Abstract
BACKGROUND:
A bioinformatics approach identified antitumor effects of tricyclic antidepressants (TCAs) in small cell lung cancer (SCLC) and other high-grade neuroendocrine carcinomas (grade 3 neuroendocrine carcinomas) (G3NEC) that was subsequently validated in preclinical models with a putative mechanism of action via inhibition of neuroendocrine signaling pathways. This study was undertaken to reposition the candidate TCA desipramine in a clinical trial in SCLC and G3NEC.
METHODS:
In this prospective, phase IIa intrapatient dose escalation clinical trial, patients were required to have failed at least one prior chemotherapy for metastatic SCLC or G3NEC. Treatment with desipramine began at 75 mg nightly with escalation in increments of 75 mg weekly to a maximum of 450 mg daily.
RESULTS:
Six patients were enrolled, 3 with SCLC, and 3 with G3NEC (lung, rectal, and pancreas). Tolerability of desipramine was worse than predicted. Of the 6 patients enrolled: 1 patient achieved 300 mg daily, 2 patients reached 150 mg dailly, 1 patient reached 75 mg daily, and 2 patients were unable to tolerate any stable dose. Reasons for discontinuation included drug-related grade 3 colon pseudo-obstruction, unrelated GI bleed, and grade 1–2 neurocognitive adverse events. Median clinical or radiographic progression free survival was 1.2 months (range 0.2–3.3) and median overall survival from study entry was 2.7 months (range 1.3–5.6).
CONCLUSIONS:
No clinical or radiographic benefit was observed using desipramine to treat SCLC and G3NEC, so this trial was terminated. Intolerable low and medium grade neurocognitive side effects led to intermittent treatment and early discontinuation in most patients; given this limitation, doses achieved may be inadequate compared to the preclinical studies.
MICROABSTRACT:
A bioinformatics approach previously identified a potential antitumor effect of tricyclic antidepressants (TCAs) in small cell lung cancer (SCLC) and other high-grade neuroendocrine carcinomas (grade 3 neuroendocrine carcinoma) (G3NEC), which was validated in preclinical models.
In this prospective, phase IIa clinical trial, patients were required to have failed at least one prior chemotherapy for metastatic SCLC or G3NEC (Ki-67 ≥ 20% or ≥ 20 mitoses/10 HPF). Treatment with desipramine began at 75 mg nightly with escalation by 75 mg weekly to a maximum dose of 450 mg daily. Six patients were enrolled on this clinical trial, 3 with SCLC, and 3 with G3NEC (lung, rectal, and pancreatic).Tolerability of desipramine was worse than predicted. In the 6 patients enrolled: 1 patient achieved 300 mg daily, 2 patients reached 150 mg daily, 1 patient reached 75 mg daily, and 2 patients were unable to tolerate any stable dose. Reasons for discontinuation included drug-related grade 3 colon pseudo-obstruction, unrelated GI bleed, and grade 1–2 drug related dizziness, confusion, and somnolence. Though numbers are small, median clinical or radiographic progression free survival was 1.2 months (range 0.2–3.3) and median overall survival from study entry was 2.7 months (range 1.3–5.6). Although preclinical evidence was promising, no clinical or radiographic benefit was observed using desipramine to treat SCLC and G3NEC, so this trial was terminated.
Keywords: Small Cell Lung Cancer, Drug Repositioning, Neuroendocrine Tumors
Introduction
Small cell lung cancer (SCLC) is a high-grade neuroendocrine carcinoma of the lung that comprises approximately 15% of all lung cancers [1]. Until recently, little had changed in the treatment of small cell lung cancer over the past 30 years [2]. Standard first-line treatment was until recently platinum-based chemotherapy (either cisplatin or carboplatin combined with either etoposide or irinotecan). The addition of PD-L1 inhibitors atezolizumab or durvalumab to platinum and etoposide modestly improves overall survival compared to chemotherapy alone, and this is now the contemporary standard of care [3]. Despite initial high response rates to first-line treatment most patients invariably relapse and rapidly progress. In Second line platinum refractory disease, treatment typically consists of a camptothecin (irinotecan or topotecan), or other single-agent chemotherapeutic agents or immune checkpoint blockade if not received in first line, but response rates are generally low [4–6]. Given the aggressive, incurable nature of metastatic SCLC and the poor survival, new treatments are desperately needed.
