Abstract
Diversity Oriented Clicking (DOC) is a unified click-approach for the modular synthesis of lead-like structures through application of the wide family of click transformations. DOC evolved from the concept of achieving “diversity with ease” by combining classic C-C -bond click chemistry with recent developments in connective SuFEx-technologies. We showcase 2-Substituted-Alkynyl-1-Sulfonyl Fluorides (SASFs) as a new class of connective hub in concert with a diverse selection of click-cycloaddition processes. Through the stereoselective DOC of SASFs with a range of dipoles and dienes, we report a diverse click-library of 173 unique functional molecules in minimal synthetic steps. The SuFExable library comprises 10 discrete heterocyclic core structures derived from 1,3- and 1,5-dipoles; while reaction with dienes yields several 3-dimensional Diels-Alder adducts. Growing the library to 278 discrete compounds through late-stage modification is made possible through SuFEx click derivatization of the pendant sulfonyl fluoride group in 96 well-plates — demonstrating the versatility of the DOC approach for the rapid synthesis of diverse functional structures. Screening for function against MRSA (USA300) revealed several lead hits with improved activity over methicillin.
Keywords: Diversity Oriented Clicking, SuFEx Click Chemistry, Sulfonyl Fluorides, Heterocycles, Cycloadditions
Graphical Abstract
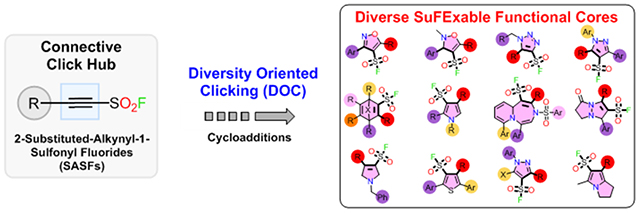
Diversity Oriented Clicking (DOC) is a unified click chemistry approach for the synthesis of diverse lead-like structures. We showcase DOC through the application of novel highly activated 2-Substituted-Alkynyl-1-Sulfonyl Fluorides (SASFs) hubs in combination with a range of selective and robust click-cycloaddition, to deliver an unprecedented SuFExable library of functional biologically active lead-structures.
Click chemistry (CC)[1,2] was launched in 2001 as a Nature-inspired synthesis approach for the discovery of functional molecules. Foremost, CC was conceived as a strategic method for the efficient combination of small modules together through heteroatom links (C-X-C); quickly evolving to be one of the most widely used and powerful technologies in modern day synthesis.[3] From materials science[4] to biology;[5] from drug discovery[6,7] to polymer synthesis,[8] CC has proven itself time and again as a uniquely powerful and enabling connective technology.[3,7]
The drive engines of CC are an expanding set of powerful “near-perfect” reactions that meet stringent requirements, being: modular, wide in scope, high yielding, stereospecific, and readily purified.[2,9] While the bar is set very high to attain this privileged click-status, the types of reactions, and especially reaction conditions were more common in the literature of 50–100 years ago. This trend is epitomized by the influence that 1,3-dipolar cycloaddition reactions have had on CC, and most notably the azide-alkyne fusion reaction recognized[10] by Professor Rolf Huisgen.[11,12] Arguably, Huisgen’s early work on cycloaddition chemistry has influenced the evolution of CC more than any other; ultimately leading to the discovery of three high-profile breakthroughs: i) target accelerated in situ CC as a tool for drug discovery;[13–17] ii) the stereoselective stepwise copper catalyzed Huisgen azide-alkyne cycloaddition (CuAAC) reaction[18,19] — a transformation with an unrivaled breadth of application;[3, 20–22] and iii) the strain promoted Huisgen 1,3-dipolar cycloaddition reaction between azide–alkyne (SPAAC, 2004),[23,24] used ubiquitously in bioconjugation applications (Figure 1).
Figure 1.
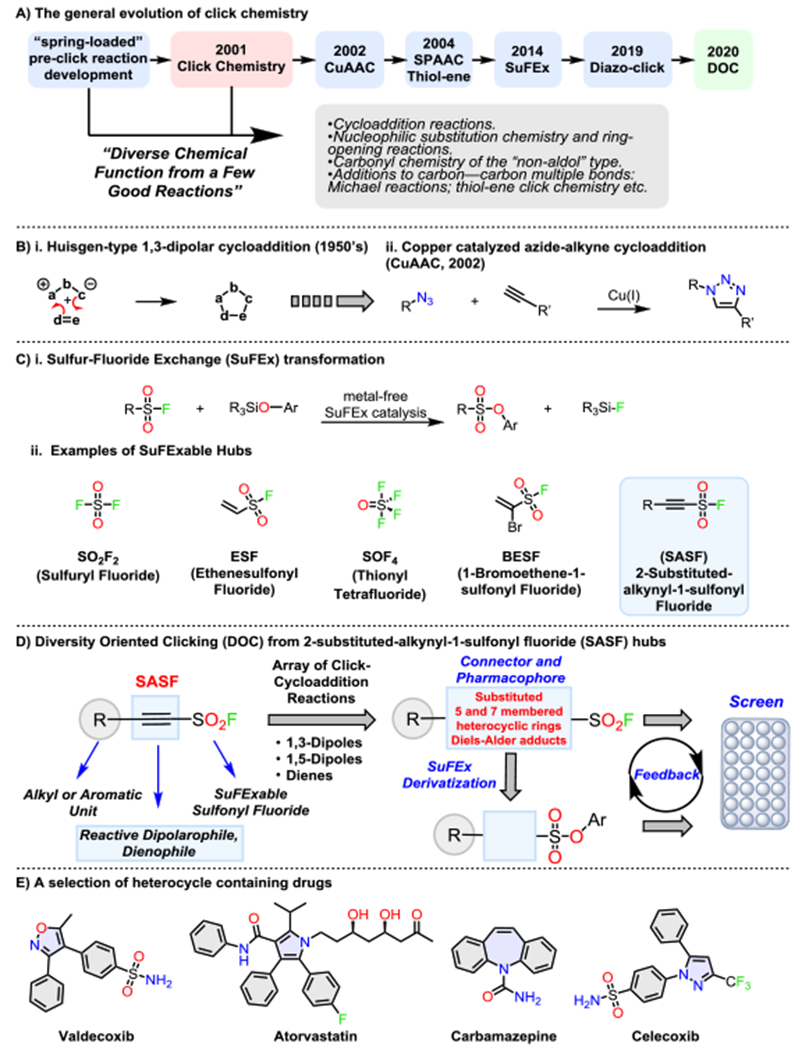
A) Timeline for the development of click chemistry; B) i. General representation of Huisgen 1,3-dipolar cycloaddition-type reactions, and ii. The stereoselective copper catalyzed azide-alkyne cycloaddition (CuAAC) reaction; C) i. The Sulfur-Fluoride Exchange (SuFEx) click-reaction, ii. Examples of connective SuFEx Hubs; D) Diversity Oriented Clicking (DOC) from 2-substituted-alkynyl-1-sulfonyl fluorides (SASFs); E) Examples of drugs comprising an aromatic heterocyclic core.
