Abstract
Background:
Suppressor of tumorigenicity 2 (ST2) is a powerful marker of prognosis and treatment response in heart failure (HF), however, it is an enzyme-linked immunosorbent assay (ELISA) which may be cumbersome and costly. A turbidimetric immunoassay (TIA) that can run on common chemistry analyzers could overcome this. We studied a novel TIA for ST2, comparing it to commercial ST2 (ELISA).
Methods:
Patients age ≥18 years meeting Framingham definition for HF were enrolled in a prospective registry (Oct 2007 - March 2015) at Henry Ford Hospital and donated blood samples. Participants with reduced ejection fraction (<50%) and available plasma samples were included and valid ST2 measurements were obtained on the same sample using both TIA and ELISA (N=721). The primary endpoint was all cause death. Correlation between the methods was quantified. The association with survival was tested using unadjusted and adjusted (for MAGGIC score and NTproBNP) Cox models and comparing the Area Under the Curve (AUC).
Results:
The inter-assay Spearman correlation coefficient was 0.77. Nonparametric regression showed no significant proportional difference (slope = 0.97) and a very small systematic difference (3.2 ng/mL). In univariate analyses both TIA and ELISA ST2 were significant associates of survival with similar effect sizes (HR 4.46 and 3.50, respectively, both p<0.001). In models adjusted for MAGGIC score both ST2 remained significant in Cox models and incrementally improved AUC vs. MAGGIC alone (MAGGIC AUC= 0.757; TIA+MAGGIC AUC=0.786, p=0.025; ELISA+MAGGIC AUC=0.793, p=0.033). In models with both MAGGIC and NTproBNP included, both ST2 still remained significant but did not improve AUC.
Conclusions:
A novel TIA method for ST2 quantification correlates highly with ELISA and offers similarly powerful risk-stratification.
Keywords: ST2, soluble ST2, TIA, ELISA, Heart Failure
1. INTRODUCTION1
Heart failure (HF) is among the leading causes of hospitalization in the United States and a major public health problem worldwide (1–3). There is a prevalence of more than 5.8 million in the United States and more than 23 million worldwide with increasing population and high rates of mortality and morbidity. This necessitates continued efforts to improve risk stratification and assessment of treatment for this challenging disease (4). Cardiac biomarkers are noninvasive means of clinical assessment that aid in diagnosis, prognosis, and management of HF and have become indispensable tools in modern clinical management (5–8).
One of the most promising biomarkers currently approved for HF is the suppressor of tumorigenicity 2 (ST2), member of the interleukin-1 receptor family, whose role was originally established in the context of inflammatory and autoimmune diseases (4). Soluble ST2 (sST2) is the circulating form of this receptor that binds interleukin-33. Elevation of sST2 is associated with worse HF functional class (9), established as a powerful marker of poor prognosis in patients with HF with reduced ejection fraction (10), associated with oxidative stress and inflammation in cardiac fibroblasts (11) and independent and additive to prognostication using natriuretic peptide (NP) levels (12). Higher levels of sST2 are also associated with increased risk of unfavorable left ventricular remodeling (13), and it is believed to function as a decoy receptor, counteracting the favorable effect(s) of interleukin-33 binding to the membrane-bound receptor isoform of ST2, ST2L. Importantly, sST2 seems to also reflect response to treatment, particularly beta-blockers. In one study of patients presenting to the emergency department with acute HF, levels of ST2 were measured at presentation and after 48 hours and those with a > 25% decrease after treatment showed improved mortality and were more likely to be treated with beta-blocker (14).
While there is mounting data regarding the clinical utility of sST2 to aid in prognostic assessment, as well as to possibly aid in directing medical therapy, a significant barrier to its adoption has been in the logistics and cost of testing. The only validated US Food and Drug Administration (FDA) cleared sST2 assay is the Presage ST2 Assay (Critical Diagnostics, San Diego, CA), which is an enzyme-linked immunosorbent assay (ELISA) (15). Like most ELISA-based assays, it requires specialized kits and has limitations regarding throughput and timing of testing. This attribute adds cost and inconvenience for most laboratories that may otherwise like to utilize the assay. A turbidimetric immunoassay (TIA), the Sequent-IA ST2 Assay (Critical Diagnostics), was released under CE mark in 2019 and has the advantages of short assay time and the ability to be completed on fully automatic biochemical analyzers that most hospital laboratories routinely utilize. Obviously, this could overcome many of the cost and inconvenience issues, but additional data is first needed to support the validity of the TIA sST2 assay. The purpose of this study was to quantify the prognostic performance of this novel sST2-TIA assay in HF with reduced ejection fraction patients both alone and when added to clinical risk stratification and then compare this to the standard Presage sST2 (ELISA-based) assay.