In addition to SCLC, high-grade neuroendocrine tumors (grade 3 neuroendocrine carcinoma - G3NEC) occur in the lung, gastrointestinal and other organ systems. Like SCLC, established therapy for metastatic first-line treatment is cisplatin or carboplatin paired with etoposide with consideration of adding a PD-L1 antibody extrapolating from recent data showing improved survival in extensive stage small cell lung cancer (ES-SCLC). No additional therapies have been proven to be extend overall survival beyond the first-line setting [7]. Prognosis for metastatic G3NEC is similarly poor.
There currently is a productivity gap between research and development costs and the number of new cancer drugs approved [8]. Using novel drug discovery technologies to find cancer treatments by repositioning older drugs approved for other indications has the potential to reduce time, costs and risks associated with drug discovery [9]. Successfully repositioned drugs in oncology include thalidomide for multiple myeloma and retinoic acid for acute promyelocytic leukemia [10, 11].
A previously published large-scale bioinformatics approach identified a potential antitumor effect of tricyclic antidepressants (TCAs) in SCLC, which was validated in cell lines, mouse models, and patientderived xenografts, with a putative mechanism of action via inhibition of G-protein coupled receptors (GPCR) inhibiting protein kinase A (PKA) and impacting neuroendocrine signaling pathways [12]. Additional studies also demonstrate potential anti-tumor activity via TCA activation of PP2A via direct binding of the PP2A Aα subunit [13]. TCAs and norepinephrine and dopamine reuptake inhibitors (NDRIs) given for depression were associated with lung cancer-specific survival in a large case-control study [14]. This study was undertaken to reposition the candidate TCA desipramine in a clinical trial in SCLC and G3NEC. Of all of the available TCAs, desipramine was chosen because of its favorable anticholinergic and non-sedating side effect profile compared with other TCAs in clinical use for the treatment of depression, and because of the ability to dose escalate beyond the standard 75 mg daily dose in adults treated for depression.
Methods
In this Stanford Cancer Institute IRB approved protocol (NCT01719861), to be eligible, patients age 18 years or older must have had metastatic small cell lung cancer or metastatic high-grade neuroendocrine carcinoma (G3NEC) of any organ system (G3NEC defined by Ki-67 ≥ 20% and/or ≥ 20 mitoses/10 (HPF)), baseline ECG with qTC by Fredericia criteria ≤ 450 msec for men and ≤ 470 msec for women, measurable disease by RECIST 1.1, Zubrod (ECOG) performance status of 0–2, adequate organ function and have received at least one line of prior chemotherapy treatment for metastatic disease.
In this intra-patient dose escalation study, patients were started at a desipramine dose of 25 mg by mouth nightly (qHS). Desipramine was then increased by 25 mg every 2–3 days as tolerated to a target dosage of 75 mg qHS by end of the first week. Dose was then increased by 75 mg PO qHS every week (−2/+ 4 days) until either unacceptable toxicity occurred or a maximum of 450 mg daily was achieved. Upon progression or intolerable toxicity a taper by 75 mg weekly was initiated to avoid withdrawal symptoms associated with the TCA. Patients were monitored closely with weekly clinical visits. Labs and ECG were required until patients remained on a stable dose for ≥ 3 weeks.
The primary outcome was efficacy of treatment as measured by response rate (percent partial response and complete response (PR +CR) with imaging to assess response every 8 weeks by RECIST 1.1 criteria). Secondary endpoints included progression-free survival (PFS) and overall survival (OS). Safety was measured in terms of type, frequency and severity of adverse event (AE) reactions according to CTCAE v4.0, Maximum tolerated dose (MTD) of each patient, established by adhering to an intrapatient dose escalation schema, and tolerability, was assessed by the incidence of AEs leading to study drug delay or discontinuation.
The study was designed to enroll 10 evaluable patients with SCLC and G3NEC with the goal of 1 or more objective response as a signal to move forward with a larger trial. The historical response rates for SCLC and other G3NEC after first line platinum chemotherapy are approximately 10%. If the true partial response rate was between 10 and 20%, the probability of observing at least 1 partial response among the 10 patients we planned to enroll is between 65% and 89%.
