Abstract
Microglia are the resident immune cells of the center nervous system and participate in various neurological diseases. Here we determined the function of microglia in epileptogenesis using microglial ablation approaches. Three different microglia-specific genetic tools were used, CX3CR1CreER/+:R26iDTA/+, CX3CR1CreER/+:R26iDTR/+, and CX3CR1CreER/+:Csf1rFlox/Flox mice. We found that microglial depletion led to worse kainic acid (KA)-induced status epilepticus, higher mortality rate, and increased neuronal degeneration in the hippocampus. In KA-induced chronic spontaneous recurrent seizures, microglial depletion increased seizure frequency, interictal spiking, and seizure duration. Therefore, microglia depletion aggravates the severity of KA-induced acute and chronic seizures. Interestingly, microglial repopulation reversed the effects of depletion upon KA-induced status epilepticus. Our results demonstrate a beneficial role of microglia in suppressing both acute and chronic seizures, suggesting that microglia are a potential therapeutic target for the management of epilepsy.
Introduction
Epilepsy is a chronic seizure disorder affecting more than 65 million people worldwide (Bhandare et al., 2017; Devinsky et al., 2013; Eyo et al., 2017; Moshe et al., 2015). This disorder is more likely to occur in young children and geriatric patients, and increase their risk for sudden death 15–20 fold (Ficker et al., 1998; Nilsson et al., 1999). Despite there being a variety of available antiepileptic drugs (AEDs), approximately one-third of patients are refractory to these treatments (Kwan and Brodie, 2000; Perucca et al., 2007). Thus, a deeper understanding of the pathogenic events and molecular changes that occur during the course of epileptogenesis is needed to develop more efficacious treatments of these patients.
Accumulating evidence indicates that the neuroinflammation is common to both experimental animal and clinical human epilepsies (Jimenez-Pacheco et al., 2016; Leal et al., 2017; Strauss and Elisevich, 2016; Vezzani et al., 2011a; Vezzani et al., 2011b). As the resident immune cells of the central nervous system (CNS), microglia are critical regulators of brain homeostasis and immune reactions (Wolf et al., 2017; Wu et al., 2015; Zhao et al., 2018a). Microglia can be rapidly activated following various CNS insults (e.g., infection, ischemia, epilepsy) (Avignone et al., 2008; Brown and Neher, 2010; Eyo et al., 2014; Qin et al., 2019) and can produce numerous pro-inflammatory cytokines and chemokines, such as interleukin (IL) 1 beta, tumor necrosis factor alpha (TNF-α), interferon gamma (IFN-γ) and BDNF (Fu et al., 2014; Gu et al., 2016; Zhou et al., 2019). These pro-inflammatory factors can increase neuronal activity and decrease seizure threshold (Galic et al., 2012; Vezzani et al., 2011a; Vezzani et al., 2013), which is favorable to chronic neuronal network hyperexcitability and development of spontaneous recurrent seizures (SRS) (Hu et al., 2014; Kan et al., 2012; Zhao et al., 2018b). Activated microglia can also induce neuronal atrophy in epileptic brains (Pitkanen and Sutula, 2002; Tian et al., 2017). Furthermore, anti-inflammatory drugs and inhibition of inflammatory microglia exhibit significant anticonvulsant effects (Vezzani et al., 2010). For example, minocycline, an inhibitor of microglial activation, has been reported to exert an anticonvulsant and neuroprotective in various animal models of seizures (Abraham et al., 2012; Heo et al., 2006; Wang et al., 2015). Thus, pro-inflammatory microglia could be an etiological driver of epileptogenesis.
However, microglia may also have beneficial function in epilepsy. Recent studies have shown that preconditioning microglia with lipopolysaccharide (LPS) can produce protective effects in rodent seizure models that can then be reversed by local microglia depletion (Mirrione et al., 2010). Other studies indicate that microglia are neuroprotective in viral encephalitis-induced epilepsy, since microglial depletion with PLX-5622, a specific inhibitor of the CSF1 receptor, accelerated the occurrence of seizures and exacerbated hippocampal damage (Waltl et al., 2018). Additionally, PLX-5622 induced microglia depletion within a Theiler’s murine encephalomyelitis virus (TMEV) induced seizure model resulted in fatal viral encephalitis and exacerbated demyelination and axonal damage (Sanchez et al., 2019). These results indicate that microglia may have beneficial functions in seizures and epilepsy. However, the definitive role of microglia in acute and chronic seizures remains unclear. Consequently, we investigated the effects of microglial depletion via three different genetic methods within KA induced status epilepticus and spontaneous chronic seizures in mice. Our results show that microglial depletion worsened acute seizures and neuronal degeneration as well as increased the frequency of spontaneous recurrent seizures (SRS) and duration chronically.
Results
Microglia depletion and re-population.
The chemokine receptor CX3CR1 is primarily expressed on microglia in the CNS (Jung et al., 2000). As such, genetic modulation of microglia using CX3CR1-Cre lines has become a common method to investigate microglial function (Zhao et al., 2019). To begin our investigation, we depleted CX3CR1+ cells including microglia using two genetic mouse models. First, we inducibly expressed diphtheria toxin A (DTA) in CX3CR1+ cells using CX3CR1CreER/+: R26iDTA/+ mice, henceforth designated iDTA mice. Second, we inducibly knocked out colony-stimulating factor 1 receptor (Csf1r), which is critical for microglia survival using CX3CR1CreER/+:Csf1rFlox/Flox mice, henceforth designated Csf1r mice. Tamoxifen (TM) dosing was scheduled for every 3 days to induce DTA expression or Csf1r knockout in CX3CR1-expressing microglia and thus their depletion (Figure 1a). In iDTA mice, approximately 83%, 92%, and 90% Iba-1+ microglial cells, were depleted at 6d, 9d, and 12d after tamoxifen treatment, respectively (Figure 1b–d). Interestingly, re-population of Iba-1+ microglial cells occurred between days 12 and 18 even though tamoxifen treatment was continued (Figure 1b–d) and did not exhibit obvious regional heterogeneity. Compared to other brain regions, the hippocampus neither the last nor first region where microglia are fully repopulated. The similar depletion efficiency and re-population phenomenon was also observed in Csf1r mice (Figure 1e–g). To further investigate the identity of these repopulated cells in the brain, we stained for CD11b, a general marker of myeloid cells, and P2Y12, a specific marker of microglia. In both iDTA and Csf1r mice, CD11b and P2Y12 was completely co-expressed at each time point (Figure 2), suggesting that the repopulated cells were likely derived from residual microglia and not from infiltrated monocytes.
Figure 1. Microglia depletion and re-population in the mouse brain.
