Abstract
Stress is a major risk factor for the development and exacerbation of mood and anxiety disorders, and recent studies have suggested inflammatory contributions to the pathogenesis of depression. Interestingly, pharmacological inhibition of cyclooxygenase-2 (COX-2) has shown promise in the treatment of affective disorders in small scale clinical studies; however, the mechanisms by which COX-2 inhibition affects behavioral domains relevant to affective disorders are not well understood. Here, we examined the effects of pharmacological inhibition of COX-2 with the highly selective inhibitor Lumiracoxib (LMX) on anxiety-like behavior and in vivo basolateral amygdala (BLA) neural activity in response to acute restraint stress exposure. In male mice, pretreatment with LMX prevented the increase in BLA calcium transients induced by restraint stress and prevented anxiogenic behavior seen after restraint stress exposure. Specifically, acute injection of LMX 5mg kg−1 reduced anxiety-like behavior in the light-dark box (LD) and elevated-zero maze (EZM). In addition, in vivo fiber photometry studies showed that acute stress increased calcium transients and the predicted action potential frequency of BLA neurons, which was also normalized by acute LMX pretreatment. These findings indicate pharmacological inhibition of COX-2 can prevent acute stress-induced increase in BLA cellular activity and anxiety-like behavior and provides insights into the neural mechanisms by which COX-2 inhibition could affect anxiety domain symptoms in patients with affective disorders.
Keywords: depression, stress, anxiety, basolateral amygdala, fiber photometry
1. Introduction
Stress is a major risk factor for the development and exacerbation of mood and anxiety disorders (Galatzer-Levy et al., 2018; McEwen, 2004), and recent studies have proposed inflammation as a mechanism by which stress can contribute to depression and anxiety (Fleshner et al., 2017). Interestingly, some clinical studies have shown efficacy of COX-2 inhibitor augmentation in patients with depression (Sethi et al., 2019), and studies using rodent models show inhibition of COX-2 decreases anxiety-like and depressive-like behaviors (Gamble-George et al., 2016; Morgan et al., 2019; Myint et al., 2007; Wang et al., 2018). Despite these data, the mechanisms by which COX-2 inhibition affects behavioral domains relevant to affective disorders are not well understood.
In the CNS, COX-2 acts to generate pro-inflammatory prostaglandins (PGs) from free arachidonic acid (AA). COX-2 is constitutively expressed in the CNS at low levels (Kaufmann et al., 1996; Yamagata et al., 1993), and typically localizes postsynaptically to dendrites and dendritic spines (Cristino et al., 2008; Yamagata et al., 1993), but is also found in astrocytes, microglia, and endothelial cells (Tzeng, 2005). Anatomical data indicate basal expression of COX-2 in the brain is high within stress-related regions, including the amygdala (Breder et al., 1995; Kaufmann et al., 1996), a brain region crucially involved in processing and repsonding to stress (Janak and Tye, 2015). In clinical populations, improvement seen following pharmacological and psychological interventions for anxiety and stress-related disorders is associated with reduced activity in the amygdala, and it has been suggested that reduced amygdala activity may be a final common pathway for successful therapeutic interventions (Fredrikson and Faria, 2013).
Here, we report the effects of pharmacological inhibition of COX-2 on BLA cellular activity and anxiety-like behavior induced by acute restraint stress in mice. Pretreatment with the COX-2 inhibitor LMX prevented an increase in calcium transients in the BLA observed during restraint stress and prevented anxiogenic behavior seen immediately following stress exposure in male mice. These findings indicate pharmacological inhibition of COX-2 can prevent acute stress- induced increases in BLA cellular activity and the subsequent expression of anxiety-like behavior, providing insight into the neural mechanisms by which COX-2 inhibition could affect anxiety domain symptoms in patients with affective disorders.
