Abstract
Background
Ketamine is a dissociative anesthetic with analgesic properties. Ketamine’s analgesic properties have been suggested to result from its dissociative properties. To our knowledge, this postulate is unsubstantiated. We hypothesize that the dissociative and analgesic properties of ketamine are independent.
Methods
We conducted a single-site, open-label study of ketamine anesthesia (2mg/kg) in 15 healthy subjects. Midazolam was administered at a pre-specified time point to attenuate dissociation. We longitudinally assessed pre-calibrated cuff pain intensity and quality using Patient-Reported Outcomes Measurement Information System questionnaires, and dissociation, using the Clinician Administered Dissociative States Scale. Mixed effects models were used to assess whether dissociation accounted for the effect of ketamine on pain intensity and quality.
Results
The dissociation model demonstrated an inverted “U” shaped quadratic relationship between time and dissociation scores. Additive to this effect, midazolam reduced the dissociation adjusted means by 10.3 [95%CI: 3.4 to 17.1] points (p = 0.005). The pain intensity model also demonstrated a “U” shaped quadratic relationship between time and pain intensity. When the pain intensity model was reanalyzed with dissociation scores as an additional covariate, the dissociation term was not retained in the model, and the other effects were preserved in direction and strength. This result was conserved for nociceptive and neuropathic pain quality.
Conclusion
Ketamine’s analgesic properties are not exclusively caused by dissociation. Thus, ketamine may be used as a probe to advance our knowledge of dissociation independent neural circuits that encode pain.
Clinical Trial Number:
Introduction
Ketamine is a phencyclidine analog with anesthetic and analgesic properties.1–4 At low doses, ketamine is associated with analgesia and dissociative symptoms, and at higher doses, it is associated with loss of responsiveness.5,6 Dissociative symptoms are characterized by distortion of visual and auditory stimuli, and feelings of detachment from the environment and self. The channel blocking activity of ketamine at N-methyl-D-aspartate (NMDA) receptors may explain these dissociative symptoms.7 This is because NMDA receptor blockade is associated with markedly dysregulated pyramidal neuronal activity that manifests as electroencephalogram high frequency oscillations when ketamine is administered alone5,6 or as a part of balanced general anesthetic technique.8–10 Ketamine also interacts with opioid, monoaminergic, cholinergic, nicotinic and muscarinic receptors.11,12
The dissociative properties of ketamine have limited its widespread use as an analgesic medication. A recent consensus guideline for the use of intravenous ketamine in acute pain management explicitly states that ketamine’s profound analgesic properties are intricately bound to its dissociative properties.13 This line of reasoning is largely supported by the following: 1) NMDA receptor-mediated activity plays a key role in sensory information processing; and, 2) analgesia and dissociation are both considered states of altered sensory perception, implying a common mechanistic basis.14,15 To our knowledge, the viewpoint that ketamine’s analgesic properties are caused by its dissociative properties has not been investigated. Thus, the analgesic and dissociative properties of ketamine analgesia may be independent. This insight may benefit future investigations of ketamine analgesia and neural circuits that encode pain in humans.
Therefore, we conducted a single-site, open-label study of ketamine anesthesia (2mg/kg) in 15 healthy subjects. Benzodiazepines are typically administered to treat ketamine-induced dissociative symptoms.15 Therefore, we administered midazolam at a pre-specified time point to facilitate disentangling analgesic and dissociative drug properties. We longitudinally assessed pre-calibrated cuff pain intensity and quality using Patient-Reported Outcomes Measurement Information System pain intensity and quality questionnaires, and dissociation, using the Clinician Administered Dissociative States Scale.16 We hypothesized that the dissociative and analgesic properties of ketamine are independent.
Materials and methods
The Partners Institutional Review Board approved this human research study (2018P000417). The data analyzed in this manuscript were acquired during an electroencephalogram study of ketamine general anesthesia that was registered on www.ClinicalTrials.gov (NCT03553758). Data from this study have not previously been published.
Subject recruitment
Subjects were recruited and data collected between September 2018 and January 2019. Subjects underwent a complete medical history and a pre-anesthesia assessment. The primary inclusion criterion was meeting American Society of Anesthesiology Physical Status I. Key criteria for exclusion were pregnancy, personal or family history of anesthesia-related complications, suspected history of drug abuse, and neuropsychiatric diagnoses. We performed the following screening laboratory tests: complete blood count, liver function, basic metabolic panel, urine toxicology, and urine pregnancy for females. We also recorded data from a 12-lead electrocardiogram.