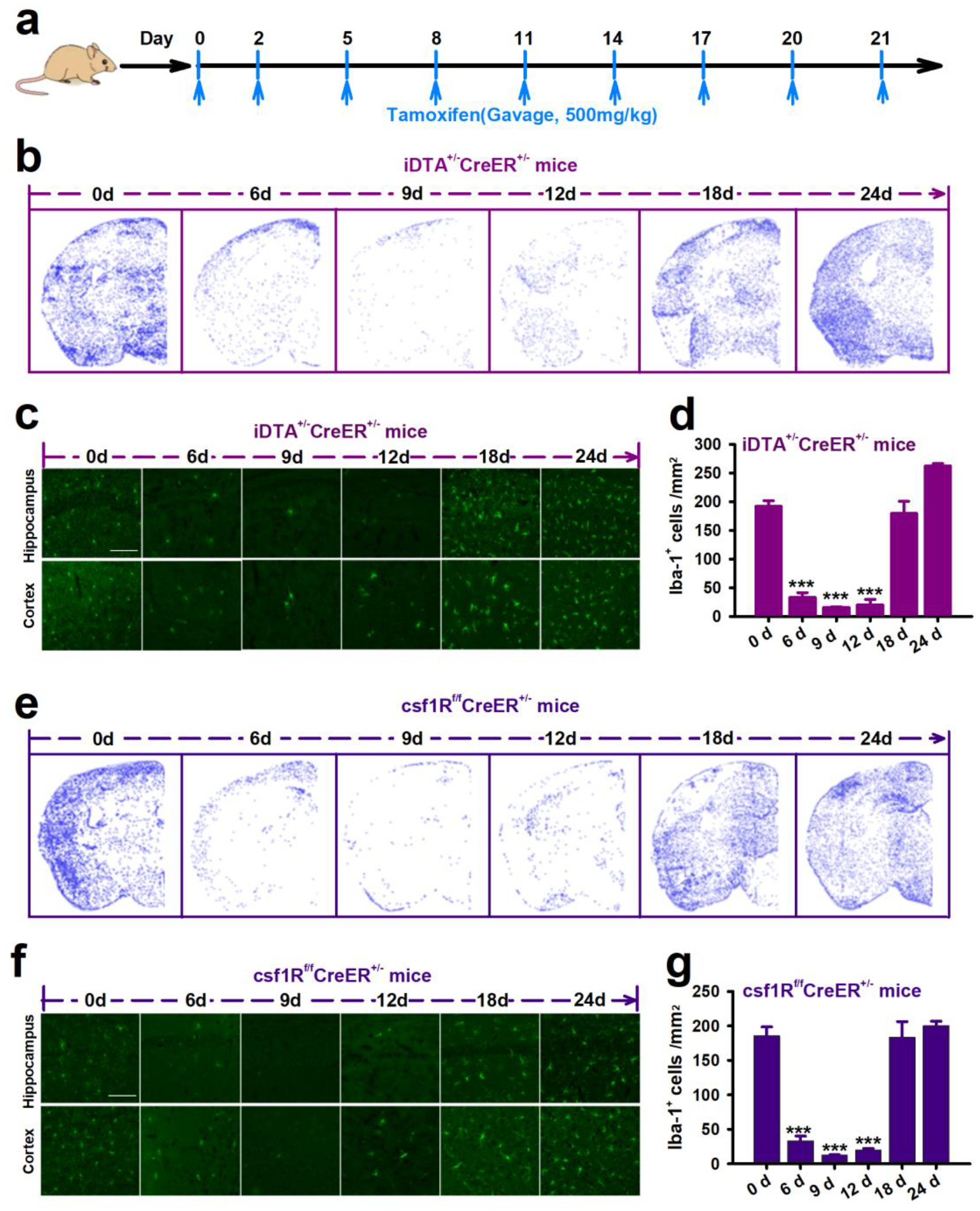
a, Tamoxifen treatment scheme. b,e, Representative images showing microglia depletion and re-population in the brain of iDTA mice (b) and Csf1r mice (e). Using Analyze Particles in ImageJ to perform automatic cell counting, each blue dot represents a microglial cell. c,f, Immunostaining images of Iba1+ microglial cells in the hippocampus and cortex of iDTA mice and Csf1r mice during the time course of depletion and re-population. Scale bar=50 μm. d,g, Quantification of Iba+ microglial cells in the brain of iDTA mice and Csf1r mice treated with tamoxifen. Data presented as means ± SEM, n=3–4, ***P<0.001 vs 0d.
Figure 2. Re-populated cells are P2Y12+ microglia.
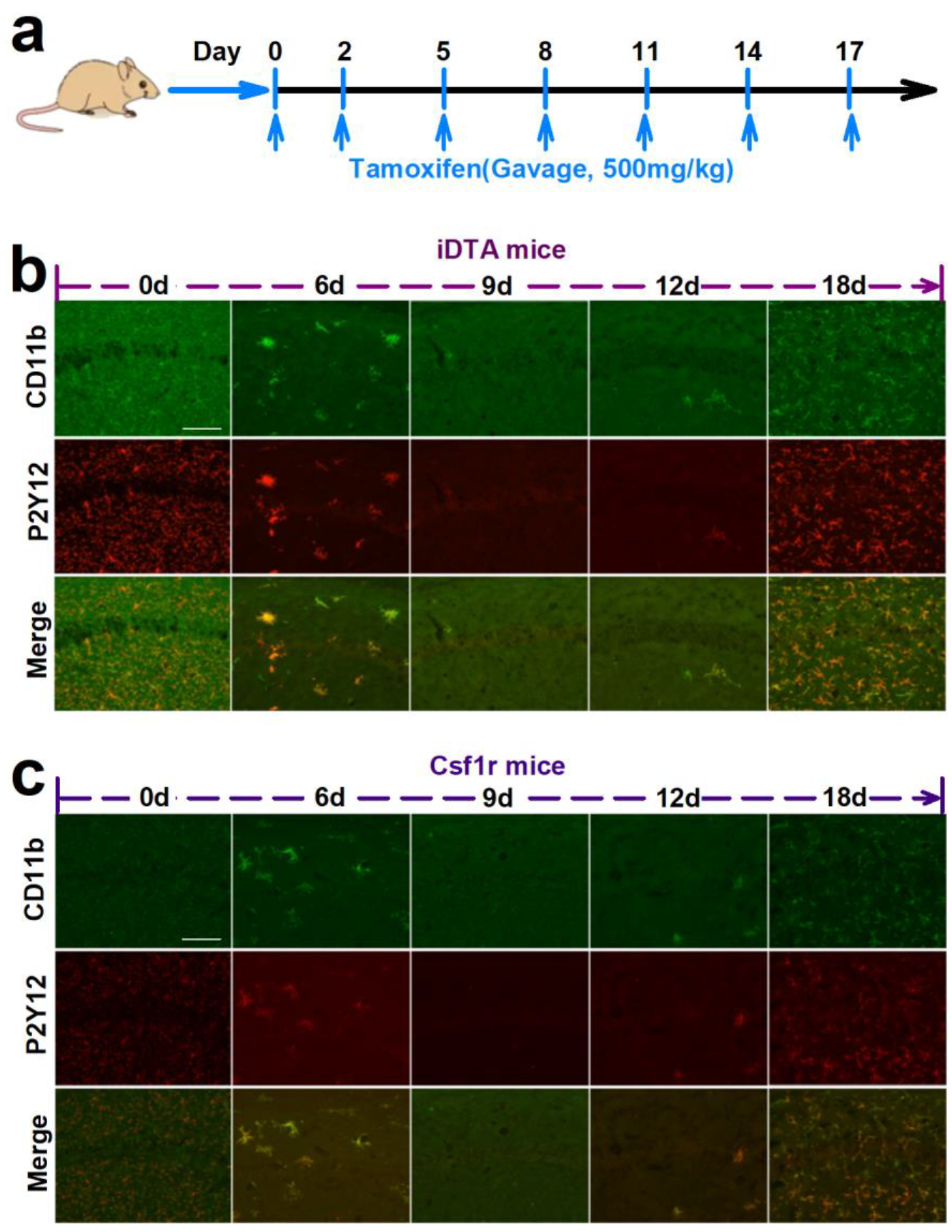
a, The scheme of tamoxifen treatment. b,c Representative images show that CD11b (green) and P2Y12 (red) are co-expressed in re-populated cells in the brain of iDTA mice (b) and Csf1r mice (c). Scale bar=50 μm.
Microglia depletion increases acute seizure severity after KA-induced status epilepticus.
Next, we investigated the role of microglia in KA-induced status epilepticus (SE) using iDTA mice and Csf1r mice. We chose 9d after tamoxifen treatment since this was the time point that displayed maximal microglia depletion. Intracerebroventricular (ICV) KA injection was performed to induce SE and seizure scores were evaluated using a modified Racine scale (Figure 3a). Our results showed that CX3CR1+ cells depletion in iDTA mice significantly increased acute seizure severity (Figure 3b). In addition, depletion of CX3CR1+ cells significantly increased the mortality rate induced by ICV KA (Figure 3c). Consistently, we found that microglia depletion in Csf1r mice also worsened seizure severity and increased mortality (Figure 3d–e).
Figure 3. Microglia depletion increases acute seizure in response to KA treatment.