2. Materials and Methods
Male and female ICR (CD-1) mice were used for all behavioral experiments (Envigo; Indianapolis, IN). All studies were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Vanderbilt University Institutional Animal Care and Use Committee (#M1600213–01). Elevated-plus and- zero mazes (EPM and EZM), Open-field test (OFT), and Light-dark box (LD) were performed exactly as described previously (Morgan et al., 2019). To examine the effects of COX-2 inhibition on stress-induced anxiety-like behaviors, we utilized the selective and well-characterized COX-2 inhibitor LMX, which has been shown to reduce PGE2 levels in human plasma (Mangold et al., 2004). Moreover, within the dose ranges of LMX used here (5–10 mg kg−1), LMX has been shown to inhibit peripheral PGE2 synthesis in rodents (Esser et al., 2005) and reduce brain levels of PGE2 in transgenic neuronal COX-2 overexpressing mice (Morgan et al., 2018). Data were analyzed via 2-way ANOVA followed by Sidaks post hoc multiple comparisons tests. F-scores andp-values for ANOVAs and post hoc tests are shown in relevant panels and graphs. Detailed methods can be found in the supplementary materials.
3. Results
In order to determine the effects of COX-2 inhibition on acute stress-induced anxiety-like behavior, we utilized an acute restraint stress paradigm (see Fig. S1). Acute LMX (5 and 10 mg kg−1) did not affect baseline anxiety-like behaviors in naive male mice in the EPM or LD box compared to vehicle (Fig. S2), consistent with previous studies indicating little effect of COX-2 inhibition on anxiety-like behaviors under basal conditions (Morgan et al., 2019). We next examined the effects of COX-2 inhibition on anxiety-like behavior after acute stress exposure in male mice. Acute restraint stress exposure led to a significant reduction in total distance, % light distance, % light time, and light side entries in the LD box assay (Fig. 1 A-E). Importantly, pretreatment with acute LMX 5 mg kg−1 prevented these effects (Fig. 1 A-E). Similar effects were observed in the EZM. Acute restraint stress reduced total distance, % open arm distance, % open arm time, and open arm entries in the EZM (Fig. 1 F-J), while pretreatment with LMX prevented these anxiogenic-like effects of acute restraint stress (Fig. 1 F-J). Following restraint stress exposure and EZM testing, blood samples were immediately collected to analyze circulating corticosterone (CORT) levels. Restraint stress caused a significant increase in plasma CORT, but this was unaffected by LMX pretreatment (Fig. S3). We also examined the effects of LMX on locomotor activity in male mice using the open field test. LMX did not affect total distance travelled in the open field in control or stress-exposed mice (Fig. S4). Interestingly, LMX was unable to reduce acute restraint-induced anxiety-like behavior in female mice in the LD Box, suggesting a degree of sex-specificity, although future studies are clearly needed to confirm this hypothesis (Fig. S5).
Figure 1. LMX prevents anxiety-like behavior after acute restraint stress in male mice.
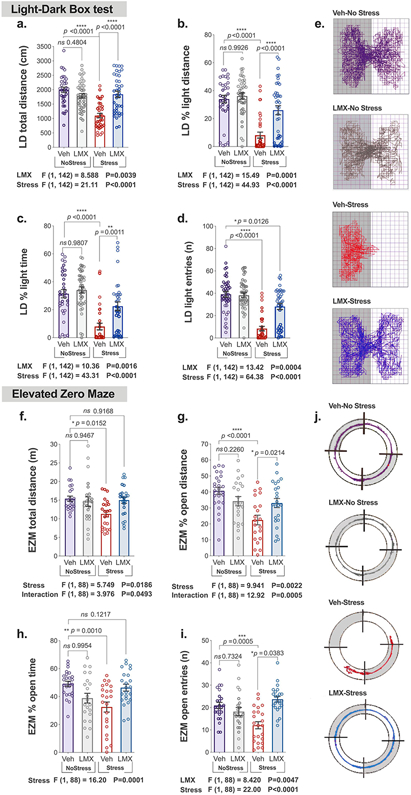
Effects of LMX 5mg kg−1 or DMSO vehicle on behavior in male mice the Light-Dark box (a-e) and Elevated Zero Maze (f-j) immediately after restraint stress. F-scores below graphs represent main effects or interactions from Two-Way ANOVAs. p-values (above bars) represent significance levels of post-hoc Holm-Sidak pairwise comparisons. Data presented as individual values and Mean ± SEM, with number of subjects per group as follows: LD box, Vehicle-No Stress n=33, LMX-No Stress n=41, Vehicle-Stress n=34, LMX-Stress n=38. In EZM: Vehicle-No Stress n=23, LMX-No Stress n=23, Vehicle-Stress n=23, LMX-Stress n=23. Track maps show total distance traveled in the (e) LD box and (j) EZM.
