Abstract
The physiological and motivational effects of heroin and other abused drugs become associated with environmental (contextual) stimuli during repeated drug use. As a result, these contextual stimuli gain the ability to elicit drug-like conditioned effects. For example, after context-heroin pairings, exposure to the heroin-paired context alone produces similar effects on peripheral immune function as heroin itself. Conditioned immune effects can significantly exacerbate the adverse health consequences of heroin use. Our laboratory has shown that exposure to a heroin-paired context suppresses lipopolysaccharide (LPS)-induced splenic nitric oxide (NO) production in male rats, and this effect is mediated in part by the dorsal hippocampus (dHpc). However, specific dHpc output regions, whose efferents might mediate conditioned immune effects, have not been identified, nor has the contribution of ventral hippocampus (vHpc) been investigated. Here, we evaluated the role of CaMKIIα-expressing neurons in the dHpc and vHpc main output regions by expressing Gi-coupled designer receptors exclusively activated by designer drugs (DREADDs) under a CaMKIIα promoter in the dorsal subiculum and CA1 (dSub, dCA1) or ventral subiculum and CA1 (vSub, vCA1). After context-heroin conditioning, clozapine-N-oxide (CNO, DREADD agonist) or vehicle was administered systemically prior to heroin-paired context (or home-cage control) exposure and LPS immune challenge. Chemogenetic inhibition of CaMKIIα-expressing neurons in dHpc, but not vHpc, output regions attenuated the expression of conditioned splenic NO suppression. These results establish that the main dHpc output regions, the dSub and dCA1, are critical for this context-heroin conditioned immune effect.
Keywords: hippocampus, heroin, learning, nitric oxide, immune conditioning, opioid, DREADD, chemogenetics
1. Introduction
Associations between environmental contexts and biologically significant events help organisms maximally adapt their behavior within that context (Maren et al., 2013). However, some contextually conditioned behaviors are maladaptive, including those learned during chronic drug use. In particular, opioids have potent unconditioned effects on the immune system. Exposure to heroin or morphine decreases splenic leukocyte proliferation, natural killer cell activity, and production of nitric oxide (NO) and proinflammatory cytokines in response to an immune challenge (Fecho et al., 1993; Fecho and Lysle, 2000; Fecho et al., 1996; Fecho et al., 2000; Lysle et al., 1993; Lysle and How, 2000; Nelson et al., 2000). After context-opioid conditioning, exposure to the opioid-paired context alone is sufficient to suppress these pathogen combating responses (Coussons et al., 1992; Lysle and Ijames, 2002). Opioid conditioned immune effects likely magnify the societal cost of opioid use disorders and prescription opioid use by disrupting normal immune responses in the presence of opioid-paired stimuli even after protracted opioid abstinence. Indeed, opioid users are well-documented to show enduring immune alterations that are thought to underlie increased incidence or severity of infection in opioid users (Govitrapong et al., 1998; Horsburgh et al., 1989; Louria et al., 1967; Risdahl et al., 1998). The broad peripheral immune effects of opioid conditioned stimuli are mediated by the central nervous system. Thus, understanding the neural mechanisms of opioid conditioned immunomodulatory effects using animal models may aid the development of therapies that mitigate this problem.
In male rats, exposure to a heroin-paired context reliably suppresses production of NO in the spleen in response to a lipopolysaccharide (LPS)-induced immune challenge (Lysle and Ijames, 2002). NO is involved in host defense immune processes (Bogdan, 2001; Lewis et al., 2010; MacMicking et al., 1995; MacMicking et al., 1997; Nathan, 1992; Nathan and Shiloh, 2000; Uehara et al., 2015) and is a useful index of both heroin-induced and heroin-conditioned immune effects. Using the LPS-induced NO model, we have determined that several brain regions, including the hippocampus, are necessary for the expression of context-heroin conditioned peripheral immune effects (Hutson et al., 2014; Szczytkowski et al., 2011; Szczytkowski et al., 2013; Szczytkowski and Lysle, 2008).
The hippocampus is critical for context encoding and contextual memory processing (Maren et al., 2013; Smith and Mizumori, 2006). Hippocampal mechanisms likely initiate the expression of context-reward associations (Lansink et al., 2009) and context-induced motivated behaviors elicited by drugs of abuse (Alizamini et al., 2018; Bossert et al., 2016; Bossert and Stern, 2014; Fuchs et al., 2007; Hitchcock and Lattal, 2018). However, the hippocampus is a heterogeneous brain region in terms of anatomical connectivity, gene expression, and function along its longitudinal axis (Bienkowski et al., 2018; Fanselow and Dong, 2010; Strange et al., 2014). Interestingly, neural inactivation of either the dorsal hippocampus (dHpc, (Fuchs et al., 2005; Ge et al., 2017; Xie et al., 2010)) or the ventral hippocampus (vHpc) and vHpc output regions (Bossert et al., 2016; Bossert and Stern, 2014; Lasseter et al., 2010) attenuates the expression of context-drug conditioned motivated behaviors. Likewise, the dHpc plays an important role in the expression of context-heroin conditioned immune effects. We have shown that GABA agonist-induced neural inactivation of the dHpc prior to heroin-paired context exposure inhibits the ability of the context to suppress LPS-induced NO production (Szczytkowski et al., 2013). This suggests that both drug contextually conditioned immune and motivational responses rely on dHpc function. However, our previous study was limited by the fact that global inactivation of the dHpc does not reveal which dHpc region(s) or cell types are critical to the expression of context-heroin conditioned immune effects. Identification of critical efferent hippocampal neurons is pivotal to testing the hypothesis that the hippocampus initiates the expression of context-induced immune effects. Furthermore, GABA agonist spread into the intermediate and/or vHpc was not characterized and thus the contribution of these adjacent hippocampal regions remains unknown. To further this line of research, the current study examined the involvement of excitatory neurons in dHpc (Experiment 1) and vHpc (Experiment 2) main output regions, the subiculum and CA1, in heroin contextually conditioned suppression of peripheral NO production using chemogenetic manipulation. These experiments provide insight into the critical hippocampal outputs for context-heroin conditioned immune effects.
2. Materials & Methods
Please see 2S. Supplemental Methods for additional methodological details.
2.1. Animals
Adult male Lewis rats weighing 225–250 g (N = 123), were purchased from Charles River Laboratories (Kingston, NY, USA). The sole use of male rats precluded assessing the biological variable of sex. However, the present study was concluded before our established paradigm to study context-heroin conditioned immune effects was validated for use with female Lewis rats (Paniccia et al., in preparation). Rats were housed individually on a reversed, 12-h light-dark cycle and all experimental procedures took place during the animals’ active dark period (7 am – 7 pm). Food and water were provided ad libitum in home cages and animals were handled regularly before experimentation. All experimental procedures were conducted in accordance with federal guidelines and with approval from the University of North Carolina at Chapel Hill Institutional Animal Care and Use Committee (IACUC).
2.2. Surgical Procedure & Virus Delivery
A viral vector containing a Gi-coupled designer receptors exclusively activated by designer drugs (DREADD) plasmid (AAV5-CaMKIIα-hM4D(Gi)-mCherry) was microinfused into dHpc or vHpc output regions using standard stereotaxic procedures. The DREADD plasmid (generously provided by Bryan Roth) was packaged in AAV5 at a titer of 4.4×1012 GC/mL (Addgene Viral prep 50477-AAV5; http://n2t.net/addgene:50477; RRID: Addgene_50477, Cambridge, MA, USA). The CaMKIIα promoter allowed for preferential virus expression in excitatory pyramidal neurons (Achterberg et al., 2014; Guo et al., 2010; Johansen et al., 2010; Liu and Jones, 1996; Tsien et al., 1996). Injectors (33 gauge, Plastics One, Roanoke, VA, USA) were lowered into dorsal subiculum (dSub; AP −6.0 mm, ML ±2.8 mm, DV −3.5 mm) or ventral subiculum (vSub; AP −6.0 mm, ML ±4.6 mm, DV −8.5 mm) using coordinates derived from the rat stereotaxic atlas (Paxinos and Watson, 2007). The virus was infused through the injectors for a total volume of 0.7 μL per hemisphere and the surgical incision was closed using sutures. All animals received at least two weeks for post-operative recovery and viral incubation prior to the start of conditioning.