Other reactions have since joined the ranks of the click-family; each with roots in the “old-school” literature, including thiol-ene CC finding wide application in polymer and materials science;[25,26] Sulfur-Fluoride Exchange (SuFEx) — a prime method for a wealth of applications,[27–39] and a powerful diazotransfer agent (diazo-click) for the guaranteed azidation of primary amines (Figure 1A).[40] While these incredibly reliable transformations (Figure 1) have become the go-to click reactions, with few exceptions,[26, 41] the development of the wider family of stereoselective “spring-loaded” click-like processes identified in the CC manifesto have been somewhat overlooked (Figure 1A).
Here we seek to address this imbalance and reinvigorate the wider family of click-cycloaddition[42] reactions in function-discovery applications, termed: Diversity Oriented Clicking (DOC) (Figure 1D).[43] DOC is an emerging strategy for combining the best of both classical and modern click-technology; reaffirming the “diversity with ease”[2] principle through minimal steps maximal modification clicking. DOC evolves beyond the primary connective function of CC and places equivalent emphasis on the 2D or 3D structures of the connectors themselves, as they are, or could be elaborated to have diverse chemical properties for addressing diverse pharmacological needs (Figure 1D).[44]
Combining the versatility of connective SuFEx hubs with classic click-cycloaddition transformations, we showcase DOC through a new class of 2-substituted-alkynyl-1-sulfonyl fluoride (SASF) connectors. The stereoselective fusion of the SASF hubs with a selection of dipoles and dienes, delivers an unprecedented library of densely substituted and diversely SuFExable lead-like compounds (Figure 1D).
Alkynyl sulfonyl fluoride hubs offer immense scope as a platform for structural diversification through several click-transformations, but until now, were noticeably absent from the series of available SuFEx connectors (Figure 1D).
Three key components were integrated into our SASF design: i) the modular incorporation of aromatic or alkyl units as a point for diversification; ii) a reactive internal alkyne for core-diversification through cycloaddition chemistry, and; iii) a sulfonyl fluoride head group — serving to both modulate the activity of the alkyne group and act as a SuFExable handle for further diversification.
Electrophilic quenching of an alkynide ion with sulfuryl fluoride (SO2F2) was considered the most direct route to the SASF modules: deprotonation of phenylacetylene 1a with nBuLi at −78 °C in THF, followed by bubbling a stream of SO2F2 gas through the solution at −110 °C, gave the 2-phenylethyne-1-sulfonyl fluoride (SASF, 2a)[45] in a 65% isolated yield (2.00 mmol scale). However, the reaction yield dropped significantly when scaled-up (37%, 10.0 mmol scale) and alternative methods were subsequently developed (See SI): Method A replaces SO2F2 with fluorosulfonic acid anhydride (FSO2-O-SO2F)[46] as the source of electrophilic “+SO2F”; whereas Method B is a one-pot-2-step protocol involving the addition of the alkynide ion into sulfur dioxide (SO2),[47] followed by electrophilic fluorination of the resulting sulfinate anion with N-fluorobenzenesulfonimide (NFSI) (Scheme 1). Through these complementary protocol’s useful quantities of the bench stable SASF hubs are available in good yields.[48] It is noteworthy that Method A performs well with electron-poor substrates but is unsatisfactory with electron-rich electron-rich substrates due to isolation problems; the converse is true for Method B (Scheme 1).
Scheme 1.
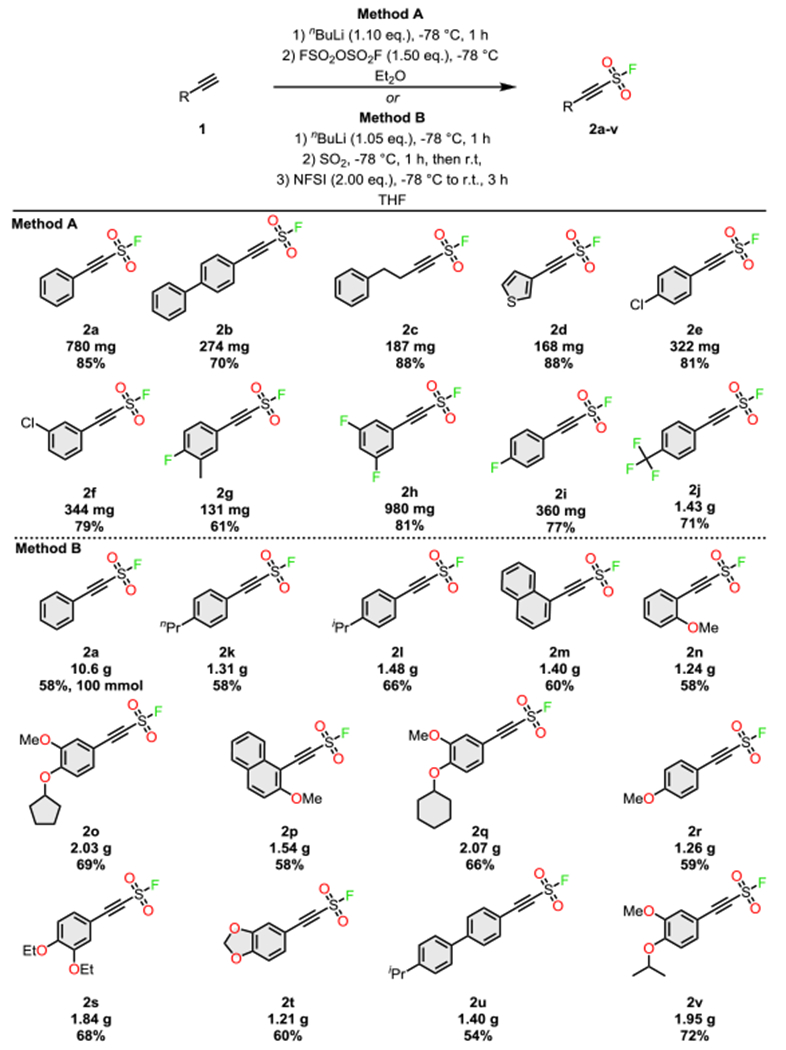
Synthesis of 2-alkynyl-1-sulfonyl fluorides (SASF).