Study Design
This was a single-site, open-label study of ketamine anesthesia in 15 healthy subjects. Figure 1 is an illustration of the study design. All study procedures were conducted at the Massachusetts General Hospital, Boston, MA. Subjects were required to avoid food and water intake for at least 8 hours prior to study onset. We performed urine toxicology and pregnancy testing prior to the administration of ketamine. We monitored blood pressure using a standard non-invasive pneumatic cuff, oxygen saturation using pulse oximetry, ventilation using capnography, and cardiac rhythm using a 5-lead electrocardiogram.
Fig. 1.
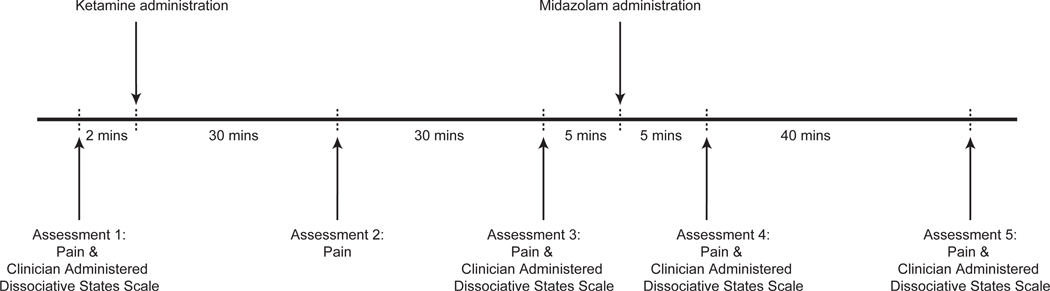
Schematic of the study protocol. We acquired data using an open-label and single-site study design in (n=15) healthy subjects. Subjects were induced to lose responsiveness with 2mg/kg of intravenous ketamine. We also administered a 2mg of intravenous midazolam to attenuate ketamine-induced dissociation. We assessed pain intensity and quality using Patient-Reported Outcomes Measurement Information System questionnaires and dissociative symptoms using the Clinician Administered Dissociative States Scale.
We rapidly (~1 minute) administered a bolus dose of intravenous ketamine (2 mg/kg) to 15 subjects and assessed pain and dissociation measures intermittently. The first assessment (pain and dissociation measures) occurred after cuff pain calibration and prior to the administration of ketamine. The second assessment (pain measures) occurred approximately 30 minutes after the administration of ketamine. The third assessment (pain and dissociation measures) occurred approximately 60 minutes after the administration of ketamine. After the third assessment, we administered 2mg of midazolam to attenuate ketamine associated dissociation. The fourth assessment (pain and dissociation measures) occurred approximately 75 minutes after the administration of ketamine. The fifth assessment (pain and dissociation measures) occurred approximately 120 minutes after the administration of ketamine. One subject did not perform assessments after recovery of responsiveness. Therefore, data from 14 subjects were analyzed in this manuscript. Of these data, only one dissociative measure datapoint was missing.
The airway was not instrumented, and all subjects breathed spontaneously throughout the study protocol without assistance. Supplemental oxygen was provided using a face mask. A board-certified anesthesiologist was present during all study procedures.
Pain and Dissociation Measures
Patient-Reported Outcomes Measurement Information System questions were used to assess for pain intensity and quality associated with the cuff pain stimulus. The National Institutes of Health Common Fund’s Patient-Reported Outcomes Measurement Information System was designed to provide patient reported outcome measurement tools developed using advances in information technology, psychometrics, and qualitative, cognitive, and health survey research to measure outcomes such as pain.17 Patient-Reported Outcomes Measurement Information System measures are normalized to a standardized t-distribution (https://www.assessmentcenter.net/ac_scoringservice). The standard t-score mean for Patient-Reported Outcomes Measurement Information System questionnaires is 50 for the population, with a standard deviation of 10. Higher scores indicate more of the concept being measured.
We assessed pain measured using the following questionnaires: Pain Intensity 1A–Numeric Rating Scale V1.0, Neuropathic Pain Quality 5a–v2.0, and Nociceptive Pain Quality 5a–v2.0. All subjects first underwent baseline pain stimuli calibration using a validated pneumatic cuff pain device (Hokanson Rapid Cuff Inflator),18–20 calibrated to 7 out of 10 pain using the Pain Intensity 1A–Numeric Rating Scale V1.0. This device, which primarily elicits pain sensitivity in muscle and other deep tissues,18 uses a computer-controlled air compressor to inflate and maintain the cuff to a pre-specified pressure. The cuff pain stimulus was delivered to the gastrocnemius area of the lower leg.
We assessed for dissociation using the Clinician Administered Dissociative States Scale, which was developed to measure perceptual, behavioral, and attentional alterations during dissociative experiences.16 Higher Clinician Administered Dissociative States Scale scores indicate more of the concept being measured.
