Abstract
Objective:
To evaluate the association between amniotomy at various time points during labor induction and maternal and neonatal outcomes among term, nulliparous women.
Study Design:
Secondary analysis of a randomized trial of term labor induction versus expectant management in low-risk, nulliparous women (2014-2017). Women met inclusion criteria if they underwent induction ≥38 weeks’ gestation using oxytocin with documented time and type of membrane rupture. Women with antepartum stillbirth or fetal anomaly were excluded. The primary outcome was cesarean delivery. Secondary outcomes included maternal and neonatal complications. Maternal and neonatal outcomes were compared among women with amniotomy versus women with intact membranes and no amniotomy at 6 2-hour time intervals: before oxytocin initiation, 0 to <2 hours after oxytocin, 2 to <4 hours after, 4 to <6 hours after, 6 to <8 hours after, and 8 to <10 hours after. Multivariable logistic regression adjusted for maternal age, BMI, race/ethnicity, modified Bishop score on admission, treatment group, and hospital (as a random effect).
Results:
Of 6,106 women in the parent trial, 2,854(46.7%) women met inclusion criteria. Of these 2,340(82.0%) underwent amniotomy, and the majority were performed between 2 and <6 hours after oxytocin. Cesarean delivery was less frequent among women with amniotomy 6 to <8 hours after oxytocin compared with women without amniotomy (21.9% vs 29.7%; aOR 0.61, 95%CI 0.42-0.89). Amniotomy at time intervals ≥ 4 hours after oxytocin was associated with lower odds of labor duration >24 hours. Amniotomy at time intervals ≥2 hours and <8 hours after oxytocin was associated with lower odds of maternal hospitalization >3 days. Amniotomy was not associated with postpartum or neonatal complications.
Conclusion:
Among a contemporary cohort of nulliparous women undergoing term labor induction, amniotomy was associated with either lower or similar odds of cesarean delivery and other adverse outcomes, compared with no amniotomy.
Keywords: amniotomy, artificial rupture of membranes, cesarean, induction, labor, nulliparous
INTRODUCTION
In the United States each year more than 1 in 5 pregnant women undergo labor induction.1 Labor induction is an important tool to expedite delivery in women with a maternal or fetal indication for delivery. Elective labor induction compared with expectant management at 39 weeks’ gestation was shown recently to reduce the risk of cesarean delivery among low-risk nulliparas without an increase in adverse neonatal outcomes.2 However, the optimal method of labor induction, including the best time to perform amniotomy, remains uncertain.
Although the majority of published randomized controlled trials and observational studies of early amniotomy in labor induction demonstrate that early amniotomy shortens the duration of labor3-10, the effect on cesarean delivery has been inconsistent.3,4,14,5,6,8-13 It is challenging to interpret available data owing to a lack of a clear definition for “early amniotomy”. Some studies use a dilation cutoff based on cervical examination,4,5,8,12-14 and others use a time cutoff based on number of hours since completion of cervical ripening10,11 or oxytocin initiation.3,6,9 Furthermore, all of these studies evaluated the effect of amniotomy only at a single point in time.
Thus, our primary objective was to assess whether amniotomy at different time points, compared with foregoing amniotomy, during labor induction in nulliparous women was associated with cesarean delivery and other adverse maternal and neonatal outcomes. We hypothesized that earlier amniotomy is not associated with an increased risk of cesarean delivery.
STUDY DESIGN
We conducted a secondary analysis of the data collected in the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units (MFMU) Network ARRIVE trial (A Randomized Trial of Induction Versus Expectant Management).2 This randomized, controlled, parallel-group trial was conducted from March 2014 through August 2017 at 41 hospitals participating in the MFMU Network to test the hypothesis that elective induction of labor at 39 weeks’ gestation among low-risk nulliparous women with a singleton gestation would result in a lower risk of a composite outcome of perinatal death or severe neonatal complications, compared with expectant management. A complete description of the study design and methodology has been previously published.2 Trained and certified research personnel abstracted data from the medical records of study participants including demographic information, medical history, and maternal and neonatal outcomes. Participants were also followed up with an interview by research personnel during their postpartum hospital stay, during which women were asked to rate their overall labor pain on a 10-point Likert scale. Briefly, the primary study found that elective induction did not significantly reduce the frequency of the composite adverse perinatal outcome (RR 0.80, 95% CI 0.64-1.00), but did reduce the risk of cesarean delivery (RR 0.84, 95% CI 0.76-0.93).2
In the parent trial, low-risk nulliparous women were enrolled between 38 weeks 0 days to 38 weeks 6 days gestation if they had a viable fetus in cephalic presentation. For this secondary analysis we included all women who underwent labor induction with oxytocin after enrolling in the parent study. Women were excluded if spontaneous rupture of membranes occurred prior to hospital admission, if there was a major fetal anomaly or antepartum stillbirth. Our primary outcome was cesarean delivery. Secondary outcomes included chorioamnionitis, duration of labor > 24 hours, overall pain score during labor > 5, maternal postpartum hospitalization > 3 days, postpartum hemorrhage, postpartum infection, neonatal intensive care unit admission, and a composite adverse perinatal outcome, as defined in the parent trial.2 Chorioamnionitis was defined as suspected or clinical diagnosis from start of labor to delivery. Overall pain score during labor was obtained by an in-person interview after delivery when women were asked to rate their labor pain on a 10-point Likert scale with higher scores indicating greater pain. Postpartum hemorrhage was defined as blood product transfusion, use of two or more uterotonics other than oxytocin, surgical interventions for excessive bleeding, non-elective hysterectomy or curettage. Postpartum infection was defined as endometritis, wound reopened for any complication, cellulitis requiring antibiotics, pneumonia, pyelonephritis, bacteremia or septic pelvic thrombosis. The composite adverse perinatal outcome included perinatal death, need for respiratory support within 72 hours after birth, Apgar score of 3 or less at 5 minutes, hypoxic-ischemic encephalopathy, seizure, infection (confirmed sepsis or pneumonia), meconium aspiration syndrome, birth trauma (bone fracture, neurologic injury, or retinal hemorrhage), intracranial or subgaleal hemorrhage, or hypotension requiring vasopressor support.
