Abstract
Background
Checkpoint inhibitor pneumonitis (CIP) is an immune-related adverse event that may complicate treatment with immune checkpoint inhibitors (ICI) and can cause significant morbidity. We sought to identify predictors for the development of CIP, and whether the use of inhaled corticosteroids (ICS) at time of ICI may be protective.
Methods
Patients with advanced cancer treated with ICI from 2011 and 2018 were included in this study. CIP attribution to ICI was determined by treating physician at time of diagnosis. Predictors were assessed by univariate and multivariable Cox proportional hazard models.
Results
We identified 837 pts treated with ICI, of whom 30 (3.6%) developed grade 2 or higher CIP. 82 patients (9.8%) were receiving ICS at time of ICI and had increased risk of developing CIP with hazard ration (HR) of 4.22 (95% CI 1.93–9.21, p < 0.001) compared to those patients not receiving ICS. Patients with age ≥ 65 years had increased risk of developing CIP (HR 2.12, 95% CI 1.02–4.40, p = 0.044), as did 209 patients with lung cancer (198 NSCLC and 11 SCLC) compared to other types of cancers (HR 3.15, 95% CI 1.54–6.46, p = 0.002). In multivariable analysis, age ≥ 65 years, lung cancer diagnosis, and ICS use remained statistically associated with the development of CIP, with adjusted HR for ICS 3.09 (95% CI 1.32–7.24, p = 0.009).
Conclusions
Patients treated with ICS at time of ICI initiation had an increased risk of developing CIP. We further identified older adults with age ≥ 65 years and lung cancers as independent risk factors for CIP.
Keywords: Pneumonitis, Immune checkpoint inhibitor (ICI), Immunotherapy, Immune-related adverse events (irAE), Inhaled corticosteroids (ICS)
Introduction
Immune checkpoint inhibitors (ICI) are an integral part of the cancer treatment landscape [1]. Antibodies targeting cytotoxic T-lymphocyte antigen-4 (CTLA-4) and programmed cell death (PD-1)/programmed cell death ligand 1 (PD-L1) pathways have led to remarkable improvements in overall survival in many cancers [1–3]. Despite the success for many patients, ICIs are associated with unique toxicities termed immune-related adverse events (irAEs) that may lead to morbidity, treatment discontinuation, and rarely death [4].
IrAEs can affect any organ system, and checkpoint inhibitor pneumonitis (CIP) has one of the highest morbidity and morbidity of all irAEs [5–8]. The underlying mechanism for CIP is poorly understood. Evidence suggests that CIP is an inflammatory infiltrative lung process brought on by the heightened immunity within the body by ICI [4, 9]. We hypothesized that inhaled corticosteroid (ICS) use may offer protection in patients receiving ICI. In this study, we studied the association between CIP and ICS, as well as other potential variables that might be predictive for CIP.
Materials and methods
We conducted a retrospective study of patients with advanced cancers treated with ICI between 2011 and 2018 at the Ohio State University. Approval was obtained from the institutional review board. Data were extracted primarily through chart review via the electronic medical record system. Research electronic data capture (REDCap) was used for data collection [10]. Medication lists at the time of ICI initiation were reviewed for the presence of ICS at time of ICI initiation by pharmacy database query and confirmed by manual chart review. The following ICS were included: fluticasone, budesonide, mometasone, beclomethasone, ciclesonide, and flunisolide. Intranasal steroids were excluded. Common Terminology Criteria for Adverse Events (CTCAE) Version 5 was used to define the severity of CIP [11]. Grade 2 or higher CIP were determined by treating physician at time of CIP diagnosis based on the clinical picture, imaging findings, exclusion of other plausible diagnoses, and response to treatment consistent with prior studies [12]. Time to CIP was defined as weeks from the start of ICI to diagnosis. Patients who never developed CIP were censored at the time of last follow-up or death.
