients should receive combination immunotherapy and which patients should receive nivolumab or pembrolizumab alone is a major clinical challenge.
Figure 4.
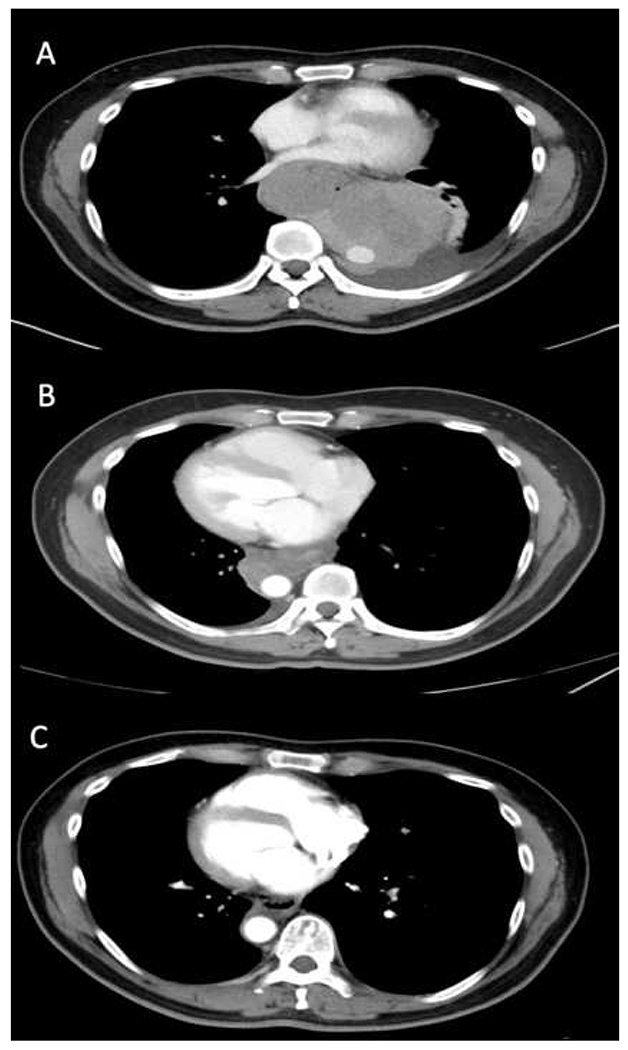
Durable antitumor response after treatment with ipilimumab and nivolumab in a patient with BRAF wildtype melanoma, metastatic to the lungs. (A) February 2016 (B) May 2016 (C) January 2018. Adverse events affecting multiple organs were observed and successfully managed with corticosteroids.
CUTANEOUS SQUAMOUS CELL CARCINOMA
Key points
Cemiplimab is the only approved CPI for cSCC.
Pembrolizumab demonstrated antitumor activity against cSCC in a phase 2 trial.
Most patients with cSCC do not respond to immunotherapy.
cSCC is the second most common cutaneous malignancy.42 Despite excellent prognosis, 4% of cSCC are unresectable and 1.5% of patients die from the disease.43 Until recently, there was no accepted standard of care for advanced cSCC. The use of CPIs in cSCC attracts considerable interest as cSCC has high mutational burden and is more commonly observed in immunosuppressed patients.18–20
In 2018, based on the results of the EMPOWER-CSCC-1 and NCT02383212 trials (Table V), cemiplimab, an anti-PD-1 agent, became the first approved CPI for cSCC (Fig. 3). The most recent update of the EMPOWER-CSCC-1 phase 2 trial44 reports a long-lasting antitumor effect and favorable safety profiles in patients with metastatic cSCC.45 The NCT02383212 phase 1 trial has also demonstrated a positive risk/benefit ratio with durable antitumor response in advanced cSCC (Table V).46
Table V.
Major studies investigating immune-checkpoint inhibitors to treat cutaneous malignancy
| Type of cutaneous malignancy | Investigating agents/Regimen | Trial identifier/Current phase | Patient population | Median follow-up | Efficacy | Adverse event | |
|---|---|---|---|---|---|---|---|
| Common | Rare/Serious | ||||||
| Cutaneous squamous cell carcinoma | Cemiplimab [Libtayo®] 3mg/kg q2w |
EMPOWER-CSCC-1 NCT02760498 Phase 2 trial | 59 patients with metastatic cSCC | 16.5 months | ORR, 49.2% CR, 6.8% PR, 42.4% SD, 13.5% PD, 37.3% PFS, 18.4 months | Diarrhea (28.8%), fatigue (25.4%), nausea (23.7%). | Cellulitis, pneumonitis, hypercalcemia, pleural effusion and death |
| Cemiplimab [Libtayo®] 3mg/kg q2w |
NCT02383212 Phase 1 trial with expansion cohort | 26 patients with locally advanced or metastatic cSCC | 11.0 months | ORR, 50.0% CR, 0.0% PR, 50.0% SD, 23.0% PD, 27.0% PFS, not reported | Fatigue (26.9%), constipation (15%), decreased appetite (15%), diarrhea (15%), nausea (15%), constipation (15%), hypercalcemia (15%), hypophosphatemia (15%), urinary tract infection (15%) | Asthenia, maculopapular rash, increased alanine aminotransferase, increased aspartate aminotransferase, adrenal insufficiency, and myalgia | |
| Merkel cell carcinoma | Avelumab [Bavencio®] 10mg/kg q2w | JAVELIN Merkel 200 NCT02155647 Phase 2 (Part A) trial | 88 patients with stage IV MCC that is refractory to chemotherapy | 16.4 months | ORR, 33.0% CR, 11.4% PR, 21.6% SD, 10.2% PD, 36.4% PFS, 2.7 months | Fatigue (24%), infusion-related reactions (17%), diarrhea (9%), nausea (9%), asthenia, (9%), rash (7%), decreased appetite (6%) | Lymphopenia (2%), increased serum creatine phosphokinase (1%), aminotransferase (1%), and cholesterol (1%) levels, enterocolitis (1%), chondrocalcinosis (1%), synovitis (1%), and interstitial nephritis (1%) |
| JAVELIN Merkel 200 NCT02155647 Phase 2 (Part B) trial | 39 patients with metastatic MCC who had not received prior systemic treatment | 5.1 months | ORR, 62.1% CR, 13.8% PR, 48.3% SD, 10.3% PD, 27.6% PFS, 9.1months | Infusion-related reactions (23.1%) | Cholangitis, elevated aspartate and alanine aminotransferase levels, paraneoplastic syndrome, gait disturbance, paraneoplastic encephalomyelitis, and polyneuropathy | ||
| Pembrolizumab [Keytruda®] 2mg/kg q3w |
KEYNOTE-017 NCT02267603 Phase 2 trial | 50 patients (26 from original cohort and 24 from expansion cohort) with advanced MCC who had not received systemic treatment | 14.9 months | ORR, 56.0% CR, 24.0% PR, 32.0% SD, 10.0% PD, 32% PFS, 16.8 months | Fatigue and laboratory abnormalities | Myocarditis, elevated liver enzyme, death | |
Abbreviations: ORR: Objective response rate; CR: Complete response; PR: Partial response; SD: Stable disease; PD: Progressive disease; PFS: Progression-free survival
Figure 3.