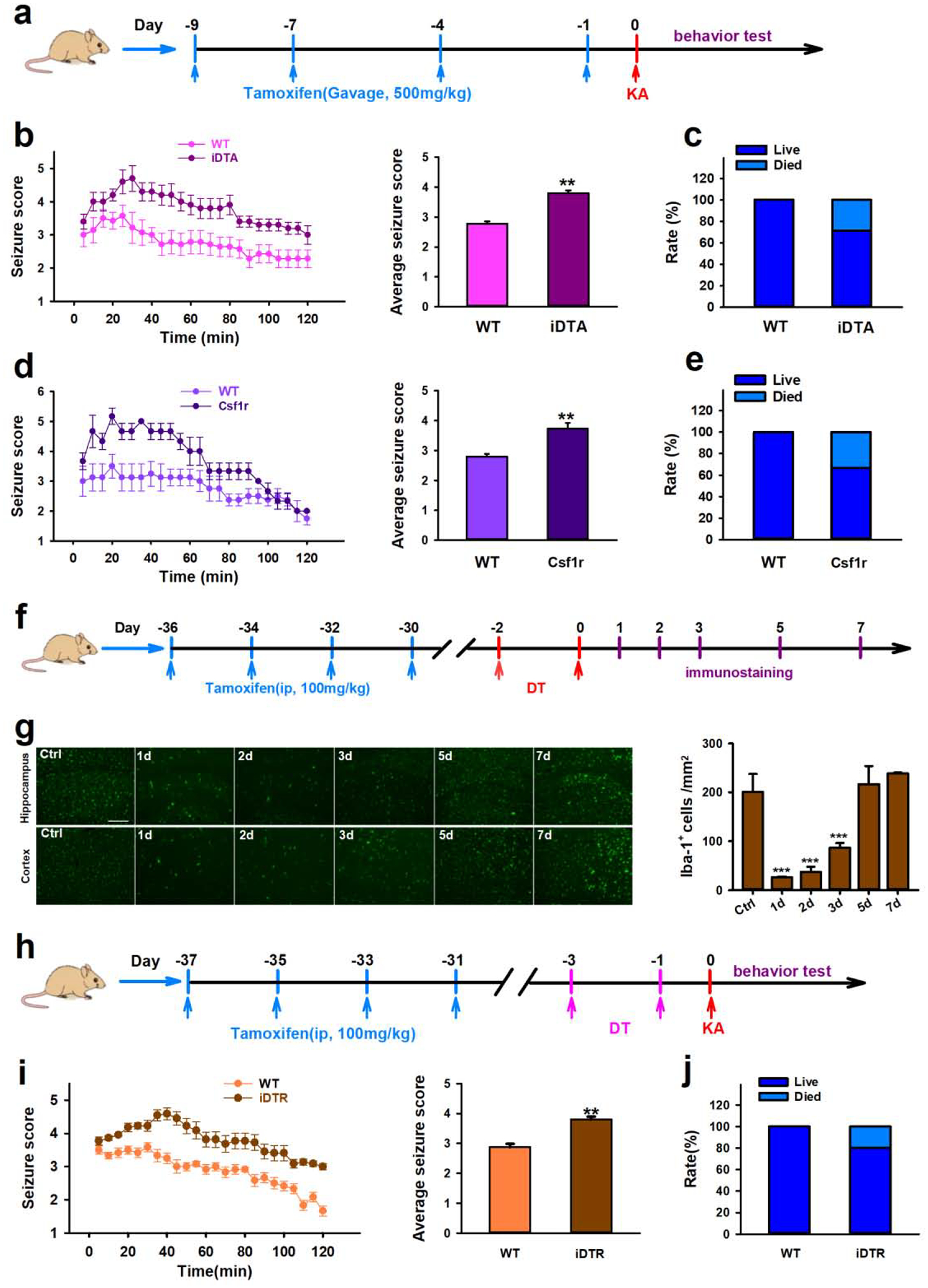
a, Drug treatment and behavior test scheme in iDTA and Csf1r mice. b–e, Quantification of seizure scores and mortality in iDTA mice (b–c) and Csf1r mice (d–e) after ICV KA. f, Drugs treatment and immunostaining scheme in iDTR mice. g, Representative images and quantification of Iba-1+ cells showing microglia depletion and re-population in iDTR mice at 1d, 2d, 3d, 5d, and 7d after tamoxifen and DT treatment. Scale bar=100 μm. h, Drugs treatment and behavior test scheme in iDTR mice. i–j, Quantification of seizure scores and mortality in iDTR mice after ICV KA. Data presented means ± SEM, n=6–8. ***P<0.001 or **P<0.01 vs control group.
While, the chemokine receptor CX3CR1 is predominantly expressed by microglia in the CNS, it can also found on subsets of monocytes, macrophages, natural killer cells, and dendritic cells (Jung et al., 2000). Consequently, to further evaluate if our iDTA and CSF1R results are microglia specific, we also evaluated KA-induced SE with CX3CR1CreER/+: R26iDTR/+ mice, henceforth designated iDTR mice. By taking advantage of the significantly slower turnover rate that microglia have compared to other CX3CR1+ myeloid cells, we could selectively express diphtheria toxin receptor (DTR) within microglia in a manner similar to previously described methods (Mo et al., 2019; Peng et al., 2016). We could then selectively deplete microglia via administration of diphtheria toxin (Figure 3f). We determined that approximately 90% of microglia were depleted 1d and 2d after the last DT injection and then rapidly re-populated by 7d (Figure 3g). In the light of this observed time course, KA was administered via ICV 1d after the last DT injection. We again determined seizure severity and mortality in iDTR mice, which were higher than non-depleted control mice (Figure 3h–j). These results from iDTR mice are consistent with those obtained from iDTA and Csf1r mice, suggesting specific microglia ablation aggravates the severity of acute seizures.
Microglia depletion increases KA-induced neuronal loss.
To test the effect of microglia depletion on neuronal degeneration after KA-induced SE, Fluoro-Jade-C (FJC) staining, which labels dying neurons, was performed on mice sacrificed at 1d and 3d after KA injection. We found that the number of FJC-positive neurons in the hippocampus was significantly increased in both iDTA and Csf1r mice when compared to their respective non-depleted controls (Figure 4a–e). In addition, FJC-positive neurons were detected in both ipsilateral and contralateral hippocampus in mice with microglia depletion (iDTA or Csf1r mice), while these dying neurons were only found in the ipsilateral hippocampus of WT mice. Curiously though, FJC-positive neurons were only present in the CA3 region of WT and Csf1r mice, but they were observed in both the CA1 and CA3 regions of iDTA mice (Figure 4b, d). When using iDTR mice to specifically ablate microglia, we also found increased FJC-positive neurons in both ipsilateral and contralateral hippocampus (Figure 4g–h). Moreover, iDTR results were consistent with iDTA in that FJC-positive neurons were present in both CA1 and CA3 regions. Taken together, our results indicate that microglia depletion worsens acute seizure and neuronal degeneration following KA-induced SE.
Figure 4. Microglia depletion increases neuronal injury in response to KA treatment.
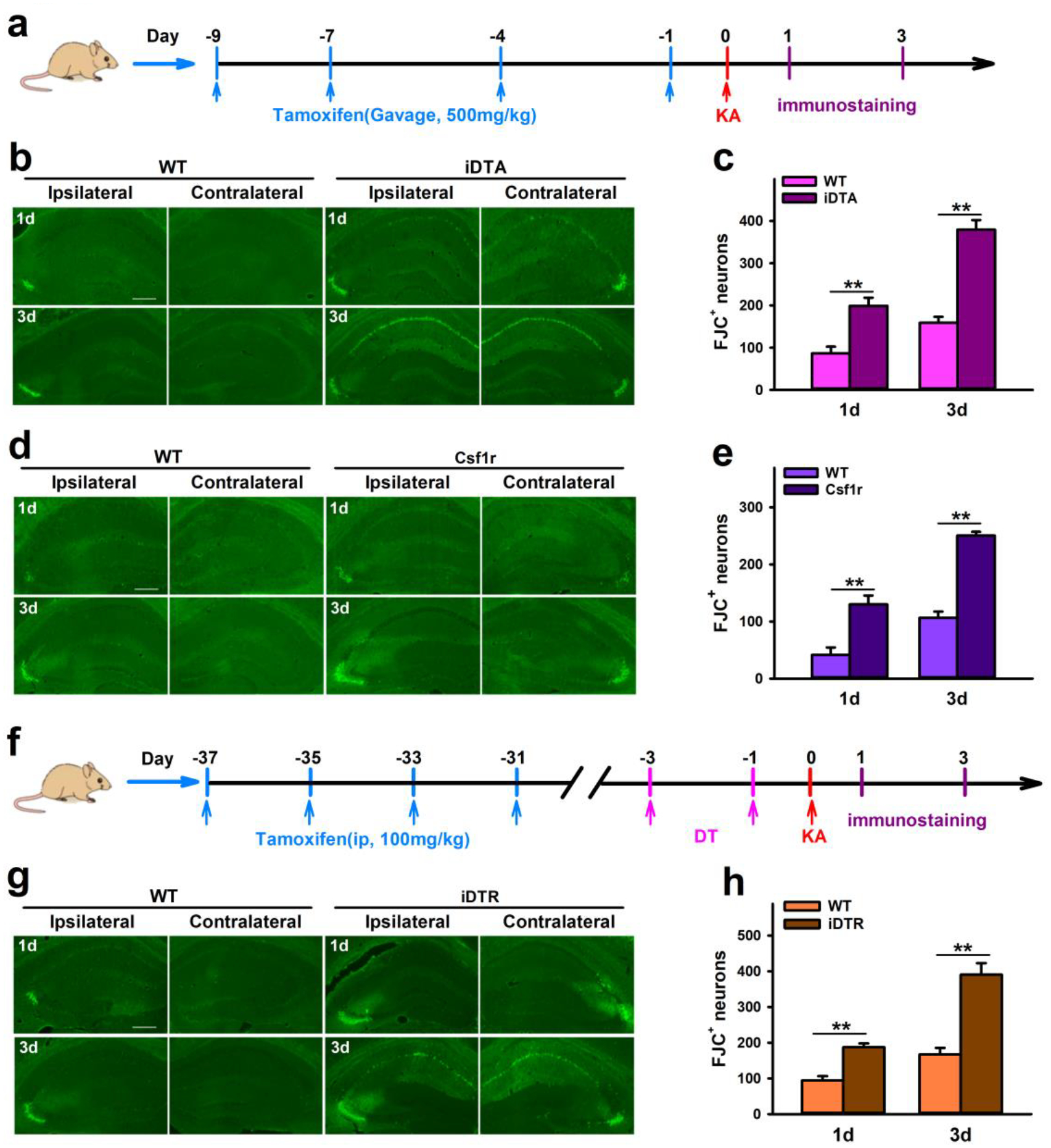
a, Drug treatment and immunostaining scheme in iDTA and Csf1r mice. b–e, Representative images and quantification of FJC+ neurons show that CX3CR1+ cell depletion worsens neuronal damage in the hippocampus of iDTA mice (b, c) and Csf1r mice (d, e) 1d and 3d after ICV KA treatment. f, Drug treatment and immunostaining scheme in iDTR mice. g–h, Representative images and quantification of FJC+ neurons show that specific microglia depletion worsens neuronal damage in the hippocampus of iDTR mice 1d and 3d after KA treatment. Scale bar=100 μm. Data presented as means ± SEM, n= 3, **P<0.01 vs control group.