Statistical Methods
We did not perform an a priori sample size calculation. Data and statistical analyses plans were defined and written after the data were accessed. Patient characteristics are presented as mean ± SD. We ran a variation of a limited backward elimination mixed random and fixed effects longitudinal analysis.21 Mixed effects models are commonly used for longitudinal analysis and contain a mixture of the standard fixed predictors and “random” terms such as subjects whose effects are not of interest per se but necessary to include to correctly account for correlated error variance, in this case within the same subject. All p-values were computed based on the two-sided tests. Significance level was set at 0.05. The fixed covariate of primary interest was the quadratic effect of time (minutes post-ketamine administration). By construct, the lower order effect of time was also included as a covariate. Additional subject-level fixed covariates were sex, age, ketamine dose (mg). Random terms were subject intercepts and the interaction of subjects with time, which were allowed to be correlated.
An initial model including all the above terms was run, as well as a limited backward elimination model excluding sequentially the least significant term (p > 0.2) until only significant terms remained or nonsignificant terms subsumed within significant higher-order terms. The above model was run separately for each of the dependent variables: Patient-Reported Outcomes Measurement Information System Pain Intensity, Patient-Reported Outcomes Measurement Information System Neuropathic Pain, Patient-Reported Outcomes Measurement Information System Nociceptive Pain, and Clinician Administered Dissociative States Scale. A time-varying fixed binary covariate of pre-midazolam versus post-midazolam was included in the Clinician Administered Dissociative States Scale model. Further models were run in which each pain measure was the dependent variable but with an additional covariate assessing dissociation to assess the possible role of dissociation in mediating the effects of ketamine on pain. Relevant differences in least squares means estimates (i.e., midazolam, sex) in the final models were reported as adjusted means. Residuals from all fit models were examined for conformance to assumptions of normality. JMP Pro 14 (Cary, NC, US) was the statistical software employed for all analyses.
Results
We obtained written informed consent from 15 subjects (8 males), age 24 ± 3.4 years, mean weight 70 ± 12 kg, and mean BMI 23.5 ± 2.4 kg/m2.Results from Patient-Reported Outcomes Measurement Information System and Clinician Administered Dissociative States Scale assessments are summarized in table 1.
Table 1.
Pain and dissociation scores
| Pre ketamine | ~30 minutes post ketamine | ~60 minutes post ketamine | ~75 minutes post ketamine; ~15 minutes post midazolam | ~120 minutes post ketamine; ~60 minutes post midazolam | |
|---|---|---|---|---|---|
| Clinician Administered Dissociation States Score, mean ± SD | 0.2 ± 0.4 | Not applicable | 22.1 ± 17 | 14.3 ± 11.6 | 5.2 ± 7.3 |
| Pain intensity, mean ± SD | 7.9 ± 0.5 | 1.6 ± 1.6 | 4.1± 2.2 | 4.3 ± 2.2 | 5.4 ± 2.3 |
| Nociceptive pain, mean ± SD | 50.6 ± 9.4 | 37.2 ± 8.6 | 41.2 ± 7.6 | 43.7 ± 9.6 | 47 ± 10.7 |
| Neuropathic pain, mean ± SD | 47.9 ± 5.6 | 39.9 ± 3.8 | 44.7 ± 4.6 | 43.7 ± 5 | 44.4 ± 6.4 |
Pain intensity and dissociation are independently modulated by ketamine
First, we assessed for differences in dissociation scores. The final backward elimination model (sex, age, and ketamine dose were eliminated) demonstrated an inverted “U” shaped quadratic relationship between time and dissociation (Figure 2a). The shape of this curvilinear relation was disrupted and lowered by the additive effect of midazolam at the point it was administered (Figure 2a). Midazolam reduced the dissociation adjusted means by 10.3 [95%CI: 3.4 to 17.1] (p = 0.005; Table 2a). Residuals from this model and all those below did not differ markedly from normality.
Fig. 2.