2.3. Conditioning & Testing Procedure
Rats received five Pavlovian conditioning sessions 48 hours apart. At the start of each session, the rats received an injection of heroin (unconditioned stimulus, US) and were immediately placed into a distinct context (conditioned stimulus, CS) for 60 minutes. Heroin (diacetylmorphine hydrochloride, 1.0 mg/mL; National Institute on Drug Abuse Drug Supply Program, Bethesda, MD, USA) was dissolved in sterile 0.9% saline and injected subcutaneously (SC) at a dose of 1.0 mg/kg. This conditioning procedure produces reliable conditioned peripheral immune effects (Lebonville et al., 2016; Paniccia et al., 2018; Szczytkowski et al., 2011; Szczytkowski et al., 2013; Szczytkowski and Lysle, 2010). Six days after the last conditioning session, the expression of the conditioned immune effect was assessed using a 2 (treatment) × 2 (context) between-subjects experimental design. The rats received a single systemic injection of clozapine-N-oxide (CNO) or vehicle. CNO (NOCD-135, NIDA Drug Supply Program, Bethesda, MD, USA; Cat# C0832, MilliporeSigma, St. Louis, MO, USA) was dissolved in 100% dimethyl sulfoxide (DMSO) then diluted with sterile 0.9% saline to a final concentration of 3.0 mg/mL CNO in 0.5% DMSO saline vehicle and was injected at a dose of 3.0 mg/kg, SC. Thirty minutes after CNO or vehicle treatment, the rats were exposed to the previously heroin-paired context (CS) for 60 minutes or remained in their home cages, both in the absence of heroin administration. Sixty minutes after CS exposure or comparable home cage stay, all rats received an LPS immune challenge (1.0 mg/kg, SC) in their home cages. LPS, derived from E. coli, serotype O55:B5, (1 mg/mL; Cat# L2880, MilliporeSigma, St. Louis, MO, USA) was dissolved in sterile 0.9% saline.
2.4. Blood and Spleen Tissue Sample Collection
Rats were sacrificed 6 hours after the LPS immune challenge by cervical dislocation without anesthesia. This euthanasia time point is optimal to detect NO production in blood plasma, based on nitrate/nitrite concentration, and in spleen, based on inducible nitric oxide synthase (iNOS) expression. In previous parametric studies, we have demonstrated that these NO measures peak around 6 hours after LPS administration (Lysle and How, 1999, 2000) and are sensitive to context-opioid conditioned suppression in our studies (Lebonville et al., 2016; Lysle and Ijames, 2002; Szczytkowski and Lysle, 2007). Thus, blood plasma and spleen tissue were harvested for iNOS RT-qPCR mRNA and ELISA protein analysis. Blood was collected in heparin-containing syringes, transferred to microcentrifuge tubes, and centrifuged at 2000 G at 4°C for 20 min. Isolated plasma was stored at −80°C until analysis. For RT-qPCR mRNA analysis, spleen tissue samples (~100 mg each) were stored in RNAlater (ThermoFisher Scientific, Waltham, MA, USA) at 4°C for up to 2 months and then at −80°C until analysis. For ELISA protein analysis, spleen tissue samples were stored in protease inhibitor buffer (Pierce™, ThermoFisher Scientific) at −80°C until analysis.
2.5. Brain Histology
Whole brains were extracted, fixed, cryoprotected, and sectioned into 40 μm coronal sections on a cryostat (Leica CM 3050 S, Leica Microsystems, Buffalo Grove, IL, USA). Sections were mounted on charged glass slides (FisherBrand Superfrost, ThermoFisher Scientific, Waltham, MA, USA) and coverslipped using HardSet VECTASHIELD mounting medium with DAPI (Vector Laboratories, Burlingame, CA, USA) for microscopy. Fluorescence microscopy (Leica DM6000 B widefield light microscope, Leica Microsystems, Buffalo Grove, IL, USA) was used to verify mCherry expression (proxy for DREADD expression) in the hippocampal target cell bodies and terminal regions. The data of rats without bilateral mCherry expression in the subiculum were eliminated from analysis.
2.6. Nitrate/Nitrite Assay
The byproducts of NO degradation in plasma, nitrate and nitrite, were measured using the Griess reagent assay as described previously (Lebonville et al., 2016; Szczytkowski and Lysle, 2007). Recovery of nitrate is greater than 95% using this assay. Total nitrate/nitrite concentration was determined based on a concurrently run standard curve.
2.7. iNOS RT-qPCR
Reverse transcription quantitative real-time polymerase chain reaction (RT-qPCR) was performed on spleen tissue samples to measure iNOS mRNA expression. RNA from spleen tissue was purified using TRI-Reagent’s manufacturer protocols with minor modifications (see 2S. Supplemental Methods). The purified RNA was dissolved in RNase Free H2O. RNA purity and concentration were assessed by spectrophotometry (Take3 microdot plate and the Epoch™ spectrophotometer, BioTek Instruments Inc., Winooski, VT, USA).
cDNA synthesis was performed on a Veriti 96-Well Fast Thermal Cycler (Applied Biosystems, ThermoFisher Scientific) using the Advantage RT-for-PCR Kit, according to the manufacturer’s protocol (Clontech/Takara Bio, Mountain View, CA, USA). Priming for the RT reaction was carried out using Oligo(dT) primers. Input RNA concentration was equalized across samples (1 μg).
qPCR was run on a QuantStudio™ 6 Flex system (AP Biosystems, ThermoFisher Scientific) using TaqMan™ Fast Advanced Master Mix (AP Biosystems) and predesigned fluorescein (FAM) assays for iNOS and reference gene L13A (ThermoFisher Scientific). Samples were analyzed in triplicate on a 384-well plate. The comparative delta delta Ct method (ΔΔCt) was used for data analysis (Livak and Schmittgen, 2001; Schmittgen and Livak, 2008).
2.8. iNOS ELISA
Spleen tissue was homogenized, and protein was extracted using freeze-thaw lysis. Total protein concentrations were quantified by Bradford Assay as described previously (Lebonville et al., 2016). Each sample was then assayed in triplicate in a rat iNOS sandwich ELISA (Cat #: abx256135, Abbexa Ltd., Cambridge, UK) following the manufacturer’s protocol. The quantity of iNOS protein in pg per μg of total protein was determined based on a concurrently run standard curve.
2.9. Statistical Analysis
All datasets were analyzed using 2 × 2 analyses of variance (ANOVAs), using SPSS Statistics 24 and 25 (IBM, Armonk, NY, USA, with context (CS or HC) and treatment (vehicle or CNO) as between-subjects factors. For all tests, alpha was set to ≤ .05. Planned comparisons were performed on A) vehicle-treated groups to test for the effect of context on expression of the conditioned response and on B) CNO-treated groups to test for attenuation of the conditioned response to context exposure. Any ancillary effects were probed with post-hoc comparisons using Tukey’s Honestly Significant Difference (HSD) test. The presence of statistical outliers was probed using Grubb’s test, and statistical outliers were removed from final data analysis. For RT-qPCR data, statistical analysis was performed on ΔΔCt values (without linear transformation) because these values tend to better meet the assumptions of an ANOVA.
3. Results
DREADD expression was characterized in the target regions based on the expression of the fluorescent reporter, mCherry. Figure 1 shows representative images of mCherry expression in dSub (Experiment 1, Figure 1A) or vSub (Experiment 2, Figure 1B) cell bodies. DREADD expression was also observed in excitatory projecting neurons, as indicated by mCherry expressing fibers in known terminal regions (see 3S. Supplemental Results).
Figure 1.