2. METHODS
2.1. Patient Data
The study was approved by the Henry Ford Hospital Institutional Review Board. Informed consent was obtained from each patient. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the institution’s human research committee. Study participants were part of the Henry Ford Pharmacogenomic Registry, which has been described in detail previously (16). Briefly, the registry enrolled patients from October 2007 to March 2015 who met Framingham criterion for HF (17), had a clinical ejection fraction measured by any modality and who were receiving care through Henry Ford Health System. The current study included patient participants with ejection fraction less than 50% who had stored plasma samples available for analysis (n = 727).
Phenotypic information (e.g., demographics, physical examination, past medical history, laboratory values, functional status, and medications) and blood samples were collected upon enrollment into the HF registry. Patient deaths were collected from the Social Security Administration Death Master File, Michigan State Division of Vital Records, and the Henry Ford Health System administrative data, through July 28, 2016.
We used clinical and registry data to calculate the Meta-analysis Global Group in Chronic Heart Failure (MAGGIC) score for each participant in the study. This was calculated using the integer tabulation as originally published. We chose the MAGGIC score as the clinical risk adjuster because it was derived from a cohort of 39,372 HF patients from 30 studies (18), has been additionally widely validated (19,20), and is tabulated using commonly available clinical data.
The N-terminal pro b-type NP (NTproBNP) values were measured in plasma from the same participants collected at baseline and stored at −70°C until use. The NTproBNP values were determined using an immunoelectrochemiluminescence assay on the Modular Analytics E 170 system (Roche Diagnostics). This assay has less than 0.001% cross-reactivity with bioactive BNP.
2.2. ST2 TIA and ELISA Assays
The Presage ST2 Assay was cleared by the US FDA in December 2011 and has been employed for clinical risk assessment of patients with HF by measuring sST2 concentrations in patients in the United States, Europe and much of Asia since 2012. The assay is a 96-well microtiter plate ELISA with a linear dynamic range of 3.125 to 200 ng/mL, with precision metrics of average coefficient of variation (CV) of 5.1% for intra-assay and 5.2% for inter-assay variation, and does not exhibit any precision bias through the defined assay dynamic concentration range (15).
The Sequent-IA™ ST2 Assay is a latex bead turbidimetric immunoassay product that is commercially available from Critical Diagnostics. Currently it is available in Europe under CE-Mark and is intended to be submitted for US FDA review. The assay uses the same pair of monoclonal antibodies that are used in the FDA cleared Presage® ST2 Assay (which is also produced and sold by Critical Diagnostics). The Sequent-IA ST2 Assay has a linear dynamic range of 15-300 ng/mL and has precision metrics of 6.5% and 8.6% for intra-assay CV and inter-assay CV, respectively, at an sST2 concentration of 26 ng/mL. At an sST2 concentration of 75 ng/mL, the CV values are 2.8% and 3.8% respectively (21,22) The assay is validated for serum and ethylenediaminetetraacetic acid plasma specimen types and is much faster than the ELISA with an on-instrument turnaround time of ~10 minutes. The Henry Ford Hospital laboratory contributed to the analytical performance assessment protocols on the Beckman AU680 which were used in the manufacturers IFU. Specifically, linearity and precision assessments were performed in the HFH laboratory.
Peripheral blood samples were collected in EDTA tubes at the time of registry enrollment, centrifuged, and plasma aliquoted and stored at −70°C until use. Upon thawing the individual plasma samples were split so that ST2 measurement was obtained with each method (TIA and ELISA) using the identical plasma sample (all samples were EDTA plasma). The assays were run in the Henry Ford Hospital Chemistry Laboratory using standardized methods as described above. Testing was performed on a Beckman AU680 instrument. The TIA assay is currently validated for use on the Beckman AU680 and AU5800 as well as the Roche Cobas c501 instrument system. Samples testing outside the recommended range of the assays were excluded from further analysis.
2.3. Statistical Analysis
We excluded data from 6 individuals who had ST2 values exceeding the maximum limit of either assay, creating a final analytic cohort of 721 patients with paired values. Participants were characterized using statistical summary measures such as means, standard deviations and counts. The amount of linear agreement between the two ST2 assays, TIA and ELISA, was examined using a nonparametric Passing and Bablok analysis to estimate the regression line. We also estimated a Spearman correlation coefficient. We also dichotomized the two assays using the cutpoint of 35 and computed the Kappa statistic, which measures agreement between the two beyond that expected by chance.