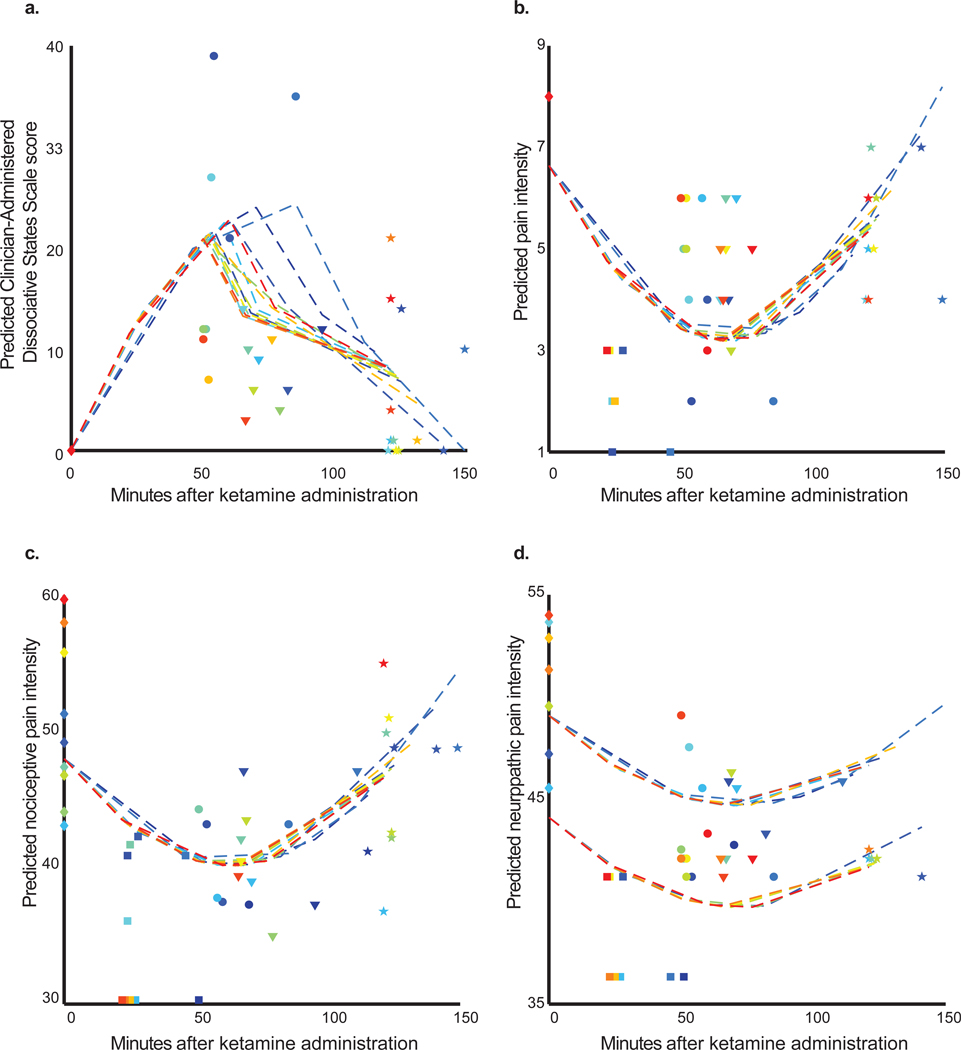
Raw and predicted data for ketamine induced dissociation and pain measures. Each dashed line connects conditional predicted values from the model for the same subject (arbitrary colors are a visual aid to differentiate subjects). Raw values are depicted with markers and aligned with the same color scheme above. Markers shapes are grouped to represent the different assessment periods. (A) Illustration of predicted inverted “U” shaped quadratic relationship between Clinician Administered Dissociative States Scale and time after ketamine administration. Also apparent in the figure is an attenuating effect of midazolam noticeable after the point of its administration for each subject. The final backward elimination models demonstrated a “U” shaped quadratic relation between time and (B) pain intensity, (C) nociceptive pain, and (D) neuropathic pain. The two distinct clusters of trajectories in (D) illustrate a main effect of sex (higher cluster corresponds to males; lower females).
Table 2.
Final Backward Elimination Models
| Term | Estimate [95% CI] | P-value |
|---|---|---|
| Table 2a. Clinician Administered Dissociated States Score final model | ||
| Intercept | −10.2 [−19.0, −1.3] | 0.026 |
| Midazolam [N] | 10.3 [3.4, 17.1] | 0.005 |
| Time (min after ketamine) | 0.6 [0.5, 0.8] | <0.0001 |
| Time (min after ketamine) x Time (min after ketamine) | −0.004 [−0.01, −0.003 ] | <0.0001 |
| Table 2b. Pain intensity final model | ||
| Intercept | 6.6 [5.7, 7.6] | <0.0001 |
| Time (min after ketamine) | −0.1 [−0.1, −0.1] | <0.0001 |
| Time (min after ketamine) x Time (min after ketamine) | 0.001 [0.001, 0.001] | <0.0001 |
| Table 2c. Nociceptive pain final model | ||
| Intercept | 48.0 [43.0, 53.0] | <0.0001 |
| Time (min after ketamine) | −0.3 [−0.4, −0.1] | 0.0002 |
| Time (min after ketamine) x Time (min after ketamine) | 0.002 [0.001, 0.003] | <0.0001 |
| Table 2d. Neuropathic pain final model | ||
| Intercept | 48.7 [45.6, 51.8] | <0.0001 |
| Time (min after ketamine) | −0.1 [−0.2, −0.02] | 0.015 |
| Time (min after ketamine) x Time (min after ketamine) | 0.001 [0.0001, 0.001] | 0.024 |
| Sex [F] | −4.5 [−8.0, −1.0] | 0.016 |
Estimate, unstandardized partial regression coefficient; for midazolam and sex, the estimate represents the difference in adjusted means; CI, confidence interval.