Our behavioral data indicate pretreatment with LMX 5 mg kg−1 prevents anxiogenic effects of restraint stress in male mice; however, the mechanisms by which COX-2 affects anxiety-related behaviors are not well understood. Amygdala activity has been linked to anxiety-related behavioral and emotional responses in humans and mice, and COX-2 is expressed within amygdala pyramidal neurons ((Breder et al., 1995; Kaufmann et al., 1996) and Fig. S6). In addition, we have shown, using ex vivo electrophysiological approaches that LMX can normalize the increase in excitatory drive to anterior BLA neurons observed after chronic corticosterone treatment (Morgan et al., 2019). Based on these data, we tested the hypothesis that acute restraint stress exposure would increase the activity of BLA neurons in vivo, and that LMX pretreatment would normalize this increase. To this end, we utilized fiber photometry-based calcium measurements using the calcium indicator GCaMP7f expressed in BLA neurons of male mice (Fig. 2).
Figure 2. LMX prevents increased BLA activity during acute restraint stress in male mice.
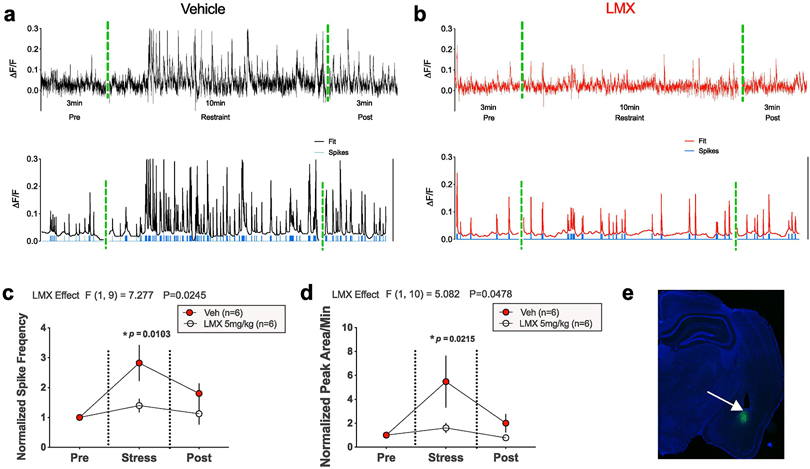
Representative traces showing changes in AF/F calcium signaling as a function of time in the BLA before, during, and immediately after acute restraint stress, in mice treated with vehicle (a top) or LMX (b top). Representative traces of filtered calcium signals and calculated action potentials underlying recorded calcium fluorescence (blue tick marks), generated using MATLAB MLspike algorithm, are shown in (a-b bottom), (c-d) Effects of LMX 5mg kg−1 on normalized spike frequency and total peak AUC as a function of time before, during, and after acute stress, (e) GCaMP7f expression in the BLA (in green, 5x magnification). Data were normalized to pre-stress time periods for all mice (baseline value of 1). P-values represent significance levels obtained via Holm-Sidak pairwise comparisons test after Two-way repeated measures ANOVA (F values shown above panels), with number of subjects per group as follows: Vehicle n=6 mice, LMX n=6 mice.