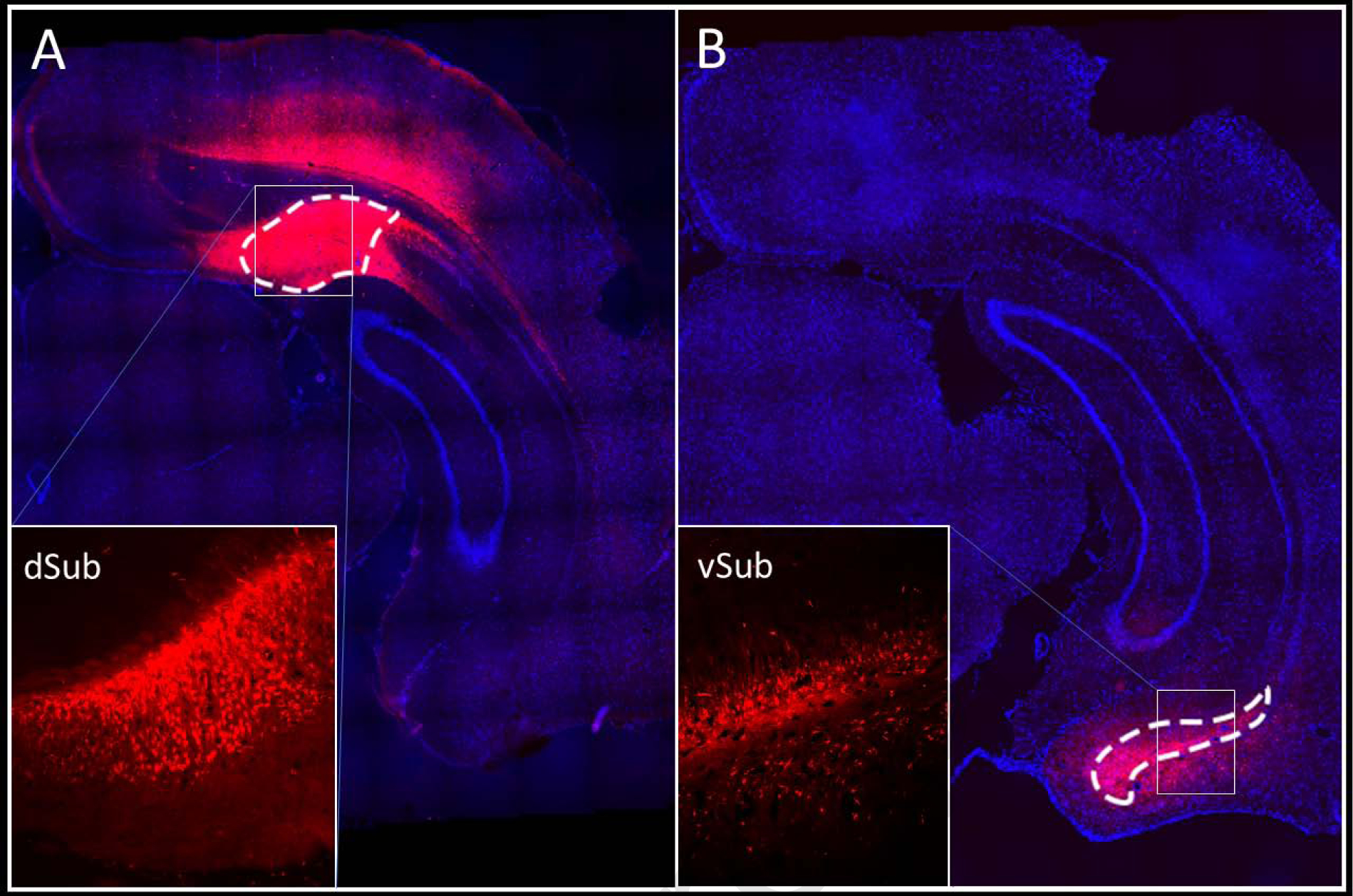
Representative DREADD (mCherry) expression in the dSub (Experiment 1; A) and vSub (Experiment 2; B). Low magnification images are composed of tiled 10X images where cell nuclei were counterstained with DAPI. The insets contain images at 10X magnification.
3.1. Experiment 1: Effects of chemogenetic inhibition of dorsal hippocampal (dHpc) output regions on the expression of heroin contextually conditioned suppression of NO production
The experimental timeline for Experiment 1 is displayed in Figure 2A. Brain templates were adapted, with permission, from the rat stereotaxic atlas (Paxinos and Watson, 2005).Virally-transduced cells, as determined by mCherry expression, were most numerous and consistently observed in the two main dHpc output regions dSub and dCA1 but were also detected in other areas of the dHpc (dDG, and dCA2), post-subiculum, overlying proximal retrosplenial granular and dysgranular cortex, and deep layers of visual cortex (Figure 2B). All subjects showed bilateral virus expression in the dSub (N = 32). All subjects also showed bilateral (N = 29) or unilateral (N = 3) virus expression in dCA1. Final group sizes for nitrate/nitrite and iNOS qPCR or ELISA analyses were n = 7–8.
Figure 2. Experiment 1: Chemogenetic inactivation of dHpc output regions dSub and dCA1 disrupts expression of heroin contextually conditioned suppression of NO.
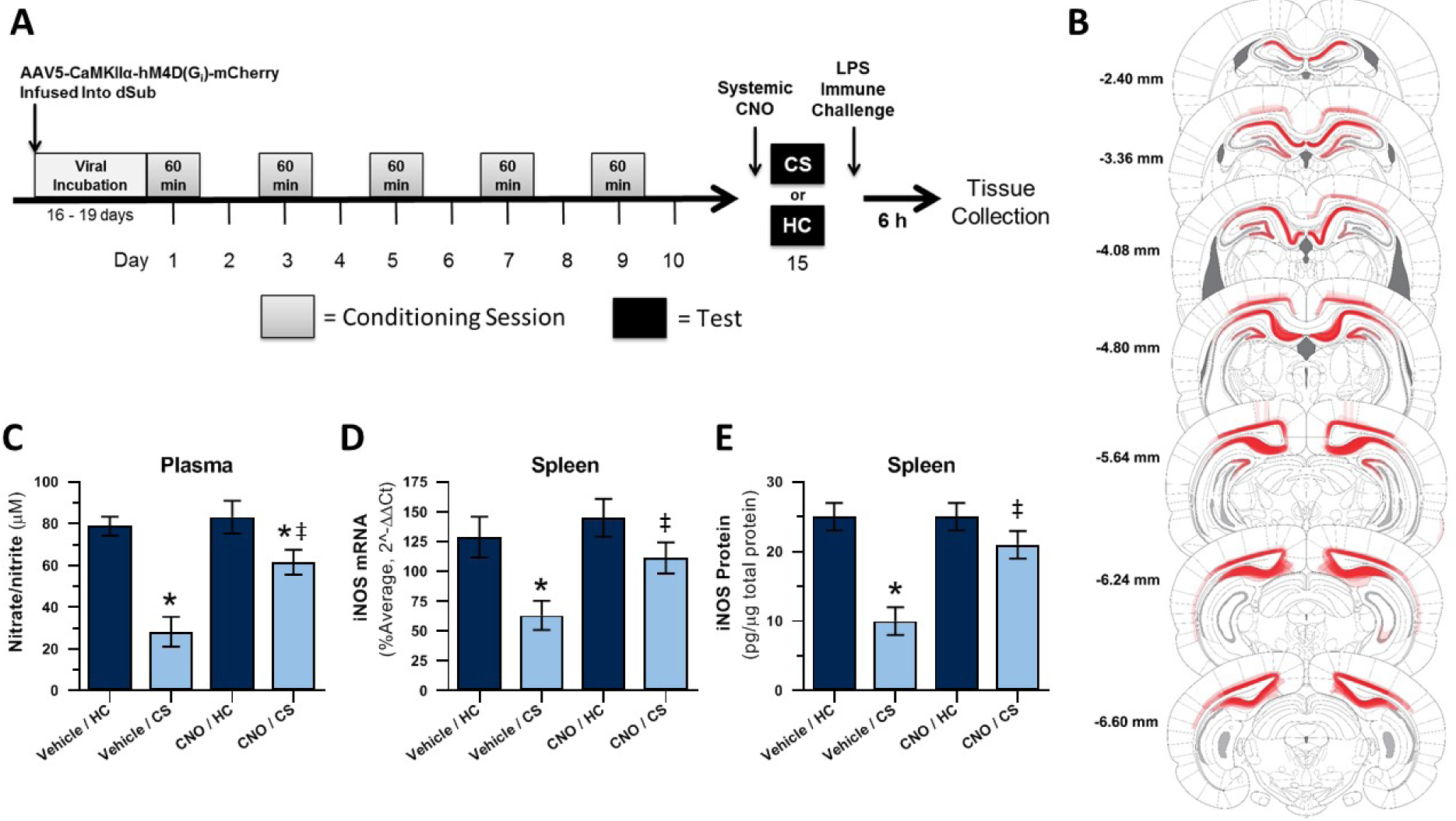
Experimental timeline (A). DREADD (mCherry) expression as a composite of all subjects (B). Effect of CNO administration on plasma nitrate/nitrite concentration (C) and splenic iNOS mRNA (D) and protein (E) expression following heroin-paired context exposure and LPS challenge. CS = heroin-paired contextual conditioned stimulus, HC = home cage; *, statistically significant difference relative to respective HC control. ‡, statistically significant difference relative to Vehicle/CS group.
Figure 2C shows the average plasma nitrate/nitrite concentration of each group 6 hours after LPS administration. A 2 × 2 ANOVA revealed a significant interaction between context (CS or HC) and treatment (CNO or Vehicle; F(1,27) = 4.725, p = .039) and significant main effects of context (F(1,27) = 29.321, p < .001) and treatment (F(1,27) = 8.002, p = .009) on plasma nitrate/nitrite concentration. Planned comparisons revealed that the vehicle-treated heroin-paired context re-exposed (Vehicle/CS) group exhibited significantly lower nitrate/nitrite concentration after LPS than the vehicle-treated home cage (Vehicle/HC) control group (p < .001), establishing the expression of conditioned suppression of NO production. The CNO-treated heroin-paired context re-exposed (CNO/CS) group demonstrated significantly lower nitrate/nitrite concentration than CNO-treated home cage (CNO/HC) control group (p = .027) but significantly higher nitrate/nitrite concentration than the Vehicle/CS control group (p < .001). Thus, CNO significantly attenuated the conditioned response upon exposure to the heroin-paired context.