Next, we assessed for differences in pain intensity scores. The final backward elimination model (sex, age, and ketamine dose were eliminated) demonstrated a “U” shaped quadratic relationship between time and pain intensity (Figure 2b; Table 2b) with a minimum value at approximately 60 minutes post ketamine. When this final pain intensity model was rerun with the dissociation term as an additional covariate, it was not retained in our model. When the dissociation term was included in the initial model for pain intensity, it was the first term removed in the backward elimination algorithm. These findings suggest that the effect of ketamine in reducing pain intensity scores is not exclusively caused by dissociation.
Nociceptive pain quality and dissociation are independently modulated by ketamine
The final nociceptive pain quality backward elimination model (sex, age, and ketamine dose were eliminated) demonstrated a “U” shaped quadratic relationship between time and nociceptive pain quality (Figure 2c, Table 2c). Similar to the pain intensity model, when the final nociceptive pain quality model was rerun with the dissociation term as an additional covariate, it was not retained in our model. When the dissociation term was included in the initial model for nociceptive pain quality, it was the first term removed in the backward elimination algorithm.
Neuropathic pain quality and dissociation are independently modulated by ketamine
The final neuropathic pain quality backward elimination model (sex and ketamine dose were eliminated) demonstrated a “U” shaped quadratic relationship between time and neuropathic pain quality (Figure 2d, Table 2d).). There was also an additive main effect of sex, whereby a reduction in adjusted means of 4.5 [95% CI, 1 to 7.8] (p = 0.016) was associated with being female. Similar to the pain intensity and quality models, when the final neuropathic pain quality model was rerun with the dissociation term as an additional covariate, it was not retained in our model. When the dissociation term was included in the initial model for nociceptive pain quality, it was the first term removed in the backward elimination algorithm.
Discussion
In this investigation, we studied whether ketamine-induced analgesia is caused by ketamine-induced dissociation. We confirmed that ketamine is associated with both analgesia and dissociation,1,2 and that midazolam attenuates ketamine-induced dissociation.22,23 However, our major finding was that ketamine-induced analgesia had no strong inherent relationship with ketamine-induced dissociation beyond being independently modulated by ketamine. Thus, ketamine or its metabolites modulate distinct neural circuits to produce dissociation and analgesia.
The channel blocking activity of ketamine at NMDA receptors may explain its dissociative properties. This is because ketamine blocks excitatory NMDA receptors on fast-spiking cortical interneurons more effectively than those on pyramidal neurons.24,25 This results in markedly dysregulated pyramidal neuronal activity because the relative inactivity of cortical interneurons leads to glutamate-mediated pyramidal-pyramidal neuronal facilitation. Consistent with this notion, lamotrigine, an antiepileptic medication that reduces cortical glutamate release and pyramidal neuron facilitation, attenuates the dissociative properties of ketamine.26,27 Similarly, midazolam also reduces pyramidal neuron facilitation by downstream activity resulting from binding at gamma amino-butyric acid receptors on pyramidal neurons. Taken together, our findings suggest that the analgesic mechanisms of ketamine are unlikely to be downstream of ketamine’s activity on fast-spiking cortical interneurons or pyramidal-pyramidal neuronal facilitation.
Our results are consistent with a recent report which states that an N-aliphatic analogue of ketamine-induced analgesia comparable to ketamine but with considerably less dissociative properties (i.e., analgesia and dissociation are separable).28 However, the mechanistic underpinnings of ketamine-induced analgesia remain unclear. Intrathecal ketamine is associated with a dense motor blockade that precedes sensory blockade to suggest that spinal motor neurons are more sensitive to the effects of ketamine.29 Intravenous ketamine is not associated with motor blockade. Thus, it is unlikely that acute analgesic properties of ketamine are mediated predominantly by drug activity in the spinal cord. We note that opioids are the mainstay of pain treatment and ketamine interacts with the opioid receptors. Thus, it is reasonable to postulate that ketamine-induced analgesia is mediated through its activity at opioid receptors.30 However, studies in laboratory models have shown that the analgesic properties of ketamine are unchanged despite the administration of naloxone, an opioid receptor antagonist.31,32 This suggests that the analgesic properties of ketamine are unrelated to its activity at opioid receptors.