To implement the click-cycloaddition DOC strategy with the SASF hubs a selection of reactive, yet selective,[2,42] dipole and diene coupling partners were chosen that would provide rapid access to a diverse array of sulfonyl fluoride functionalized ring structures of pharmaceutical importance (Figure 1E).[2,49–51] A selection of nitrogen based 1,3-dipoles, including nitrile oxides (3a), nitrones (3b), azides (3c), nitrile imines (3d), Sydnones (3e) and Münchnones (3f and 3g) smoothly coupled with the SASFs under thermal conditions (r.t. to 120 °C) giving the respective isoxazoles (4a), isoxazolines (4b), triazoles (4c),[52] pyrazoles (4d and 4e) and pyrroles (4f and 4g) in good to excellent yields (refer to SI for full substrate scope and procedures).
Particularly notable was the observed strict selectivity in every one of these highly exergonic fusions;[42] giving a single regioisomer in each case (as elucidated through detailed NMR analysis and single crystal X-ray diffraction, see SI).[53]
Further core diversity was achieved through the reaction of the SASF hubs with the thio-Münchnone (3h) — itself generated in situ [1.00 eq. of the elected 2-(benzoylthio)-2-substitutedarylacetic acid (see SI), 1.20 eq. of the elected SASF and 1.00 eq. of trifluoroacetic acid anhydride (TFAA) heating in toluene at 120 °C in a sealed tube] — giving the 2,4,5-trisubstituted thiophene-3-sulfonyl fluorides exclusively (Scheme 2, 4h).
Scheme 2.
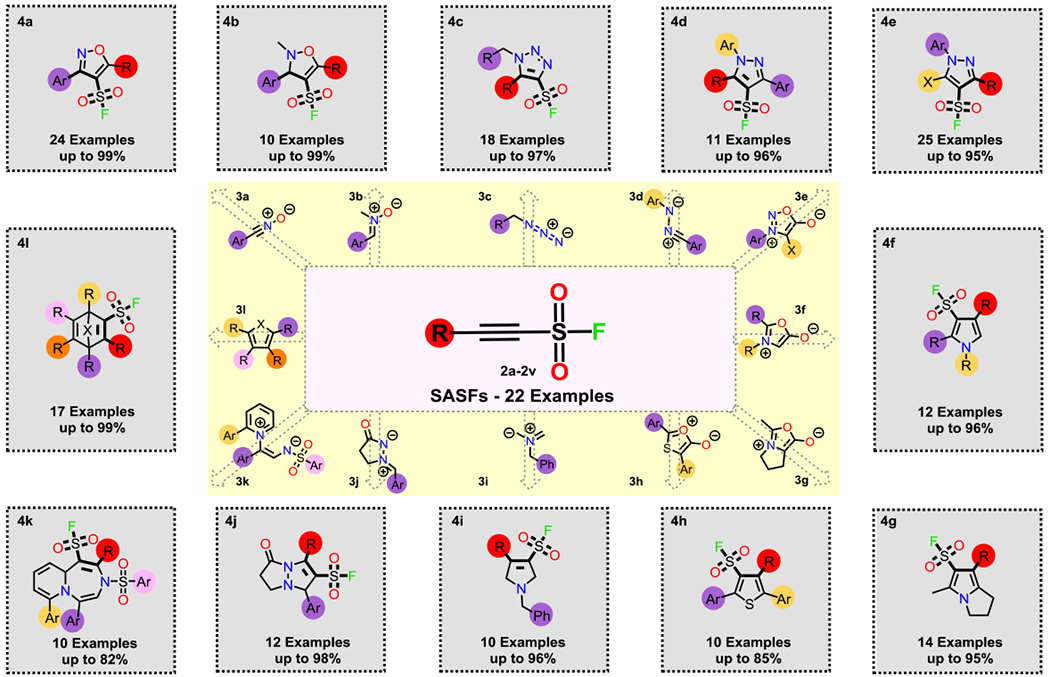
Reaction of SASF hubs with a selection of 1,3-dipoles, 1,5-dipoles, and dienes to assemble the SuFExable 173 compound DOC library 4a) 3,5-disubstituted isoxazole-4-sulfonyl fluorides [1.00 eq. SASF hub, 1.00 eq. imidoyl chloride, 2.00 eq. Et3N stirring in toluene at r.t., 10 min]; 4b) 2-methyl-3,5-disubstituted-2,3-dihydroisoxazole-4-sulfonyl fluorides [1.00 eq. SASF hub, 1.00 eq. nitrone stirring in CH2Cl2 at r.t., 30 min]; 4c) 1,5-disubstituted-1H-1,2,3-triazole-4-sulfonyl fluorides [1.00 eq. SASF hub, 1.00 eq. azide, 2.50 mol% [Rh(CO)2Cl]2 heating in DCE at 40 °C, 16 h]; 4d) 1,3,5-trisubstituted-1H-pyrazole-4-sulfonyl fluorides [2.00 eq. SASF hub, 1.00 eq. hydrazonoyl chloride, 1.50 eq. DIPEA stirring in MeCN at r.t., 1 h]; 4e) 1,3,5-trisubstituted-1H-pyrazole-4-sulfonyl fluorides [1.00 eq. SASF hub, 1.00 eq. Sydnone heating in toluene at 120 °C, 16 h]; 4f) 1,2,4-1H-pyrrole-3-sulfonyl fluorides [1.20 eq. SASF hub, 1.00 eq. substituted glycine heating in Ac2O at 120 °C, 30 min]; 4g) 5-methyl-7-substituted-2,3-dihydro-1H-pyrrolizine-6-sulfonyl fluorides [1.20 eq. SASF hub, 1.00 eq. L-proline heating in Ac2O at 120 °C, 3 h]; 4h) 2,4,5-trisubstituted thiophene-3-sulfonyl fluoride [1.20 eq. SASF hub, 1.00 eq. 2-(benzoylthio)-2-substitutedarylacetic acid and 1.00 eq. TFAA in toluene at 120 °C, 16 h]; 4i) 1-benzyl-4-substituted-2,5-dihydro-1H-pyrrole-3-sulfonyl fluoride [1.00 eq. SASF hub, 1.10 eq. N-(methoxymethyl)-N-(trimethylsilylmethyl)benzylamine and 20.0 mol% TFA in CH2Cl2 at 0 °C, 30 min]; 4j) 1,3-disubstituted-5-oxo-6,7-dihydro-1H,5H-pyrazolo[1,2-a]pyrazole-2-sulfonyl fluorides [1.00 eq. SASF hub, 1.00 eq. azomethine imine heating in CDCl3 at 100 °C (μW), 30 min]; 4k) 2,3,5,7-tetrasubstituted-3,1a-dihydropyrido[1,2-d][1,4]diazepine-1-sulfonyl fluorides [1.00 eq. SASF hub, 1.20 eq. azomethine imine stirring in DCE at r.t., 4-40 h]; 4l) 3-substituted bicyclo[2.2.1]hepta-2,5-diene-2-sulfonyl fluoride and 1,3,4,5,6-pentasubstituted-7-oxabicyclo[2.2.1]hepta-2,5-diene-2-sulfonyl fluorides [1.00 eq. SASF hub, 1.00 eq. of diene in CH2Cl2 at r.t., 2-16 h] or [1.00 eq. SASF hub, 1.00 eq. of diene heating in DCE at reflux, 16 h].