Baseline characteristics and maternal and neonatal outcomes for women who underwent amniotomy before oxytocin was initiated and within each 2-hour interval after oxytocin was initiated were compared with women who reached the corresponding time interval with intact membranes and did not undergo amniotomy during those 2 hours. Women who had spontaneous or artificial rupture of membranes prior to the specified time interval, delivery prior to the specified time interval, or spontaneous rupture of membranes during this interval were excluded from the analysis for this time interval because they were not at risk for the study exposure (i.e., amniotomy). For example, women who underwent amniotomy at 2 to <4 hours after oxytocin initiation were compared with women with intact membranes who did not undergo amniotomy at 2 to <4 hours and thus had intact membranes from 4 hours after oxytocin initiation onward. Women who were in the no amniotomy group could subsequently have spontaneous rupture of membranes or amniotomy at a later point during the labor induction. Similar methodology has been described previously evaluating outcomes associated with labor induction compared with expectant management with advancing gestational age.15
Baseline characteristics and maternal and neonatal outcomes were compared by amniotomy versus foregoing amniotomy at 6 different time intervals: before oxytocin initiation, 0 to <2 hours after oxytocin initiation, 2 to <4 hours after oxytocin initiation, 4 to <6 hours after oxytocin initiation, 6 to <8 hours after oxytocin initiation, and 8 to <10 hours after oxytocin initiation. We chose these 6 different 2-hour intervals in order to optimize power to detect a difference between the amniotomy and no amniotomy groups as well as evaluate potential differences in the effect of amniotomy at different time points during labor induction. A Cox proportional hazard regression was not used since the time to amniotomy is the study exposure and not the outcome for this analysis. Wilcoxon rank-sum test was used for continuous variables, and Chi-square or Fisher’s exact tests were used for categorical variables, as appropriate. Multivariable logistic regression models were used to adjust for covariates selected a priori including maternal age, body mass index (BMI), race/ethnicity, admission modified Bishop score (based on dilation, effacement or length, and station), treatment group, and hospital (as a random effect). We were not able to adjust for the clinical indication for amniotomy (i.e. to facilitate placement of fetal scalp electrode, in response to lack of cervical change, or routine labor practice) or the cervical examination at the time of amniotomy as these data were not collected in the parent trial. We were not able to adjust for cervical ripening as this was correlated with modified Bishop score on admission. Adjusted odds ratios with 95% confidence intervals are presented for the primary outcome and 99% confidence intervals for the secondary outcomes. Statistical significance was defined as p<0.05 for the primary outcome of cesarean delivery, and p<0.01 for secondary outcomes given multiple comparisons.16 All tests were two-tailed, and all analyses were performed with SAS (version 9.4). No imputation for missing data was performed.
Approval by the Institutional Review Board was obtained for the primary study at all participating hospitals. This secondary analysis was approved by the University of North Carolina at Chapel Hill Institutional Review Board (IRB #15-2099).
RESULTS
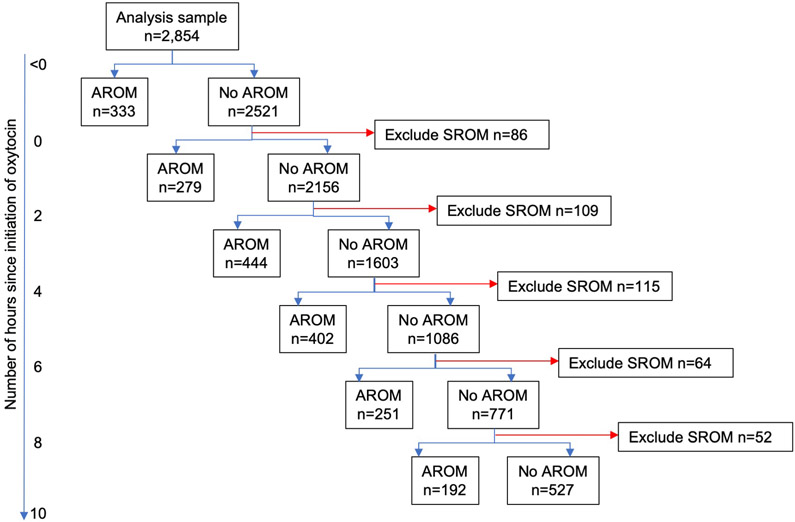
Of the 6,106 women enrolled in the ARRIVE trial, 2,854 (46.7%) women met inclusion criteria for this analysis (Figure 1). Among eligible women, 2,340 (82.0%) had amniotomy during labor induction and 514 (18.0%) had spontaneous rupture of membranes (Figure 2). The majority of women had amniotomy performed 2 to <6 hours after oxytocin initiation. Overall, most women had BMI > 25 kg/m2, were electively induced, and had an unfavorable cervix on admission (Tables 1a and 1b). Compared with women who did not have amniotomy, women with amniotomy were more likely to be non-Hispanic white. Women with amniotomy <4 hours after oxytocin initiation were also more likely to be older, married, and have private insurance, compared with women who did not have amniotomy at these time intervals (Table 1a). Women with amniotomy also were more likely to have higher mean modified Bishop score on admission and less likely to have Foley balloon cervical ripening (Tables 1a and 1b). Gestational age at delivery was similar between women with and those without amniotomy.
Figure 1.