The two ST2 measures were evaluated for their ability to predict 1-year survival using a Cox proportional hazards model. We used the Uno statistic to evaluate the area under the curve (AUC) getting both an estimate of the AUC along with its associated standard error. This methodology was utilized to evaluate if adding the ST2 variable improved the predictability of the model with MAGGIC alone. This approach was repeated to assess adding an ST2 variable to a model with both MAGGIC and NTproBNP.
A p-value less than 0.05 was considered as evidence of statistical significance. All analyses were performed in SAS software (version 9.4, SAS Institute Inc., Cary, NC).
3. RESULTS
Baseline characteristics of patient participants overall (n=721) and broken down by ST2 threshold of 35 for each assay is summarized in Table 1. The range of sST2 for ELISA was 10.6-356.6 ng/mL and for TIA was 15 to 611.7 ng/mL(<15 is below lower limit of detection). We excluded test results that had sST2-ELISA values greater than the recommended test maximum of 200 ng/ml and test results that had sST2-TIA values greater than the recommended test maximum of 300 ng/ml. This dropped 6 unique individuals giving us an analytic cohort of 721. This cohort included 66.6% males and 46.1% African Americans, and 41 deaths (5.7%) occurred over 1 year. The median sST2-TIA value was 25.6 (interquartile range 19.3-35.0) while the median ELISA value was 29.3 (interquartile range 22.4-40.2).
Table 1.
Baseline Characteristics
| Variable | Total (n=721) |
ST2-TIA ≤35 ng/mL (n=542) |
ST2-TIA >35 ng/mL (n=179) |
ST2-ELISA ≤35 ng/mL (n=476) |
ST2-ELISA >35 ng/mL (n=245) |
|---|---|---|---|---|---|
| Age | 68.0 (60,77) | 66.0 (59,76) | 71.0 (64,80) | 67.0 (59,77) | 70.0 (60,78) |
| Male | 66.6% | 63.8% | 749% | 64.7% | 70.2% |
| African American | 46.1% | 47.8% | 40.8% | 47.5% | 43.3% |
| Hypertension | 91.8% | 924% | 89.9% | 92.2% | 91.0% |
| Diabetes | 42.4% | 40.0% | 49.7% | 41.8% | 43.7% |
| COPD | 22.9% | 21.6% | 26.8% | 21.0% | 26.5% |
| Ischemic etiology | 58.5% | 57.4% | 62.0% | 56.1% | 63.3% |
| NYHA Class III-IV | 17 2% | 12.7% | 30.7% | 13.0% | 25.3% |
| Beta-blocker | 52.1% | 50.9% | 55.9% | 50.8% | 54.7% |
| ACE/ARB | 49.4% | 48.9% | 50.8% | 49.6% | 49.0% |
| Creatinine (mg/dL) | 1.01 (0.8,1.3) | 0.99 (0.78,1.20) | 1.17 (0.9,1.54) | 0.99 (0.79,1.20) | 1.10 (0.84,1.45) |
| Ejection fraction (%) | 35.0 (30,44) | 36.0 (30,45) | 35.0 (25,43) | 36.0 (30,45) | 35.0 (25,42) |
| NTproBNP (pmol/L) | 194 (79,459) | 149 (68,354) | 433 (166,852) | 148 (68,349) | 341 (123,693) |
| MAGGIC | 18.0 (13,23) | 17.0 (12,22) | 21.0 (16,27) | 17.5 (12,22) | 19.0 (14,25) |
| Follow-up (days) | 365 (365,365) | 365 (365,365) | 365 (365,365) | 365 (365,365) | 365 (365,365) |
| Death 1 year | 5.7% | 3.1% | 13.4% | 3.2% | 10.6% |
All values reported as medians and interquartile ranges. ACE, angiotensin converting enzyme; ARB, angiotensin receptor blockers; COPD, chronic obstructive pulmonary disease; ELISA, enzyme-linked immunosorbent assay; NTproBNP, N-terminal pro b-type natriuretic peptide; MAGGIC, Meta-analysis Global Group in Chronic Heart Failure, NYHA, New York Heart Association; TIA, turbidimetric immunoassay; ST2 suppression tumorigenicity 2
3.1. Inter-assay Correlation between sST2 by TIA vs. Presage ELISA
A scatter plot of sST2 TIA vs ELISA, along with the results of a nonparametric regression (Passing and Bablok) is shown in Figure 1. This demonstrated no significant proportional difference between the TIA and ELISA assays, with a slope 0.97 (95% CI 0.93, 1.01), and a very small systematic difference, with an estimated intercept of 3.16 ng/mL (95% CI 2.15, 4.37), the ELISA being slightly higher on average. We also quantified the correlation using the Spearman coefficient to compare TIA vs. ELISA values. This showed strong inter-assay correlation with estimated Spearman coefficient of 0.77. Finally, we examined dichotomized assay results, as might be used clinically, with the assay results being considered ‘elevated’ or not based on the FDA-certified cutoff level of 35ng/ml. Overall, 153 patients had both assay results elevated, 450 had both results low, while 26 had elevated TIA but low ELISA and 92 had elevated ELISA but low TIA. This yielded a kappa of 0.61, consistent with substantial agreement.