The stereoselective 1,3-dipolar cycloaddition with the in situ generated azomethine ylide (3i),[54,55] derived from N-(methoxymethyl)-N-(trimethylsilylmethyl)benzylamine,[54–56] gave the corresponding 1-benzyl-4-substituted-2,5-dihydro-1H-pyrrole-3-sulfonyl fluorides (4i) in excellent yield.
The [3+2] and [5+2] cycloaddition pathways of azomethine imines with the SASF also led to the target products (4j and 4k). For example, upon microwave irradiation at 120 °C for 30 min in CDCl3, the 3-oxopyrazolidin-1-ium-2-ides (3j) gave the corresponding N,N-bicyclic pyrazolidinone products in excellent yields (Scheme 2, 4j);[57] whereas the air-stable azomethine imine 1,5-dipoles (3k), recently reported by Yoo and co-workers,[58] delivered the bicyclic-1,4-diazepines (4k) products in good yield upon stirring at room temperature in 1,2-dichloroethane (DCE).
Further elaboration of the proof-of-concept DOC strategy was possible by exploiting the SASFs in classic [4+2] Diels-Alder cycloadditions.[2] A selection of dienes and hetero-dienes (3l) reacted with impressive stereoselectivity and excellent yield, to deliver an array of densely substituted adducts as single diastereoisomers (Scheme 2, 4l).
To assess the structural properties and novelty of the new DOC library, we performed a lead-likeness and molecular analysis using the LLAMA package.[59] Of the 173 compounds, 2.3% were deemed to be in lead-like chemical space (Alog P of -1 to 3 and RMM of 200 to 350), with 68% in ‘Lipinski’ space (Alog P <5 and RMM <500); indicating that the majority of the library could be considered “drug-like”. A principle moment of inertia (PMI) plot for the library suggests that the majority of the library lies between the linear (diacetylene) and the flat disc-like (benzene) sections— this is inherently related to the nature of the cycloaddition reactions and the sp2 rich products (see SI).
We next screened a portion (151)[60] of the DOC library for activity against the pathogenic methicillin resistant strain staphylococcus aureus USA300. The choice of screen is timely given the burgeoning antibiotic crisis, and while it does not explore the global biological potential of the library, the identification of lead hits serves to demonstrate DOC as a valid method for function discovery. Performing a preliminary screen at 200 M revealed 16 actives out of a total of 151 sulfonyl fluorides,[60] suggesting a hit rate of 11% of compounds screened (equating to 70% within the azepine subset (4k), and 82% within the iodo-substituted pyrazole (4e) subset; see SI). The minimum inhibitory concentration (MICs) for each of the 16 lead compounds was next determined, identifying 5 of the iodo-substituted pyrazole sulfonyl fluorides (4ea-4ee) with significantly improved activity over methicillin (up to 10-fold lower).[61]
It is noteworthy that although analysis of lead-like structures is a useful exercise, the active compounds from the DOC library each had a large lead-likeness penalty[62] and, in the majority of cases, are outside of ‘Lipinski’ space (81%). While the results from the narrow screen do not allow any firm conclusions to be made about specific or special properties of the DOC library per se, they further demonstrate the value of sulfonyl fluoride-based compounds in lead discovery.[29,39]
We next evaluated the SuFEx reactivity of the diverse array of structures: a number of aryl silyl ethers (5) were reacted with representative examples of the DOC library at room temperature [0.20 eq. 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU), acetonitrile(MeCN)] (Scheme 4A) [See SI for procedures]. Aside from the pyrrole derivatives that were unreactive to the nucleophilic exchange at room temperature (and only moderately upon raising the temperature to 80 °C, see SI), the remainder of representative compound classes (Scheme 2 4a-4l) (with the reactive exclusion of 4j and 4k) were amenable to late-stage SuFEx modification.
Scheme 4.