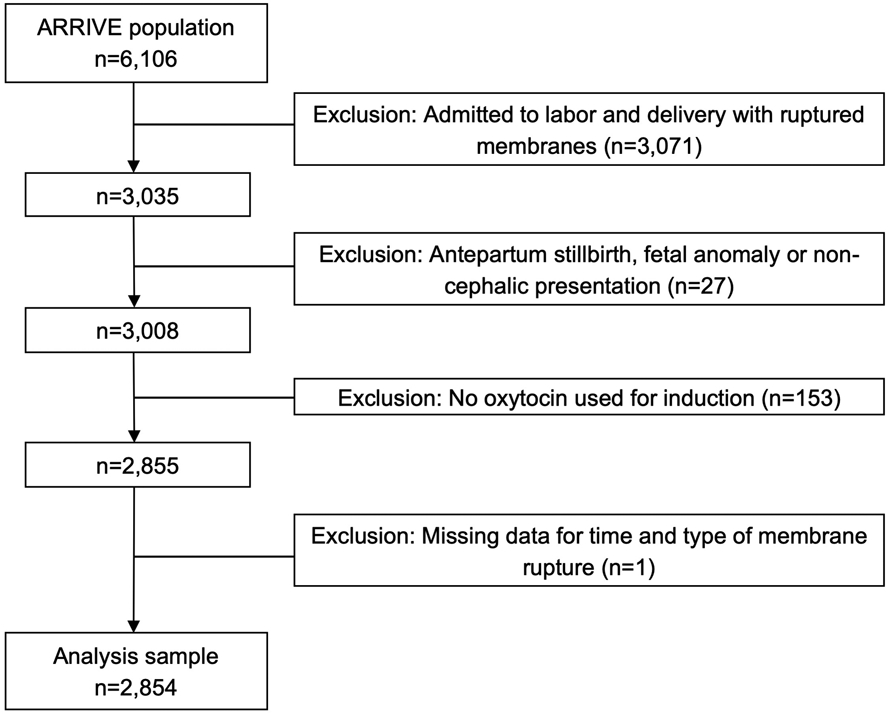
Flow diagram of study cohort
Figure 2.
Flow diagram describing comparison groups by timing of amniotomy
Abbreviations: AROM, artificial rupture of membranes; SROM, spontaneous rupture of membranes.
Note: There were 439 women that had AROM >10 hours and 88 women that had SROM >10 hours.
Table 1a.
Maternal demographic, obstetric, and neonatal characteristics by timing of amniotomy among term, nulliparous labor inductions in the ARRIVE trial (2014-2017)
| Before oxytocin | 0 to <2 hours | 2 to <4 hours | ||||
|---|---|---|---|---|---|---|
| AROM n=333 |
No AROM n=2521 |
AROM n=279 |
No AROM n=2156 |
AROM n=444 |
No AROM n=1603 |
|
| Maternal demographic characteristics | ||||||
| Maternal age (years) | 25.6 ± 5.4 | 24.4 ± 5.1 | 25.7 ± 5.4 | 24.3 ± 5.1 | 25.1 ± 5.3 | 24.1 ± 5.0 |
| BMI (kg/m2) | 26.8 ± 6.9 | 27.3 ± 6.7 | 26.5 ± 5.8 | 27.4 ± 6.8 | 26.7 ± 6.7 | 27.7 ± 6.8 |
| Race/ethnicity | ||||||
| Non-Hispanic Black | 49 (14.7) | 623 (24.7) | 36 (12.9) | 570 (26.4) | 90 (20.3) | 456 (28.4) |
| Non-Hispanic White | 157 (47.1) | 1110 (44.0) | 181 (64.9) | 896 (41.6) | 231 (52.0) | 620 (38.7) |
| Other | 29 (8.7) | 112 (4.4) | 15 (5.4) | 93 (4.3) | 20 (4.5) | 68 (4.2) |
| Hispanic | 98 (29.4) | 676 (26.8) | 47 (16.8) | 597 (27.7) | 103 (23.2) | 459 (28.6) |
| Private insurance | 167 (50.2) | 1102 (43.7) | 183 (65.6) | 875 (40.6) | 230 (51.8) | 603 (37.6) |
| Married | 222 (66.7) | 1457 (57.8) | 197 (70.6) | 1212 (56.2) | 287 (64.6) | 869 (54.2) |
| Tobacco use | 13 (3.9) | 230 (9.1) | 17 (6.1) | 203 (9.4) | 36 (8.1) | 157 (9.8) |
| Obstetric characteristics | ||||||
| Gestational hypertension or preeclampsia | 35 (10.5) | 399 (15.8) | 35 (12.5) | 354 (16.4) | 56 (12.6) | 275 (17.2) |
| Gestational age at delivery (weeks) | 39.7 ± 0.7 | 39.7 ± 0.7 | 39.7 ± 0.7 | 39.7 ± 0.7 | 39.7 ± 0.7 | 39.7 ± 0.7 |
| Indication for induction | ||||||
| Elective | 224 (67.3) | 1776 (70.4) | 203 (72.8) | 1504 (69.8) | 301 (67.8) | 1133 (70.7) |
| Postdates | 54 (16.2) | 333 (13.2) | 35 (12.5) | 288 (13.4) | 69 (15.5) | 204 (12.7) |
| Fetal* | 29 (8.7) | 193 (7.7) | 17 (6.1) | 174 (8.1) | 37 (8.3) | 123 (7.7) |
| Maternal† | 26 (7.8) | 219 (8.7) | 24 (8.6) | 190 (8.8) | 37 (8.3) | 143 (8.9) |
| Modified Bishop score on admission | 4 (3–5) | 5 (3–6) | 5 (4–7) | 4 (3–6) | 5 (4–6) | 4 (3–5) |
| Cervical dilation on admission (cm) | 1 (1–2) | 1 (1–2) | 1 (1–2.5) | 1 (1–2) | 1 (1–2) | 1 (0.5–2) |
| Foley balloon for cervical ripening | 150 (45.0) | 1433 (56.8) | 121 (43.4) | 1270 (58.9) | 215 (48.4) | 1001 (62.4) |
| Prostaglandins for cervical ripening | 240 (72.1) | 1083 (43.0) | 146 (52.3) | 874 (40.5) | 188 (42.3) | 632 (39.4) |
| Hours from start of oxytocin to membrane rupture | −2.6 ± 3.0 | 6.9 ± 6.3 | 1.1 ± 0.6 | 7.9 ± 6.3 | 3.0 ± 0.6 | 9.6 ± 6.5 |
| Epidural anesthesia | 328 (98.5) | 2403 (95.3) | 272 (97.5) | 2049 (95.0) | 420 (94.6) | 1534 (95.1) |
| Neonatal characteristics | ||||||
| Male neonate | 154 (46.2) | 1253 (49.7) | 147 (52.7) | 1066 (49.4) | 221 (49.8) | 790 (49.3) |
| Birthweight (grams) | 3356 ± 408 | 3378 ± 398 | 3394 ± 407 | 3375 ± 397 | 3382 ± 411 | 3378 ± 394 |
Data presented as n (%), mean +/− standard deviation, or median (IQR).