Figure 1.
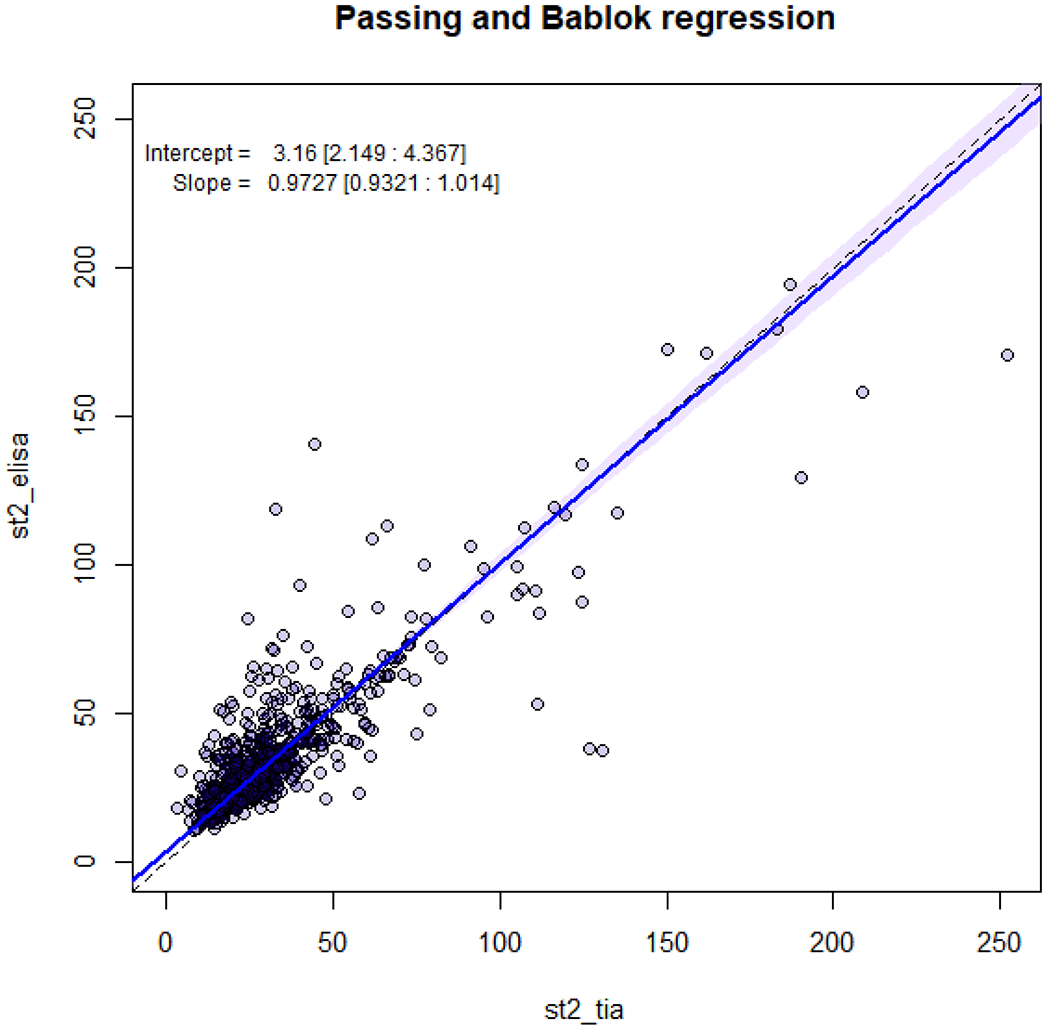
Passing and Bablok Regression Analysis
3.2. Mortality Prediction by sST2-TIA and sST2-ELISA
In analyses with only ST2 as the only variable, the TIA and ELISA values were both significant associates of survival time with similar effect sizes (Table 2). In Cox models (with sST2 as a continuous variable), the hazard ratio (HR) for sST2-TIA was 1.33 (for an increment of 15 ng/ml) and was statistically significant (p = 0.001), a result very similar to the sST2-ELISA that showed a HR of 1.34 (p < 0.001). The AUC calculated from these models also showed the two assays to produce similar overall risk prediction (Supplemental Table 1), both generating fair discrimination; the AUC for sST2-TIA was 0.729 (95% CI 0.66, 0.80) and for ELISA was 0.716 (95% CI 0.62, 0.81). We also tested sST2 by each assay as a dichotomized variable at the FDA approved cut-point of 35 (i.e. > 35 vs. ≤ 35). In this format, both assays again showed significant risk prediction with HR of 4.46 for TIA and 3.50 for the standard ELISA assay (Table 3), and similar (but poor) discrimination by AUC criteria (Supplemental Table 2).