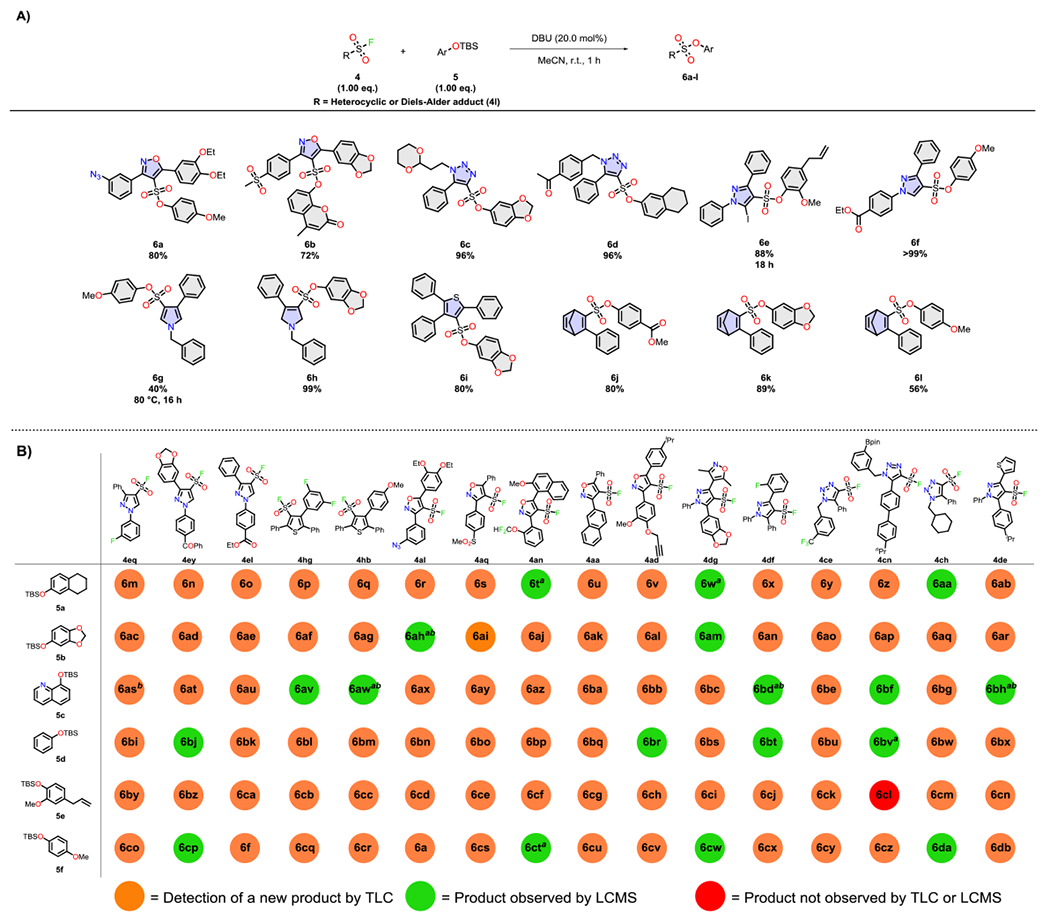
A) SuFEx reactions on select examples of the DOC library; B) Parallel SuFEx synthesis of sulfonate derivatives performed in 96 well plate format (3.00 μmoles of sulfonyl fluoride, 3.00 μmoles of TBS ether, 20.0 mol% DBU in 500 μL MeCN agitated at r.t. for 16 h; [a] The product was not distinguishable from the starting materials by TLC and was instead identified by LCMS; [b] Small amounts of starting material remained.
Competition experiments suggest that the order of SuFEx reactivity follows: triazole 4c > pyrazole 4e (X=I) ≥ isoxazoline 4b ≈ isoxazole 4a ≈ thiophene 4h > pyrazole 4e (X=H) > pyrazole 4d > Diels-Alder adduct 4l > pyrroles 4f/4g > N,N-bicyclic pyrazolidinone 4j/bicyclic-1,4-diazepine 4k (see SI).[63]
This avenue to increased library diversity was demonstrated by performing SuFEx reactions in parallel (96 well-plates) with a combination of 16 sulfonyl fluorides and 6 aryl silyl ethers (Scheme 4B) to give a focused library of 95 sulfonate derivatives upon simple agitation for 16 hours. The almost “guaranteed” outcome of SuFEx-reactions conveniently allowed thin layer chromatography (TLC) to be employed as a means to monitor the course of the reaction array.[64] In the few cases where reaction products were indistinguishable from the starting materials by TLC, LCMS analysis was performed as a back-up option.
In summary, we have described Diversity Oriented Clicking (DOC) as a strategy for the rapid synthesis of structurally diverse compounds. DOC evolves CC beyond a connective technology and places equal emphasis on the structure and function of the diverse array of linkers. To showcase the potential of DOC and pay tribute to the pioneering work of Professor Rolf Huisgen, we have qualified two aspects of click chemistry inspired ideology to synthesize a focused library of lead-like compounds: i) the application of the recently introduced connective SuFEx hub concept, and ii) the availability of a range of click-cycloaddition transformations. We have further reported a new class of versatile 2-substituted-alkynyl-1-sulfonyl fluoride (SASF) SuFEx hubs (22 examples), that undergo an array of stereoselective click-cycloaddition reactions to generate a structurally diverse library of 173 unprecedented sulfonyl fluoride containing heterocycles as lead structures, which themselves can be further diversified through late-stage SuFEx modification with an array of aryl silyl ethers. In total, we have qualified the syntheses of 300 novel compounds, including SASFs, aromatic heterocyclic sulfonyl fluorides and sulfonates. The functionality of the library was demonstrated through screening against MRSA, leading to the identification of 16 hit compounds (11%).
DOC is a powerful discovery method that takes full advantage of the wider family of click reactions to generate structurally diverse connections. Given the simplicity, reliability and selectivity of the DOC approach, we believe it will find wide application in lead-discovery endeavors.
Supplementary Material
Figure 2.