Note: Bolded values represent statistical significance defined as p<0.05.
Fetal indications include intrauterine growth restriction, oligohydramnios, non-reassuring fetal status, and macrosomia.
Maternal indications include abruption, gestational hypertension or preeclampsia, and chorioamnionitis.
Number of missing values: BMI (n=16), insurance (n=1), modified Bishop score (n=211), cervical dilation on admission (n=147).
Table 1b.
Maternal demographic, obstetric, and neonatal characteristics by timing of amniotomy among term, nulliparous labor inductions in the ARRIVE trial (2014-2017)
| 4 to <6 hours | 6 to <8 hours | 8 to <10 hours | ||||
|---|---|---|---|---|---|---|
| AROM n=402 |
No AROM n=1086 |
AROM n=251 |
No AROM n=771 |
AROM n=192 |
No AROM n=527 |
|
| Maternal demographic characteristics | ||||||
| Maternal age (years) | 24.0 ± 5.0 | 24.1 ± 5.0 | 24.0 ± 5.2 | 24.2 ± 5.1 | 24.0 ± 4.9 | 24.3 ± 5.1 |
| BMI (kg/m2) | 27.1 ± 6.8 | 28.1 ± 7.0 | 28.4 ± 7.5 | 28.0 ± 6.9 | 28.1 ± 7.3 | 28.2 ± 6.9 |
| Race/ethnicity | ||||||
| Non-Hispanic Black | 115 (28.6) | 322 (29.7) | 64 (25.5) | 242 (31.4) | 67 (34.9) | 164 (31.1) |
| Non-Hispanic White | 176 (43.8) | 393 (36.2) | 94 (37.5) | 279 (36.2) | 87 (45.3) | 171 (32.4) |
| Other | 19 (4.7) | 42 (3.9) | 11 (4.4) | 26 (3.4) | 6 (3.1) | 19 (3.6) |
| Hispanic | 92 (22.9) | 329 (30.3) | 82 (32.7) | 224 (29.1) | 32 (16.7) | 173 (32.8) |
| Private insurance | 166 (41.4) | 385 (35.5) | 93 (37.1) | 272 (35.3) | 74 (38.5) | 177 (33.6) |
| Married | 239 (59.5) | 558 (51.4) | 133 (53.0) | 391 (50.7) | 96 (50.0) | 266 (50.5) |
| Tobacco use | 41 (10.2) | 106 (9.8) | 25 (10.0) | 76 (9.9) | 23 (12.0) | 51 (9.7) |
| Obstetric characteristics | ||||||
| Gestational hypertension or preeclampsia | 76 (18.9) | 179 (16.5) | 36 (14.3) | 134 (17.4) | 33 (17.2) | 92 (17.5) |
| Gestational age at delivery (weeks) | 39.7 ± 0.7 | 39.7 ± 0.7 | 39.6 ± 0.7 | 39.7 ± 0.7 | 39.7 ± 0.7 | 39.7 ± 0.6 |
| Indication for induction | ||||||
| Elective | 266 (66.2) | 791 (72.8) | 185 (73.7) | 558 (72.4) | 128 (66.7) | 394 (74.8) |
| Postdates | 59 (14.7) | 120 (11.0) | 28 (11.2) | 87 (11.3) | 25 (13.0) | 58 (11.0) |
| Fetal* | 30 (7.5) | 84 (7.7) | 20 (8.0) | 59 (7.7) | 17 (8.9) | 37 (7.0) |
| Maternal† | 47 (11.7) | 91 (8.4) | 18 (7.2) | 67 (8.7) | 22 (11.5) | 38 (7.2) |
| Modified Bishop score on admission | 5 (4 – 6) | 4 (3 – 5) | 5 (4 – 6) | 4 (2 – 5) | 4 (3 – 5) | 4 (2 – 5) |
| Cervical dilation on admission (cm) | 1 (1 – 2) | 1 (0.5 – 2) | 1 (1 – 2) | 1 (0 – 2) | 1 (0.5 – 2) | 1 (0 – 1.5) |
| Foley balloon for cervical ripening | 212 (52.7) | 725 (66.8) | 150 (59.8) | 540 (70.0) | 136 (70.8) | 377 (71.5) |
| Prostaglandins for cervical ripening | 154 (38.3) | 412 (37.9) | 86 (34.3) | 295 (38.3) | 58 (30.2) | 209 (39.7) |
| Hours from start of oxytocin to membrane rupture | 4.9 ± 0.6 | 11.9 ± 6.9 | 7.0 ± 0.5 | 13.9 ± 7.2 | 8.9 ± 0.6 | 16.2 ± 7.7 |
| Epidural anesthesia | 385 (95.8) | 1028 (94.7) | 242 (96.4) | 728 (94.4) | 183 (95.3) | 495 (93.9) |
| Neonatal characteristics | ||||||
| Male neonate | 185 (46.0) | 547 (50.4) | 131 (52.2) | 387 (50.2) | 93 (48.4) | 269 (51.0) |
| Birthweight (grams) | 3362 ± 397 | 3382 ± 399 | 3377 ± 397 | 3388 ± 404 | 3360 ± 391 | 3404 ± 410 |
Data presented as n (%), mean +/− standard deviation, or median (IQR).