Figure 2D shows the average splenic iNOS mRNA expression of each group 6 hours after LPS administration. A 2 × 2 ANOVA revealed significant main effects of context (CS or HC; F(1,28) = 13.178, p = .001) and treatment (CNO or Vehicle; F(1,28) = 7.346, p = .011), without interaction between context and treatment (F(1,28) = 3.117, p = .088), on splenic iNOS mRNA expression. Planned comparisons revealed that the Vehicle/CS group demonstrated significantly lower iNOS mRNA expression in response to LPS than the Vehicle/HC control group (p = .001), verifying the presence of conditioned suppression of NO production. Furthermore, the CNO/CS group showed similar iNOS mRNA expression in response to LPS as the CNO/HC control group (p = .181) but higher iNOS mRNA expression than the Vehicle/CS control group (p = .018). These results again suggest that CNO attenuated the conditioned response to the heroin-paired context.
Figure 2E shows the average splenic iNOS protein expression for each group 6 hours after LPS administration. A 2 × 2 ANOVA revealed a significant interaction between context (CS or HC) and treatment (CNO or Vehicle) (F(1,28) = 9.145, p = .005), and significant main effects of context (F(1,28) = 25.081, p < .001) and treatment (F(1,28) = 7.288, p = .012), on iNOS protein expression. Planned comparisons revealed that the Vehicle/CS group demonstrated significantly lower iNOS protein expression than the Vehicle/HC control group (p < .001), confirming the expression of conditioned suppression of NO production. The CNO/CS group showed similar iNOS protein expression in response to LPS as the CNO/HC control group (p = .172) and significantly higher iNOS mRNA expression than the Vehicle/CS control group (p = .002). Thus, CNO attenuated the conditioned response upon exposure to the heroin-paired context.
Overall, Experiment 1 results provide strong evidence that chemogenetic inhibition of putative excitatory, CaMKIIα-expressing cells in dHpc output regions dSub and dCA1 attenuates the conditioned suppression of LPS-induced peripheral NO production after exposure to a heroin-paired context.
3.2. Experiment 2: Effects of chemogenetic inhibition of ventral hippocampal (vHpc) output regions on the expression of heroin contextually conditioned suppression of NO production
The experimental timeline for Experiment 2 is displayed in Figure 3A. Brain templates were adapted, with permission, from the rat stereotaxic atlas (Paxinos and Watson, 2005). Virally-transduced cells, as indicated by mCherry expression, were most numerous and consistently observed in the two main vHpc output regions vSub and vCA1. However, transduced cells were also detected in other areas of the vHpc/intermediate hippocampus (DG and CA3), parasubiculum, and adjacent cortex (Figure 3B). Twenty-nine of 38 subjects showed bilateral virus expression in the vSub (N = 29) and, of these, all but one showed bilateral (N = 22) or unilateral (N = 6) virus expression in vCA1. The final group sizes for the analyses were as follows: nitrate/nitrite and iNOS qPCR, n = 5–8; iNOS ELISA, n = 6–8.
Figure 3. Experiment 2: Chemogenetic inactivation of vHpc output regions vSub and vCA1 does not disrupt expression of heroin contextually conditioned suppression of NO.
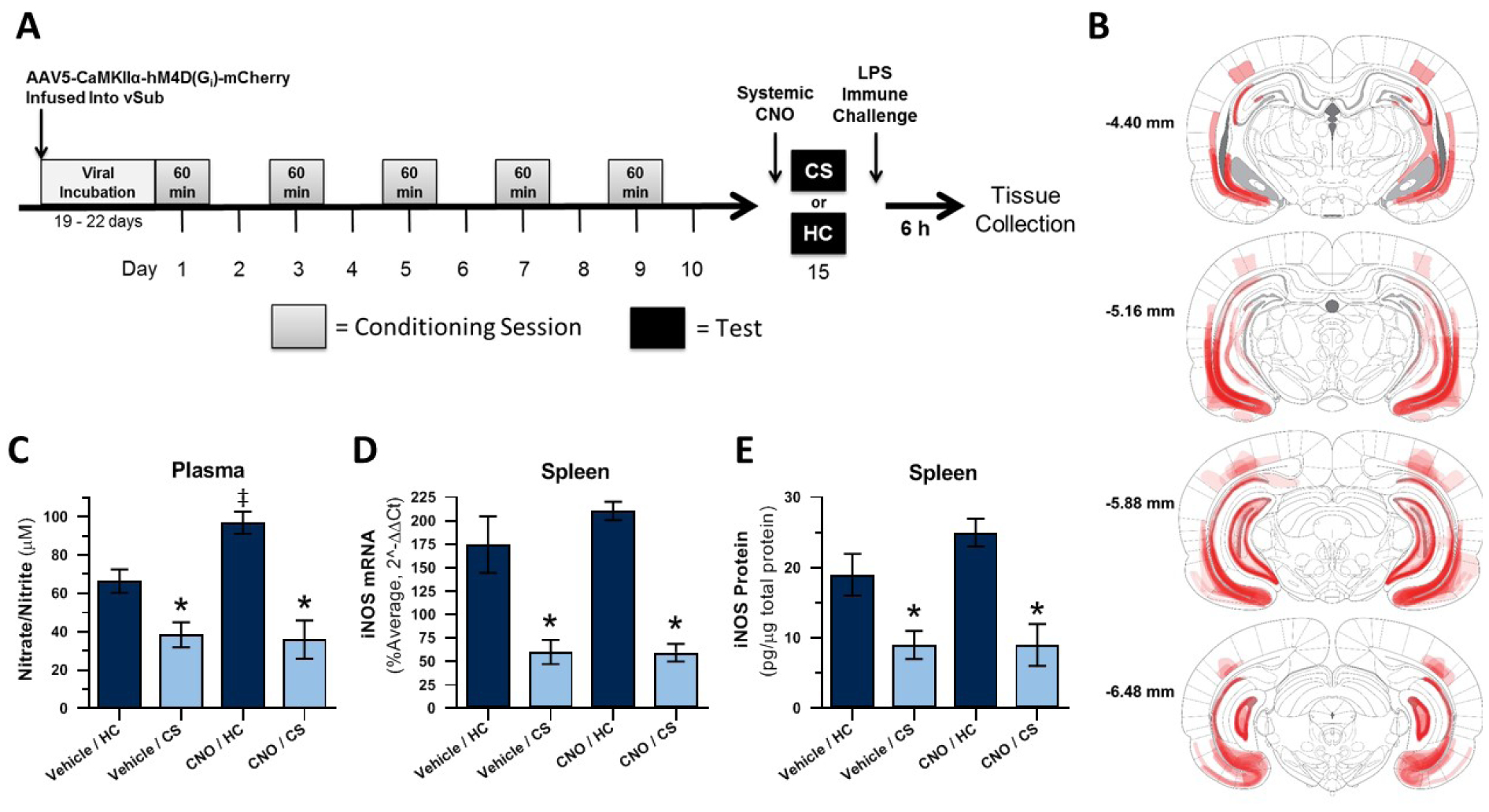
Experimental timeline (A). DREADD (mCherry) expression (B). Effect of CNO administration on plasma nitrate/nitrite concentration (C) and splenic iNOS mRNA (D) and protein (E) expression following heroin-paired context exposure and LPS challenge. CS = heroin-paired contextual conditioned stimulus, HC = home cage; *, statistically significant difference relative to respective HC control. ‡, statistically significant difference relative to Vehicle/HC group.
Figure 3C shows the average plasma nitrate/nitrite concentration for each group 6 hours after LPS administration. A 2 × 2 ANOVA revealed a significant interaction between context (CS or HC) and treatment (CNO or Vehicle; F(1,24) = 5.361, p = .029), a significant main effect of context (F(1,24) = 38.893, p < .001), and a trend for a main effect of treatment (F(1,24) = 3.839, p = .062) on plasma nitrate/nitrite concentration. Planned comparison revealed that the Vehicle/CS group demonstrated significantly lower nitrate/nitrite concentration after LPS than the Vehicle/HC control group (p = .008), confirming the expression of conditioned suppression of NO production. Similarly, the CNO/CS group demonstrated significantly lower nitrate/nitrite concentration than the CNO/HC control group (p < .001) and did not differ in nitrate/nitrite concentration compared to Vehicle/CS control group (p = .994). indicating that CNO failed to alter the conditioned response upon exposure to the heroin-paired context. Interestingly, the CNO/HC control group demonstrated significantly higher nitrate/nitrite concentration than the Vehicle/HC control group (p = .027). Thus, CNO itself increased LPS-induced nitrate/nitrite concentration in the spleen relative to vehicle.