Bilateral interventions to the locus coeruleus in laboratory models have demonstrated the existence of locus coeruleus-noradrenergic pain circuitry,33,34 and several lines of evidence suggest that the noradrenergic system is fundamental to ketamine-induced analgesia. First, ketamine is a non-competitive inhibitor of the norepinephrine transporter, and thus targets locus coeruleus auto-receptors.35–37 Second, ketamine-induced antinociception was found to be absent after lesion of the spinal dorsolateral funiculus containing descending noradrenergic axons.38 Third, intrathecal yohimbine, an α2-adrenoceptor antagonist, has been demonstrated to diminish the analgesic properties of ketamine.39 Fourth, a correlation between locus coeruleus norepinephrine and ketamine analgesia has been demonstrated in rats with a targeted degeneration of nerve terminal projections originating from the locus coeruleus.40 Thus, ketamine-induced analgesia may be mediated through locus coeruleus-noradrenergic pain circuitry.
Functional magnetic resonance imaging studies have recently implicated the descending antinociceptive pathway, which is chiefly modulated by monoamines, in ketamine-induced analgesia from neuropathic pain.41,42 Our current finding is expected to further motivate future studies (e.g., ultra-high field functional magnetic resonance imaging) to elucidate how ketamine acts in the brainstem and cortical circuits to produce neuropathic and nociceptive analgesia. The strengths of our study include the structured and longitudinal pain and dissociation assessments, and pharmacological modulation of dissociation with midazolam. However, a key limitation is that our study population was healthy without chronic pain disorders (e.g., fibromyalgia). Further, the clinical significance of the sex difference we found in neuropathic pain is unclear. We note that there may be components of dissociation that are not captured by the Clinician Administered Dissociative States Scale. However, our results were conserved when we analyzed amnesia, depersonalization, and derealization subscales of the Clinician Administered Dissociative States Scale (Supplementary Figure 1). Validating our methodological approach, and consistent with clinical intuition, midazolam disrupted the shapes of the curvilinear relation of the depersonalization and derealization subscales, but not the amnesia subscale (i.e., midazolam does not reduce amnesia). Further, our sample size was relatively modest. Finally, our inferences may be limited to pneumatic cuff pain. However, cuff pain is preferential to deep tissue nociceptors, and is clinically relevant because most clinical pain originates in deep tissue nociceptors rather than cutaneous nociceptors. and is clinically relevant because most clinical pain originates in deep tissue nociceptors rather than cutaneous nociceptors.
Taken together, our results suggest that the ketamine-induced dissociation and analgesic properties are independent. Thus, ketamine may be used as a probe to advance our knowledge of dissociation independent neural circuits that encode pain.
Supplementary Material
Acknowledgments
Funding Statement: NIH NIA RO1AG053582 to OA; and, Innovation funds from the Department of Anesthesia, Critical Care, and Pain Medicine, Massachusetts General Hospital to OA. Funds from Department of Anesthesiology, School of Medicine, Pontificia Universidad Católica de Chile to JP.
Footnotes
Conflicts of Interest: OA has received speaker’s honoraria from Masimo Corporation and is listed as an inventor on pending patents on EEG monitoring and oral dexmedetomidine that are assigned to Massachusetts General Hospital. All other authors declare that no competing interests exist.
References
- 1.Domino EF: Taming the ketamine tiger. 1965. Anesthesiology 2010; 113: 678–84 [DOI] [PubMed] [Google Scholar]
- 2.Li L, Vlisides PE: Ketamine: 50 Years of Modulating the Mind. Front Hum Neurosci 2016; 10: 612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peltoniemi MA, Hagelberg NM, Olkkola KT, Saari TI: Ketamine: A Review of Clinical Pharmacokinetics and Pharmacodynamics in Anesthesia and Pain Therapy. Clin Pharmacokinet 2016; 55: 1059–77 [DOI] [PubMed] [Google Scholar]
- 4.Nowacka A, Borczyk M: Ketamine applications beyond anesthesia - A literature review. Eur J Pharmacol 2019; 860: 172547. [DOI] [PubMed] [Google Scholar]