The MLspike algorithm was used in MATLAB to estimate the number of action potentials (i.e., spikes) responsible for generating observed calcium transients (Deneux et al., 2016; Harris et al., 2018). Representative data of raw (top) and processed calcium signals (bottom) from vehicle and LMX-treated mice are shown in Figure 2A-B. Clear increases in the frequency of transients, as well as predicted spikes (see Supplementary Methods) are observable during the restraint stress period relative to the pre-stress period in vehicle-treated mice (Fig. 2A). This increase was significantly reduced in LMX-pretreated mice (Fig. 2B). Two-way repeated measures ANOVA revealed a significant main effect of LMX pretreatment for both spike frequency and AUC (Fig. 2 C-D). Post-hoc Holm-Sidak comparisons showed differences in BLA calcium signals between vehicle and LMX-treated mice during the stress, but not post-stress, period (Fig. 2 C-D). A representative photomicrograph of GCaMP7f expression in the BLA is shown in Fig. 2 E.
4. Discussion
The main findings of this study are that pharmacological inhibition of COX-2 with LMX prevented avoidance behavior measured using the LD Box and EZM assays, without affecting locomotor activity, following acute restraint stress in male mice. Importantly, LMX had no effect on behavior in the absence of stress, which is consistent with previous findings (Gamble-George et al., 2016; Morgan et al., 2019). We also found that increases in BLA activity during restraint stress can be prevented by pharmacological inhibition of COX-2 with LMX. These data are consistent with previous data demonstrating LMX can normalize the increase in excitatory postsynaptic current frequency onto anterior BLA neurons observed after chronic corticosterone treatment (Morgan et al., 2019). These data are also in line with previous work demonstrating that another COX-2 inhibitor, LM-4131, reduced the intrinsic excitability of BLA neurons ex vivo and BLA single unit activity in vivo specifically when mice transition out of the closed arms of the EPM (Gamble-George et al., 2016). Taken together, these data suggest COX-2 inhibition can reduce amygdala activity and stress-induced avoidance behavior, and support the notion that COX- 2 inhibition could represent a novel approach to the treatment of anxiety domain symptoms in patients with affective disorders.
Understanding the mechanisms subserving the conditional efficacy of COX-2 inhibition may have significant clinical implications, as a recent study clinical study suggested the therapeutic effect of the COX-2 inhibitor Celecoxib for the treatment of depression may be related to the degree of gliosis observed in patients (Attwells et al., 2020). We have previously noted the conditional efficacy of COX-2 inhibition to reduce anxiety-like behaviors only in stressed, but not naive, male mice. Specifically, we have shown LMX has no effect on anxiety-like behavior under basal conditions; however, LMX does decrease anxiety-like behavior after footshock stress and chronic corticosterone treatment (Gamble-George et al., 2016; Morgan et al., 2019). Here, we again show LMX had no effect on anxiety-like behavior in unstressed mice but prevented restraint stress-induced increases in anxiety-like behavior in the EZM and LD Box. The reduction in avoidance behavior and normalized calcium signaling seen with LMX treatment were not likely attributable to a reduction in systemic stress response, as LMX did not affect corticosterone levels after restraint stress, consistent with previous studies (Madrigal et al., 2003). Our previous studies examined the effects of COX-2 inhibition hours or days after acute stress or chronic corticosterone treatment, which suggested the possibility that increased COX-2 expression induced by stress could explain the conditional efficacy of COX-2 inhibition in these models (Gamble-George et al., 2016; Morgan et al., 2019). However, in the current study, behavioral measures were taken 30 minutes after stress onset, which is not sufficient time for COX-2 protein upregulation. These data therefore suggest the conditional efficacy may be related to the role of COX-2 and related PG signaling in the regulation of stress-induced BLA glutamatergic transmission and/or neuronal activity, which may only become relevant under conditions of increased BLA excitation which can occur even during acute stress exposure (see Fig. 2).