Note: Bolded values represent statistical significance defined as p<0.05.
Fetal indications include intrauterine growth restriction, oligohydramnios, non-reassuring fetal status, and macrosomia.
Maternal indications include abruption, gestational hypertension or preeclampsia, and chorioamnionitis.
Number of missing values: BMI (n=16), insurance (n=1), modified Bishop score (n=211), cervical dilation on admission (n=147).
The frequency of cesarean delivery was lower among women with amniotomy at 2 to <4 hours after oxytocin (20.9% vs 26.0%) and at 6 to <8 hours (21.9% v 29.7%) after oxytocin, compared with those with no amniotomy; cesarean delivery was similar at all other time intervals (Tables 2a and 2b). Maternal-reported overall pain score > 5 and hospitalization > 3 days also were either less frequent or of similar frequency in women with amniotomy, compared with no amniotomy, at all time intervals (Tables 2a and 2b). However, women with amniotomy before oxytocin initiation more commonly had chorioamnionitis (20.7% vs 13.8%), duration of labor > 24 hours (62.2% vs 53.9%), and neonatal intensive care unit admission (18.0% vs 12.5%), compared with those who did not have amniotomy prior to oxytocin (Table 2a). There were no differences in the frequency of the composite adverse perinatal outcome among women with amniotomy, compared with no amniotomy, at all time intervals (Tables 2a and 2b).
Table 2a.
Maternal and neonatal outcomes by timing of amniotomy among term, nulliparous labor inductions in the ARRIVE trial (2014-2017)
| Before oxytocin | 0 to <2 hours | 2 to <4 hours | ||||
|---|---|---|---|---|---|---|
| Outcomes | AROM n=333 |
No AROM n=2521 |
AROM n=279 |
No AROM n=2156 |
AROM n=444 |
No AROM n=1603 |
| Cesarean delivery | 79 (23.7) | 596 (23.6) | 62 (22.2) | 517 (24.0) | 93 (20.9) | 416 (26.0) |
| Chorioamnionitis | 69 (20.7) | 347 (13.8) | 25 (9.0) | 309 (14.3) | 58 (13.1) | 245 (15.3) |
| Duration of labor > 24 hours | 207 (62.2) | 1358 (53.9) | 133 (47.7) | 1166 (54.1) | 200 (45.0) | 920 (57.4) |
| Overall pain score > 5 | 196 (60.7) | 1654 (67.8) | 156 (57.8) | 1440 (69.1) | 268 (63.5) | 1102 (70.9) |
| Maternal hospitalization > 3 days | 99 (29.7) | 843 (33.4) | 60 (21.5) | 762 (35.3) | 106 (23.9) | 636 (39.7) |
| Postpartum hemorrhage† | 22 (6.6) | 137 (5.4) | 10 (3.6) | 123 (5.7) | 22 (5.0) | 99 (6.2) |
| Postpartum infection‡ | 4 (1.2) | 57 (2.3) | 2 (0.7) | 52 (2.4) | 8 (1.8) | 43 (2.7) |
| Neonatal ICU admission | 60 (18.0) | 314 (12.5) | 27 (9.7) | 279 (12.9) | 77 (17.3) | 194 (12.1) |
| Composite adverse perinatal outcome § | 17 (5.1) | 128 (5.1) | 8 (2.9) | 115 (5.3) | 33 (7.4) | 78 (4.9) |
Data presented as n (%).
Note: Bolded values denote statistical significance (p<0.05 for primary outcome of cesarean delivery and p<0.01 for secondary outcomes).
Postpartum hemorrhage included transfusion, use of two or more uterotonics other than oxytocin, surgical interventions for excessive bleeding, and non-elective hysterectomy.
Postpartum infection included endometritis, wound reopened for any complication, cellulitis requiring antibiotics, pneumonia, pyelonephritis, bacteremia or septic pelvic thrombosis.
Composite adverse perinatal outcome included perinatal death, respiratory support, Apgar score ≤ 3 at 5 min, hypoxic ischemic encephalopathy, seizure, infection, meconium aspiration syndrome, birth trauma, intracranial or subgaleal hemorrhage, and hypotension requiring pressor support.
Number of missing values: maternal pain score (n=92).
Table 2b.
Maternal and neonatal outcomes by timing of amniotomy among term, nulliparous labor inductions in the ARRIVE trial (2014-2017)
| 4 to <6 hours | 6 to <8 hours | 8 to <10 hours | ||||
|---|---|---|---|---|---|---|
| Outcomes | AROM n=402 |
No AROM n=1086 |
AROM n=251 |
No AROM n=771 |
AROM n=192 |
No AROM n=527 |
| Cesarean delivery | 93 (23.1) | 299 (27.5) | 55 (21.9) | 229 (29.7) | 55 (28.6) | 160 (30.4) |
| Chorioamnionitis | 52 (12.9) | 175 (16.1) | 42 (16.7) | 128 (16.6) | 24 (12.5) | 94 (17.8) |
| Duration of labor > 24 hours | 181 (45.0) | 675 (62.2) | 113 (45.0) | 524 (68.0) | 100 (52.1) | 392 (74.4) |
| Overall pain score > 5 | 254 (65.8) | 773 (73.1) | 173 (70.9) | 550 (73.3) | 133 (71.1) | 379 (73.7) |
| Maternal hospitalization > 3 days | 114 (28.4) | 484 (44.6) | 80 (31.9) | 384 (49.8) | 77 (40.1) | 288 (54.6) |
| Postpartum hemorrhage† | 23 (5.7) | 66 (6.1) | 15 (6.0) | 47 (6.1) | 10 (5.2) | 35 (6.6) |
| Postpartum infection‡ | 13 (3.2) | 26 (2.4) | 6 (2.4) | 19 (2.5) | 5 (2.6) | 14 (2.7) |
| Neonatal ICU admission | 47 (11.7) | 133 (12.2) | 27 (10.8) | 101 (13.1) | 20 (10.4) | 77 (14.6) |
| Composite adverse perinatal outcome § | 20 (5.0) | 53 (4.9) | 12 (4.8) | 38 (4.9) | 4 (2.1) | 32 (6.1) |
Data presented as n (%).