Table 2.
ST2 TIA and ST2 ELISA predicting 1-year mortality as a continuous variable in Cox models
| ST2 TIA | ST2 ELISA | |||
|---|---|---|---|---|
| Model | HR (95% CI) | P value | HR (95% CI) | P value |
| ST2 | 1.33 (1.22, 1.45) | <0.001 | 1.34 (1.22, 1.48) | <0.001 |
| ST2 | 1.25 (1.14, 1.37) | <0.001 | 1.28 (1.15, 1,42) | <0.001 |
| MAGGIC | 1.11 (1.08, 1.16) | <0.001 | 1.12 (1.07, 1.17) | <0.001 |
| ST2 | 1.16 (1.04, 1.29) | 0.008 | 1.16 (1.03, 1.31) | 0.018 |
| MAGGIC | 1.07 (1.02, 1.12) | <0.001 | 1.07 (1.02, 1.12) | <0.001 |
| NTproBNP | 1.74 (1.32, 2.29) | <0.001 | 1.75 (1.32, 2.31) | <0.001 |
ST2 HR is given per 15ng/mL difference in value. NTproBNP HR is per 400 ng/L difference in value. ELISA, enzyme-linked immunosorbent assay; HR, hazard ratio; MAGGIC, Meta-analysis Global Group in Chronic Heart Failure; NTproBNP, N-terminal pro b-type natriuretic peptide; P, p-value; TIA, turbidimetric immunoassay; sST2, soluble suppression tumorigenicity 2
Table 3.
ST2 TIA and ST2 ELISA predicting 1-year mortality as a dichotomous variable (> 35 vs not)
| ST2 TIA | ST2 ELISA | |||
|---|---|---|---|---|
| Model | HR (95% CI) | P value | HR (95% CI) | P value |
| ST2 | 4.46 (2.40, 8.31) | <0.001 | 3.50 (1.85, 6.60) | <0.001 |
| ST2 | 2.80 (1.46, 5.40) | 0.002 | 2.49 (1.29, 4.79) | 0.006 |
| MAGGIC | 1.11 (1.06, 1.16) | <0.001 | 1.12 (1.07, 1.17) | <0.001 |
| ST2 | 2.02 (1.03, 3.96) | 0.040 | 1.87 (0.96, 3.63) | 0.066 |
| MAGGIC | 1.06 (1.01, 1.11) | 0.01 | 1.07 (1.02, 1.12) | 0.007 |
| NTproBNP | 1.83 (1.41, 2.38) | <0.001 | 1.86 (1.44, 2.41) | <0.001 |
NTproBNP HR is per 400 ng/L difference in value. ELISA, enzyme-linked immunosorbent assay; HR, hazard ratio; MAGGIC, Meta-analysis Global Group in Chronic Heart Failure; NTproBNP, N-terminal pro b-type natriuretic peptide; P, p-value; TIA, turbidimetric immunoassay; sST2, soluble suppression tumorigenicity 2
3.3. Incremental Value of sST2-TIA and sST2-ELISA When Added to MAGGIC and NTproBNP
In order to assess the incremental value of the new sST2 TIA assay when added to other available risk stratification methods, we tested models with each sST2 assay adjusted for a comprehensive and validated clinical risk score (the MAGGIC score) and then adjusted for both MAGGIC and NTproBNP. The results of these models are summarized in Tables 2, 3, Supplemental Tables 1, 2. When tested as a continuous variable (Table 2), both versions of sST2 remained a significant predictor of survival in Cox models on top of MAGGIC score and even in addition to MAGGIC and NTproBNP simultaneously. When tested as a dichotomous variable, the results (Table 3) were similar with sST2 remaining statistically significant in all models except the ELISA added to both MAGGIC and NTproBNP, in which case it was of borderline significance (p = 0.066).
We also examined overall risk discrimination using AUC comparisons. In this case, both versions of sST2 again performed similarly when tested as a continuous variable and significantly improved the AUCs in similar magnitude when added to MAGGIC score alone (Supplemental Table 1). For sST2-TIA the AUC improved to 0.786 (p = 0.025) compared to MAGGIC only (AUC = 0.757). For sST2-ELISA the AUC increased to 0.793 (p = 0.033). On the other hand, both sST2 measures did not significantly improve the AUC in models with both MAGGIC and NTproBNP included. When tested as a dichotomized variable, sST2 did not significantly improve model performance (Supplemental Table 2).