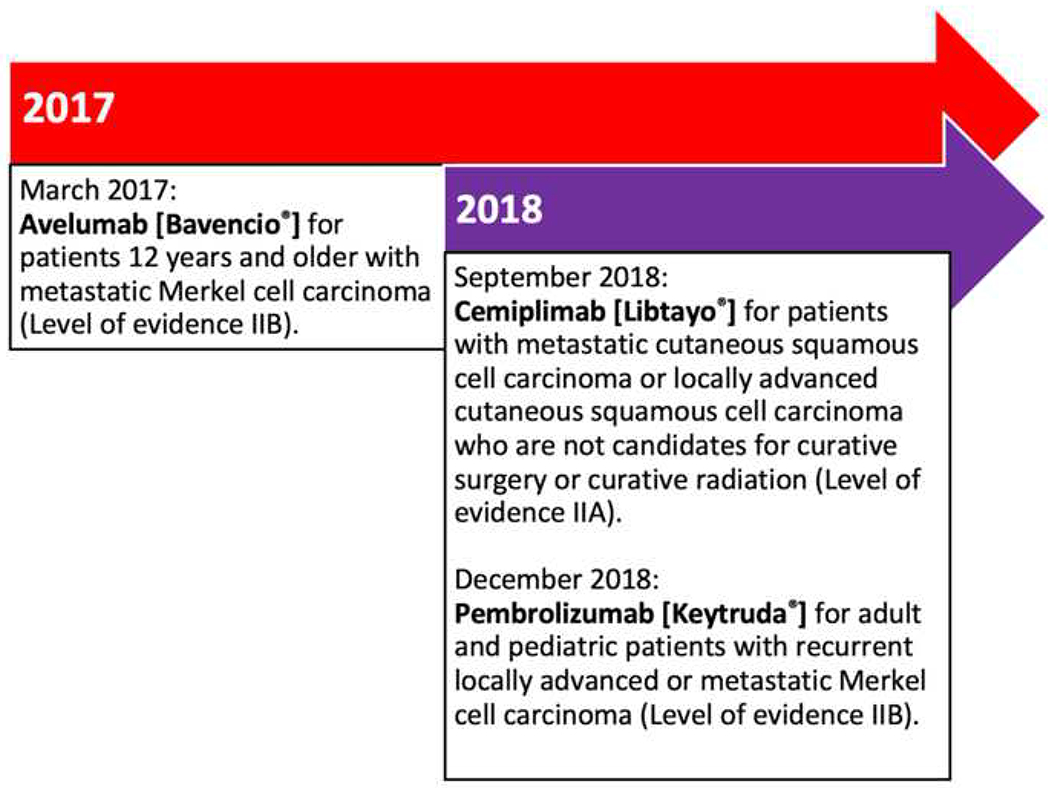
Timeline history of approved immune-checkpoint inhibitors to treat cutaneous squamous cell carcinoma and Merkel cell carcinoma
Level IA evidence includes evidence from meta-analysis of randomized controlled trials.
level IB evidence includes evidence from at least one randomized controlled trial.
Level IIA evidence includes evidence from at least one controlled study without randomization.
Level IIB evidence includes evidence from at least one other type of experimental study.
Level III evidence includes evidence from nonexperimental descriptive studies (i.e. comparative, correlation & case-control).
Level IV evidence includes evidence from expert committee reports or opinions or clinical experience of respected authorities, or both.
Pembrolizumab is being evaluated as first-line therapy in patients with unresectable cSCC in the NCT02883556 trial.17 Initial results showed a promising objective response rate (ORR) of 38.5% at 15 weeks of with a median PFS of 8.4 months. AEs occurred in 67% of patients and caused discontinuation in 10% of patients. Eight percent of patients had severe AEs, including cholestasis and colitis. Retrospective studies and case reports of pembrolizumab for cSCC have shown varying responses.15,47–52 The efficacy of CPIs in immunosuppressed patients is not well studied.53 Favorable responses to CPIs have been reported in transplant recipients either with or without graft rejection.47,48 Optimal immunosuppressive regimens that promote graft preservation without dampening CPI antitumor activity would greatly benefit this group of patients.
Nivolumab for cSCC has only been studied in case reports, showing benefit in recurrent cSCC. AEs include weight loss, nausea, fatigue, hyponatremia, hip pain, and hyperglycemia with one death due to arrhythmia.50,51,54,55 Data on ipilimumab for cSCC is limited, with one case report demonstrating some efficacy when used in conjunction with radiotherapy in a patient with metastatic cSCC and metastatic melanoma.56 Chemotherapy and radiotherapy used concurrently with CPIs have shown efficacy in refractory cSCC55,57 and could be utilized to further improve the antitumor activities of immunotherapy.
MERKEL CELL CARCINOMA
Key points
Avelumab and pembrolizumab are approved for MCC.
Nivolumab showed efficacy against MCC with favorable safety profile in an ongoing trial.
The NCCN recommends avelumab, pembrolizumab and nivolumab as first-line therapies for advanced MCC, prior to chemotherapy.
MCC is a rare and aggressive neuroendocrine skin cancer associated with Merkel cell polyomavirus (MCPyV), ultraviolet radiation exposure, immunosuppression, and advanced age.58 Excision followed by radiotherapy is considered the first-line treatment for primary MCC. Before immunotherapy, chemotherapy was the only systemic treatment available for advanced MCC,58 which despite a good initial response in nearly 90% of patients, has a short-lived efficacy (~90 days). Currently, CPIs have emerged as front-line therapies for advanced MCC with about 50% of patients demonstrating a durable response, although not without considerable toxicity.