Results
Six patients were enrolled on this intra-patient dose escalation study of desipramine in SCLC and G3NEC between December 2012 and October 2013. Pertinent demographics and clinical outcomes for each patient are summarized in Table 1. Though numbers are small in this analysis, median clinical or radiographic PFS was 1.2 months (range0.2–3.3) and median OS from study entry was 2.7 months (range 1.3–5.6) (Figure 1).
Table 1.
Dem and Clinical Outcomes of SCLC and other G3NEC patients treated with Desipramine
| Patient | Gender | Age | Number of Prior Treatments | CNS Metastases | Diagnosis | Best Response* | PFS (mo.) | OS (mo.) |
|---|---|---|---|---|---|---|---|---|
| 1 | M | 61 | 4 | Y | SCLC | PD | 1.4 | 3 |
| 2 | M | 59 | 3 | N | Rectal G3NEC | PD | 1.5 | 5.6 |
| 3 | F | 75 | 1 | N | SCLC | NE | 0.2 | 1.3 |
| 4 | M | 74 | 3 | Y | SCLC | NE | 0.9 | 1.3 |
| 5 | M | 49 | 4 | N | pancreatic G3NEC | NE | 3.3 | 5.1 |
| 6 | F | 51 | 9 | Y | lung G3NEC | PD | 1.1 | 2.4 |
(PD=Progressive Disease, NE = Not Evaluable for Response).
Figure 1.
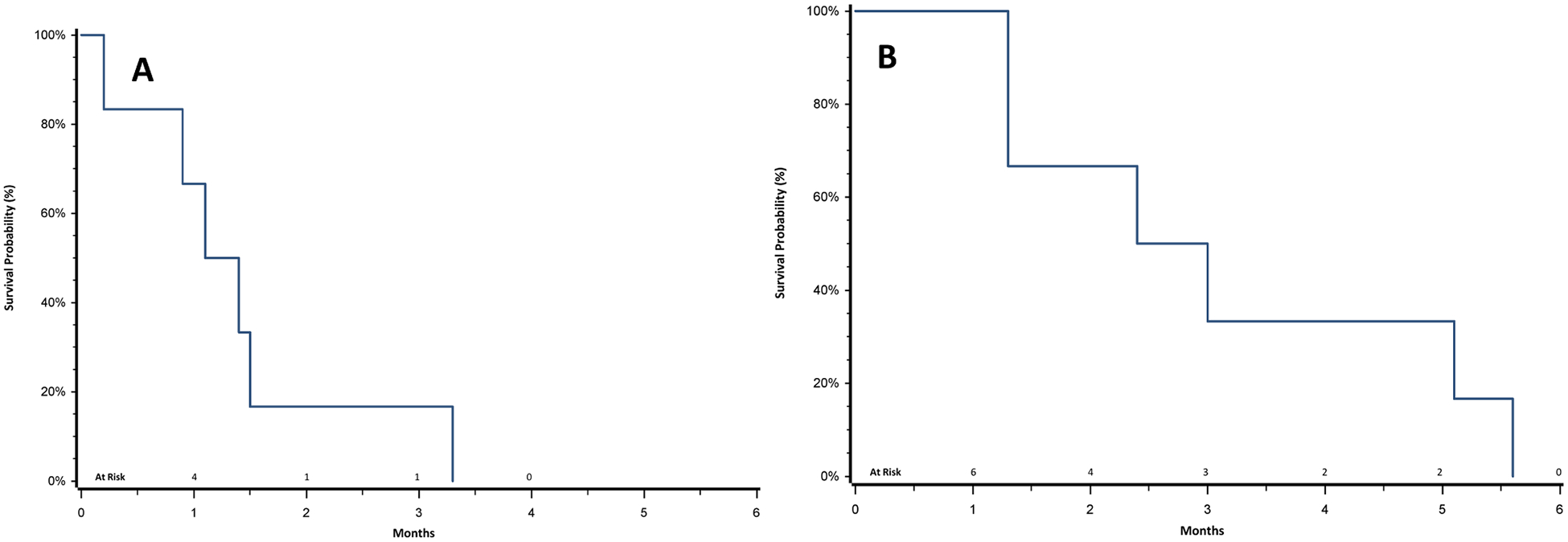
Progression-Free Survival (A) and Overall Survival (B) of SCLC and G3NEC Patients Treated with Desipramine
AEs attributable to study drug were mainly neurocognitive (100%). Though none were above grade 3, 50% of patients had grade 2 neurocognitive AEs. There was one grade 3 AE; a large bowel obstruction likely related to anticholinergic effects of desipramine that occurred in the patient with high-grade neuroendocrine rectal tumor (Table 2). Among all patients, the maximum dose achieved (median) was 112.5 mg and only 3 patients were able to escalate beyond 75 mg qHS. One patient was able to dose-escalate to 300 mg desipramine QHS. Due to lack off efficacy and low to medium grade neurocognitive side effects limiting dose escalation of desipramine, the trial was closed to accrual early after 6 patients.