4. DISCUSSION
HF continues to be a major source of morbidity and mortality worldwide. Therefore, risk stratification and assessment of response to treatments is crucial for optimal management of HF patients (23,24). The NPs are established and useful markers in patients with HF; however, there remains room for further improvement. sST2 has been previously shown to add independent prognostic information, is less affected by impaired kidney function and age, and appears to reflect favorable drug response, particularly for beta-blockers. sST2 is therefore emerging as a valuable addition to the cardiac biomarker armamentarium (25). Our data explores a novel TIA-based assay and shows that it compares very favorably to the standard Presage ELISA sST2, with high correlation and comparable risk stratification. This opens the door to potentially use this valuable assay in many more hospitals and clinics that may have encountered barriers to entry based on cost, timing, and the number of tests being ordered.
These data should be interpreted in the context of other HF biomarker studies. Our study cohort was very diverse in terms of race and gender, and also featured a substantial prevalence of comorbid conditions such as hypertension and diabetes mellitus. This reflects well the true HF population and the ‘real world’ nature of our registry. Our findings, which show sST2 being predictive of survival by itself and after adjustment for a validated clinical risk score (MAGGIC) is consistent with other studies of sST2. When NTproBNP was also included, sST2 remained statistically significant in Cox models though the magnitude of association was attenuated (HR = 1.16 per 15 ng/dL increment). Our data differ from some previous efforts in that we included adjustment for both a validated clinical model (MAGGIC score) and NTproBNP. This comprehensive risk adjustment clearly makes it even more difficult to provide incremental stratification for any tool or marker, yet ST2 in both formats achieved this in the Cox models though the AUC did not improve from the already high value of 0.80.
Our study adds to the current knowledge base by helping to establish the validity and utility of the TIA assay, which may allow greater access to sST2 measurement, adding unique clinical information in HF patients that is not captured by the other typical risk assessment methods. sST2 also has emerging data for utility in a variety of other settings. Unlike NPs, sST2 is known to reflect inflammatory state, which is critical in the setting of HF, but also plays important roles in other disease states such as in adults with congenital heart disease and even diabetes mellitus (26,27). sST2 has been elucidated to have several roles, especially in cardiovascular disease process as a stress hormone, being involved in both cardiac and vascular remodeling (12). sST2 has emerging data for prediction of sudden cardiac death (28). In the biomarker community, a consensus is slowly forming that a single marker is unlikely to be adequately predictive of all clinically important phenotypes and that a combination of markers that measure differing areas of pathophysiology represents the future of medical care and biomarkers (29).
A study similar to ours, comparing the ASPECT-PLUS sST2 test (a rapid quantitative lateral flow immunoassay for measurement) to the Presage ST2 ELISA and the MBL (Medical & Biological Laboratories International) ST2 ELISA, has previously been reported.(30) While the overall concordance between the lateral flow and Presage was good, there was significant high bias with the lateral flow and the precision of the lateral flow (~20% CV), was not as good as the Presage (<10%)(21) nor as what we found with the TIA assay (~5%). It is also important to bear in mind that the MBL ELISA is a research-only assay primarily used before creation of the Presage assay, and which was never validated and cleared by FDA (unlike Presage). Consequently, the calibration is different than that with Presage, which is currently the only FDA certified ST2 assay available.
Our study has a few limitations to note. First is the overall lower risk of our patients with a relatively modest number of deaths in the first year of follow up. However, despite this we were able to show strong and consistent risk prediction for both sST2 assays, suggesting that we had adequate power. Another consideration is that we had 6 subjects/samples that were excluded due to being beyond the range of detection of the assays. The reason for these improbably large values remains unknown. While alternative analytics approaches, such as imputing these at the maximum value, could have been considered, we felt that the most sound statistical analysis was to exclude them completely since it seemed possible that these represent a sample-specific problem. These potential weaknesses are outweighed by the strengths of our study, which include using identical samples for both assays, adequate cohort size of > 700 participants, detailed clinical characterization of the HF patients, and rigorous analysis including adjustment for clinical risk model and NPs.
5. CONCLUSIONS
This observation registry of HF patients demonstrates that a TIA assay for sST2 is correlated to the FDA approved Presage assay and has strong risk prediction even incremental to clinical risk score that is as good as or better than the standard ELISA based assay. This new assay may improve access and cost when seeking to use sST2 for assessment of HF and risk stratification.
Supplementary Material
HIGHLIGHTS.
Suppressor of tumorigenicity 2 (ST2) is a powerful marker of prognosis and treatment response in heart failure (HF), but is currently available only as enzyme-linked immunosorbent assay (ELISA) which may be cumbersome and costly.