In 2017, on the basis of durable responses and favorable safety profiles observed in the JAVELIN Merkel 200 trial part A, avelumab became the first approved treatment for metastatic MCC (Table V);59,60 and recently, part B of this trial showed good tolerance of the anti-PD-L1 agent as first-line therapy for metastatic MCC (Table V).61 In 2018, pembrolizumab was approved for first-line treatment of advanced MCC in the KEYNOTE-017 trial62 (Table V), which in addition to positive CPI-associated antitumor efficacy and safety outcomes, also resulted in glucocorticoids having no effect on tumor response among patients with severe AEs.62 The expanded NCT02267603 trial further strengthened the efficacy of pembrolizumab as first-line treatment for advanced MCC (Fig. 5).63 The CheckMate 358 trial with 25 patients investigated nivolumab for advanced MCC, resulting in a 68% ORR and more than two thirds with AEs.64 In the above studies, PD-L1 expression and MCPyV status did not appear to correlate with clinical responses.59,60,62,64
Figure 5.
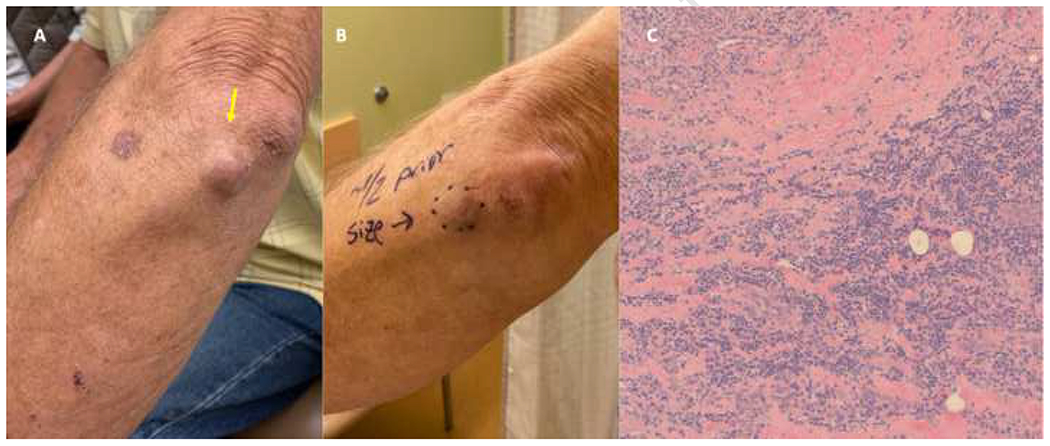
(A, B) Complete clinicopathologic response at three weeks after the first dose of pembrolizumab in a patient with Merkel cell carcinoma. (C) Findings on histopathology reveal dermal fibrosis and mixed lymphocytic inflammation with negative synaptophysin and chromogranin stains (not shown), both of which were expressed at pre-treatment with pembrolizumab.
The use of avelumab, pembrolizumab, and nivolumab for advanced metastatic MCC has also been reported in cases studies, with varying responses.65–74 Serious AEs include central diabetes insipidus66, pneumonia, autoimmune hepatitis,68 cytokine release syndrome,74 and thrombocytopenia.75 Ipilimumab has been studied less frequently against MCC, with inconclusive antitumor activity.76 In addition, ipilimumab did not demonstrate activity as adjuvant therapy for resected MCC.77 Despite the success of CPIs in treating MCC, many patients do not respond, or develop resistant disease following an initial response; however, the use of combinatorial or sequential CPIs has shown activation of antitumor immunity in a subset of non-responders,78 which represents a promising therapeutic approach for patients who do not persistently benefit from CPI treatment in this population.
OTHER CUTANEOUS NEOPLASMS
Key points
There is no CPI approved for BCC, cutaneous lymphomas, cutaneous sarcomas, or CAC.
In small studies and case reports, anti-PD-1 therapy appears to be efficacious in BCC, certain subsets of cutaneous lymphomas, and cutaneous sarcomas.
Basal cell carcinoma
BCC is the most common human cancer with increasing incidence. A small subset of BCC progresses to locally advanced and metastatic tumors and requires aggressive systemic treatments.79,80 Immunotherapy is anticipated to be effective in BCC as it bears the highest mutational burden of any human cancer.81
Pembrolizumab showed antitumor activity against advanced BCC in a phase 1b trial, in which nine patients received pembrolizumab monotherapy and seven patients received pembrolizumab plus vismodegib.82 The ORRs at 18 weeks were 44% and 29%, and the one-year PFSs were 62% and 83% for the monotherapy versus dual therapy group, respectively. Thus, the RR of the dual therapy was not superior to the monotherapy group. Pembrolizumab was well tolerated with dermatitis and fatigue being the most common AEs.82 The use of pembrolizumab in BCC has also been reported in five case reports with clinical responses ranging from DP83 to PR16,84,85 and CR.83,86 There was only one report of subclinical hypothyroidism84 and sarcoid-like reaction.16 Cemiplimab87 and nivolumab88,89 have also shown efficacy against advanced BCC without serious AEs.
Cutaneous lymphomas
Cutaneous T cell lymphomas (CTCL) involve extensive infiltration of malignant T cells into the skin and lack effective treatment for advanced disease.90 Mycosis fungoides (MF) and Sezary syndrome (SS) are the most common CTCL subtypes, with cells expressing high level of PD-1, PD-L1 and CTLA-4, suggesting a role of CPIs in targeting the disease.91,92
As demonstrated by a 15% ORR in 13 patients with MF and 0% ORR in 2 patients with SS in a phase 1b trial, nivolumab has a limited antitumor activity against CTCL.93 AEs occurred in 65% of patients, with 15% discontinuing treatment due to severe AEs, including pneumonitis, sepsis, and myositis. A phase 2 study of pembrolizumab for 24 patients with advanced CTCL demonstrated a 38% ORR.94,95 While there was no significant association between tumor response and the expression of PD-1, PD-L1, or infiltrating CD8+ T cells, pembrolizumab was well-tolerated; serious AEs included grade 2 pneumonitis and grade 3 diarrhea secondary to steroid-refractory duodenitis.94 Curiously, 53% patients with SS experienced skin flare reactions, characterized by a transient worsening of erythroderma and pruritus.95 This reaction correlated with PD-1 expression on Sezary cells but did not associate with subsequent clinical responses. The use of ipilimumab for CTCL has been reported in only two case reports with conflicting responses and requires further investigation.96,97
Cutaneous sarcomas
Cutaneous sarcomas are a rare and heterogenous group of skin mesenchymal spindle cell tumors with good prognosis for early disease. There is a lack of effective therapy for patients with advanced diseases.98 In a phase 2 trial,99 pembrolizumab did not show benefit in patients with undifferentiated pleomorphic sarcoma (UPS). In the NCT01295827 trial with 10 UPS patients, there was 10% CR, 30% PR, 30% SD, and 30% PD.100 Among the 10 patients with liposarcoma in the same trial, there was 0% CR, 2% PR, 40% SD, and 40% PD. The most frequent grade 3 or worse AEs were anemia and other hematologic abnormalities, and 6% of patients discontinued therapy due to toxicity, including nephritis and pneumonitis.