Microglia depletion aggravates chronic spontaneous seizure.
To further study the effect of microglia depletion, we next examined its influence on the development of spontaneous recurrent seizures (SRS). However, previous studies including ours have shown that ICV KA cannot induce reliable SRS (Tian et al., 2017), the characteristics of epilepsy in clinics. To this end, we used hippocampal KA injection which is known to induce chronic spontaneous seizures 28 days after insults (Dugladze et al., 2007; Klement et al., 2019). We performed EEG recordings for chronic seizure measurements. In iDTA and Csf1r mice, tamoxifen was injected 20d after intra-hippocampal KA, and EEG recordings were performed between days 28–31 (when microglia are mostly depleted) after KA (Figure 5a). We found that microglia depletion resulted in increased seizure frequency and duration, as well as, interictal spiking when compared to respective controls (Figure 5b–e). Similarly, EEG recordings in iDTR mice found that microglia only depletion also increased chronic seizures (Figure 5g–h). These results demonstrate that the depletion of microglia promotes KA-induced SRS.
Figure 5. Microglia depletion aggravates spontaneous seizure induced by KA.
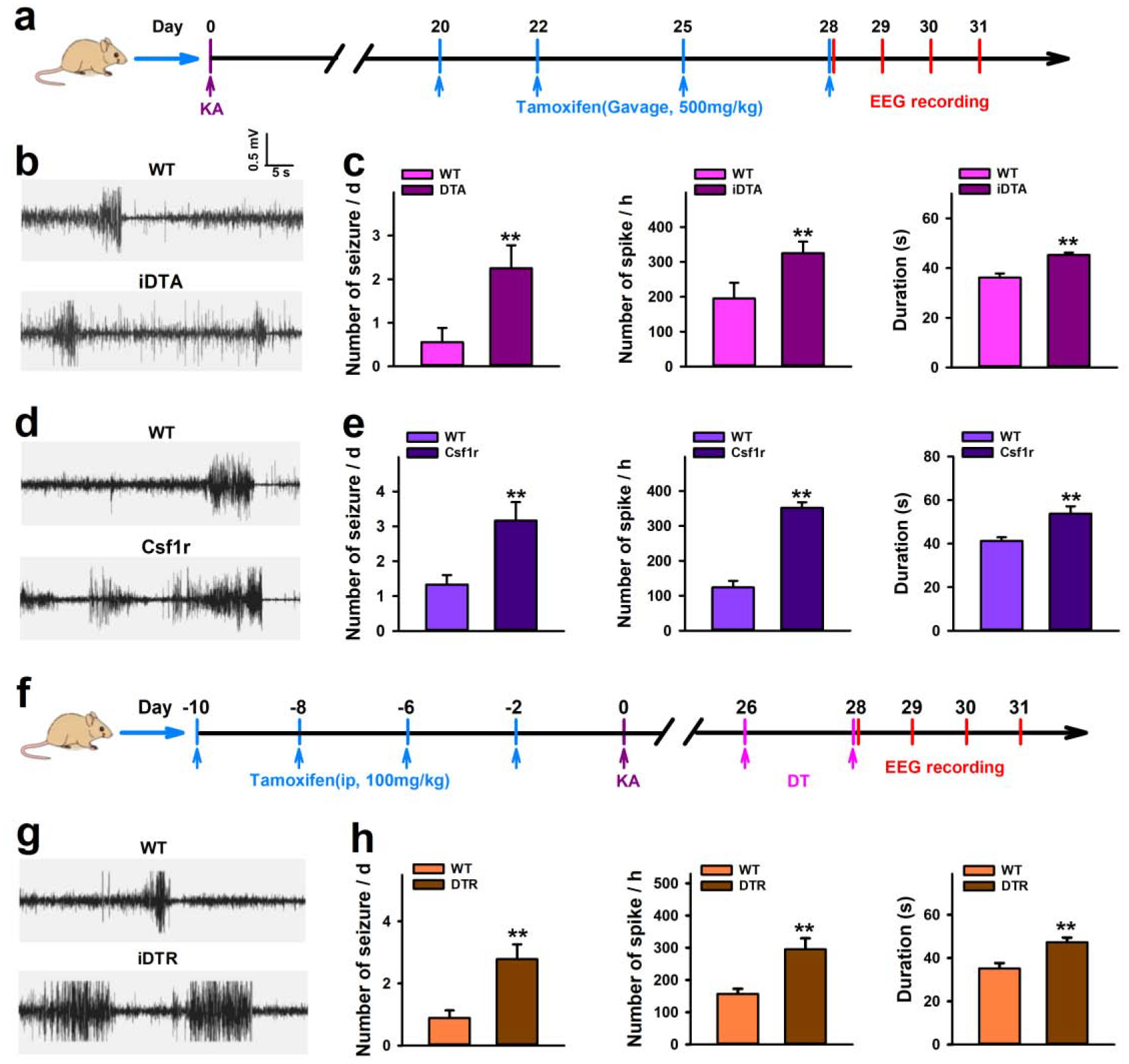
a, Drug treatment and EEG recording scheme in iDTA and Csf1r mice. b–e, Representative traces and quantification of EEG recordings show that CX3CR1+ cell ablation aggravates spontaneous seizures in iDTA mice (b, c) and Csf1r mice (d, e) starting 28 days after KA treatment. f, Drug treatment and EEG recording scheme in iDTR mice. g–h, Representative traces and quantification of EEG recordings show that specific microglia depletion increases spontaneous seizures in iDTR mice starting 28 days after KA treatment. Data presented as means ± SEM, n=3–4. **P<0.01 vs control group.
Re-populated microglia reverse the effects on SE induced by microglial depletion.
Since microglia depletion worsened SE, we wanted to determine what effects re-populated microglia would have on SE. Thus, tamoxifen was administered to iDTA mice and Csf1r mice to deplete microglia, and then tamoxifen administration was ceased to allow microglia re-population prior to KA-induced SE (Figure 6a). Microglia were fully replenished in the brain 10d after the last tamoxifen injection in both iDTA and Csf1r mice (Figure 6b–c). Accordingly, ICV KA-induced SE was induced at this time point. We found that iDTA and Csf1r mice with re-populated microglia showed no significant difference in seizure scores when compared to their respective controls (Figure 6d–e). Thus, the detrimental effects of microglial depletion on the seizure induction was reversed when microglia were re-populated. Therefore, the results suggest that microglia are beneficial in finely control the KA-induced seizure severity.