A novel turbidimetric immunoassay (TIA) has very strong correlation (coefficient = 0.85) and offers similarly powerful risk-stratification when compared to ELISA in 721 HF patients.
This assay can run on standard chemistry analyzers, potentially improving access to testing.
Acknowledgments
Funding Sources: This research was supported by the National Heart, Lung, and Blood Institute (Lanfear R01HL103871, R01HL132154; Williams R01HL118267, R01HL141845; Sabbah P01HL074237, R01HL132154) and by a research grant from Critical Diagnostics. Dr. Williams is also supported by the National Institute of Allergy and Infectious Diseases (R01AI079139) and the National Institute of Diabetes and Digestive and Kidney Diseases (R01DK064695, R01DK113003).
Declarations of Interests:
Dr Snider works for Critical Diagnostics, the provider of the sST2 assay.
Dr. Lanfear is a consultant for Amgen, Janssen, Ortho Diagnostics and DCRI (Novartis) and has participated in research with Amgen, Bayer, and Janssen.
Dr. Pinto holds less than 5% share in ACS Biomarker Services.
Dr. Cook reports research grants from Abbott Diagnostics, Beckman Coulter, Critical Diagnostics, Roche Diagnostics and is a consultant to Beckman Coulter, and Roche Diagnostics.
Dr. McCord reports research support from Beckman, Abbott Diagnostics, Roche Diagnostics, and Siemens and is a consultant to Beckman Coulter, Roche Diagnostics, and Siemens.
All other authors have no relevant relationships to disclose.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Abbreviations: AUC, area under the curve; ELISA, enzyme-linked immunosorbent assay; CV, coefficient of variation; FDA, Food and Drug Administration; HF, heart failure; HR, hazard ratio; MAGGIC, Meta-analysis Global Group in Chronic Heart Failure; NP, natriuretic peptide; NTproBNP, N-terminal pro b-type natriuretic peptide; SD, standard deviation; sST2, soluble suppressor of tumorigenicity 2; ST2, suppressor of tumorigenicity 2; TIA, turbidimetric immunoassay
REFERENCES
- 1.Lindenauer PK, Remus D, Roman S et al. Public reporting and pay for performance in hospital quality improvement. The New England journal of medicine 2007;356:486–96. [DOI] [PubMed] [Google Scholar]
- 2.Cleland JG, Chiswell K, Teerlink JR et al. Predictors of postdischarge outcomes from information acquired shortly after admission for acute heart failure: a report from the Placebo-Controlled Randomized Study of the Selective A1 Adenosine Receptor Antagonist Rolofylline for Patients Hospitalized With Acute Decompensated Heart Failure and Volume Overload to Assess Treatment Effect on Congestion and Renal Function (PROTECT) Study. Circulation Heart failure 2014;7:76–87. [DOI] [PubMed] [Google Scholar]
- 3.Rosamond W, Flegal K, Furie K et al. Heart disease and stroke statistics--2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 2008;117:e25–146. [DOI] [PubMed] [Google Scholar]
- 4.McCarthy CP, Januzzi JL Jr. Soluble ST2 in heart failure. Heart failure clinics 2018;14:41–48. [DOI] [PubMed] [Google Scholar]
- 5.Paul S, Harshaw-Ellis K. Evolving use of biomarkers in the management of heart failure. Cardiology in review 2019;27:153–159. [DOI] [PubMed] [Google Scholar]
- 6.Kociol RD, Horton JR, Fonarow GC et al. Admission, discharge, or change in B-type natriuretic peptide and long-term outcomes: data from Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF) linked to Medicare claims. Circulation Heart failure 2011;4:628–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Naffaa M, Makhoul BF, Tobia A et al. Brain natriuretic peptide at discharge as a predictor of 6-month mortality in acute decompensated heart failure. The American journal of emergency medicine 2014;32:44–9. [DOI] [PubMed] [Google Scholar]
- 8.Salah K, Kok WE, Eurlings LW et al. A novel discharge risk model for patients hospitalised for acute decompensated heart failure incorporating N-terminal pro-B-type natriuretic peptide levels: a European coLlaboration on Acute decompeNsated Heart Failure: ELAN-HF Score. Heart (British Cardiac Society) 2014;100:115–25. [DOI] [PubMed] [Google Scholar]
- 9.Yucel O, Gul I, Zararsiz A et al. Association of soluble ST2 with functional capacity in outpatients with heart failure. Herz 2018;43:455–460. [DOI] [PubMed] [Google Scholar]
- 10.Aimo A, Vergaro G, Ripoli A et al. Meta-analysis of soluble suppression of tumorigenicity-2 and prognosis in acute heart failure. JACC Heart failure 2017;5:287–296. [DOI] [PubMed] [Google Scholar]