Kaposi sarcoma (KS) is often observed in immunosuppressed patients, suggesting that it might be a good target for CPIs. In a series of 9 HIV positive KS patients who received nivolumab (8) or pembrolizumab (1), the ORR was 66%. The most common AEs included fatigue, pruritus, muscle/joint aches, abdominal discomfort, and onycholysis.101 Pembrolizumab also has antitumor activity against HIV-negative, classic KS.69,102 Nivolumab is also effective in HIV-negative KS patients with the only notable AE being hyponatremia due to low cortisol level.103 Pembrolizumab has also been attempted in two separate cases of angiosarcoma in which the patients either achieved CR104 or durable PR with autoimmune hepatitis that required prednisone treatment.105 There are no data regarding the efficacy of CPIs against dermatofibrosarcoma protuberans or cutaneous leiomyosarcoma.
Cutaneous adnexal carcinomas
CACs are a heterogenous group of malignant neoplasms that display differentiation towards skin-primary adnexal structures, and which currently have limited effective treatment for metastasis.106 High expression levels of PD-L1 have been reported in sebaceous carcinoma.73,107 In two case reports, the use of pembrolizumab with or without chemotherapy demonstrated clinical efficacy against metastatic sebaceous carcinoma.108,109 One patient remained on pembrolizumab despite requiring systemic corticosteroids due to secondary adrenal insufficiency.108
FUTURE DIRECTIONS AND CONCLUSIONS
As the field of immunotherapeutics continues to revolutionize the treatment of cutaneous malignances, blocking antibodies to CTLA-4 and PD-1/PD-L1 have improved survival for many patients. For melanoma, ipilimumab in combination with nivolumab or either nivolumab or pembrolizumab alone are standard front-line treatment options. Several trials are in development to investigate the role of anti-PD-L1 agents in metastatic melanoma,110,111 including atezolizumab and avelumab.
Cemiplimab is the only approved CPI for cSCC, and there is a critical need for improved therapies that can better target the advanced stage of this cutaneous malignancy. Although pembrolizumab has demonstrated antitumor activity against cSCC in a phase 2 trial, most patients do not respond to immunotherapy. For MCC, the NCCN guidelines recommend avelumab, pembrolizumab, and nivolumab as first-line therapies, ahead of chemotherapy. Although the data is limited and there is no CPI approved for BCC, cutaneous lymphoma, cutaneous sarcoma or CACs,112 evidence from small observational studies and case reports suggest the potential utility of anti-PD-1 therapy in BCC and certain subsets of cutaneous lymphoma and cutaneous sarcomas.
Despite exceptional clinical benefits observed with CPIs in cutaneous malignancies, their associated irAEs require careful monitoring. As such, expanding immunotherapy clinical research efforts can lead to identifying new CPI regimens that improve antitumor responses and reduce the incidence and severity of irAEs. Furthermore, striving to achieve a more concrete understanding of predictive markers of response and mechanisms of resistance to anti-CTLA-4 and anti-PD-1/PD-L1 therapies, may help identify subsets of patients who are more likely to respond to therapy with these agents.
Acknowledgments
Funding Source: This study was supported in part by the NIH/NCI Cancer Center Support Grant P30 CA008748 and NIH/NIAMS grant U01AR07751 (MEL) and 5P01CA225517 (TA & PN). Funders/sponsors were not involved in the design and conduct of the study; collection, management, analysis and interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
Conflict of Interest Disclosure(s): DMB, MHD, GSP, and TA have nothing to disclose. MAP receives consulting fees (2015-Present) from BMS; Merck; Array BioPharma; Novartis; Incyte; NewLink Genetics and Aduro. Dr. Postow also receives Honoraria from BMS and Merck; and institutional support from RGenix; Infinity; BMS; Merck; Array BioPharma; Novartis and AstraZeneca. PN receives consulting fees from EMD Serono; Merck and Gegeneron/Sanofi/Genzyme. Dr. Ngheim also receives research support to his institution from BMS and EMD Serono. MEL has consultant/speaking roles with ADC Therapeutics America, Inc.; Apricity Health, LLC; Azitra, Inc.; Deciphera; Johnson and Johnson; NCODA; Novocure Inc., Kyowa Kirin, Inc.; Janssen Research & Development LLC; Menlo Therapeutics; Novartis Pharmaceuticals Corporation; QED Therapeutics; F. Hoffmann-La Roche AG; Amgen Inc., Astrazeneca Pharmaceuticals LP; Genentech Inc.; Seattle Genetics; Lutris; Paxman Coolers; Teva Mexico; Parexel; OnQuality Pharmaceuticals Ltd; Oncodermatology and Takeda Millenium. Dr. Lacouture also receives research funding from Lutris; Paxman; Novocure Inc.; US Biotest and Veloce.
ABBREVIATIONS USED:
- AE
Adverse event
- BCC
Basal cell carcinoma
- CPI
Checkpoint inhibitor
- CR
Complete response
- cSCC
Cutaneous squamous cell carcinoma
- CTLA-4
Cytotoxic T-lymphocyte-associated protein-4
- FDA
Food and Drug Administration
- irAE
Immune-related adverse event
- MCC
Merkel cell carcinoma
- ORR
Objective response rate
- PD
Progressive disease
- PD-1
Programmed cell death-1
- PD-L1
Programmed cell death ligand-1
- PFS
Progression-free survival
- PR
Partial response
- RR
Response rate
- QoL
Quality of Life
- SD
Stable disease
- TRAE
Treatment-related adverse event
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Prior presentation: The contents of this manuscript are not under consideration for publication elsewhere, have not been copyrighted or published previously, and will not be copyrighted, submitted, or published elsewhere while acceptance by your journal is under consideration.