Note: Bolded values denote statistical significance (p<0.05 for primary outcome of cesarean delivery and p<0.01 for secondary outcomes).
Postpartum hemorrhage included transfusion, use of two or more uterotonics other than oxytocin, surgical interventions for excessive bleeding, and non-elective hysterectomy.
Postpartum infection included endometritis, wound reopened for any complication, cellulitis requiring antibiotics, pneumonia, pyelonephritis, bacteremia or septic pelvic thrombosis.
Composite adverse perinatal outcome included perinatal death, respiratory support, Apgar score ≤ 3 at 5 min, hypoxic ischemic encephalopathy, seizure, infection, meconium aspiration syndrome, birth trauma, intracranial or subgaleal hemorrhage, and hypotension requiring pressor support.
Number of missing values: maternal pain score (n=92).
After adjustment for confounding factors, amniotomy at 6 to <8 hours after oxytocin initiation was associated with lower odds of cesarean delivery (aOR 0.61, 95% CI 0.42-0.89). There were no statistically significant associations between amniotomy and cesarean delivery at other time points (Figure 3). Amniotomy was associated with lower odds of labor duration > 24 hours when it was performed at 4 to <6 hours after oxytocin (aOR 0.59, 99% CI 0.41-0.86), 6 to <8 hours after oxytocin (aOR 0.38, 99% CI 0.24-0.61), and 8 to <10 hours after oxytocin (aOR 0.37, 99% CI 0.21-0.64) (Figure 3). Similarly, amniotomy was associated with lower odds of maternal postpartum hospitalization >3 days when it was performed at 2 to <4 hours after oxytocin (aOR 0.53, 99% CI 0.37-0.78), 4 to <6 hours after oxytocin (aOR 0.59, 99% CI 0.40-0.86), and 6 to <8 hours after oxytocin (aOR 0.47, 99% CI 0.30-0.75). There were no significant associations between amniotomy and chorioamnionitis, postpartum hemorrhage, infection, neonatal intensive care unit admission, or the composite adverse perinatal outcome (Figure 3).
Figure 3.
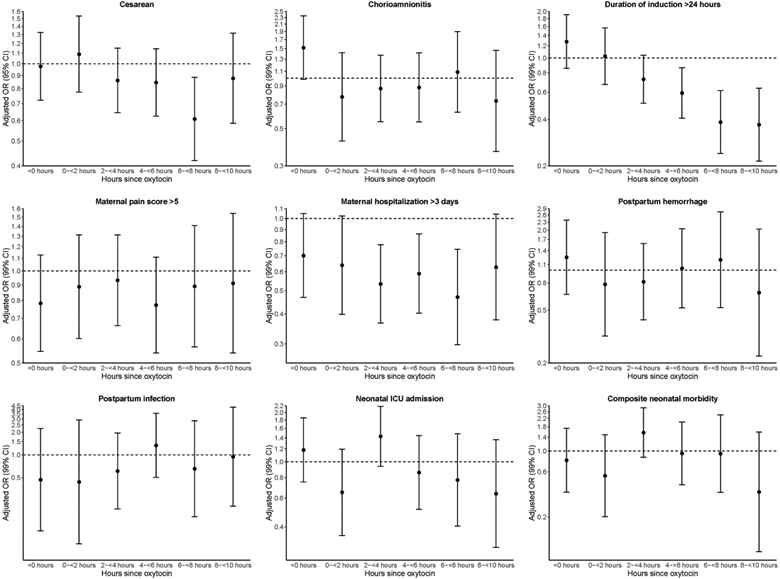
Multivariable logistic regression of maternal and neonatal outcomes by timing of amniotomy among term, nulliparous labor inductions in the ARRIVE trial (2014-2017)
Adjusted for maternal age, BMI, race/ethnicity, modified Bishop score on admission, treatment group, and hospital (as a random effect).
Statistical significance defined as p<0.05 for primary outcome of cesarean delivery and p<0.01 for secondary outcomes.
Figures display 95% confidence intervals for primary outcome of cesarean delivery and 99% confidence intervals for secondary outcomes.
COMMENT
In this secondary analysis of the ARRIVE trial including nulliparous women who underwent labor induction with oxytocin, women who had amniotomy after oxytocin initiation were less likely to have several maternal complications compared with those who did not undergo amniotomy. Specifically, women who had amniotomy at 6 to <8 hours after oxytocin initiation were less likely to have cesarean delivery, compared with not having amniotomy at that time. Amniotomy at 4 to <6 and 6 to <8 hours also was associated with shorter labor and maternal postpartum hospitalization duration. The differences in these maternal outcomes are clinically significant as well as statistically significant. For example, maternal hospitalization > 3 days occurred approximately 1.5 times more often among women who did not have amniotomy at 4 to <6 hours, compared with amniotomy at 4 to <6 hours after oxytocin. Overall, there were no statistically significant associations between amniotomy timing and adverse neonatal outcomes.