- 11.Matilla L, Ibarrola J, Arrieta V et al. Soluble ST2 promotes oxidative stress and inflammation in cardiac fibroblasts: an in vitro and in vivo study in aortic stenosis. Clinical science (London, England: 1979) 2019;133:1537–1548. [DOI] [PubMed] [Google Scholar]
- 12.Sugano A, Seo Y, Ishizu T et al. Soluble ST2 and brain natriuretic peptide predict different mode of death in patients with heart failure and preserved ejection fraction. Journal of cardiology 2019;73:326–332. [DOI] [PubMed] [Google Scholar]
- 13.Aleksova A, Paldino A, Beltrami AP et al. Cardiac biomarkers in the emergency department: the role of soluble ST2 (sST2) in acute heart failure and acute coronary syndrome-there is meat on the bone. Journal of clinical medicine 2019;8:270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Breidthardt T, Balmelli C, Twerenbold R et al. Heart failure therapy-induced early ST2 changes may offer long-term therapy guidance. J Card Fail 2013;19:821–8. [DOI] [PubMed] [Google Scholar]
- 15.Dieplinger B, Januzzi JL Jr., Steinmair M et al. Analytical and clinical evaluation of a novel high-sensitivity assay for measurement of soluble ST2 in human plasma--the Presage ST2 assay. Clinica chimica acta; international journal of clinical chemistry 2009;409:33–40. [DOI] [PubMed] [Google Scholar]
- 16.Luzum JA, Peterson E, Li J et al. Race and beta-blocker survival benefit in patients with heart failure: an investigation of self-reported race and proportion of African genetic ancestry. Journal of the American Heart Association 2018;7:e007956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. N Engl J Med 1971;285:1441–6. [DOI] [PubMed] [Google Scholar]
- 18.Pocock SJ, Ariti CA, McMurray JJ et al. Predicting survival in heart failure: a risk score based on 39 372 patients from 30 studies. Eur Heart J 2013;34:1404–13. [DOI] [PubMed] [Google Scholar]
- 19.Radjef R, Peterson EL, Michaels A et al. Performance of the Meta-Analysis Global Group in Chronic Heart Failure Score in Black Patients Compared With Whites. Circ Cardiovasc Qual Outcomes 2019;12:e004714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Michaels A, Aurora L, Peterson E et al. Risk prediction in transition: MAGGIC score performance at discharge and incremental utility of natriuretic peptides. Journal of cardiac failure 2020;26:52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Critical Diagnostics. Presage® ST2 Assay - instructions for use. 2018.
- 22.Critical Diagnostics. SEQUENT-IA™ ST2 Assay Test. Dublin, Ireland: Critical Diagnostics Limited, 2019. [Google Scholar]
- 23.Go AS, Mozaffarian D, Roger VL et al. Executive summary: heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation 2014;129:399–410. [DOI] [PubMed] [Google Scholar]
- 24.Echouffo-Tcheugui JB, Greene SJ, Papadimitriou L et al. Population risk prediction models for incident heart failure: a systematic review. Circulation Heart failure 2015;8:438–47. [DOI] [PubMed] [Google Scholar]
- 25.Ciccone MM, Cortese F, Gesualdo M et al. A novel cardiac bio-marker: ST2: a review. Molecules (Basel, Switzerland) 2013;18:15314–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Geenen LW, Baggen VJM, van den Bosch AE et al. Prognostic value of soluble ST2 in adults with congenital heart disease. Heart (British Cardiac Society) 2019;105:999–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ruocco G, Evangelista I, Franci B et al. Combination of ST2 and B-type natriuretic peptide in diabetic patients with acute heart failure: relation with ventricular stiffness and outcome. Journal of cardiovascular medicine (Hagerstown, Md) 2019;20:81–90. [DOI] [PubMed] [Google Scholar]
- 28.Lupon J, Cediel G, Moliner P et al. A bio-clinical approach for prediction of sudden cardiac death in outpatients with heart failure: the ST2-SCD score. International journal of cardiology 2019;293:148–152. [DOI] [PubMed] [Google Scholar]
- 29.Huang Z, Zhong J, Ling Y et al. Diagnostic value of novel biomarkers for heart failure : a meta-analysis. Herz 2020;45:65–78. [DOI] [PubMed] [Google Scholar]
- 30.Dieplinger B, Egger M, Gegenhuber A, Haltmayer M, Mueller T. Analytical and clinical evaluation of a rapid quantitative lateral flow immunoassay for measurement of soluble ST2 in human plasma. Clin Chim Acta 2015;451:310–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