Figure 6. Re-populated microglia did not alter seizures induced by KA.
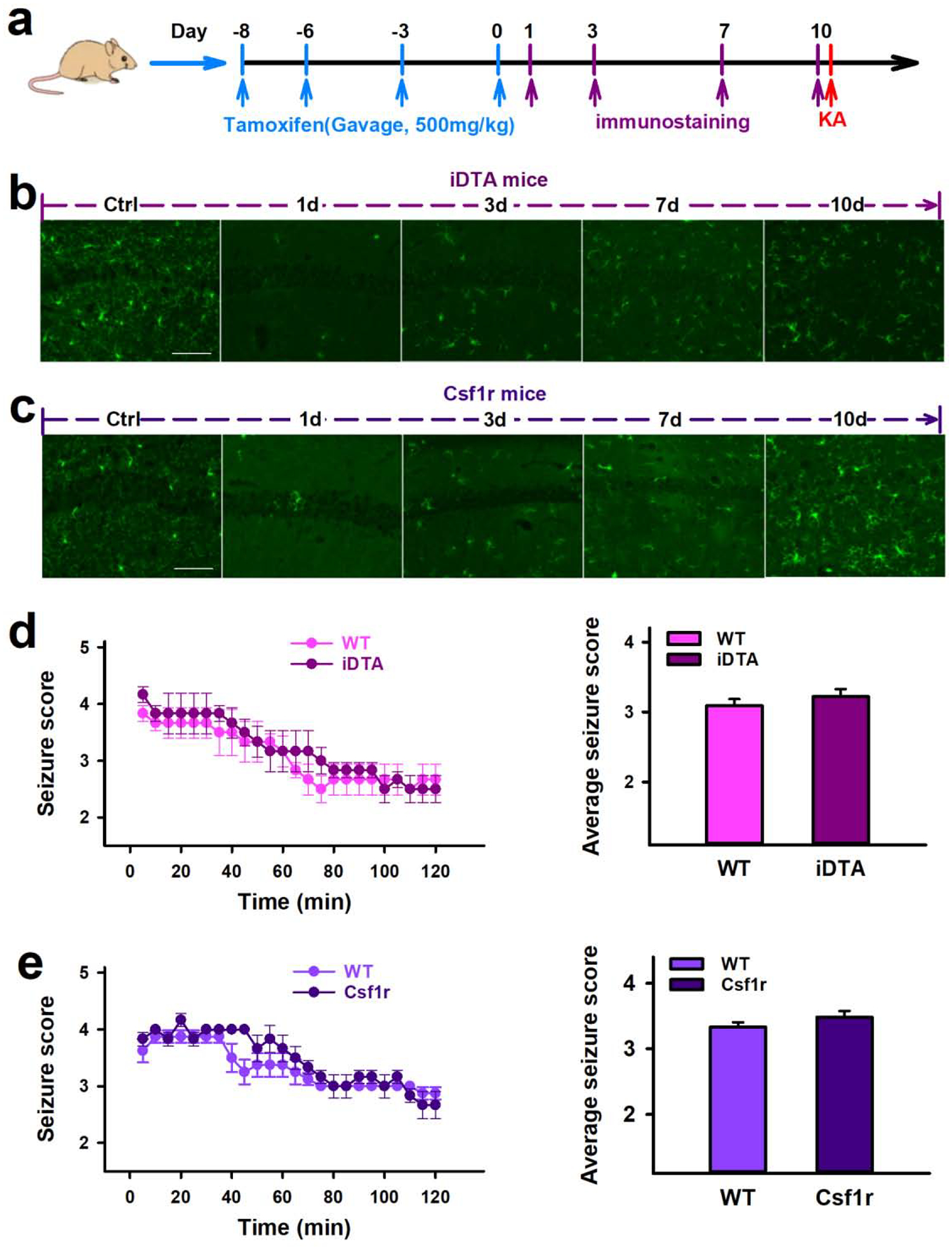
a, Drug treatment, immunostaining and behavior test scheme in iDTA and Csf1r mice. b–c, Representative images showing the time course of microglia repopulation in the hippocampus following rapid depletion in iDTA mice (b) and Csf1r mice (c). Scale bar =50 μm, n=3. d–e, Quantification of seizure scores show that iDTA mice and Csf1r mice with repopulated microglia have similar seizure scores compared with their respective controls. Data presented as means ± SEM, n=6–8.
Discussion
Microglia participate in many important physiological processes such as modulating neurotransmission, promoting neuronal survival, clearing apoptotic neurons, and regulating synaptic function (Baalman et al., 2015; Eyo and Wu, 2019; Schafer et al., 2013; Shigemoto-Mogami et al., 2014; Sipe et al., 2016; Wu, 2013). However, the role that microglia have in the pathologies of epilepsy is less studied (Eyo et al., 2017). To investigate this, we used three genetics-based microglia depletion methods to determine the importance of microglia in KA-induced seizures. We found that microglial depletion promoted both acute SE and chronic SRS. Microglia re-population then reversed the effect of microglial depletion on acute seizures. Consequently, our results suggest that microglia are overall protective and play a beneficial role in controlling KA-induced seizures and epilepsy.
It is increasingly suggested that microglia have opposing roles in seizures, being beneficial during the acute phase and deleterious during the chronic phase (Bhandare et al., 2017). However, contrary to some reports that showed preventing microglia activity during that chronic phase was beneficial (Bhandare et al., 2017; Wang et al., 2015), we found microglia depletion increased the frequency of SRS and seizure duration. It should be noted however, many of these studies utilize minocycline, an inhibitor of microglial activation, which can also positively influence neuronal, astrocytic, and oligodendrocyte activities (Huang et al., 2010; Moller et al., 2016). More recently, a study using PLX-3397, a specific inhibitor of Csf1r, demonstrated anti-epileptic effects in acute and chronic seizure models (Srivastava et al., 2018). That study used only one-sixth of the dose for microglia depletion (Elmore et al., 2014; Sosna et al., 2018), impacting microglial morphology and activation without reducing cell survival (Srivastava et al., 2018). This is in contrast to other studies using PLX-5622 to deplete microglia, which showed microglia have neuroprotective effects (Sanchez et al., 2019; Waltl et al., 2018). These results suggest that while inhibition of microglial Csf1r may have an anti-seizure effect, microglia deletion leads to worse seizures.
A recent study using iDTR mice suggests increased inflammation after microglia ablation (Rubino et al., 2018). If this is the case, the observed effects upon seizure severity might not be directly attributable to the microglial protective function. However, there are several caveats to the published report (Rubino et al., 2018), such as administration scheme and dosage of DT. Three daily i.p. doses of 1.0 μg DT were used, which could result in high concentrations of circulating DT. Although DT receptor is not expressed on native cells in mice, there could be chance for DT to enter into cells under high dose exposure (Yamaizumi et al., 1978). Therefore, DT could potentially do harm to even WT mice. In addition, our deletion model using Csf1r knockout was consistent with iDTA and iDTR results, suggesting the increased seizures may not be due to the elevated inflammation. Future studies are needed to definitively exclude the possibility that microglia ablation-associated inflammation might be partially responsible for aggravated seizures.
Memory and cognitive impairment are a common comorbidity of epilepsy, especially for epilepsy after viral encephalitis (Liu, 2019), which are associated with SRS and structural brain lesions (Helmstaedter and Witt, 2017). Hippocampus is the major structure involved in the learning, memory and affective behavior. Recurrent seizures can cause hippocampal damage, resulting in cognitive deficits (Vrinda et al., 2019). Our previous study indeed showed that KA-induced seizures lead to memory deficits in mice (Tian et al., 2017). Here we found that microglia depletion aggravated seizures and increased hippocampal neuronal loss in SE. Therefore, these microglia-depleted mice might exhibit worse memory loss compared with control mice after ICV KA, suggesting microglia could be beneficial for protecting cognitive function in KA-induced seizure. Further efforts will be made to investigate neuronal loss in chronic seizure and its effect on memory and cognitive function.