Figure 3D shows the average splenic iNOS mRNA expression for each group 6 hours after LPS administration. A 2 × 2 ANOVA revealed a significant main effect of context (CS or HC; F(1,23) = 26.310, p < .001) but no significant main effect of treatment (CNO or Veh; F(1,23) = 1.143, p = .296) or interaction between context and treatment (F(1,23) = .036, p = .851). Planned comparison revealed that the Vehicle/CS group demonstrated significantly lower iNOS mRNA expression in response to LPS than the Vehicle/HC control group (p = .001), establishing the expression of conditioned suppression of NO production. The CNO/CS group also showed lower iNOS mRNA expression compared to the CNO/HC control group (p < .001) and similar iNOS mRNA expression compared to Vehicle/CS control group (p = .929). These results indicate CNO failed to alter LPS-induced iNOS expression upon exposure to either context.
Figure 3E shows the average splenic iNOS protein expression for each group 6 hours after LPS administration. A 2 × 2 ANOVA revealed a significant main effect of context (CS or HC; F(1,25) = 24.059, p < .001) but no significant main effect of treatment (CNO or Vehicle; F(1,23) = 1.339, p = .258) or interaction between context and treatment (F(1,25) = .845, p = .367). Planned comparison revealed that the Vehicle/CS group demonstrated significantly lower iNOS protein expression in response to LPS than the Vehicle/HC control group (p = .006), confirming the expression of conditioned suppression of NO production. Similarly, the CNO/CS group showed significantly lower iNOS protein expression than the CNO/HC control group (p = .001) and no difference in iNOS protein expression compared to the Vehicle/CS control group (p = .998). These results also indicate that CNO failed to alter LPS-induced iNOS expression upon exposure to either context.
Overall, Experiment 2 results provide strong evidence that chemogenetic inhibition of putative excitatory, CaMKIIα-expressing cells in vHpc output regions (vSub, vCA1) does not attenuate the conditioned suppression of LPS-induced peripheral NO production after exposure to a heroin-paired context.
4. Discussion
Our primary finding is that CaMKIIα-expressing neurons in dHpc, but not vHpc, projection areas - specifically in the dSub and dCA1 - are necessary for the expression of context-heroin conditioned suppression of LPS-induced peripheral NO production in male Lewis rats. This study confirms our previous finding that the functional integrity of the dHpc is critical for context-heroin conditioned suppression of peripheral NO production and extends this research by revealing cell-and region-specific contributions of the dHpc. These new findings will inform future research into specific dHpc circuit-level mechanisms of heroin conditioned immune effects and expand our understanding of how the central nervous system initiates these effects to heroin-paired contextual cues. We have focused on the role of NO production in this phenomenon in the current and previous studies, because in vivo measures of NO production are reliably suppressed by heroin (Lysle and How, 2000; Saurer et al., 2009) or heroin-paired contexts (Lysle and Ijames, 2002; Szczytkowski et al., 2011; Szczytkowski et al., 2013; Szczytkowski and Lysle, 2007, 2008, 2010), and NO plays a central role in immune function [for review, see (Uehara et al., 2015)]. The time point for tissue collection was optimized for the detection of an LPS-induced increase in NO production (Lysle and How, 1999), heroin suppression of NO production (Lysle and How, 2000; Saurer et al., 2009), and conditioned suppression of NO production (Lysle and Ijames, 2002) in the spleen, liver, and lung. Thus, while assessment of immune function at a single time point could not capture potential time-dependent alterations in vulnerability or resistance to disease and infection, we believe that assessment of NO production at the 6-hour time point is a reliable and meaningful measure of immune function. Nonetheless, it will be necessary to demonstrate the clinical relevance of changes in NO production in models of infectious disease, as has been extensively done with unconditioned opioid immune effects [for review, see (Eisenstein, 2019)].
Recent research by our team has shown that, similar to male rats, female Lewis rats display heroin-induced and context-heroin conditioned suppression of splenic NO production (Paniccia et al., in preparation) using similar procedures as those used in the current study. Since the present study was completed prior to the validation of the model in females, we were unable to assess potential sex differences in hippocampal mechanism of opioid conditioned immune effects. Based on known sex-differences in hippocampal function (Koss and Frick, 2017), it will be imperative to systematically explore sex differences in opioid conditioned immune effects. The results will likely be relevant for addressing the needs of both male and female opioid users.
While vHpc output via the vSub or vCA1 were not necessary in the conditioned immune effect evaluated in the present study, these output regions are required for heroin-and alcohol-conditioned motivational responses (Bossert et al., 2016; Bossert et al., 2013; Bossert and Stern, 2014; Marchant et al., 2016). Similarly, we have shown that dHpc neuroimmune mechanisms mediate heroin-conditioned suppression of peripheral NO production but not heroin-conditioned place preference (Paniccia et al., 2018). These contrasting results suggest that the hippocampal mechanism recruited for the expression of drug-conditioned contextual motivational and immune responses diverge. Such mechanistic divergence between these heroin-conditioned responses has important implications for combatting the harmful effects of heroin.
Our findings add to a growing literature that indicates functional differences between the dHpc and vHpc. The dHpc is thought to primarily support spatial, navigational, and episodic learning and memory, whereas the vHpc is thought to be involved in aversive fear/anxiety and appetitive reward learning and memory (“emotional memory”) (Bannerman et al., 2004; Bienkowski et al., 2018; Fanselow and Dong, 2010; Strange et al., 2014). However, not all evidence supports such a clear functional distinction between these hippocampal regions. Similar to the role of the vHpc in appetitive memory, opioid agonists administered directly into the dHpc generate conditioned motivational responses [(Corrigall and Linseman, 1988; Stevens et al., 1991) but see (Olmstead and Franklin, 1997)]. Neurons in the dCA1 have also been shown to encode reward independent of context encoding and could support motivated behavior (Gauthier and Tank, 2018). Furthermore, the dHpc is critical for some conditioned contextual fear responses (Matus-Amat et al., 2004; Roy et al., 2017). Similar to the dHpc, the vHpc is involved in the acquisition of spatial memory under certain training conditions or spatial demands [eg. (de Hoz et al., 2003; Ferbinteanu et al., 2003)]. Thus, the dHpc and vHpc, and their respective output regions, can be recruited to mediate common functions depending on task requirements (Bannerman et al., 2004).
A plausible explanation for the critical involvement of the dHpc in heroin conditioned immune effects is that the hippocampus regulates immune function per se. Several studies have shown that lesion or stimulation of the dHpc can alter components of the immune system, including levels of circulating antibodies and splenocyte counts (Devi et al., 1993; Devi et al., 2000; Devi et al., 2004). Similar effects have been seen following lesions of the vHpc (Devi and Namasivayam, 1990, 1991; Devi et al., 2004), suggesting that the vHpc may mediate other immune components than those examined in the present study. In support of the idea that mechanisms for specific immune effects diverge, dopamine D1-like receptor antagonism in the medial NAc shell blocks heroin’s suppression of LPS-induced NK cell activity and NO production but fails to alter lymphocyte proliferation (Saurer et al., 2009).
In addition to the hippocampus, several other brain regions have been implicated in both context-drug conditioned immune or motivational responses, including the basolateral amygdala (BLA), ventral tegmental area (VTA), and nucleus accumbens (NAc) (Bossert et al., 2006; Bossert et al., 2004; Chaudhri et al., 2009; Chaudhri et al., 2013; Fuchs et al., 2005; Hutson et al., 2014; Saurer et al., 2008a; Szczytkowski et al., 2011; Szczytkowski and Lysle, 2008; Xu et al., 2012). Signaling in the medial NAc shell might be a common mechanism by which context-drug associations regulate both immune and motivational behavior (Bossert et al., 2007; Marchant et al., 2019; Saurer et al., 2008b; Szczytkowski et al., 2011). Consistent with this idea, the current study (see 3S. Supplemental Results) and others reveal anatomical projections from the dHpc to the rostromedial NAc shell. Furthermore, it has been shown that dCA1 pyramidal neuronal projections to the rostromedial NAc shell are necessary for spatial memory and motivated behavior (Trouche et al., 2019). Future studies will need to investigate whether dHpc efferents to the NAc are similarly relevant to opioid contextually conditioned immune effects.