Although previous clinical studies have utilized the FDA approved COX-2 inhibitor Celecoxib, we used LMX for our experiments as Celecoxib has poor brain penetration in humans (Dembo et al., 2005) and rodents (Paulson et al., 2000), and we have previously reported LMX is detected at high levels in brain after i.p. administration at 5mg kg−1 (Morgan et al., 2018). Moreover, LMX has 500-fold selectivity for COX-2 over COX-1 (Tannenbaum, 2004), and we have shown LMX 5mg kg−1 reduces levels of PGE2 in whole brain of transgenic neuronal COX-2 overexpressing mice (Morgan et al., 2018). In our recent work using a chronic stress model, both acute and repeated injections of 5mg kg−1 of LMX in male ICR mice prevented anxiety-like behavior and prevented stress-driven increases in excitatory glutamatergic signaling in the BLA (Morgan et al., 2019). Furthermore, LMX at doses between 0.3 and 10 mg kg−1 is antiinflammatory in several rodents models (Esser et al., 2005). These data support the notion that behavioral and neuronal activity modulating effects of LMX occur within dose ranges shown to have peripheral anti-inflammatory effects, further supporting the potential therapeutic relevance of our pharmacological experiments. Future studies comparing diverse COX-2 inhibitors could further establish COX-2 as a relevant molecular target for the treatment of affective and stress- related disorders.
Interestingly, the present study also found no effect of LMX on stress-induced avoidance behavior in female mice. However, we have previously reported inhibition of COX-2 was able to prevent anxiety-like behavior after stress in female mice, in the novelty-induced hypophagia (NIH) test (Gamble-George et al., 2016), suggesting a complex interaction between COX-2 inhibition and sex in the regulation of emotional behavior as a function of behavior, type of stressor and duration of stress. Interestingly, one study has reported sex-dependent effects of COX-2 gene deletion on systemic inflammation and inflammatory pain (Chillingworth et al., 2006). Furthermore, previous studies have shown sex-dependent differences in stress-induced neurotransmitter level changes in the BLA of rats (Mitsushima et al., 2006), and electrophysiological studies have shown sex-and estrus-dependent alterations in excitation and inhibition patterns of BLA neurons related to fear learning (Blume et al., 2017). Future studies should be aimed at determining the mechanisms underlying potential sex-dependent differences in neuronal and behavioral responses to COX-2 inhibition.
One limitation of our work is related to the route of administration of LMX. Although we have shown direct effects of LMX on amygdala neuron physiology (Gamble-George et al., 2016; Morgan et al., 2019) and BLA neuron activity in vivo (Fig. 2), the effects of systemic LMX on multiple interconnected brain regions likely contributes to our observed behavioral effects. Indeed, although COX-2 is basally expressed at high levels in the BLA, it is expressed in other stress-responsive brain regions, including the hippocampus and parts of the prefrontal cortex (See Fig. S6 and (Breder et al., 1995; Yamagata et al., 1993)), suggesting multiple sites of action may subserve the behavioral effects of LMX on stress-induced avoidance behaviors. Future studies examining the effects local BLA infusions of COX-2 inhibitors could be used to address this important issue.
In conclusion, our results indicate acute LMX pretreatment prevents avoidance behavior induced by acute restraint stress. Our fiber photometry data indicate acute restraint stress increases neuronal activity in the BLA, which is also prevented by LMX treatment. Taken together, these findings provide insight into the neural mechanisms by which COX-2 inhibition could affect anxiety-like avoidance behavior and exert anxiolytic effects in patients with mood and anxiety disorders.
Supplementary Material
COX-2 inhibition reduced restraint-stress induced avoidance behavior in male mice
COX-2 inhibition reduced amygdala calcium transients during restraint stress exposure
COX-2 inhibition did not affect locomotor activity
ACKNOWLEDGEMENTS
This research was supported by NIH Grants 4T32MH065215–14 (AM) and R01MH100096 (SP). Behavioral studies were carried out at the Vanderbilt Neurobehavioral Core (supported by the EKS NICHD Award U54HD083211).