Table 2.
Adverse events in G3NEC and SCLC Patients Treated with Desipramine
| Adverse Events Possibly, Probably or Definitely Related to Desipramine | Grade 1–2 % (N) | Grade 3 (N) |
|---|---|---|
| Fatigue | 50% (3/6) | - |
| Dry Mouth | 33% (2/6) | - |
| Dizziness | 83%% (5/6) | - |
| Confusion | 33% (2/6) | - |
| Somnolence | 33% (2/6) | - |
| Headache | 17% (1/6) | - |
| Palpitations | 17% (1/6) | - |
| Nausea | 17% (1/6) | - |
| Large Bowel Pseudo-obstruction | - | 17% (1/6) |
Discussion
This trial demonstrates the feasibility of rapidly translating a drug repositioning bioinformatics approach previously validated in preclinical models into a clinical study, in order to identify FDA approved candidate drugs for other indications to treat SCLC and G3NEC. The repositioning of FDA approved drugs for other indications has the potential to accelerate the drug development timeline. Although in vitro and in vivo preclinical evidence was promising with imipramine and other TCAs, no clinical or radiographic benefit was observed using desipramine to treate SCLC and G3NEC, which led to early termination of the study after no responses were noted in the first 6 patients enrolled. PFS and OS were poor, consistent with the dismal prognosis of these tumors after progressive disease on platinum-based treatments.
Further work from bench to bedside to bench evaluation of TCAs in SCLC and G3NEC suggest that differences in antitumor activity between desipramine and other TCAs such as imipramine against relevant G-protein coupled receptors (which are hypothesized to induce cytotoxicity in these neuroendocrine malignancies) may be responsible for the discordant in vivo findings in SCLC mouse models (data not shown). This study also illustrates the challenges associated with drug repositioning including the difficulties achieving higher therapeutic doses potentially needed for antitumor activity than what is typically used for the FDA approved indication for depression and how differences in relevant patient populations (typically young adults with depression versus older, heavily pretreated neuroendocrine cancer patients often with multiple medical comorbidities) can impact drug dosing and tolerability.
Intolerability of SCLC and G3NEC patients to desipramine with low and medium grade neurocognitive side effects led to intermittent treatment interruption, inability to dose escalate, and early discontinuation in most patients. Therefore the doses achieved may be inadequate compared to the efficacious doses achieved in the preclinical models. Screening compounds that selectively target G-coupled receptors and the PKA signaling pathway implicated in antitumor activity of SCLC and G3NEC could be a more promising approach.
Conclusion
Although promising pre-clincial data on TCAs emerged from a drug repositioning bioinformatics approach in SCLC and G3NEC, no clinical benefit was observed using desipramine to treat patients with SCLC and G3NEC and substantial toxicity was observed. Given these findings, this trial was closed before the accrual goal of 10 patients after 6 patients failed to achieve an objective response. Intolerable low and medium grade neurocognitive side effects (usually not seen in patients treated with similar drugs for depression) led to intermittent treatment and early discontinuation in most patients, so doses achieved may be inadequate compared to the preclinical studies demonstrating activity in SCLC. Selectively targeting G-coupled receptors implicated in antitumor activity of SCLC and G3NEC could be a more promising approach to achieve adequate target inhibition.