Similar to the NAc, the lateral septum (LS) receives direct input from the dHpc and vHpc (Risold and Swanson, 1997). The LS in turn projects to the hypothalamus, NAc, amygdala, and VTA, regulating behavioral arousal, emotional states, and motivated behaviors (Risold and Swanson, 1997; Sheehan et al., 2004; Swanson and Cowan, 1979). Disconnection of dHpc-LSVTA circuit elements using either GABA agonist-or DREADD-induced inhibition of neural activity impairs context-induced reinstatement of extinguished cocaine-seeking behavior, whereas manipulations of vHpc-LS circuits do not (Luo et al., 2011; McGlinchey and Aston-Jones, 2018). Thus, future studies will need to investigate the extent to which computations relevant for context-heroin conditioned immune effects are relayed through dHpc-LS circuits.
The present study used a DREADD expressed under a CaMKIIα promoter to target excitatory projection neurons within the dHpc and vHpc. Our results indicate that dSub and/or dCA1 neurons play a role in heroin contextually conditioned immune effects. Determining the specific contributions of dSub and dCA1 is challenging due to the proximity of these subregions, and to do so may require identification of dSub-or dCA1-specific promoters. We also did not characterize the phenotypes of DREADD-expressing neurons. Since DREADD expression was controlled by a CaMKIIα promoter, chemogenetic inhibition of excitatory pyramidal neurons was very likely responsible for the observed attenuation of the conditioned immune effect. However, GABAergic neurons can also express transgenes under the CaMKIIα promoter, albeit at lower rates (Calu et al., 2013; Johansen et al., 2010). Thus, it has yet to be determined whether our results are primarily due to the manipulation of excitatory or inhibitory projection and/or local circuit neurons in the dSub or dCA1. Additional research using other promoters to control DREADD expression and/or terminal administration of CNO will be important to enhance our understanding of the exact dHpc circuits involved in context-heroin conditioned immune effects.
Clozapine, a metabolite of CNO, can stimulate endogenous receptors, including histamine, serotonin, muscarinic acetylcholine, and dopamine receptors in addition to DREADDs [(Gomez et al., 2017) but see (Mahler and Aston-Jones, 2018)]. Importantly, however, it is unlikely that CNO alone had a non-specific effect on NO production in the group that expressed the DREADD in the dHpc because the CNO-treated group that expressed the DREADD in the vHpc did not show a similar attenuation of the conditioned response. Interestingly, there seemed to be a CNO-induced increase in NO production in the home cage control group that expressed the DREADD in the vHpc. However, since CNO failed to alter NO production a) in the group that was exposed to the heroin-paired context in the same experiment and b) in the home cage control group that expressed the DREADD in the dHpc, the results mitigate the possibility of a DREADD-independent CNO effect on NO production. Instead, it is possible that the slight enhancement in LPS-induced splenic NO production following vHpc inactivation was obscured in the presence of a heroin-paired context. This possibility should be explored further using DREADD ligands, like Compound 21, with no CNO-like off-target effects or κ-opioid-derived DREADDs [KORD; (Mahler and Aston-Jones, 2018)]. Future studies will also need to investigate whether Gs-or Gq-coupled DREADD signaling is sufficient to elicit or enhance opioid contextually conditioned immune responses.
Findings from the present study suggest that environmental contexts associated with drug use can influence health outcomes by modulating immune responses to pathogens. Through putative excitatory neurons in its main output regions, the dSub and dCA1, the dHpc plays a critical role in opioid contextually conditioned suppression of NO production in response to an immune challenge. Conversely, similar neuronal populations in vHpc output regions, the vSub and vCA1, do not seem to be critical for this phenomenon. It will be important from a therapeutic perspective to continue identifying the specific circuits and cellular mechanisms by which the dHpc contributes to the ability of environmental conditioned stimuli to regulate immune function in individuals chronically exposed to prescription opioids and in those suffering from opioid use disorders.
Supplementary Material
Highlights:
The hippocampus mediates heroin context conditioned suppression of nitric oxide.
Dorsal CA1 and subiculum neurons mediate this conditioned immune effect.
Ventral CA1 and subiculum do not play a vital role in this effect.
Dorsal hippocampal regions likely communicate with extra-hippocampal areas.
Funding and Disclosure:
This research was supported by National Institute on Drug Abuse grants R01 DA034721 (DTL and RFL), T32 DA007244 (CLL, JEP, SVP), and F31 DA047054 (JEP), a National Science Foundation grant DGE-1144081 (CLL), and awards from The University of North Carolina at Chapel Hill including a David Bray Peele Memorial Research Award (LMW), a Lindquist Undergraduate Research Award (LMW), a Richard A. King Research Excellence Award (CLL), a Chase Dashiell & Crane Graduate Student Award (CLL), and a Dean’s Graduate Fellowship (CLL). The authors declare no potential conflict of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary Information: Supplementary methods and results accompanies this paper online.
References
- Achterberg KG, Buitendijk G, Kool MJ, Goorden S, Post L, Slump DE, Silva AJ, van Woerden GM, Kushner SA, Elgersma Y, 2014. Temporal and Region-Specific Requirements of αCaMKII in Spatial and Contextual Learning. The Journal of Neuroscience 34, 11180–11187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alizamini MM, Kavianpour M, Karimi-Haghighi S, Fatahi Z, Haghparast A, 2018. Intra hippocampal administration of orexin receptor antagonists dose-dependently attenuates reinstatement of morphine seeking behavior in extinguished rats. Peptides 110, 40–46. [DOI] [PubMed] [Google Scholar]
- Bannerman DM, Rawlins JN, McHugh SB, Deacon RM, Yee BK, Bast T, Zhang WN, Pothuizen HH, Feldon J, 2004. Regional dissociations within the hippocampus--memory and anxiety. Neuroscience and biobehavioral reviews 28, 273–283. [DOI] [PubMed] [Google Scholar]
- Bienkowski MS, Bowman I, Song MY, Gou L, Ard T, Cotter K, Zhu M, Benavidez NL, Yamashita S, Abu-Jaber J, Azam S, Lo D, Foster NN, Hintiryan H, Dong H-WW, 2018. Integration of gene expression and brain-wide connectivity reveals the multiscale organization of mouse hippocampal networks. Nat Neurosci 21, 1628–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdan C, 2001. Nitric oxide and the immune response. Nature immunology 2, 907. [DOI] [PubMed] [Google Scholar]
- Bossert JM, Adhikary S, Laurent RS, Marchant NJ, Wang HL, Morales M, Shaham Y, 2016. Role of projections from ventral subiculum to nucleus accumbens shell in context-induced reinstatement of heroin seeking in rats. Psychopharmacology 233, 1991–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Gray SM, Lu L, Shaham Y, 2006. Activation of group II metabotropic glutamate receptors in the nucleus accumbens shell attenuates context-induced relapse to heroin seeking. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 31, 2197–2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Liu SY, Lu L, Shaham Y, 2004. A role of ventral tegmental area glutamate in contextual cue-induced relapse to heroin seeking. The Journal of neuroscience : the official journal of the Society for Neuroscience 24, 10726–10730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Marchant NJ, Calu DJ, Psychopharmacology S-Y, 2013. The reinstatement model of drug relapse: recent neurobiological findings, emerging research topics, and translational research. Psychopharmacology. [DOI] [PMC free article] [PubMed]