Another interesting aspect of our study was the re-population of microglia within each of our depletion methods. This is consistent with previous reports showing pharmacologically and genetically mediated microglial depletion can be reversed within a relatively short period (Elmore et al., 2014; Han et al., 2017; Varvel et al., 2012). Interestingly, in iDTA and Csf1r mice also show complete microglial repopulation even in the presence of tamoxifen. Therefore, the repopulated microglia may come from insufficient ablation (without iDTA expression or Csf1r deficiency) due to the efficiency of CX3CR1 cre recombinase. Our results also suggest that the origin of repopulated cells was derived from residual microglia because those re-populated microglia are mostly expressing P2Y12 receptor. This is in line with a recent study showing repopulated microglia are from residual resident microglia (Huang et al., 2018), other than from nestin-positive microglia progenitors (Elmore et al., 2014). Another study suggests a dual origin of re-populated microglia, local microglial proliferation combined with infiltration of BM-derived precursors (Lund et al., 2018). It is possible that different depletion methods could result in different routes of microglia re-population. We further found that the microglia repopulation reversed the effect of microglial depletion on the seizures, strengthening the idea that microglia are beneficial in controlling seizures.
In summary, we effectively depleted microglia using three different genetic models and found that microglia depletion increases the severity of seizure and neuronal degeneration following acute seizures, which can be reserved by microglial re-population. Additionally, microglia depletion worsened chronic SRS. Taken together, our study demonstrated that microglia have important protective roles in both the acute and chronic phase of epileptogenesis.
Methods
Animals:
Six to twelve weeks old male mice were used for all experiments in accordance with institutional guidelines as approved by the Mayo Clinic Institutional Animal Care and Use Committee. CX3CR1CreER/+:R26iDTA/+ and CX3CR1CreER/+:R26iDTR/+ mice were generated by crossing CX3CR1CreER/CreER mice [B6.129P2(Cg)-CX3CR1tm2.1(cre/ERT2)Litt/WganJ; Jackson Labs, Bar Harbor, ME] with either R26iDTA/+ [B6.129P2-Gt(ROSA)26Sortm1(DTA)Lky/J; Jackson Labs] or R26iDTR/+ [C57BL/6-Gt(ROSA)26Sortm1(HBEGF)Awai/J; Jackson Labs]. CX3CR1CreER/+: Csf1rFlox/Flox mice were generated by crossing CX3CR1CreER/+: Csf1rFlox/Flox mice with Csf1rFlox/Flox mice (B6.Cg-Csf1rtm1.2Jwp/J; Jackson Labs). Littermate, CX3CR1CreER/+ mice or Csf1rFlox/Flox mice treated with tamoxifen were used as respective controls. Experimenters were blind to genotypes.
Ablation induction:
For CX3CR1CreER/+: R26iDTA/+ and CX3CR1CreER/+: Csf1rFlox/Flox mice, two initial doses of tamoxifen (500 mg/kg, 20 mg/ml in corn oil) was administered orally in 48 h intervals, followed by an equal repeated dose every third day. For CX3CR1CreER/+: R26iDTR/+ mice, tamoxifen (150 mg/kg, 20 mg/ml in corn oil) was administered by i.p. injection in 48 h intervals. 3 days after the last tamoxifen treatment, two doses of diphtheria toxin (DT, 50μg/kg) were given by i.p. injection in 48 h intervals. For specific microglia depletion, the interval between the last tamoxifen and the first DT was 4 weeks. Respective control mice were administered with corresponding dose of tamoxifen and DT.
Status epilepticus induction:
KA-induced SE was performed as previously described (Eyo et al., 2014; Tian et al., 2017). Briefly, animals were anesthetized with isoflurane and a 26-gauge, stainless steel guide cannula was implanted into the intracerebroventricular (ICV) space (−0.2 mm anteroposterior (AP), +1.0 mm mediolateral (ML), −2.0 mm dorsoventral (DV)). 24 h later kainic acid (KA; 0.15 μg in 5 μl of artificial CSF) was then administered. Seizure behavior was monitored using a modified Racine scale: (1) freezing behavior; (2) rigid posture with raised tail; (3) continuous head bobbing and forepaws shaking; (4) rearing, falling, and jumping; (5) continuous level 4; and (6) loss of posture and generalized convulsion activity (Avignone et al., 2008; Racine, 1972).
Spontaneous recurrent seizure induction and EEG recording:
Animals were anesthetized with isoflurane and 70 ng of KA in 2 μl of aCSF was injected into the hippocampus (−1.6 mm anteroposterior (AP), +1.8 mm mediolateral (ML), −1.6 mm dorsoventral (DV)). Electroencephalogram (EEG) electrodes and pre-amplifier (Pinnacle Technologies) were then implanted for cortical recording. Briefly, four bone screw/recoding electrodes were placed onto the dura through burr holes in the skull. Dental cement was used to permanently affix the electrodes and pre-amplifier to the skull. After 4 weeks animals were connected to the recording system and EEG were recorded continuously for 3 days. EEG data was analyzed with Matlab2018a. Seizure spells were defined as high-frequency>5Hz, high-voltage synchronized poly-spikes or paroxysmal sharp waves with amplitude >2-fold of background and lasting >6 s. Interictal spikes were defined as paroxysmal electrical sharp activities with amplitude >2-fold of background, frequency< 5 Hz, duration < 100 ms.
Fluorescent immunostaining and analysis:
Mice were deeply anaesthetized with isoflurane (5% in O2) and transcardially perfused with 20 ml PBS followed by 20 ml of cold 4% paraformaldehyde (PFA) in PBS containing 1.5% picric acid. Brains were then removed and post-fixed with the same 4% PFA solution for 4–6 h at 4°C. The samples were then transferred to 30% sucrose in PBS overnight. Sample sections (14 μm in thickness) were prepared on gelatin-coated glass slide via cryosection. Sections were blocked with 5% goat serum and 0.3% Triton X-100 in TBS for 60 min, and then incubated overnight at 4°C with primary antibody for Iba1 (019–19741; 1:1,000; Wako Chemicals, Richmond, VA), CD11b (101202; 1:200; Biolegend, San Diego, CA), or P2Y12 (66043A; 1:500; Anaspec, Fremont, CA). The sections were then washed with TBS and incubated with secondary antibodies (1:500 Alexa Fluor 594 and Alexa Fluor 488; Life Technologies, Carlsbad, CA) for 60 min at room temperature before mounting with Fluoromount-G (SouthernBiotech, Birmingham, AL). Fluorescent images were obtained with an EVOS florescent microscope (ThermoFisher Scientific, Waltham, MA). Cell numbers were quantified using ImageJ (National Institutes of Health, Bethesda, MD).
Fluoro-Jade C staining and analysis:
Fluoro-Jade C (FJC) was used to investigate neuronal degeneration. Briefly, previously collected tissue sections were brought to room temperature and air dried. Slides were next washed with PBS, incubated in a solution of 80% EtOH and 1% NaOH for 5 mins, then washed with 70% EtOH followed by ddiH2O. Slides were then immersed in 0.006% potassium permanganate solution for 15 mins, then again washed with ddiH2O. Slides were then placed in a solution of 0.001% FJC (Millipore-Sigma, Burlington, MA) in 0.1% acetic acid for 30 min. Finally, sections were dehydrated and cleared with xylenes, air dried, and mounted with a coverslip. FJC-positive cells were quantified using ImageJ (National Institutes of Health). Three sections per mouse were selected. The numbers of FJC+ neurons from 9 sections (3 sections per mouseⅹ3 mice) were averaged and the results were reported as the number of FJC+ neurons / hippocampus per brain section.
Statistical analysis:
Data is expressed as means ± SEM. Two-sided and unpaired Student’s t test were used to evaluate differences. P<0.05 was considered statistically significant.
Highlights.
Three microglia-specific genetic tools were used, CX3CR1CreER/+:R26iDTA/+, CX3CR1CreER/+:R26iDTR/+, and CX3CR1CreER/+:Csf1rFlox/Flox mice to study microglia depletion in epilepsy.
Microglial depletion worsened KA-induced status epilepticus, higher mortality rate, and increased neuronal degeneration.
Microglial depletion increased seizure frequency, interictal spiking, and seizure duration in KA-induced chronic spontaneous recurrent seizures.
Acknowledgements
This work is supported by National Institute of Health (R01NS088627, R01NS112144).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The authors declare no competing financial interests.