Our findings confirm and extend previously published data on the timing of amniotomy in labor induction. Previous studies evaluating the timing of amniotomy in labor induction have characterized the timing of amniotomy with two main approaches: using a dilation cutoff based on cervical examination8,12 or using a time cutoff based on the number of hours since completion of cervical ripening10,11 or oxytocin initiation.3,6 Despite the differences in definition used, almost all studies show a reduction in the duration of labor with early amniotomy.3,5,6,8,10
The association of early amniotomy with route of delivery, however, has been inconsistent. Some studies have demonstrated an increased risk of cesarean3,4,11,12,14 while others have reported no significant difference,6,8-10,17 and one noted a decreased risk.5 Variation in study design and adoption of a more modern labor curve likely contributes to these discrepant outcomes. Overall, previous retrospective cohort studies were more likely to demonstrate an increase in cesarean compared with randomized controlled trials. It is possible that the observational studies were not able to adjust for the clinical indication for early amniotomy such as protracted labor or non-reassuring fetal status. If this indication for amniotomy were also associated with cesarean delivery, then any observed association would be confounded by indication. A second theory to explain the inconsistencies in results of previous studies is different definitions of early amniotomy. Randomized clinical trials that defined early amniotomy as prior to or concurrent with oxytocin initiation found an increased risk of cesarean3,11 whereas those that used a cervical examination cutoff found a decrease or no difference in cesarean delivery.4,5,8 Lastly, differences in study populations differences such as in maternal BMI, parity, and labor management practices may also explain inconsistencies in existing literature. Our analysis did not find an increased odds of cesarean delivery with amniotomy at any time point during labor induction. Instead, our results suggest amniotomy is either no different than foregoing amniotomy or was associated with a reduced chance of cesarean delivery.
Biological explanations have been proposed to explain the association of early amniotomy with shortened labor duration or with increased risk of cesarean delivery. Stimulation of prostaglandin E2 release and increase in endogenous oxytocin levels with amniotomy may accelerate uterine contractions and subsequently cervical dilation.18,19 Furthermore, observational studies suggest that about one-third of women in whom labor is attempted with oxytocin administration without concurrent amniotomy will remain undelivered 2-3 days after the beginning of the induction attempt, thereby increasing the risk for maternal morbidity.20 Nevertheless, randomized clinical trials as well as observational studies are needed to assess both efficacy and effectiveness of amniotomy in nulliparous labor induction.
Our unique study design allowed us to evaluate the association between amniotomy at multiple time points during induction and maternal and neonatal outcomes. Another strength of this analysis is that we had a large study sample of approximately 2,500 low-risk nulliparas with rigorously collected data on demographic and obstetric characteristics as well as maternal and neonatal outcomes. Our findings are generalizable to low-risk, nulliparous women in the U.S. who present for term labor induction as women in the trial were enrolled at 41 different U.S. sites across different geographic regions with diverse racial and ethnic backgrounds. The findings of this analysis, however, should be interpreted within the context of the study design. While our findings are applicable to term, low-risk nulliparous women undergoing inductions, we did not evaluate the timing of amniotomy among multiparous women, women with pre-existing medical comorbidities, or women requiring inductions before term. Although we considered many factors associated with the outcomes of interest, there may be other variables, such as the cervical examination at time of amniotomy, the contraction pattern and oxytocin rate at time of amniotomy, and the clinical indication for amniotomy that could not be quantified. For example, if women who underwent amniotomy had more favorable cervical examinations than those who did not undergo amniotomy, our results may have overstated the association between amniotomy and cesarean delivery. In contrast, if women who underwent amniotomy had artificial rupture of membranes performed because labor was not progressing well or there were signs of non-reassuring fetal status, our results may underestimate the association. Lastly, our power to detect significant differences among rare components of the neonatal composite outcome was limited.
In summary, we found that amniotomy in term low-risk nulliparous women undergoing labor induction was associated with either lower or similar odds of cesarean delivery and other adverse maternal outcomes, compared with foregoing amniotomy at various time points during labor induction, and also may be associated with a shorter duration of labor.
Supplementary Material
Acknowledgements:
The authors thank Gail Mallett, R.N., M.S., C.C.R.C. and Kim Hill, R.N., B.S.N. for protocol development and coordination between clinical research centers; Lindsay Doherty, M.S. for protocol and data management; and Elizabeth A. Thom, Ph.D. and Madeline M. Rice, Ph.D. for protocol development and oversight.
Funding: Supported by grants (HD40512, HD36801, HD27869, HD34208, HD68268, HD40485, HD40500, HD53097, HD40560, HD40545, HD27915, HD40544, HD34116, HD68282, HD87192, HD68258, HD87230) from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the National Center for Advancing Translational Sciences (UL1TR001873). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Disclosure statement: The authors report no conflict of interest.
Presentation: Presented in the oral plenary at the 39th annual meeting of the Society for Maternal-Fetal Medicine, Las Vegas, NV, Feb 11-16, 2019.