Footnotes
DECLARATION OF COMPETING INTEREST
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. S.P. is a paid consultant for Psy Therapeutics and Lundbeck Pharmaceuticals unrelated to the current work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Attwells S, Setiawan E, Rusjan PM, Xu C, Hutton C, Rafiei D, Varughese B, Kahn A, Kish SJ, Vasdev N, Houle S, Meyer JH, 2020. Translocator Protein Distribution Volume Predicts Reduction of Symptoms During Open-Label Trial of Celecoxib in Major Depressive Disorder. Biological Psychiatry. [DOI] [PubMed] [Google Scholar]
- Blume SR, Freedberg M, Vantrease JE, Chan R, Padival M, Record MJ, DeJoseph MR, Urban JH, Rosenkranz JA, 2017. Sex- and Estrus-Dependent Differences in Rat Basolateral Amygdala. JNeurosci 37, 10567–10586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breder CD, Dewitt D, Kraig RP, 1995. Characterization of inducible cyclooxygenase in rat brain. J Comp Neurol 355, 296–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chillingworth NL, Morham SG, Donaldson LF, 2006. Sex differences in inflammation and inflammatory pain in cyclooxygenase-deficient mice. Am J Physiol Regul Integr Comp Physiol 291, R327–334. [DOI] [PubMed] [Google Scholar]
- Cristino L, Starowicz K, De Petrocellis L, Morishita J, Ueda N, Guglielmotti V, Di Marzo V, 2008. Immunohistochemical localization of anabolic and catabolic enzymes for anandamide and other putative endovanilloids in the hippocampus and cerebellar cortex of the mouse brain. Neuroscience 151, 955–968. [DOI] [PubMed] [Google Scholar]
- Dembo G, Park SB, Kharasch ED, 2005. Central nervous system concentrations of cyclooxygenase-2 inhibitors in humans. Anesthesiology 102, 409–415. [DOI] [PubMed] [Google Scholar]
- Deneux T, Kaszas A, Szalay G, Katona G, Lakner T, Grinvald A, Rozsa B, Vanzetta T, 2016. Accurate spike estimation from noisy calcium signals for ultrafast three-dimensional imaging of large neuronal populations in vivo. Nat Commun 7, 12190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esser R, Berry C, Du Z, Dawson J, Fox A, Fujimoto RA, Haston W, Kimble EF, Koehler J, Peppard J, Quadros E, Quintavalla J, Toscano K, Urban L, van Duzer J, Zhang X, Zhou S, Marshall PJ, 2005. Preclinical pharmacology of lumiracoxib: a novel selective inhibitor of cyclooxygenase-2. Br J Pharmacol 144, 538–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleshner M, Frank M, Maier SF, 2017. Danger Signals and Inflammasomes: Stress-Evoked Sterile Inflammation in Mood Disorders. Neuropsychopharmacology 42, 36–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredrikson M, Faria V, 2013. Neuroimaging in anxiety disorders. Mod Trends Pharmacopsychiatry 29, 47–66. [DOI] [PubMed] [Google Scholar]
- Galatzer-Levy IR, Huang SH, Bonanno GA, 2018. Trajectories of resilience and dysfunction following potential trauma: A review and statistical evaluation. Clin Psychol Rev 63, 41–55. [DOI] [PubMed] [Google Scholar]
- Gamble-George J, Baldi R, Halladay L, Kocharian A, Hartley N, Silva C, Roberts H, Haymer A, Marnett LJ, Holmes A, Patel S, 2016. Cyclooxygenase-2 inhibition reduces stress-induced affective pathology. eLife 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris NA, Isaac AT, Gunther A, Merkel K, Melchior J, Xu M, Eguakun E, Perez R, Nabit BP, Flavin S, Gilsbach R, Shonesy B, Hein L, Abel T, Baumann A, Matthews R, Centanni SW, Winder DG, 2018. Dorsal BNST a. JNeurosci 38, 8922–8942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janak PH, Tye KM, 2015. From circuits to