References
- Abraham J, Fox PD, Condello C, Bartolini A, Koh S, 2012. Minocycline attenuates microglia activation and blocks the long-term epileptogenic effects of early-life seizures. Neurobiology of disease 46, 425–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avignone E, Ulmann L, Levavasseur F, Rassendren F, Audinat E, 2008. Status epilepticus induces a particular microglial activation state characterized by enhanced purinergic signaling. J Neurosci 28, 9133–9144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baalman K, Marin MA, Ho TS, Godoy M, Cherian L, Robertson C, Rasband MN, 2015. Axon initial segment-associated microglia. J Neurosci 35, 2283–2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhandare AM, Kapoor K, Powell KL, Braine E, Casillas-Espinosa P, O’Brien TJ, Farnham MMJ, Pilowsky PM, 2017. Inhibition of microglial activation with minocycline at the intrathecal level attenuates sympathoexcitatory and proarrhythmogenic changes in rats with chronic temporal lobe epilepsy. Neuroscience 350, 23–38. [DOI] [PubMed] [Google Scholar]
- Brown GC, Neher JJ, 2010. Inflammatory neurodegeneration and mechanisms of microglial killing of neurons. Molecular neurobiology 41, 242–247. [DOI] [PubMed] [Google Scholar]
- Devinsky O, Vezzani A, Najjar S, De Lanerolle NC, Rogawski MA, 2013. Glia and epilepsy: excitability and inflammation. Trends Neurosci 36, 174–184. [DOI] [PubMed] [Google Scholar]
- Dugladze T, Vida I, Tort AB, Gross A, Otahal J, Heinemann U, Kopell NJ, Gloveli T, 2007. Impaired hippocampal rhythmogenesis in a mouse model of mesial temporal lobe epilepsy. Proceedings of the National Academy of Sciences of the United States of America 104, 17530–17535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmore MR, Najafi AR, Koike MA, Dagher NN, Spangenberg EE, Rice RA, Kitazawa M, Matusow B, Nguyen H, West BL, Green KN, 2014. Colony-stimulating factor 1 receptor signaling is necessary for microglia viability, unmasking a microglia progenitor cell in the adult brain. Neuron 82, 380–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyo UB, Murugan M, Wu LJ, 2017. Microglia-Neuron Communication in Epilepsy. Glia 65, 5–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyo UB, Peng J, Swiatkowski P, Mukherjee A, Bispo A, Wu LJ, 2014. Neuronal Hyperactivity Recruits Microglial Processes via Neuronal NMDA Receptors and Microglial P2Y12 Receptors after Status Epilepticus. J Neurosci 34, 10528–10540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyo UB, Wu LJ, 2019. Microglia: Lifelong patrolling immune cells of the brain. Prog Neurobiol, 101614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ficker DM, So EL, Shen WK, Annegers JF, O’Brien PC, Cascino GD, Belau PG, 1998. Population-based study of the incidence of sudden unexplained death in epilepsy. Neurology 51, 1270–1274. [DOI] [PubMed] [Google Scholar]
- Fu R, Shen Q, Xu P, Luo JJ, Tang Y, 2014. Phagocytosis of microglia in the central nervous system diseases. Molecular neurobiology 49, 1422–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galic MA, Riazi K, Pittman QJ, 2012. Cytokines and brain excitability. Frontiers in neuroendocrinology 33, 116–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu N, Peng J, Murugan M, Wang X, Eyo UB, Sun D, Ren Y, DiCicco-Bloom E, Young W, Dong H, Wu LJ, 2016. Spinal Microgliosis Due to Resident Microglial Proliferation Is Required for Pain Hypersensitivity after Peripheral Nerve Injury. Cell Rep 16, 605–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J, Harris RA, Zhang XM, 2017. An updated assessment of microglia depletion: current concepts and future directions. Molecular brain 10, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmstaedter C, Witt JA, 2017. Epilepsy and cognition - A bidirectional relationship? Seizure 49, 83–89. [DOI] [PubMed] [Google Scholar]
- Heo K, Cho YJ, Cho KJ, Kim HW, Kim HJ, Shin HY, Lee BI, Kim GW, 2006. Minocycline inhibits caspase-dependent and -independent cell death pathways and is neuroprotective against hippocampal damage after treatment with kainic acid in mice. Neuroscience letters 398, 195–200. [DOI] [PubMed] [Google Scholar]
- Hu MH, Huang GS, Wu CT, Lin JJ, Hsia SH, Wang HS, Lin KL, Group CS, 2014. Analysis of plasma multiplex cytokines for children with febrile seizures and severe acute encephalitis. Journal of child neurology 29, 182–186.23674232 [Google Scholar]
- Huang WC, Qiao Y, Xu L, Kacimi R, Sun X, Giffard RG, Yenari MA, 2010. Direct protection of cultured neurons from ischemia-like injury by minocycline. Anat Cell Biol 43, 325–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Xu Z, Xiong S, Sun F, Qin G, Hu G, Wang J, Zhao L, Liang YX, Wu T, Lu Z, Humayun MS, So KF, Pan Y, Li N, Yuan TF, Rao Y, Peng B, 2018. Repopulated microglia are solely derived from the proliferation of residual microglia after acute depletion. Nat Neurosci 21, 530–540. [DOI] [PubMed] [Google Scholar]
- Jimenez-Pacheco A, Diaz-Hernandez M, Arribas-Blazquez M, Sanz-Rodriguez A, Olivos-Ore LA, Artalejo AR, Alves M, Letavic M, Miras-Portugal MT, Conroy RM, Delanty N, Farrell MA, O’Brien DF, Bhattacharya A, Engel T, Henshall DC, 2016. Transient P2X7 Receptor Antagonism Produces Lasting Reductions in Spontaneous Seizures and Gliosis in Experimental Temporal Lobe Epilepsy. The Journal of neuroscience : the official journal of the Society for Neuroscience 36, 5920–5932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung S, Aliberti J, Graemmel P, Sunshine MJ, Kreutzberg GW, Sher A, Littman DR, 2000. Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol Cell Biol 20, 4106–4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan AA, de Jager W, de Wit M, Heijnen C, van Zuiden M, Ferrier C, van Rijen P, Gosselaar P, Hessel E, van Nieuwenhuizen O, de Graan PN, 2012. Protein expression profiling of inflammatory mediators in human temporal lobe epilepsy reveals co-activation of multiple chemokines and cytokines. Journal of neuroinflammation 9, 207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klement W, Blaquiere M, Zub E, deBock F, Boux F, Barbier E, Audinat E, Lerner-Natoli M, Marchi N, 2019. A pericyte-glia scarring develops at the leaky capillaries in the hippocampus during seizure activity. Epilepsia 60, 1399–1411. [DOI] [PubMed] [Google Scholar]
- Kwan P, Brodie MJ, 2000. Early identification of refractory epilepsy. The New England journal of medicine 342, 314–319. [DOI] [PubMed] [Google Scholar]
- Leal B, Chaves J, Carvalho C, Rangel R, Santos A, Bettencourt A, Lopes J, Ramalheira J, Silva BM, da Silva AM, Costa PP, 2017. Brain expression of inflammatory mediators in Mesial Temporal Lobe Epilepsy patients. Journal of neuroimmunology 313, 82–88. [DOI] [PubMed] [Google Scholar]
- Liu Y, Zhou WJ, 2019. Clinical features and surgical treatment of epilepsy after viral encephalitis. Brain Science Advances 5, 41–50. [Google Scholar]
- Lund H, Pieber M, Parsa R, Han J, Grommisch D, Ewing E, Kular L, Needhamsen M, Espinosa A, Nilsson E, Overby AK, Butovsky O, Jagodic M, Zhang XM, Harris RA, 2018. Competitive repopulation of an empty microglial niche yields functionally distinct subsets of microglia-like cells. Nature communications 9, 4845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirrione MM, Konomos DK, Gravanis I, Dewey SL, Aguzzi A, Heppner FL, Tsirka SE, 2010. Microglial ablation and lipopolysaccharide preconditioning affects pilocarpine-induced seizures in mice. Neurobiol Dis 39, 85–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo M, Eyo UB, Xie M, Peng J, Bosco DB, Umpierre AD, Zhu X, Tian DS, Xu P, Wu LJ, 2019. Microglial P2Y12 Receptor Regulates Seizure-Induced Neurogenesis and Immature Neuronal Projections. J Neurosci 39, 9453–9464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller T, Bard F, Bhattacharya A, Biber K, Campbell B, Dale E, Eder C, Gan L, Garden GA, Hughes ZA, Pearse DD, Staal RG, Sayed FA, Wes PD, Boddeke HW, 2016. Critical data-based re-evaluation of minocycline as a putative specific microglia inhibitor. Glia 64, 1788–1794. [DOI] [PubMed] [Google Scholar]