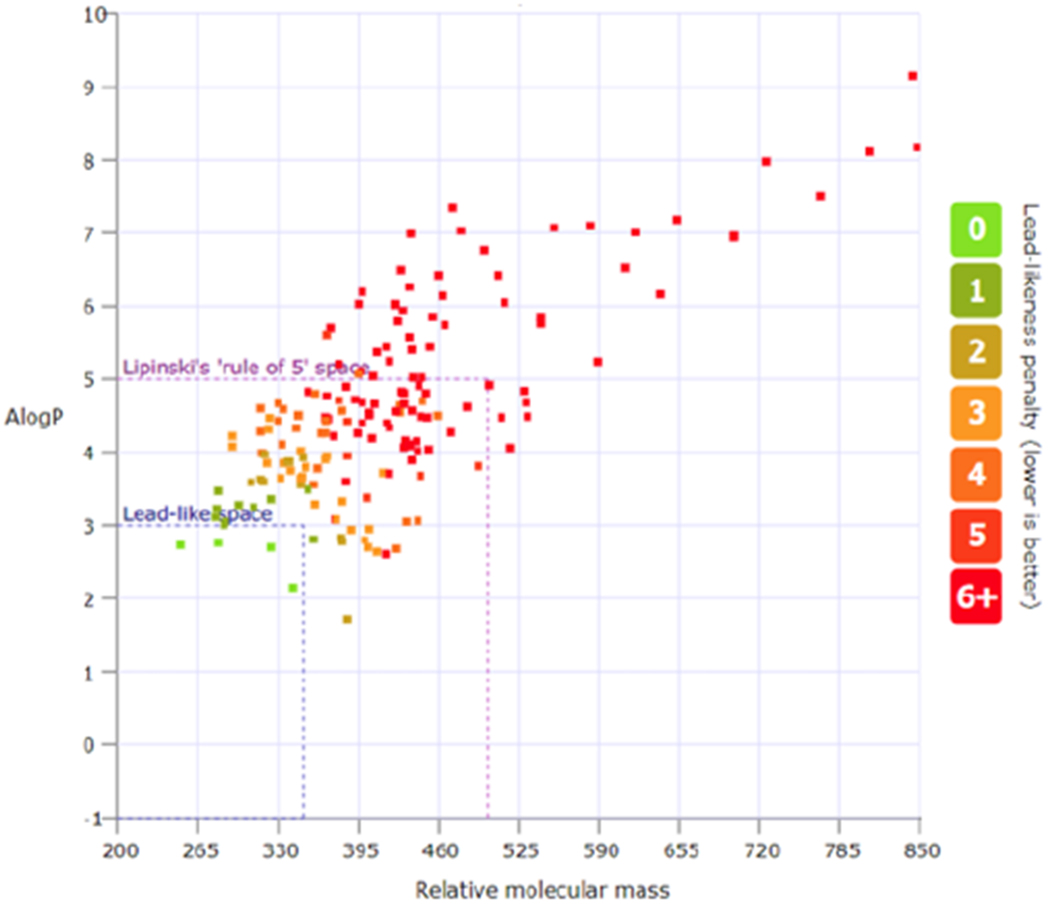
A) LLAMA analysis of 173 member sulfonyl fluoride DOC library.
Scheme 3.
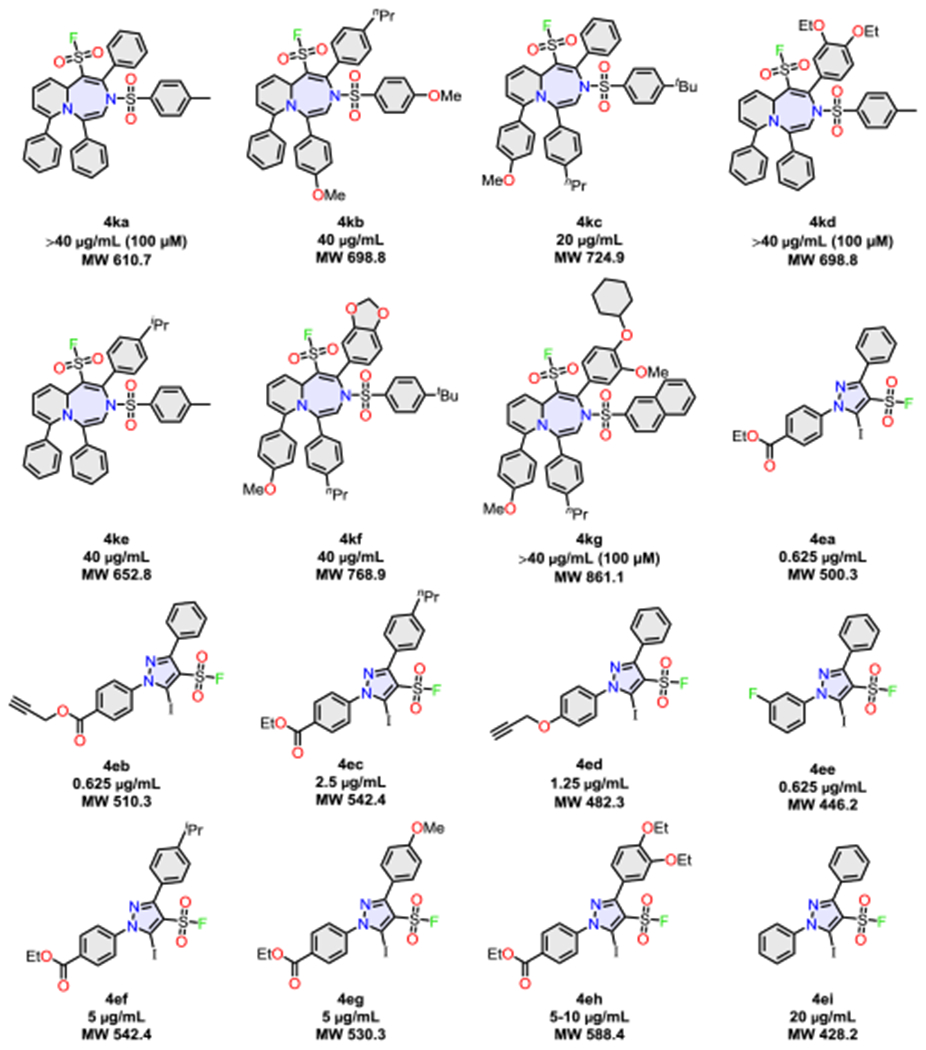
Lead hit compounds identified from the library screen of 151 compounds at 200 μM MRSA and MICs.
Acknowledgements
We thank the ARC (J.E.M) (Future Fellowship; FT170100156), and the NIH (K.B.S) (P50 GM103368, R01 GM117145) for financial support. We thank the National Mass Spectrometry Facility at Swansea University. We thank Jie Sun and the State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, CAS, for performing X-ray crystallography.
Footnotes
Supporting information for this article is given via a link at the end of the document.
Dedicated to the memory of Professor Rolf Huisgen and the great contribution of his pioneering work to the development of click chemistry
References
- [1].The term click chemistry (CC) was first coined by K. Barry Sharpless in 1998 and was later published as a concept in 2001 see ref: [2].
- [2].Kolb HC, Finn MG, Sharpless KB, Angew. Chem. Int. Ed 2001, 40, 2004–2021. [DOI] [PubMed] [Google Scholar]
- [3].Moses JE, Moorhouse AD, Chem. Soc. Rev 2007, 36, 1249–1262. [DOI] [PubMed] [Google Scholar]
- [4].Xi W, Scott TF, Kloxin CJ, Bowman CN, Adv. Funct. Mater 2014, 24, 2572–2590. [Google Scholar]
- [5].Best MD, Biochemistry 2009, 48, 6571–6584. [DOI] [PubMed] [Google Scholar]
- [6].Thirumurugan P, Matosiuk D, Jozwiak K, Chem. Rev 2013, 113, 4905–4979. [DOI] [PubMed] [Google Scholar]
- [7].Moorhouse AD, Moses JE, ChemMedChem 2008, 3, 715–723. [DOI] [PubMed] [Google Scholar]
- [8].Martens S, Holloway JO, Du Prez FE, Macromol. Rapid Commun 2017, 38, 1700469. [DOI] [PubMed] [Google Scholar]
- [9].The aspirational criteria of a click reaction provide a template to help guide the discovery of perfect reactions.