REFERENCES
- 1.Ribas A. Tumor immunotherapy directed at PD-1. N Engl J Med. 2012;366(26):2517–2519. [DOI] [PubMed] [Google Scholar]
- 2.Alsaab HO, Sau S, Alzhrani R, et al. PD-1 and PD-L1 Checkpoint Signaling Inhibition for Cancer Immunotherapy: Mechanism, Combinations, and Clinical Outcome. Front Pharmacol. 2017;8:561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boussiotis VA. Molecular and Biochemical Aspects of the PD-1 Checkpoint Pathway. N Engl J Med. 2016;375(18):1767–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kluger HM, Zito CR, Turcu G, et al. PD-L1 Studies Across Tumor Types, Its Differential Expression and Predictive Value in Patients Treated with Immune Checkpoint Inhibitors. Clin Cancer Res. 2017;23(15):4270–4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coit DG, Thompson JA, Albertini MR, et al. Cutaneous Melanoma, Version 2.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2019;17(4):367–402. [DOI] [PubMed] [Google Scholar]
- 6.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N Engl J Med. 2019;381(16):1535–1546. [DOI] [PubMed] [Google Scholar]
- 7.Hodi FS, Chiarion-Sileni V, Gonzalez R, et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol. 2018;19(11):1480–1492. [DOI] [PubMed] [Google Scholar]
- 8.Zaragoza J, Caille A, Beneton N, et al. High neutrophil to lymphocyte ratio measured before starting ipilimumab treatment is associated with reduced overall survival in patients with melanoma. Br J Dermatol. 2016;174(1):146–151. [DOI] [PubMed] [Google Scholar]
- 9.Kelderman S, Heemskerk B, van Tinteren H, et al. Lactate dehydrogenase as a selection criterion for ipilimumab treatment in metastatic melanoma. Cancer Immunol Immunother. 2014;63(5):449–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosner S, Kwong E, Shoushtari AN, et al. Peripheral blood clinical laboratory variables associated with outcomes following combination nivolumab and ipilimumab immunotherapy in melanoma. Cancer Med. 2018;7(3):690–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Coana YP, Wolodarski M, Poschke I, et al. Ipilimumab treatment decreases monocytic MDSCs and increases CD8 effector memory T cells in long-term survivors with advanced melanoma. Oncotarget. 2017;8(13):21539–21553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ouwerkerk W, van den Berg M, van der Niet S, Limpens J, Luiten RM. Biomarkers, measured during therapy, for response of melanoma patients to immune checkpoint inhibitors: a systematic review. Melanoma Res. 2019;29(5):453–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Byrne EH, Fisher DE. Immune and molecular correlates in melanoma treated with immune checkpoint blockade. Cancer. 2017;123(S11):2143–2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weber JS, Hamid O, Chasalow SD, et al. Ipilimumab increases activated T cells and enhances humoral immunity in patients with advanced melanoma. J Immunother. 2012;35(1):89–97. [DOI] [PubMed] [Google Scholar]
- 15.Stevenson ML, Wang CQ, Abikhair M, et al. Expression of Programmed Cell Death Ligand in Cutaneous Squamous Cell Carcinoma and Treatment of Locally Advanced Disease With Pembrolizumab. JAMA Dermatol. 2017;153(4):299–303. [DOI] [PubMed] [Google Scholar]
- 16.Winkler JK, Schneiderbauer R, Bender C, et al. Anti-programmed cell death-1 therapy in nonmelanoma skin cancer. Br J Dermatol. 2017;176(2):498–502. [DOI] [PubMed] [Google Scholar]
- 17.Maubec E, Boubaya M, Petrow P, et al. Pembrolizumab as first-line therapy in patients with unresectable cutaneous squamous cell carcinoma (cSCC): Phase 2 results from CARSKIN. 2019;37(15_suppl):9547–9547. [Google Scholar]
- 18.Chalmers ZR, Connelly CF, Fabrizio D, et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med. 2017;9(1):34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Euvrard S, Kanitakis J, Claudy A. Skin cancers after organ transplantation. N Engl J Med. 2003;348(17):1681–1691. [DOI] [PubMed] [Google Scholar]
- 20.Pickering CR, Zhou JH, Lee JJ, et al. Mutational landscape of aggressive cutaneous squamous cell carcinoma. Clin Cancer Res. 2014;20(24):6582–6592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wolchok JD, Neyns B, Linette G, et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol. 2010;11(2):155–164. [DOI] [PubMed] [Google Scholar]
- 23.Ascierto PA, Del Vecchio M, Robert C, et al. Ipilimumab 10 mg/kg versus ipilimumab 3 mg/kg in patients with unresectable or metastatic melanoma: a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2017;18(5):611–622. [DOI] [PubMed] [Google Scholar]
- 24.Eggermont AM, Chiarion-Sileni V, Grob JJ, et al. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2015;16(5):522–530. [DOI] [PubMed] [Google Scholar]
- 25.Eggermont AM, Chiarion-Sileni V, Grob JJ. Correction to Lancet Oncol 2015; 16: 522–30. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2015;16(6):e262. [DOI] [PubMed] [Google Scholar]
- 26.Eggermont AMM, Chiarion-Sileni V, Grob JJ, et al. Adjuvant ipilimumab versus placebo after complete resection of stage III melanoma: long-term follow-up results of the European Organisation for Research and Treatment of Cancer 18071 double-blind phase 3 randomised trial. Eur J Cancer. 2019;119:1–10. [DOI] [PubMed] [Google Scholar]
- 27.Raedler LA. Keytruda (Pembrolizumab): First PD-1 Inhibitor Approved for Previously Treated Unresectable or Metastatic Melanoma. Am Health Drug Benefits. 2015;8(Spec Feature):96–100. [PMC free article] [PubMed] [Google Scholar]
- 28.Robert C, Ribas A, Wolchok JD, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet. 2014;384(9948):1109–1117. [DOI] [PubMed] [Google Scholar]
- 29.Robert C, Schachter J, Long GV, et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med. 2015;372(26):2521–2532. [DOI] [PubMed] [Google Scholar]
- 30.Eggermont AMM, Blank CU, Mandala M, et al. Adjuvant Pembrolizumab versus Placebo in Resected Stage III Melanoma. N Engl J Med. 2018;378(19):1789–1801. [DOI] [PubMed] [Google Scholar]
- 31.Weber JS, D’Angelo SP, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16(4):375–384. [DOI] [PubMed] [Google Scholar]