- Bossert JM, Poles GC, Wihbey KA, Koya E, Shaham Y, 2007. Differential effects of blockade of dopamine D1-family receptors in nucleus accumbens core or shell on reinstatement of heroin seeking induced by contextual and discrete cues. The Journal of neuroscience : the official journal of the Society for Neuroscience 27, 12655–12663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Stern AL, 2014. Role of ventral subiculum in context-induced reinstatement of heroin seeking in rats. Addiction biology 19, 338–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calu DJ, Kawa AB, Marchant NJ, Navarre BM, Henderson MJ, Chen B, Yau H-JJ, Bossert JM, Schoenbaum G, Deisseroth K, Harvey BK, Hope BT, Shaham Y, 2013. Optogenetic inhibition of dorsal medial prefrontal cortex attenuates stress-induced reinstatement of palatable food seeking in female rats. The Journal of Neuroscience 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhri N, Sahuque LL, Janak PH, 2009. Ethanol seeking triggered by environmental context is attenuated by blocking dopamine D1 receptors in the nucleus accumbens core and shell in rats. Psychopharmacology 207, 303–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhri N, Woods CA, Sahuque LL, Gill TM, Janak PH, 2013. Unilateral inactivation of the basolateral amygdala attenuates context-induced renewal of Pavlovian-conditioned alcohol-seeking. The European journal of neuroscience 38, 2751–2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigall WA, Linseman M, 1988. Conditioned place preference produced by intra hippocampal morphine. Pharmacol Biochem Be 30, 787–789. [DOI] [PubMed] [Google Scholar]
- Coussons ME, Dykstra LA, Lysle DT, 1992. Pavlovian conditioning of morphine-induced alterations of immune status. Journal of neuroimmunology 39, 219–230. [DOI] [PubMed] [Google Scholar]
- de Hoz L, Knox J, Morris RGM, 2003. Longitudinal axis of the hippocampus: both septal and temporal poles of the hippocampus support water maze spatial learning depending on the training protocol. Hippocampus 13. [DOI] [PubMed] [Google Scholar]
- Devi RS, Namasivayam A, 1990. Neuro immuno modulation by ventral hippocampus. Indian journal of physiology and pharmacology 34, 85–93. [PubMed] [Google Scholar]
- Devi RS, Namasivayam A, 1991. Modulation of specific immunity by ventral hippocampal formation in albino rats. Journal of neuroimmunology 33, 1–6. [DOI] [PubMed] [Google Scholar]
- Devi RS, Namasivayam A, Prabhakaran K, 1993. Modulation of non-specific immunity by hippocampal stimulation. Journal of neuroimmunology 42, 193–197. [DOI] [PubMed] [Google Scholar]
- Devi RS, Namasivayam A, Sivaprakash RM, 2000. Neuro-immunomodulation by dorsolateral hippocampus--role of macrophages,T and B cells. Indian journal of physiology and pharmacology 44, 136–142. [PubMed] [Google Scholar]
- Devi SR, Sivaprakash ARM, Namasivayam A, 2004. Rat hippocampus and primary immune response. Indian journal of physiology and pharmacology 48, 329–336. [PubMed] [Google Scholar]
- Eisenstein TK, 2019. The Role of Opioid Receptors in Immune System Function. Front Immunol 10, 2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS, Dong HW, 2010. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron 65, 7–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fecho K, Dykstra LA, Lysle DT, 1993. Evidence for beta adrenergic receptor involvement in the immunomodulatory effects of morphine. The Journal of pharmacology and experimental therapeutics 265, 1079–1087. [PubMed] [Google Scholar]
- Fecho K, Lysle DT, 2000. Heroin-induced alterations in leukocyte numbers and apoptosis in the rat spleen. Cell Immunol 202, 113–123. [DOI] [PubMed] [Google Scholar]
- Fecho K, Maslonek KA, Dykstra LA, Lysle DT, 1996. Assessment of the involvement of central nervous system and peripheral opioid receptors in the immunomodulatory effects of acute morphine treatment in rats. Journal of Pharmacology and Experimental Therapeutics 276, 626–636. [PubMed] [Google Scholar]
- Fecho K, Nelson CJ, Lysle DT, 2000. Phenotypic and functional assessments of immune status in the rat spleen following acute heroin treatment. Immunopharmacology 46, 193–207. [DOI] [PubMed] [Google Scholar]
- Ferbinteanu J, Ray C, McDonald RJ, 2003. Both dorsal and ventral hippocampus contribute to spatial learning in Long–Evans rats. Neuroscience letters 345. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Eaddy JL, Su ZI, Bell GH, 2007. Interactions of the basolateral amygdala with the dorsal hippocampus and dorsomedial prefrontal cortex regulate drug context-induced reinstatement of cocaine-seeking in rats. European Journal of Neuroscience 26, 487–498. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Evans AK, Ledford CC, Parker MP, Case JM, Mehta RH, See RE, 2005. The Role of the Dorsomedial Prefrontal Cortex, Basolateral Amygdala, and Dorsal Hippocampus in Contextual Reinstatement of Cocaine Seeking in Rats. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 30, 296. [DOI] [PubMed] [Google Scholar]
- Gauthier JL, Tank DW, 2018. A Dedicated Population for Reward Coding in the Hippocampus. Neuron 99, 179–1930000000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge F, Wang N, Cui C, Li Y, Liu Y, Ma Y, Liu S, Zhang H, Sun X, 2017. Glutamatergic Projections from the Entorhinal Cortex to Dorsal Dentate Gyrus Mediate Context-Induced Reinstatement of Heroin Seeking. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 42, 1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez JL, Bonaventura J, Lesniak W, Mathews WB, Sysa-Shah P, Rodriguez LA, Ellis RJ, Richie CT, Harvey BK, Dannals RF, Pomper MG, Bonci A, Michaelides M, 2017. Chemogenetics revealed: DREADD occupancy and activation via converted clozapine. Science 357, 503–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govitrapong P, Suttitum T, Kotchabhakdi N, Uneklabh T, 1998. Alterations of immune functions in heroin addicts and heroin withdrawal subjects. Journal of Pharmacology and Experimental Therapeutics 286, 883–889. [PubMed] [Google Scholar]
- Guo JY, Wang JY, Luo F, 2010. Dissection of placebo analgesia in mice: the conditions for activation of opioid and non-opioid systems. J Psychopharmacol 24, 1561–1567. [DOI] [PubMed] [Google Scholar]
- Hitchcock LN, Lattal KM, 2018. Involvement of the dorsal hippocampus in expression and extinction of cocaine-induced conditioned place preference. Hippocampus 28, 226–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsburgh CR, Anderson JR, Boyko EJ, 1989. Increased Incidence of Infections in Intravenous Drug-Users. Infect Cont Hosp Ep 10, 211–215. [DOI] [PubMed] [Google Scholar]
- Hutson LW, Szczytkowski JL, Saurer TB, Lebonville C, Fuchs RA, Lysle DT, 2014. Region-specific contribution of the ventral tegmental area to heroin-induced conditioned immunomodulation. Brain, behavior, and immunity 38, 118–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen JP, Hamanaka H, Monfils MH, Behnia R, Deisseroth K, Blair HT, LeDoux JE, 2010. Optical activation of lateral amygdala pyramidal cells instructs associative fear learning. Proceedings of the National Academy of Sciences 107, 12692–12697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koss WA, Frick KM, 2017. Sex differences in hippocampal function. Journal of neuroscience research 95, 539–562. [DOI] [PubMed] [Google Scholar]
- Lansink CS, Goltstein PM, Lankelma JV, McNaughton BL, Pennartz CM, 2009. Hippocampus leads ventral striatum in replay of place-reward information. PLoS biology 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasseter HC, Xie X, Ramirez DR, Fuchs RA, 2010. Sub-Region Specific Contribution of the Ventral Hippocampus to Drug Context-Induced Reinstatement of Cocaine-Seeking Behavior in Rats. Neuroscience 171, 830–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebonville CL, Jones ME, Hutson LW, Cooper LB, Fuchs RA, Lysle DT, 2016. Acquisition of heroin conditioned immunosuppression requires IL-1 signaling in the dorsal hippocampus. Brain, behavior, and immunity 56, 325–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis ND, Asim M, Barry DP, Singh K, de Sablet T, Boucher J-L, Gobert AP, Chaturvedi R, Wilson KT, 2010. Arginase II Restricts Host Defense to Helicobacter pylori by Attenuating Inducible Nitric Oxide Synthase Translation in Macrophages. The Journal of Immunology 184, 2572–2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X-B, Jones EG, 1996. Localization of alpha type II calcium calmodulin-dependent protein kinase at glutamatergic but not gamma-aminobutyric acid (GABAergic) synapses in thalamus and cerebral cortex. Proceedings of the National Academy of Sciences 93, 7332–7336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD, 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods (San Diego, Calif.) 25, 402–408. [DOI] [PubMed] [Google Scholar]
- Louria DB, Hensle T, Rose J, 1967. The major medical complications of heroin addiction. Annals of internal medicine 67, 1–22. [DOI] [PubMed] [Google Scholar]
- Luo AH, Tahsili-Fahadan P, Wise RA, Lupica CR, Aston-Jones G, 2011. Linking context with reward: a functional circuit from hippocampal CA3 to ventral tegmental area. Science (New York, N.Y.) 333, 353–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lysle DT, Coussons ME, Watts VJ, Bennett EH, Dykstra LA, 1993. Morphine-induced alterations of immune status: dose dependency, compartment specificity and antagonism by naltrexone. The Journal of pharmacology and experimental therapeutics 265, 1071–1078. [PubMed] [Google Scholar]