- 5.Akeju O, Song AH, Hamilos AE, Pavone KJ, Flores FJ, Brown EN, Purdon PL: Electroencephalogram signatures of ketamine anesthesia-induced unconsciousness. Clin Neurophysiol 2016; 127: 2414–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vlisides PE, Bel-Bahar T, Nelson A, Chilton K, Smith E, Janke E, Tarnal V, Picton P, Harris RE, Mashour GA: Subanaesthetic ketamine and altered states of consciousness in humans. Br J Anaesth 2018; 121: 249–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akeju O, Brown EN: Neural oscillations demonstrate that general anesthesia and sedative states are neurophysiologically distinct from sleep. Curr Opin Neurobiol 2017; 44: 178–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chamadia S, Pedemonte JC, Hahm EY, Mekonnen J, Ibala R, Gitlin J, Ethridge BR, Qu J, Vazquez R, Rhee J, Liao ET, Brown EN, Akeju O: Delta oscillations phase limit neural activity during sevoflurane anesthesia. Commun Biol 2019; 2: 415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akeju O, Hamilos AE, Song AH, Pavone KJ, Purdon PL, Brown EN: GABAA circuit mechanisms are associated with ether anesthesia-induced unconsciousness. Clin Neurophysiol 2016; 127: 2472–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hayashi K, Tsuda N, Sawa T, Hagihira S: Ketamine increases the frequency of electroencephalographic bicoherence peak on the alpha spindle area induced with propofol. Br J Anaesth 2007; 99: 389–95 [DOI] [PubMed] [Google Scholar]
- 11.Mion G, Villevieille T: Ketamine pharmacology: an update (pharmacodynamics and molecular aspects, recent findings). CNS Neurosci Ther 2013; 19: 370–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zanos P, Moaddel R, Morris PJ, Riggs LM, Highland JN, Georgiou P, Pereira EFR, Albuquerque EX, Thomas CJ, Zarate CA Jr., Gould TD: Ketamine and Ketamine Metabolite Pharmacology: Insights into Therapeutic Mechanisms. Pharmacol Rev 2018; 70: 621–660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwenk ES, Viscusi ER, Buvanendran A, Hurley RW, Wasan AD, Narouze S, Bhatia A, Davis FN, Hooten WM, Cohen SP: Consensus Guidelines on the Use of Intravenous Ketamine Infusions for Acute Pain Management From the American Society of Regional Anesthesia and Pain Medicine, the American Academy of Pain Medicine, and the American Society of Anesthesiologists. Reg Anesth Pain Med 2018; 43: 456–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oye I, Paulsen O, Maurset A: Effects of ketamine on sensory perception: evidence for a role of N-methyl-D-aspartate receptors. J Pharmacol Exp Ther 1992; 260: 1209–13 [PubMed] [Google Scholar]
- 15.Brown EN, Purdon PL, Van Dort CJ: General anesthesia and altered states of arousal: a systems neuroscience analysis. Annu Rev Neurosci 2011; 34: 601–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bremner JD, Krystal JH, Putnam FW, Southwick SM, Marmar C, Charney DS, Mazure CM: Measurement of dissociative states with the Clinician-Administered Dissociative States Scale (CADSS). J Trauma Stress 1998; 11: 125–36 [DOI] [PubMed] [Google Scholar]
- 17.Reeve BB, Hays RD, Bjorner JB, Cook KF, Crane PK, Teresi JA, Thissen D, Revicki DA, Weiss DJ, Hambleton RK, Liu H, Gershon R, Reise SP, Lai JS, Cella D, Group PC: Psychometric evaluation and calibration of health-related quality of life item banks: plans for the Patient-Reported Outcomes Measurement Information System (PROMIS). Med Care 2007; 45: S22–31 [DOI] [PubMed] [Google Scholar]
- 18.Polianskis R, Graven-Nielsen T, Arendt-Nielsen L: Computer-controlled pneumatic pressure algometry--a new technique for quantitative sensory testing. European journal of pain 2001; 5: 267–77 [DOI] [PubMed] [Google Scholar]
- 19.Polianskis R, Graven-Nielsen T, Arendt-Nielsen L: Pressure-pain function in desensitized and hypersensitized muscle and skin assessed by cuff algometry. The journal of pain : official journal of the American Pain Society 2002; 3: 28–37 [DOI] [PubMed] [Google Scholar]
- 20.Polianskis R, Graven-Nielsen T, Arendt-Nielsen L: Spatial and temporal aspects of deep tissue pain assessed by cuff algometry. Pain 2002; 100: 19–26 [DOI] [PubMed] [Google Scholar]
- 21.Locascio JJ, Atri A: An overview of longitudinal data analysis methods for neurological research. Dement Geriatr Cogn Dis Extra 2011; 1: 330–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suzuki M, Tsueda K, Lansing PS, Tolan MM, Fuhrman TM, Sheppard RA, Hurst HE, Lippmann SB: Midazolam attenuates ketamine-induced abnormal perception and thought process but not mood changes. Can J Anaesth 2000; 47: 866–74 [DOI] [PubMed] [Google Scholar]