- 32.Hazarika M, Chuk MK, Theoret MR, et al. U.S. FDA Approval Summary: Nivolumab for Treatment of Unresectable or Metastatic Melanoma Following Progression on Ipilimumab. Clin Cancer Res. 2017;23(14):3484–3488. [DOI] [PubMed] [Google Scholar]
- 33.Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372(4):320–330. [DOI] [PubMed] [Google Scholar]
- 34.Beaver JA, Theoret MR, Mushti S, et al. FDA Approval of Nivolumab for the First-Line Treatment of Patients with BRAF(V600) Wild-Type Unresectable or Metastatic Melanoma. Clin Cancer Res. 2017;23(14):3479–3483. [DOI] [PubMed] [Google Scholar]
- 35.Weber J, Mandala M, Del Vecchio M, et al. Adjuvant Nivolumab versus Ipilimumab in Resected Stage III or IV Melanoma. N Engl J Med. 2017;377(19):1824–1835. [DOI] [PubMed] [Google Scholar]
- 36.Yamazaki N, Kiyohara Y, Uhara H, et al. Long-term follow up of nivolumab in previously untreated Japanese patients with advanced or recurrent malignant melanoma. Cancer Sci. 2019;110(6):1995–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamazaki N, Kiyohara Y, Uhara H, et al. Efficacy and safety of nivolumab in Japanese patients with previously untreated advanced melanoma: A phase II study. Cancer Sci. 2017;108(6):1223–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ascierto PA, Long GV, Robert C, et al. Survival Outcomes in Patients With Previously Untreated BRAF Wild-Type Advanced Melanoma Treated With Nivolumab Therapy: Three-Year Follow-up of a Randomized Phase 3 Trial. JAMA Oncol. 2019;5(2):187–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Postow MA, Chesney J, Pavlick AC, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372(21):2006–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med. 2015;373(1):23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N Engl J Med. 2017;377(14):1345–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Que SKT, Zwald FO, Schmults CD. Cutaneous squamous cell carcinoma: Incidence, risk factors, diagnosis, and staging. J Am Acad Dermatol. 2018;78(2):237–247. [DOI] [PubMed] [Google Scholar]
- 43.Karia PS, Han J, Schmults CD. Cutaneous squamous cell carcinoma: estimated incidence of disease, nodal metastasis, and deaths from disease in the United States, 2012. J Am Acad Dermatol. 2013;68(6):957–966. [DOI] [PubMed] [Google Scholar]
- 44.Migden MR, Rischin D, Schmults CD, et al. PD-1 Blockade with Cemiplimab in Advanced Cutaneous Squamous-Cell Carcinoma. N Engl J Med. 2018;379(4):341–351. [DOI] [PubMed] [Google Scholar]
- 45.Guminski AD, Lim AML, Khushalani NI, et al. Phase 2 study of cemiplimab, a human monoclonal anti-PD-1, in patients (pts) with metastatic cutaneous squamous cell carcinoma (mCSCC; Group 1): 12-month follow-up. 2019;37(15_suppl):9526–9526. [Google Scholar]
- 46.Owonikoko TK, Papadopoulos KP, Johnson ML, et al. Phase 1 study of cemiplimab, a human monoclonal anti-PD-1, in patients with unresectable locally advanced or metastatic cutaneous squamous cell carcinoma (CSCC): Final efficacy and safety data. 2018;36(15_suppl):9557–9557. [Google Scholar]
- 47.Lipson EJ, Bagnasco SM, Moore J Jr., et al. Tumor Regression and Allograft Rejection after Administration of Anti-PD-1. N Engl J Med. 2016;374(9):896–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sadaat M, Jang S. Complete Tumor Response to Pembrolizumab and Allograft Preservation in Renal Allograft Recipient on Immunosuppressive Therapy. J Oncol Pract. 2018;14(3):198–199. [DOI] [PubMed] [Google Scholar]
- 49.Assam JH, Powell S, Spanos WC. Unresectable cutaneous squamous cell carcinoma of the forehead with MLH1 mutation showing dramatic response to Programmed Cell Death Protein 1 Inhibitor Therapy. Clin Skin Cancer. 2016;1(1):26–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tran DC, Colevas AD, Chang AL. Follow-up on Programmed Cell Death 1 Inhibitor for Cutaneous Squamous Cell Carcinoma. JAMA Dermatol. 2017;153(1):92–94. [DOI] [PubMed] [Google Scholar]
- 51.Borradori L, Sutton B, Shayesteh P, Daniels GA. Rescue therapy with anti-programmed cell death protein 1 inhibitors of advanced cutaneous squamous cell carcinoma and basosquamous carcinoma: preliminary experience in five cases. Br J Dermatol. 2016;175(6):1382–1386. [DOI] [PubMed] [Google Scholar]
- 52.Chang AL, Kim J, Luciano R, Sullivan-Chang L, Colevas AD. A Case Report of Unresectable Cutaneous Squamous Cell Carcinoma Responsive to Pembrolizumab, a Programmed Cell Death Protein 1 Inhibitor. JAMA Dermatol. 2016;152(1):106–108. [DOI] [PubMed] [Google Scholar]
- 53.Cippa PE, Schiesser M, Ekberg H, et al. Risk Stratification for Rejection and Infection after Kidney Transplantation. Clin J Am Soc Nephrol. 2015;10(12):2213–2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Blum V, Muller B, Hofer S, et al. Nivolumab for recurrent cutaneous squamous cell carcinoma: three cases. Eur J Dermatol. 2018;28(1):78–81. [DOI] [PubMed] [Google Scholar]
- 55.Chen A, Ali N, Boasberg P, Ho AS. Clinical Remission of Cutaneous Squamous Cell Carcinoma of the Auricle with Cetuximab and Nivolumab. J Clin Med. 2018;7(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Day F, Kumar M, Fenton L, Gedye C. Durable Response of Metastatic Squamous Cell Carcinoma of the Skin to Ipilimumab Immunotherapy. J Immunother. 2017;40(1):36–38. [DOI] [PubMed] [Google Scholar]