- Moshe SL, Perucca E, Ryvlin P, Tomson T, 2015. Epilepsy: new advances. Lancet 385, 884–898. [DOI] [PubMed] [Google Scholar]
- Nilsson L, Farahmand BY, Persson PG, Thiblin I, Tomson T, 1999. Risk factors for sudden unexpected death in epilepsy: a case-control study. Lancet 353, 888–893. [DOI] [PubMed] [Google Scholar]
- Peng J, Gu N, Zhou L, U BE, Murugan M, Gan WB, Wu LJ, 2016. Microglia and monocytes synergistically promote the transition from acute to chronic pain after nerve injury. Nature communications 7, 12029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perucca E, French J, Bialer M, 2007. Development of new antiepileptic drugs: challenges, incentives, and recent advances. The Lancet. Neurology 6, 793–804. [DOI] [PubMed] [Google Scholar]
- Pitkanen A, Sutula TP, 2002. Is epilepsy a progressive disorder? Prospects for new therapeutic approaches in temporal-lobe epilepsy. The Lancet. Neurology 1, 173–181. [DOI] [PubMed] [Google Scholar]
- Qin C, Zhou LQ, Ma XT, Hu ZW, Yang S, Chen M, Bosco DB, Wu LJ, Tian DS, 2019. Dual Functions of Microglia in Ischemic Stroke. Neurosci Bull 35, 921–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racine RJ, 1972. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol 32, 281–294. [DOI] [PubMed] [Google Scholar]
- Rubino SJ, Mayo L, Wimmer I, Siedler V, Brunner F, Hametner S, Madi A, Lanser A, Moreira T, Donnelly D, Cox L, Rezende RM, Butovsky O, Lassmann H, Weiner HL, 2018. Acute microglia ablation induces neurodegeneration in the somatosensory system. Nature communications 9, 4578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez JMS, DePaula-Silva AB, Doty DJ, Truong A, Libbey JE, Fujinami RS, 2019. Microglial cell depletion is fatal with low level picornavirus infection of the central nervous system. Journal of neurovirology 25, 415–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer DP, Lehrman EK, Stevens B, 2013. The “quad-partite” synapse: Microglia-synapse interactions in the developing and mature CNS. Glia 61, 24–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigemoto-Mogami Y, Hoshikawa K, Goldman JE, Sekino Y, Sato K, 2014. Microglia enhance neurogenesis and oligodendrogenesis in the early postnatal subventricular zone. The Journal of neuroscience : the official journal of the Society for Neuroscience 34, 2231–2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipe GO, Lowery RL, Tremblay ME, Kelly EA, Lamantia CE, Majewska AK, 2016. Microglial P2Y12 is necessary for synaptic plasticity in mouse visual cortex. Nature communications 7, 10905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosna J, Philipp S, Albay R 3rd, Reyes-Ruiz JM, Baglietto-Vargas D, LaFerla FM, Glabe CG, 2018. Early long-term administration of the CSF1R inhibitor PLX3397 ablates microglia and reduces accumulation of intraneuronal amyloid, neuritic plaque deposition and pre-fibrillar oligomers in 5XFAD mouse model of Alzheimer’s disease. Molecular neurodegeneration 13, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava PK, van Eyll J, Godard P, Mazzuferi M, Delahaye-Duriez A, Van Steenwinckel J, Gressens P, Danis B, Vandenplas C, Foerch P, Leclercq K, Mairet-Coello G, Cardenas A, Vanclef F, Laaniste L, Niespodziany I, Keaney J, Gasser J, Gillet G, Shkura K, Chong SA, Behmoaras J, Kadiu I, Petretto E, Kaminski RM, Johnson MR, 2018. A systems-level framework for drug discovery identifies Csf1R as an anti-epileptic drug target. Nature communications 9, 3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss KI, Elisevich KV, 2016. Brain region and epilepsy-associated differences in inflammatory mediator levels in medically refractory mesial temporal lobe epilepsy. Journal of neuroinflammation 13, 270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian DS, Peng J, Murugan M, Feng LJ, Liu JL, Eyo UB, Zhou LJ, Mogilevsky R, Wang W, Wu LJ, 2017. Chemokine CCL2-CCR2 Signaling Induces Neuronal Cell Death via STAT3 Activation and IL-1beta Production after Status Epilepticus. J Neurosci 37, 7878–7892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varvel NH, Grathwohl SA, Baumann F, Liebig C, Bosch A, Brawek B, Thal DR, Charo IF, Heppner FL, Aguzzi A, Garaschuk O, Ransohoff RM, Jucker M, 2012. Microglial repopulation model reveals a robust homeostatic process for replacing CNS myeloid cells. Proceedings of the National Academy of Sciences of the United States of America 109, 18150–18155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezzani A, Balosso S, Maroso M, Zardoni D, Noe F, Ravizza T, 2010. ICE/caspase 1 inhibitors and IL-1beta receptor antagonists as potential therapeutics in epilepsy. Current opinion in investigational drugs 11, 43–50. [PubMed] [Google Scholar]
- Vezzani A, French J, Bartfai T, Baram TZ, 2011a. The role of inflammation in epilepsy. Nat Rev Neurol 7, 31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezzani A, Friedman A, Dingledine RJ, 2013. The role of inflammation in epileptogenesis. Neuropharmacology 69, 16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezzani A, Maroso M, Balosso S, Sanchez MA, Bartfai T, 2011b. IL-1 receptor/Toll-like receptor signaling in infection, inflammation, stress and neurodegeneration couples hyperexcitability and seizures. Brain, behavior, and immunity 25, 1281–1289. [DOI] [PubMed] [Google Scholar]
- Vrinda M, Arun S, Srikumar BN, Kutty BM, Shankaranarayana Rao BS, 2019. Temporal lobe epilepsy-induced neurodegeneration and cognitive deficits: Implications for aging. Journal of chemical neuroanatomy 95, 146–153. [DOI] [PubMed] [Google Scholar]
- Waltl I, Kaufer C, Gerhauser I, Chhatbar C, Ghita L, Kalinke U, Loscher W, 2018. Microglia have a protective role in viral encephalitis-induced seizure development and hippocampal damage. Brain, behavior, and immunity 74, 186–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, Mi X, Gao B, Gu J, Wang W, Zhang Y, Wang X, 2015. Minocycline inhibits brain inflammation and attenuates spontaneous recurrent seizures following pilocarpine-induced status epilepticus. Neuroscience 287, 144–156. [DOI] [PubMed] [Google Scholar]
- Wolf SA, Boddeke HW, Kettenmann H, 2017. Microglia in Physiology and Disease. Annu Rev Physiol 79, 619–643. [DOI] [PubMed] [Google Scholar]
- Wu LJ, Stevens B, Duan S, MacVicar BA, 2013. Microglia in neuronal circuits. Neural Plast 2013, 586426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Dissing-Olesen L, MacVicar BA, Stevens B, 2015. Microglia: Dynamic Mediators of Synapse Development and Plasticity. Trends Immunol 36, 605–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaizumi M, Mekada E, Uchida T, Okada Y, 1978. One molecule of diphtheria toxin fragment A introduced into a cell can kill the cell. Cell 15, 245–250. [DOI] [PubMed] [Google Scholar]
- Zhao X, Eyo UB, Murugan M, Wu LJ, 2018a. Microglial interactions with the neurovascular system in physiology and pathology. Dev Neurobiol 78, 604–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Liao Y, Morgan S, Mathur R, Feustel P, Mazurkiewicz J, Qian J, Chang J, Mathern GW, Adamo MA, Ritaccio AL, Gruenthal M, Zhu X, Huang Y, 2018b. Noninflammatory Changes of Microglia Are Sufficient to Cause Epilepsy. Cell Rep 22, 2080–2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao XF, Alam MM, Liao Y, Huang T, Mathur R, Zhu X, Huang Y, 2019. Targeting Microglia Using Cx3cr1-Cre Lines: Revisiting the Specificity. eNeuro 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou LJ, Peng J, Xu YN, Zeng WJ, Zhang J, Wei X, Mai CL, Lin ZJ, Liu Y, Murugan M, Eyo UB, Umpierre AD, Xin WJ, Chen T, Li M, Wang H, Richardson JR, Tan Z, Liu XG, Wu LJ, 2019. Microglia Are Indispensable for Synaptic Plasticity in the Spinal Dorsal Horn and Chronic Pain. Cell Rep 27, 3844–3859 e3846. [DOI] [PMC free article] [PubMed] [Google Scholar]