behaviour in the amygdala. Nature 517, 284–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann WE, Worley PF, Pegg J, Bremer M, Isakson P, 1996. COX-2, a synaptically induced enzyme, is expressed by excitatory neurons at postsynaptic sites in rat cerebral cortex. Proc Natl Acad Sci U S A 93, 2317–2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madrigal JL, Garcia-Bueno B, Moro MA, Lizasoain T, Lorenzo P, Leza JC, 2003. Relationship between cyclooxygenase-2 and nitric oxide synthase-2 in rat cortex after stress. Eur JNeurosci 18, 1701–1705. [DOI] [PubMed] [Google Scholar]
- Mangold JB, Gu H, Rodriguez LC, Bonner J, Dickson J, Rordorf C, 2004. Pharmacokinetics and metabolism of lumiracoxib in healthy male subjects. Drug Metab Dispos 32, 566–571. [DOI] [PubMed] [Google Scholar]
- McEwen BS, 2004. Protection and damage from acute and chronic stress: allostasis and allostatic overload and relevance to the pathophysiology of psychiatric disorders. Ann N Y Acad Sci 1032, 1–7. [DOI] [PubMed] [Google Scholar]
- Mitsushima D, Yamada K, Takase K, Funabashi T, Kimura F, 2006. Sex differences in the basolateral amygdala: the extracellular levels of serotonin and dopamine, and their responses to restraint stress in rats. Eur J Neurosci 24, 3245–3254. [DOI] [PubMed] [Google Scholar]
- Morgan A, Kondev V, Bedse G, Baldi R, Marcus D, Patel S, 2019. Cyclooxygenase-2 inhibition reduces anxiety-like behavior and normalizes enhanced amygdala glutamatergic transmission following chronic oral corticosterone treatment. Neurobiol Stress 11, 100190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan AJ, Kingsley PJ, Mitchener MM, Altemus M, Patrick TA, Gaulden AD, Mamett LJ, Patel S, 2018. Detection of Cyclooxygenase-2-Derived Oxygenation Products of the Endogenous Cannabinoid 2-Arachidonoylglycerol in Mouse Brain. ACS Chem Neurosci 9, 1552–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myint AM, Steinbusch HW, Goeghegan L, Luchtman D, Kim YK, Leonard BE, 2007. Effect of the COX-2 inhibitor celecoxib on behavioural and immune changes in an olfactory bulbectomised rat model of depression. Neuroimmunomodulation 14, 65–71. [DOI] [PubMed] [Google Scholar]
- Paulson SK, Zhang JY, Breau AP, Hribar JD, Liu NW, lessen SM, Lawal YM, Cogburn JN, Gresk CJ, Markos CS, Maziasz TJ, Schoenhard GL, Burton EG, 2000. Pharmacokinetics, tissue distribution, metabolism, and excretion of celecoxib in rats. Drug Metab Dispos 28, 514–521. [PubMed] [Google Scholar]
- Sethi R, Gomez-Coronado N, Walker AJ, D’Arcy Robertson O, Agustini B, Berk M, Dodd S, 2019. Neurobiology and Therapeutic Potential of Cyclooxygenase-2 (COX-2) Inhibitors for Inflammation in Neuropsychiatric Disorders. Frontiers in Psychiatry 10, 605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannenbaum H, 2004. Lumiracoxib. Drugs 64, 2. [Google Scholar]
- Tzeng S-F a.H. H-Y a.M. O-T, 2005. Prostaglandins and cyclooxygenases in glial cells during brain inflammation. Current Drug Targets: Inflammation and Allergy 4, 335–340. [DOI] [PubMed] [Google Scholar]
- Wang M, Duan F, Wu J, Min Q, Huang Q, Luo M, He Z, 2018. Effect of cyclooxygenase-2 inhibition on the development of post-traumatic stress disorder in rats. Mol Med Rep 17, 4925–4932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata K, Andreasson KI, Kaufmann WE, Barnes CA, Worley PF, 1993. Expression of a mitogen-inducible cyclooxygenase in brain neurons: regulation by synaptic activity and glucocorticoids. Neuron 11, 371–386. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