- 57.Vaidya P, Mehta A, Ragab O, Lin S, In GK. Concurrent radiation therapy with programmed cell death protein 1 inhibition leads to a complete response in advanced cutaneous squamous cell carcinoma. JAAD Case Rep. 2019;5(9):763–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bichakjian CK, Olencki T, Aasi SZ, et al. Merkel Cell Carcinoma, Version 1.2018, NCCN Clinical Practice Guidelines in Oncology. 2018;16(6):742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kaufman HL, Russell J, Hamid O, et al. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: a multicentre, single-group, open-label, phase 2 trial. Lancet Oncol. 2016;17(10):1374–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kaufman HL, Russell JS, Hamid O, et al. Updated efficacy of avelumab in patients with previously treated metastatic Merkel cell carcinoma after >/=1 year of follow-up: JAVELIN Merkel 200, a phase 2 clinical trial. J Immunother Cancer. 2018;6(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.D’Angelo SP, Russell J, Lebbe C, et al. Efficacy and Safety of First-line Avelumab Treatment in Patients With Stage IV Metastatic Merkel Cell Carcinoma: A Preplanned Interim Analysis of a Clinical Trial. JAMA Oncol. 2018;4(9):e180077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nghiem PT, Bhatia S, Lipson EJ, et al. PD-1 Blockade with Pembrolizumab in Advanced Merkel-Cell Carcinoma. N Engl J Med. 2016;374(26):2542–2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nghiem P, Bhatia S, Lipson EJ, et al. Durable Tumor Regression and Overall Survival in Patients With Advanced Merkel Cell Carcinoma Receiving Pembrolizumab as First-Line Therapy. J Clin Oncol. 2019;37(9):693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Topalian SL, Bhatia S, Hollebecque A, et al. Abstract CT074: Non-comparative, open-label, multiple cohort, phase 1/2 study to evaluate nivolumab (NIVO) in patients with virus-associated tumors (CheckMate 358): Efficacy and safety in Merkel cell carcinoma (MCC). 2017;77(13 Supplement):CT074–CT074. [Google Scholar]
- 65.Eshghi N, Lundeen TF, MacKinnon L, Avery R, Kuo PH. 18F-FDG PET/CT for Monitoring Response of Merkel Cell Carcinoma to the Novel Programmed Cell Death Ligand 1 Inhibitor Avelumab. Clin Nucl Med. 2018;43(5):e142–e144. [DOI] [PubMed] [Google Scholar]
- 66.Zhao C, Tella SH, Del Rivero J, et al. Anti-PD-L1 Treatment Induced Central Diabetes Insipidus. J Clin Endocrinol Metab. 2018;103(2):365–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mantripragada K, Birnbaum A. Response to Anti-PD-1 Therapy in Metastatic Merkel Cell Carcinoma Metastatic to the Heart and Pancreas. Cureus. 2015;7(12):e403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Walocko FM, Scheier BY, Harms PW, Fecher LA, Lao CD. Metastatic Merkel cell carcinoma response to nivolumab. J Immunother Cancer. 2016;4:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Patnaik A, Kang SP, Rasco D, et al. Phase I Study of Pembrolizumab (MK-3475; Anti-PD-1 Monoclonal Antibody) in Patients with Advanced Solid Tumors. Clin Cancer Res. 2015;21(19):4286–4293. [DOI] [PubMed] [Google Scholar]
- 70.Cugley DR, Roberts-Thomson SJ, McNab AA, Pick Z. Biopsy-Proven Metastatic Merkel Cell Carcinoma to the Orbit: Case Report and Review of Literature. Ophthalmic Plast ReconstrSurg. 2018;34(3):e86–e88. [DOI] [PubMed] [Google Scholar]
- 71.Winkler JK, Bender C, Kratochwil C, Enk A, Hassel JC. PD-1 blockade: a therapeutic option for treatment of metastatic Merkel cell carcinoma. Br J Dermatol. 2017;176(1):216–219. [DOI] [PubMed] [Google Scholar]
- 72.Haug V, Behle V, Benoit S, et al. Pembrolizumab-associated mucous membrane pemphigoid in a patient with Merkel cell carcinoma. Br J Dermatol. 2018;179(4):993–994. [DOI] [PubMed] [Google Scholar]
- 73.Xu MJ, Wu S, Daud AI, Yu SS, Yom SS. In-field and abscopal response after short-course radiation therapy in patients with metastatic Merkel cell carcinoma progressing on PD-1 checkpoint blockade: a case series. J Immunother Cancer. 2018;6(1):43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Barker CA, Kim SK, Budhu S, Matsoukas K, Daniyan AF, D’Angelo SP. Cytokine release syndrome after radiation therapy: case report and review of the literature. J Immunother Cancer. 2018;6(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kratzsch D, Simon JC, Ponitzsch I, Ziemer M. Lethal thrombocytopenia in a patient treated with avelumab for metastatic Merkel cell carcinoma. J Dtsch Dermatol Ges. 2019;17(1):73–75. [DOI] [PubMed] [Google Scholar]
- 76.Winkler JK, Dimitrakopoulou-Strauss A, Sachpekidis C, Enk A, Hassel JC. Ipilimumab has efficacy in metastatic Merkel cell carcinoma: a case series of five patients. J Eur Acad Dermatol Venereol. 2017;31(9):e389–e391. [DOI] [PubMed] [Google Scholar]
- 77.Becker JC, Hassel JC, Menzer C, et al. Adjuvant ipilimumab compared with observation in completely resected Merkel cell carcinoma (ADMEC): A randomized, multicenter DeCOG/ADO study. 2018;36(15_suppl):9527–9527. [Google Scholar]
- 78.LoPiccolo J, Schollenberger MD, Dakhil S, et al. Rescue therapy for patients with anti-PD-1-refractory Merkel cell carcinoma: a multicenter, retrospective case series. J Immunother Cancer. 2019;7(1):170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cameron MC, Lee E, Hibler BP, et al. Basal cell carcinoma: Epidemiology; pathophysiology; clinical and histological subtypes; and disease associations. J Am Acad Dermatol. 2019;80(2):303–317. [DOI] [PubMed] [Google Scholar]
- 80.Cameron MC, Lee E, Hibler BP, et al. Basal cell carcinoma: Contemporary approaches to diagnosis, treatment, and prevention. J Am Acad Dermatol. 2019;80(2):321–339. [DOI] [PubMed] [Google Scholar]
- 81.Jayaraman SS, Rayhan DJ, Hazany S, Kolodney MS. Mutational landscape of basal cell carcinomas by whole-exome sequencing. J Invest Dermatol. 2014;134(1):213–220. [DOI] [PubMed] [Google Scholar]
- 82.Chang ALS, Tran DC, Cannon JGD, et al. Pembrolizumab for advanced basal cell carcinoma: An investigator-initiated, proof-of-concept study. J Am Acad Dermatol. 2019;80(2):564–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cannon JGD, Russell JS, Kim J, Chang ALS. A case of metastatic basal cell carcinoma treated with continuous PD-1 inhibitor exposure even after subsequent initiation of radiotherapy and surgery. JAAD Case Rep. 2018;4(3):248–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lipson EJ, Lilo MT, Ogurtsova A, et al. Basal cell carcinoma: PD-L1/PD-1 checkpoint expression and tumor regression after PD-1 blockade. J Immunother Cancer. 2017;5:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fischer S, Hasan Ali O, Jochum W, Kluckert T, Flatz L, Siano M. Anti-PD-1 Therapy Leads to Near-Complete Remission in a Patient with Metastatic Basal Cell Carcinoma. Oncol Res Treat. 2018;41(6):391–394. [DOI] [PubMed] [Google Scholar]