- Lysle DT, How T, 1999. Endogenous opioids regulate the expression of inducible nitric oxide synthase by splenocytes. The Journal of pharmacology and experimental therapeutics 288, 502–508. [PubMed] [Google Scholar]
- Lysle DT, How T, 2000. Heroin modulates the expression of inducible nitric oxide synthase. Immunopharmacology 46, 181–192. [DOI] [PubMed] [Google Scholar]
- Lysle DT, Ijames SG, 2002. Heroin-associated environmental stimuli modulate the expression of inducible nitric oxide synthase in the rat. Psychopharmacology 164, 416–422. [DOI] [PubMed] [Google Scholar]
- MacMicking JD, Nathan C, Hom G, Chartrain N, Fletcher DS, Trumbauer M, Stevens K, Xie QW, Sokol K, Hutchinson N, et al. , 1995. Altered responses to bacterial infection and endotoxic shock in mice lacking inducible nitric oxide synthase. Cell 81, 641–650. [DOI] [PubMed] [Google Scholar]
- MacMicking JD, North RJ, LaCourse R, Mudgett JS, Shah SK, Nathan CF, 1997. Identification of nitric oxide synthase as a protective locus against tuberculosis. Proceedings of the National Academy of Sciences 94, 5243–5248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Aston-Jones G, 2018. CNO Evil? Considerations for the Use of DREADDs in Behavioral Neuroscience. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 43, 934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant NJ, Campbell EJ, Pelloux Y, Bossert JM, Shaham Y, 2019. Context-induced relapse after extinction versus punishment: similarities and differences. Psychopharmacology 236, 439–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant NJ, Campbell EJ, Whitaker LR, Harvey BK, Kaganovsky K, Adhikary S, Hope BT, Heins RC, Prisinzano TE, Vardy E, Bonci A, Bossert JM, Shaham Y, 2016. Role of Ventral Subiculum in Context-Induced Relapse to Alcohol Seeking after Punishment-Imposed Abstinence. The Journal of neuroscience : the official journal of the Society for Neuroscience 36, 3281–3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Phan KL, Liberzon I, 2013. The contextual brain: implications for fear conditioning, extinction and psychopathology. Nature reviews. Neuroscience 14, 417–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matus-Amat P, Higgins EA, Barrientos RM, Rudy JW, 2004. The role of the dorsal hippocampus in the acquisition and retrieval of context memory representations. The Journal of neuroscience : the official journal of the Society for Neuroscience 24, 2431–2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlinchey EM, Aston-Jones G, 2018. Dorsal Hippocampus Drives Context-Induced Cocaine Seeking via Inputs to Lateral Septum. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 43, 987–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C, 1992. Nitric oxide as a secretory product of mammalian cells. The FASEB Journal 6, 3051–3064. [PubMed] [Google Scholar]
- Nathan C, Shiloh MU, 2000. Reactive oxygen and nitrogen intermediates in the relationship between mammalian hosts and microbial pathogens. Proceedings of the National Academy of Sciences 97, 8841–8848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson CJ, Schneider GM, Lysle DT, 2000. Involvement of central mu-but not delta-or kappa-opioid receptors in immunomodulation. Brain, behavior, and immunity 14, 170–184. [DOI] [PubMed] [Google Scholar]
- Olmstead MC, Franklin KBJ, 1997. The development of a conditioned place preference to morphine: Effects of microinjections into various CNS sites. Behavioral neuroscience 111, 1324. [DOI] [PubMed] [Google Scholar]
- Paniccia JE, Lebonville CL, Jones ME, Parekh SV, Fuchs RA, Lysle DT, 2018. Dorsal hippocampal neural immune signaling regulates heroin-conditioned immunomodulation but not heroin-conditioned place preference. Brain, behavior, and immunity 73, 698–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C, 2005. The rat brain in stereotaxic coordinates. Elsevier Academic Press, Amsterdam ; Boston. [Google Scholar]
- Paxinos G, Watson C, 2007. The rat brain in stereotaxic coordinates. Academic Press/Elsevier, Amsterdam ; Boston: ;. [Google Scholar]
- Risdahl JM, Khanna KV, Peterson PK, Molitor TW, 1998. Opiates and infection. Journal of neuroimmunology 83, 4–18. [DOI] [PubMed] [Google Scholar]
- Risold PY, Swanson LW, 1997. Connections of the rat lateral septal complex. Brain research. Brain research reviews 24, 115–195. [DOI] [PubMed] [Google Scholar]
- Roy DS, Kitamura T, Okuyama T, Ogawa SK, Sun C, Obata Y, Yoshiki A, Tonegawa S, 2017. Distinct Neural Circuits for the Formation and Retrieval of Episodic Memories. Cell 170, 1000–689963008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saurer TB, Ijames SG, Carrigan KA, Lysle DT, 2008a. Neuroimmune mechanisms of opioid-mediated conditioned immunomodulation. Brain, behavior, and immunity 22, 89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saurer TB, Ijames SG, Lysle DT, 2009. Evidence for the nucleus accumbens as a neural substrate of heroin-induced immune alterations. The Journal of pharmacology and experimental therapeutics 329, 1040–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saurer TB, James SG, Carrigan KA, Lysle DT, 2008b. Neuroimmune mechanisms of opioid-mediated conditioned immunomodulation. Brain Behavior and Immunity 22, 89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ, 2008. Analyzing real-time PCR data by the comparative CT method. Nature protocols 3, 1101–1108. [DOI] [PubMed] [Google Scholar]
- Sheehan TP, Chambers RA, Russell DS, 2004. Regulation of affect by the lateral septum: implications for neuropsychiatry. Brain research. Brain research reviews 46, 71–117. [DOI] [PubMed] [Google Scholar]
- Smith DM, Mizumori SJ, 2006. Hippocampal place cells, context, and episodic memory. Hippocampus 16, 716–729. [DOI] [PubMed] [Google Scholar]
- Stevens KE, Shiotsu G, Stein L, 1991. Hippocampal μ-receptors mediate opioid reinforcement in the CA3 region. Brain research 545, 8–16. [DOI] [PubMed] [Google Scholar]
- Strange BA, Witter MP, Lein ES, Moser EI, 2014. Functional organization of the hippocampal longitudinal axis. Nature reviews. Neuroscience 15, 655–669. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Cowan WM, 1979. The connections of the septal region in the rat. Journal of Comparative Neurology. [DOI] [PubMed]
- Szczytkowski JL, Fuchs RA, Lysle DT, 2011. Ventral tegmental area-basolateral amygdala-nucleus accumbens shell neurocircuitry controls the expression of heroin-conditioned immunomodulation. Journal of neuroimmunology 237, 47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczytkowski JL, Lebonville C, Hutson L, Fuchs RA, Lysle DT, 2013. Heroin-induced conditioned immunomodulation requires expression of IL-1beta in the dorsal hippocampus. Brain, behavior, and immunity 30, 95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczytkowski JL, Lysle DT, 2007. Conditioned effects of heroin on the expression of inducible nitric oxide synthase in the rat are susceptible to extinction and latent inhibition. Psychopharmacology 191, 879–889. [DOI] [PubMed] [Google Scholar]
- Szczytkowski JL, Lysle DT, 2008. Conditioned effects of heroin on proinflammatory mediators require the basolateral amygdala. The European journal of neuroscience 28, 1867–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczytkowski JL, Lysle DT, 2010. Dopamine D1 receptors within the basolateral amygdala mediate heroin-induced conditioned immunomodulation. Journal of neuroimmunology 226, 38–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trouche S, Koren V, Doig NM, Ellender TJ, El-Gaby M, Lopes-dos-Santos V, Reeve HM, Perestenko PV, Garas FN, Magill PJ, Sharott A, Dupret D, 2019. A Hippocampus-Accumbens Tripartite Neuronal Motif Guides Appetitive Memory in Space. Cell 176, 1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien JZ, Chen D, Gerber D, Tom C, Mercer EH, Anderson DJ, Mayford M, Kandel ER, Tonegawa S, 1996. Subregion-and Cell Type–Restricted Gene Knockout in Mouse Brain. Cell 87, 1317–1326. [DOI] [PubMed] [Google Scholar]
- Uehara E, de Shida B, de Brito C, 2015. Role of nitric oxide in immune responses against viruses: beyond microbicidal activity. Inflamm Res 64, 845–852. [DOI] [PubMed] [Google Scholar]
- Xie XH, Ramirez DR, Lasseter HC, Fuchs RA, 2010. Effects of mGluR1 antagonism in the dorsal hippocampus on drug context-induced reinstatement of cocaine-seeking behavior in rats. Psychopharmacology 208, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Lv X-F, Cui C-L, Ge F-F, Li Y-J, Zhang H-L, 2012. Essential role of NR2B-containing NMDA receptor–ERK pathway in nucleus accumbens shell in morphine-associated contextual memory. Brain research bulletin 89, 22–30. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