Clinical Practice Points.
Though preclinical data was promising, no clinical benefit was observed using desipramine to treat patients with small cell lung cancer and grade 3 neuroendocrine carcinoma and substantial toxicity was seen.
Doses achieved may be inadequate relative to the preclinical studies demonstrating activity, mainly due to mainly neurocognitive side effects during dose escalation.
Acknowledgements
The project described was supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant 5 KL2 RR025743 (JWR) and a Stanford Cancer Institute Developmental Cancer Research Award, through a National Institute Cancer Center Support Grant P30CA124435 (JWR and JWN). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NCI and NIH. We are grateful to Alex McMillan, Ph.D. for biostatistical support.
Footnotes
Declaration of Competing Interest
Nadine Jahchan, PhD is currently an employee of Pionyr. Julien Sage receives research funding from Stemcentrx/Abbvie and Pfizer on small cell lung cancer, and licensed a patent on small cell lung cancer to Forty Seven Inc/Gilead. Otherwise the authors have no relevant conflicts of interest.
References
- [1].Govindan R, et al. , Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database, J Clin Oncol 24 (28) (2006) 4539–4544. [DOI] [PubMed] [Google Scholar]
- [2].Riess JW, Lara PN Jr., Left behind? Drug discovery in extensive-stage small-cell lung cancer, Clin Lung Cancer 15 (2) (2014) 93–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Horn L, et al. , First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer, N Engl J Med 379 (23) (2018) 2220–2229. [DOI] [PubMed] [Google Scholar]
- [4].Spigel DR, Greco FA, Evolving role of irinotecan in small-cell lung cancer, Clin Adv Hematol Oncol 1 (8) (2003) 482–485. [PubMed] [Google Scholar]
- [5].Ardizzoni A, et al. , Topotecan, a new active drug in the second-line treatment of small-cell lung cancer: a phase II study in patients with refractory and sensitive disease. The European Organization for Research and Treatment of Cancer Early Clinical Studies Group and New Drug Development Office, and the Lung Cancer Cooperative Group, J Clin Oncol 15 (5) (1997) 2090–2096. [DOI] [PubMed] [Google Scholar]
- [6].Antonia SJ, et al. , Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial, Lancet Oncol 17 (7) (2016) 883–895. [DOI] [PubMed] [Google Scholar]
- [7].Strosberg JR, et al. , The NANETS consensus guidelines for the diagnosis and management of poorly differentiated (high-grade) extrapulmonary neuroendocrine carcinomas, Pancreas 39 (6) (2010) 799–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].DiMasi JA, Hansen RW, Grabowski HG, The price of innovation: new estimates of drug development costs, J Health Econ 22 (2) (2003) 151–185. [DOI] [PubMed] [Google Scholar]
- [9].Ashburn TT, Thor KB, Drug repositioning: identifying and developing new uses for existing drugs, Nat Rev Drug Discov 3 (8) (2004) 673–683. [DOI] [PubMed] [Google Scholar]
- [10].Pujol JL, et al. , Phase III double-blind, placebo-controlled study of thalidomide in extensive-disease small-cell lung cancer after response to chemotherapy: an intergroup study FNCLCC cleo04 IFCT 00–01, J Clin Oncol 25 (25) (2007) 3945–3951. [DOI] [PubMed] [Google Scholar]
- [11].Coombs CC, Tavakkoli M, Tallman MS, Acute promyelocytic leukemia: where did we start, where are we now, and the future, Blood Cancer J 5 (2015) e304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Jahchan NS, et al. , A drug repositioning approach identifies tricyclic antidepressants as inhibitors of small cell lung cancer and other neuroendocrine tumors, Cancer Discov 3 (12) (2013) 1364–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].McClinch K, et al. , Small-Molecule Activators of Protein Phosphatase 2A for the Treatment of Castration-Resistant Prostate Cancer, Cancer Res 78 (8) (2018) 2065–2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Zingone A, et al. , Relationship between anti-depressant use and lung cancer survival, Cancer Treat Res Commun 10 (2017) 33–39. [DOI] [PMC free article] [PubMed] [Google Scholar]