- 86.Moreira A, Kirchberger MC, Toussaint F, Erdmann M, Schuler G, Heinzerling L. Effective anti-programmed death-1 therapy in a SUFU-mutated patient with Gorlin-Goltz syndrome. Br J Dermatol. 2018;179(3):747–749. [DOI] [PubMed] [Google Scholar]
- 87.Falchook GS, Leidner R, Stankevich E, et al. Responses of metastatic basal cell and cutaneous squamous cell carcinomas to anti-PD1 monoclonal antibody REGN2810. J Immunother Cancer. 2016;4:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cohen PR, Kato S, Goodman AM, Ikeda S, Kurzrock R. Appearance of New Cutaneous Superficial Basal Cell Carcinomas during Successful Nivolumab Treatment of Refractory Metastatic Disease: Implications for Immunotherapy in Early Versus Late Disease. Int J Mol Sci. 2017;18(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ikeda S, Goodman AM, Cohen PR, et al. Metastatic basal cell carcinoma with amplification of PD-L1: exceptional response to anti-PD1 therapy. NPJ Genom Med. 2016;1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wilcox RA. Cutaneous T-cell lymphoma: 2017 update on diagnosis, risk-stratification, and management. Am J Hematol. 2017;92(10):1085–1102. [DOI] [PubMed] [Google Scholar]
- 91.Dai J, Almazan T, Kim Y, Khodadoust MJAoL. Pembrolizumab in systemic and cutaneous T-cell lymphoma. 2018. 2018;2(4). [Google Scholar]
- 92.Wong HK, Wilson AJ, Gibson HM, et al. Increased expression of CTLA-4 in malignant T-cells from patients with mycosis fungoides -- cutaneous T cell lymphoma. J Invest Dermatol. 2006;126(1):212–219. [DOI] [PubMed] [Google Scholar]
- 93.Lesokhin AM, Ansell SM, Armand P, et al. Nivolumab in Patients With Relapsed or Refractory Hematologic Malignancy: Preliminary Results of a Phase Ib Study. J Clin Oncol. 2016;34(23):2698–2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Khodadoust M, Rook AH, Porcu P, et al. Pembrolizumab for Treatment of Relapsed/Refractory Mycosis Fungoides and Sezary Syndrome: Clinical Efficacy in a Citn Multicenter Phase 2 Study. Blood 2016;128(22):181–181. [Google Scholar]
- 95.Khodadoust MS, et al. (2020). “Pembrolizumab in Relapsed and Refractory Mycosis Fungoides and Sezary Syndrome: A Multicenter Phase II Study.” J Clin Oncol 38(1): 20–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bar-Sela G, Bergman R. Complete regression of mycosis fungoides after ipilimumab therapy for advanced melanoma. JAAD Case Rep. 2015;1(2):99–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sekulic A, Liang WS, Tembe W, et al. Personalized treatment of Sezary syndrome by targeting a novel CTLA4:CD28 fusion. Mol Genet Genomic Med. 2015;3(2):130–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kohlmeyer J, Steimle-Grauer SA, Hein R. Cutaneous sarcomas. J Dtsch Dermatol Ges. 2017;15(6):630–648. [DOI] [PubMed] [Google Scholar]
- 99.Toulmonde M, Penel N, Adam J, et al. Use of PD-1 Targeting, Macrophage Infiltration, and IDO Pathway Activation in Sarcomas: A Phase 2 Clinical Trial. JAMA Oncol. 2018;4(1):93–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tawbi HA, Burgess M, Bolejack V, et al. Pembrolizumab in advanced soft-tissue sarcoma and bone sarcoma (SARC028): a multicentre, two-cohort, single-arm, open-label, phase 2 trial. Lancet Oncol. 2017;18(11):1493–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Galanina N, Goodman AM, Cohen PR, Frampton GM, Kurzrock R. Successful Treatment of HIV-Associated Kaposi Sarcoma with Immune Checkpoint Blockade. Cancer Immunol Res. 2018;6(10):1129–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Saller J, Walko CM, Millis SZ, Henderson-Jackson E, Makanji R, Brohl AS. Response to Checkpoint Inhibitor Therapy in Advanced Classic Kaposi Sarcoma: A Case Report and Immunogenomic Study. J Natl Compr Canc Netw. 2018;16(7):797–800. [DOI] [PubMed] [Google Scholar]
- 103.Delyon J, Bizot A, Battistella M, Madelaine I, Vercellino L, Lebbe C. PD-1 blockade with nivolumab in endemic Kaposi sarcoma. Ann Oncol. 2018;29(4):1067–1069. [DOI] [PubMed] [Google Scholar]
- 104.Hamacher R, Kampfe D, Ahrens M, et al. 1506PPD-L1 inhibition – a new therapeutic opportunity in cutaneous angiosarcoma? Annals of Oncology. 2017;28(suppl_5). [Google Scholar]
- 105.Sindhu S, Gimber LH, Cranmer L, McBride A, Kraft AS. Angiosarcoma treated successfully with anti-PD-1 therapy - a case report. J Immunother Cancer. 2017;5(1):58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Martinez SR, Barr KL, Canter RJ. Rare tumors through the looking glass: an examination of malignant cutaneous adnexal tumors. Arch Dermatol. 2011;147(9):1058–1062. [DOI] [PubMed] [Google Scholar]
- 107.Kandl TJ, Sagiv O, Curry JL, et al. High expression of PD-1 and PD-L1 in ocular adnexal sebaceous carcinoma. Oncoimmunology. 2018;7(9):e1475874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Domingo-Musibay E, Murugan P, Giubellino A, et al. Near complete response to Pembrolizumab in microsatellite-stable metastatic sebaceous carcinoma. J Immunother Cancer. 2018;6(1):58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kodali S, Tipirneni E, Gibson PC, Cook D, Verschraegen C, Lane KA. Carboplatin and Pembrolizumab Chemoimmunotherapy Achieves Remission in Recurrent, Metastatic Sebaceous Carcinoma. Ophthalmic Plast Reconstr Surg. 2018;34(5):e149–e151. [DOI] [PubMed] [Google Scholar]
- 110.Hamid O, Molinero L, Bolen CR, et al. Safety, Clinical Activity, and Biological Correlates of Response in Patients with Metastatic Melanoma: Results from a Phase I Trial of Atezolizumab. Clin Cancer Res. 2019. [DOI] [PubMed] [Google Scholar]
- 111.Keilholz U, Mehnert JM, Bauer S, et al. Avelumab in patients with previously treated metastatic melanoma: phase 1b results from the JAVELIN Solid Tumor trial. J Immunother Cancer. 2019;7(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Choi FD, et al. (2020). “Programmed cell death 1 protein and programmed death-ligand 1 inhibitors in the treatment of nonmelanoma skin cancer: A systematic review.” J Am Acad Dermatol 82(2): 440–459. [DOI] [PubMed] [Google Scholar]