Contributor Information
Ashley N. Battarbee, Department of Obstetrics and Gynecology of University of North Carolina at Chapel Hill, Chapel Hill, NC
Grecio Sandoval, Department of Obstetrics and Gynecology the George Washington University Biostatistics Center, Washington, DC
William A. Grobman, Department of Obstetrics and Gynecology of Northwestern University, Chicago, IL
Uma M. Reddy, Departments of Obstetrics and Gynecology of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, MD
Alan T.N. Tita, Department of Obstetrics and Gynecology of University of Alabama at Birmingham, Birmingham, AL
Robert M. Silver, Department of Obstetrics and Gynecology of University of Utah Health Sciences Center, Salt Lake City, UT
Yasser Y. El-Sayed, Department of Obstetrics and Gynecology of Stanford University, Stanford, CA
Ronald J. Wapner, Department of Obstetrics and Gynecology of Columbia University, New York, NY
Dwight J. Rouse, Department of Obstetrics and Gynecology of Brown University, Providence, RI
George R. Saade, Department of Obstetrics and Gynecology of University of Texas Medical Branch, Galveston, TX
Suneet P. Chauhan, Department of Obstetrics and Gynecology of University of Texas Health Science Center at Houston-Children’s Memorial Hermann Hospital, Houston, TX
Jay D. Iams, Department of Obstetrics and Gynecology of The Ohio State University, Columbus, OH
Edward K. Chien, Department of Obstetrics and Gynecology of MetroHealth Medical Center-Case Western Reserve University, Cleveland, OH
Brian M. Casey, Department of Obstetrics and Gynecology of University of Texas Southwestern Medical Center, Dallas, TX
Ronald S. Gibbs, Department of Obstetrics and Gynecology of University of Colorado School of Medicine, Anschutz Medical Campus, Aurora, CO
Sindhu K. Srinivas, Department of Obstetrics and Gynecology of University of Pennsylvania, Philadelphia, PA
Geeta K. Swamy, Department of Obstetrics and Gynecology of Duke University, Durham, NC
Hyagriv N. Simhan, Department of Obstetrics and Gynecology of University of Pittsburgh, Pittsburgh, PA
REFERENCES
- 1.Osterman MJK, Martin JA. Recent declines in induction of labor by gestational age. NCHS Data Brief. 2014;(155):1–8. http://www.ncbi.nlm.nih.gov/pubmed/24941926.Accessed May 1, 2019. [PubMed] [Google Scholar]
- 2.Grobman WA, Rice MM, Reddy UM, et al. Labor Induction versus Expectant Management in Low-Risk Nulliparous Women. N Engl J Med. 2018;379(6):513–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bala A, Bagga R, Kalra J, Dutta S. Early versus delayed amniotomy during labor induction with oxytocin in women with Bishop’s score of ≥6: a randomized trial. J Matern Fetal Neonatal Med. 2018;31(22):2994–3001. [DOI] [PubMed] [Google Scholar]
- 4.Ghafarzadeh M, Moeininasab S, Namdari M. Effect of early amniotomy on dystocia risk and cesarean delivery in nulliparous women: a randomized clinical trial. Arch Gynecol Obstet. 2015;292(2):321–325. [DOI] [PubMed] [Google Scholar]
- 5.Makarem MH, Zahran KM, Abdellah MS, Karen MA. Early amniotomy after vaginal misoprostol for induction of labor: A randomized clinical trial. Arch Gynecol Obstet. 2013;288(2):261–265. [DOI] [PubMed] [Google Scholar]
- 6.Gagnon-Gervais K, Bujold E, Iglesias MH, et al. Early versus late amniotomy for labour induction: A randomized controlled trial. J Matern Neonatal Med. 2012;25(11):2326–2329. [DOI] [PubMed] [Google Scholar]
- 7.Tanno K, Okazaki M, Saito B, Uchibe H. Analytical methods for residues of tetracyclines in the liver of swine. (Studies on analytical methods for residues of antibiotics in livestock products. II). J Food Hyg Soc Japan. 1982;23(3):259–264. [Google Scholar]
- 8.Macones GA, Cahill A, Stamilio DM, Odibo AO. The efficacy of early amniotomy in nulliparous labor induction: a randomized controlled trial. Am J Obstet Gynecol. 2012;207(5):403.e1–403.e5. [DOI] [PubMed] [Google Scholar]
- 9.Mercer BM, McNanley T, O’Brien JM, Randal L, Sibai BM. Early versus late amniotomy for labor induction: a randomized trial. Am J Obstet Gynecol. 1995;173(4):1321–1325. [DOI] [PubMed] [Google Scholar]
- 10.Battarbee AN, Palatnik A, Peress DA, Grobman WA. Association of Early Amniotomy after Foley Balloon Catheter Ripening and Duration of Nulliparous Labor Induction. Obstet Gynecol. 2016;128(3). [DOI] [PubMed] [Google Scholar]
- 11.Levy R, Ferber A, Ben-Arie A, et al. A randomised comparison of early versus late amniotomy following cervical ripening with a Foley catheter. BJOG An Int J Obstet Gynaecol. 2002;109(2):168–172. [DOI] [PubMed] [Google Scholar]
- 12.Pasko D, Miller K, Jauk V, Subramaniam A. Pregnancy Outcomes after Early Amniotomy among Class III Obese Gravidas Undergoing Induction of Labor. Am J Perinatol. November2018. [DOI] [PubMed] [Google Scholar]
- 13.Parrish MM, Kuper SG, Jauk VC, Baalbaki SH, Tita AT, Harper LM. Does Early Artificial Rupture of Membranes Speed Labor in Preterm Inductions? Am J Perinatol. 2018;35(8):716–720. [DOI] [PubMed] [Google Scholar]
- 14.Lee SM, Park JW, Park CW, Yoon BH. “Early rupture of membranes” during induced labor as a risk factor for cesarean delivery in term nulliparas. PLoS One. 2012;7(6):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bailit JL, Grobman W, Zhao Y, et al. Nonmedically indicated induction vs expectant treatment in term nulliparous women. Am J Obstet Gynecol. 2015;212(1):103.e1–103.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davis CE. Secondary endpoints can be validly analyzed, even if the primary endpoint does not provide clear statistical significance. Control Clin Trials. 1997;18(6):557–560; discussion 561-7. [DOI] [PubMed] [Google Scholar]
- 17.Bostancı E, Eser A, Yayla Abide C, Kılıccı C, Kucukbas M. Early amniotomy after dinoprostone insert used for the induction of labor: a randomized clinical trial. J Matern Neonatal Med. 2018;31(3):352–356. [DOI] [PubMed] [Google Scholar]
- 18.Dreifuss J Oxytocin in reproductive biology: newly discovered sites of production and of action. In: Campana A (Ed)Reproductive Health. ; 1993:71–74. [Google Scholar]
- 19.Mitchell MD, Flint AP, Bibby J, Brunt J, Arnold JM, Anderson AB TA. Rapid increases in plasma prostaglandin concentrations after vaginal examination and amniotomy. - PubMed - NCBI. Br Med J. 1977;2(6096):1183–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keirse MJNC. Natural prostaglandins for induction of labor and preinduction cervical ripening. Clin Obstet Gynecol. 2006;49(3):609–626. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.