- [10].For the original report on the azide–alkyne reaction, see:; Michael A, J. Prakt. Chem 1893, 48, 94–95. [Google Scholar]
- [11].Huisgen R, Angew. Chem. Int. Ed 1963, 2, 565–598. [Google Scholar]
- [12].Huisgen R, Angew. Chem. Int. Ed 1963, 2, 633–645. [Google Scholar]
- [13].Lewis WG, Green LG, Grynszpan F, Radić Z, Carlier PR, Taylor P, Finn MG, Sharpless KB, Angew. Chem. Int. Ed 2002, 41, 1053–1057. [DOI] [PubMed] [Google Scholar]
- [14].Manetsch R, Krasiński A, Radić Z, Raushel J, Taylor P, Sharpless KB, Kolb HC, J. Am. Chem. Soc 2004, 126, 12809–12818. [DOI] [PubMed] [Google Scholar]
- [15].Millward SW, Agnew HD, Lai B, Lee SS, Lim J, Nag A, Pitram S, Rohde R, Heath JR, Integr. Biol 2013, 5, 87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Mamidyala SK, Finn MG, Chem. Soc. Rev 2010, 39, 1252–1261. [DOI] [PubMed] [Google Scholar]
- [17].Sharpless KB, Manetsch R, Expert Opin. Drug Discov 2006, 1, 525–538. [DOI] [PubMed] [Google Scholar]
- [18].Rostovtsev VV, Green LG, Fokin VV, Sharpless KB, Angew. Chem. Int. Ed 2002, 41, 2596–2599. [DOI] [PubMed] [Google Scholar]
- [19].Tornøe CW, Christensen C, Meldal M, J. Org. Chem 2002, 67, 3057–3064. [DOI] [PubMed] [Google Scholar]
- [20].Jiang X, Hao X, Jing L, Wu G, Kang D, Liu X, Zhan P, Expert Opin. Drug Discov 2019, 14, 779–789. [DOI] [PubMed] [Google Scholar]
- [21].Li L, Zhang Z, Molecules 2016, 21, 1393. [Google Scholar]
- [22].Neumann S, Biewend M, Rana S, Binder WH, Macromol. Rapid Commun 2020, 41, 1900359. [DOI] [PubMed] [Google Scholar]
- [23].For the original report on the azide-strained alkyne reaction, see; Wittig G, Krebs A, Chem. Ber 1961, 94, 3260–3275. [Google Scholar]
- [24].Agard NJ, Prescher JA, Bertozzi CR, J. Am. Chem. Soc 2004, 126, 15046–15047. [DOI] [PubMed] [Google Scholar]
- [25].Kade MJ, Burke DJ, Hawker CJ, J. Polym. Sci. A: Polym. Chem 2010, 48, 743–750. [Google Scholar]
- [26].Hoyle CE, Bowman CN, Angew. Chem. Int. Ed 2010, 49, 1540–1573. [DOI] [PubMed] [Google Scholar]
- [27].Dong J, Krasnova L, Finn MG, Sharpless KB, Angew. Chem. Int. Ed 2014, 53, 9430–9448. [DOI] [PubMed] [Google Scholar]
- [28].Barrow AS, Smedley CJ, Zheng Q, Li S, Dong J, Moses JE, Chem. Soc. Rev 2019, 48, 4731–4758. [DOI] [PubMed] [Google Scholar]
- [29].Baker BR, Hurlbut JA, J. Med. Chem 1968, 11, 241–245. [DOI] [PubMed] [Google Scholar]
- [30].Narayanan A, Jones LH, Chem. Sci 2015, 6, 2650–2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Smedley CJ, Giel M-C, Molino A, Barrow AS, Wilson DJD, Moses JE, Chem. Commun 2018, 54, 6020–6023. [DOI] [PubMed] [Google Scholar]
- [32].Smedley CJ, Zheng Q, Gao B, Li S, Molino A, Duivenvoorden HM, Parker BS, Wilson DJD, Sharpless KB, Moses JE, Angew. Chem. Int. Ed 2019, 58, 4552–4556. [DOI] [PubMed] [Google Scholar]
- [33].Smedley CJ, Barrow AS, Spiteri C, Giel M-C, Sharma P, Moses JE, Chem. Eur. J 2017, 23, 9990–9995. [DOI] [PubMed] [Google Scholar]
- [34].Chinthakindi PK, Arvidsson PI, Eur. J. Org. Chem 2018, 3648–3666. [Google Scholar]
- [35].Krutak JJ, Burpitt RD, Moore WH, Hyatt JA, J. Org. Chem 1979, 44, 3847–3858. [Google Scholar]
- [36].Hedrick RM, US Patent 2653973, 1953.