- 23.Sener S, Eken C, Schultz CH, Serinken M, Ozsarac M: Ketamine with and without midazolam for emergency department sedation in adults: a randomized controlled trial. Ann Emerg Med 2011; 57: 109–114 e2 [DOI] [PubMed] [Google Scholar]
- 24.Olney JW, Farber NB: Glutamate receptor dysfunction and schizophrenia. Arch Gen Psychiatry 1995; 52: 998–1007 [DOI] [PubMed] [Google Scholar]
- 25.Seamans J: Losing inhibition with ketamine. Nat Chem Biol 2008; 4: 91–3 [DOI] [PubMed] [Google Scholar]
- 26.Kornhall D, Nielsen EW: Failure of ketamine anesthesia in a patient with lamotrigine overdose. Case Rep Crit Care 2014; 2014: 916360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anand A, Charney DS, Oren DA, Berman RM, Hu XS, Cappiello A, Krystal JH: Attenuation of the neuropsychiatric effects of ketamine with lamotrigine: support for hyperglutamatergic effects of N-methyl-D-aspartate receptor antagonists. Arch Gen Psychiatry 2000; 57: 270–6 [DOI] [PubMed] [Google Scholar]
- 28.Dimitrov IV, Harvey MG, Voss LJ, Sleigh JW, Bickerdike MJ, Denny WA: Ketamine esters and amides as short-acting anaesthetics: Structure-activity relationships for the side-chain. Bioorg Med Chem 2019; 27: 1226–1231 [DOI] [PubMed] [Google Scholar]
- 29.Hawksworth C, Serpell M: Intrathecal anesthesia with ketamine. Reg Anesth Pain Med 1998; 23: 283–8 [DOI] [PubMed] [Google Scholar]
- 30.Smith DJ, Bouchal RL, deSanctis CA, Monroe PJ, Amedro JB, Perrotti JM, Crisp T: Properties of the interaction between ketamine and opiate binding sites in vivo and in vitro. Neuropharmacology 1987; 26: 1253–60 [DOI] [PubMed] [Google Scholar]
- 31.Maurset A, Skoglund LA, Hustveit O, Oye I: Comparison of ketamine and pethidine in experimental and postoperative pain. Pain 1989; 36: 37–41 [DOI] [PubMed] [Google Scholar]
- 32.Mikkelsen S, Ilkjaer S, Brennum J, Borgbjerg FM, Dahl JB: The effect of naloxone on ketamine-induced effects on hyperalgesia and ketamine-induced side effects in humans. Anesthesiology 1999; 90: 1539–45 [DOI] [PubMed] [Google Scholar]
- 33.Brightwell JJ, Taylor BK: Noradrenergic neurons in the locus coeruleus contribute to neuropathic pain. Neuroscience 2009; 160: 174–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pertovaara A, Hamalainen MM, Kauppila T, Mecke E, Carlson S: Dissociation of the alpha 2-adrenergic antinociception from sedation following microinjection of medetomidine into the locus coeruleus in rats. Pain 1994; 57: 207–15 [DOI] [PubMed] [Google Scholar]
- 35.Hara K, Yanagihara N, Minami K, Ueno S, Toyohira Y, Sata T, Kawamura M, Bruss M, Bonisch H, Shigematsu A, Izumi F: Ketamine interacts with the noradrenaline transporter at a site partly overlapping the desipramine binding site. Naunyn Schmiedebergs Arch Pharmacol 1998; 358: 328–33 [DOI] [PubMed] [Google Scholar]
- 36.Nishimura M, Sato K, Okada T, Yoshiya I, Schloss P, Shimada S, Tohyama M: Ketamine inhibits monoamine transporters expressed in human embryonic kidney 293 cells. Anesthesiology 1998; 88: 768–74 [DOI] [PubMed] [Google Scholar]
- 37.Smith DJ, Azzaro AJ, Zaldivar SB, Palmer S, Lee HS: Properties of the optical isomers and metabolites of ketamine on the high affinity transport and catabolism of monoamines. Neuropharmacology 1981; 20: 391–6 [DOI] [PubMed] [Google Scholar]
- 38.Crisp T, Perrotti JM, Smith DL, Stafinsky JL, Smith DJ: The local monoaminergic dependency of spinal ketamine. Eur J Pharmacol 1991; 194: 167–72 [DOI] [PubMed] [Google Scholar]
- 39.Kawamata T, Omote K, Sonoda H, Kawamata M, Namiki A: Analgesic mechanisms of ketamine in the presence and absence of peripheral inflammation. Anesthesiology 2000; 93: 520–8 [DOI] [PubMed] [Google Scholar]
- 40.Kushikata T, Yoshida H, Kudo M, Kudo T, Kudo T, Hirota K: Role of coerulean noradrenergic neurones in general anaesthesia in rats. Br J Anaesth 2011; 107: 924–9 [DOI] [PubMed] [Google Scholar]
- 41.Bosma RL, Cheng JC, Rogachov A, Kim JA, Hemington KS, Osborne NR, Venkat Raghavan L, Bhatia A, Davis KD: Brain Dynamics and Temporal Summation of Pain Predicts Neuropathic Pain Relief from Ketamine Infusion. Anesthesiology 2018; 129: 1015–1024 [DOI] [PubMed] [Google Scholar]
- 42.Rogachov A, Bhatia A, Cheng JC, Bosma RL, Kim JA, Osborne NR, Hemington KS, Venkatraghavan L, Davis KD: Plasticity in the dynamic pain connectome associated with ketamine-induced neuropathic pain relief. Pain 2019; 160: 1670–1679 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