- [37].Giel M-C, Smedley CJ, Mackie ERR, Guo T, Dong J, Soares da Costa TP, Moses JE, Angew. Chem. Int. Ed 2020, 59, 1181–1186. [DOI] [PubMed] [Google Scholar]
- [38].Liu Z, Li J, Li S, Li G, Sharpless KB, Wu P, J. Am. Chem. Soc 2018, 140, 2919–2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Zheng Q, Woehl JL, Kitamura S, Santos-Martins D, Smedley CJ, Li G, Forli S, Moses JE, Wolan DW, Barry Sharpless K, Proc. Natl. Acad. Sci. U.S.A 2019, 116, 18808–18814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Meng G, Guo T, Ma T, Zhang J, Shen Y, Sharpless KB, Dong J, Nature 2019, 574, 86–89. [DOI] [PubMed] [Google Scholar]
- [41].Tasdelen MA, Polym. Chem 2011, 2, 2133–2145. [Google Scholar]
- [42].Mayr H, Ofial AR, Angew. Chem. Int. Ed 2006, 45, 1844–1854. [DOI] [PubMed] [Google Scholar]
- [43].Diversity Oriented Clicking (DOC) falls under the wider umbrella of Diversity Oriented Synthesis (DOS). However, DOC is a purely click-chemistry oriented approach, while DOS (first described by S. L. Schreiber) is a broader strategy for the efficient, simultaneous synthesis of structurally diverse compounds, see:; Schreiber SL, Science 2000, 287, 1964–1969. [DOI] [PubMed] [Google Scholar]; For selected references relating to the topic of DOS, see; a) Kidd SL, Osberger TJ, Mateu N, Sore HF, Spring DR, Front. Chem 2018, 6, 460. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Mortensen KT, Osberger TJ, King TA, Sore HF, Spring DR, Chem. Rev 2019, 119, 10288–10317; [DOI] [PubMed] [Google Scholar]; c) Pavlinov I, Gerlach EM, Aldrich LN, Org. Biomol. Chem 2019, 17, 1608–1623 [DOI] [PubMed] [Google Scholar]; d) Muncipinto G, Cycloaddition reactions in Diversity-Oriented Synthesis In Diversity-Oriented Synthesis, Trabocchi A (Ed.), 2013 [Google Scholar]; e) Wang Y, Madsen AØ, Diness F, Meldal M, Chem. Eur. J 2017, 23, 13869–13874. [DOI] [PubMed] [Google Scholar]
- [44].Lesch John E.. The First Miracle Drugs: How the Sulfa Drugs Transformed Medicine. New York: Oxford University Press, 2006. [Google Scholar]
- [45].One synthesis of a related 2-chloroethyne-1-sulfonyl fluoride has been reported, see:; Gladshtein BM, Soborovskii LZ, Zh. Org. Khim 1960, 30, 1574–1577. [Google Scholar]
- [46].Warning: Fluorosulfonic acid anhydride is a highly reactive and must be handled with care. It releases the superacid, fluorosulfonic acid upon hydrolysis.
- [47].While this synthetic approach does not meet click-ideals and gives lower yields of the SASF hubs (2a, 2k-2v) — likely due to the decomposition of the sulfinic acid intermediate — we find the chemistry to be operationally more convenient due to the ready availability of sulfur dioxide and NFSI. In any click synthesis, the lowest yielding step should ideally be the first step.
- [48].Method B was amenable to scales of 100 mmol (see compound 2a).
- [49].Vitaku E, Smith DT, Njardarson JT, J. Med. Chem 2014, 57, 10257–10274. [DOI] [PubMed] [Google Scholar]
- [50].Zhang X-W, Hu W-L, Chen S, Hu X-G, Org. Lett 2018, 20, 860–863. [DOI] [PubMed] [Google Scholar]
- [51].Gomtsyan A, Chem. Heterocycl. Compd 2012, 48, 7–10. [Google Scholar]
- [52].Reactions were performed using a modified Rhodium(I)-Catalyzed Azide-Alkyne Cycloaddition (RhAAC) procedure developed by Song and co-workers see:; Song W, Zheng N, Li M, Dong K, Li J, Ullah K, Zheng Y, Org. Lett 2018, 20, 6705–6709. [DOI] [PubMed] [Google Scholar]
- [53].Regiochemical outcomes are largely predictable and consistent with the literature, see:; Woodward RB, Hoffmann R, Angew. Chem. Int. Ed 1969, 8, 781–853; [Google Scholar]; for nitrile oxides see:; Christl M, Huisgen R, Sustmann R, Chem. Ber 1973, 106, 3275–3290; [Google Scholar]; for nitrile imines see:; Kawai H, Yuan Z, Tokunaga E, Shibata N, Org. Lett 2012, 14, 5330–5333; [DOI] [PubMed] [Google Scholar]; for azides see: [52]; for Sydnones see:; Croce PD, La Rosa, Zecchi G, J. Chem. Soc. Perkin Trans. 1 1985, 2621–2624; [Google Scholar]; for Münchnones see:; Park WKC, Kennedy RM, Larsen SD, Miller S, Roth BD, Song Y, Steinbaugh BA, Sun K, Tait BD, Kowala MC, et al. , Bioorg. Med. Chem. Lett 2008, 18, 1151–1156. [DOI] [PubMed] [Google Scholar]
- [54].Terao Y, Kotaki H, Imai N, Achiwa K, Chem. Pharm. Bull 1985, 33, 896–898. [Google Scholar]
- [55].Terao Y, Kotaki H, Imai N, Achiwa K, Chem. Pharm. Bull 1985, 33, 2762–2766. [Google Scholar]
- [56].Mykhalchuk VL, Yarmolchuk VS, Doroschuk RO, Tolmachev AA, Grygorenko OO, Eur. J. Org. Chem 2018, 2870–2876. [Google Scholar]
- [57].Dorn H, Otto A, Angew. Chem. Int. Ed 1968, 7, 214–215. [Google Scholar]
- [58].The 1,5-dipoles can be prepared in excellent yield by the Rh(II) catalyzed reaction of 1-sulfonyl-1,2,3-triazoles and 2-arylpyridines, see:; Lee DJ, Han HS, Shin J, Yoo EJ, J. Am. Chem. Soc 2014, 136, 11606–11609. [DOI] [PubMed] [Google Scholar]
- [59].LLAMA is an open access tool for assessing the lead-likeness of compounds see:; Colomer I, Empson CJ, Craven P, Owen Z, Doveston RG, Churcher I, Marsden SP, Nelson A, Chem. Commun 2016, 52, 7209–7212. [DOI] [PubMed] [Google Scholar]
- [60].Compounds 4i and 4j were not tested due to degradation.
- [61].To mitigate solubility for assessing the antibacterial activity, agar-based methods were avoided. The assays were performed in 96-well plate liquid cultures where all compounds readily dissolved in DMSO. As compounds may sometimes aggregate and/or precipitate out of solution upon dilution into the bacterial media; to compensate for this possibility, the compound stock dilutions were performed in DMSO first and then added to the bacterial media.
- [62].Note: Until recently, sulfonyl fluoride functional groups have been rarely found in lead structures and drugs. However, their unusual stability and compatibility with biological systems may not warrant a negative scoring associated with electrophilic groups. For selected references of sulfonyl fluorides as lead structures see [38] and [39]. For more information on LLAMA scoring see [59].
- [63].Compound 4i was not used in the study due to degradation.
- [64].Click chemistry is a democratizing technology for synthesis and discovery. By virtue of their design and development, click reactions are incredibly reliable and selective, if not specific; they more often than not give guaranteed reaction outcomes. In such cases, reaction analyses and monitoring using thin layer chromatography (TLC) is adequate for high throughput synthesis.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