Cox proportional hazard models were used to assess univariate associations between potential predictors for CIP. Cumulative hazard functions were plotted for significant predictors (p < 0.05) in univariate analysis. These predictors were entered into a multivariable Cox proportional hazard model. Adjusted hazard ratios (HR) and 95% confidence intervals (CI) were reported. Kaplan–Meier method was used to estimate median overall survival (OS) and log-rank test was used to compare OS between patients with and without CIP. A p value of < 0.05 was considered statistically significant. All analyses were conducted using the SAS system, version 9.4 (SAS Institute Inc., Cary, NC).
Results
Out of 837 patients with advanced cancer treated with ICI in our study, 30 (3.6%) patients developed CIP. Overall, 477 (57.0%) were male and median age was 62.5 years. The median time to the diagnosis of CIP was 11.4 (interquartile range 4.2–23.5) weeks. Out of 30 patients with CIP, 12 (40.0%) had grade 2, 14 (46.7%) had grade 3, 1 (3.3%) had grade 4, and 3 (10%) had grade 5 CIP. (Table 1).
Table 1.
Patient demographics
| Patient | Percent (%) | Patient | Percent (%) | ||
|---|---|---|---|---|---|
| Age | Gender | ||||
| < 65 years | 483 | 57.7 | Male | 477 | 57.0 |
| ≥ 65 years | 354 | 42.3 | Female | 360 | 43.0 |
| CIP grade | ICI | ||||
| 2 | 12 | 1.4 | Nivolumab | 376 | 44.9 |
| 3 | 14 | 1.7 | Ipilimumab | 180 | 21.5 |
| 4 | 1 | 0.1 | Pembrolizumab | 141 | 16.8 |
| 5 | 3 | 0.4 | Nivo + Ipi | 61 | 7.3 |
| Other | 79 | 9.4 | |||
| Line of therapy | Cancer types | ||||
| 1st | 287 | 34.3 | Melanoma | 310 | 37 |
| 2nd | 259 | 30.9 | NSCLC | 198 | 23.7 |
| ≥ 3rd | 272 | 32.5 | RCC | 73 | 8.7 |
| Unknown | 19 | 2.3 | Other | 256 | 30.6 |
| ICS | COPD | ||||
| No | 755 | 90.2 | No | 703 | 84.0 |
| Yes | 82 | 9.8 | Yes | 134 | 16.0 |
| Ever smoked | Prior chemo | ||||
| No | 348 | 41.6 | No | 325 | 38.8 |
| Yes | 488 | 58.3 | Yes | 386 | 46.1 |
| Unknown | 1 | 0.1 | Unknown | 126 | 15.1 |
Univariate analysis (Fig. 1 and Table 2)
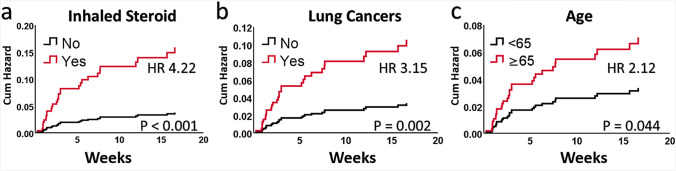
Fig. 1.
Univariate cumulative hazard plots of developing checkpoint inhibitor pneumonitis for a inhaled corticosteroid, b lung cancer, and c age in univariate analysis. Inhaled corticosteroid, lung cancer, and age remained statistically significant in multivariate analysis
Table 2.
Univariate and multivariate analyses of each variable in Cox proportional hazard models with hazard ratio and p value
| HR | 95% CI | p | ||
|---|---|---|---|---|
| Univariate analysis | ||||
| Age | ||||
| < 65 years | ||||
| ≥ 65 years | 2.12 | 1.02 | 4.40 | 0.044 |
| Gender | ||||
| Female | ||||
| Male | 1.51 | 0.71 | 3.22 | 0.291 |
| ICI | ||||
| Ipilimumab | ||||
| Nivolumab | 2.63 | 0.76 | 9.17 | |
| Pembrolizumab | 3.63 | 0.96 | 13.70 | |
| Ipi + Nivo | 2.79 | 0.56 | 13.81 | |
| Other | 1.62 | 0.27 | 9.69 | 0.395 |
| Cancer type | ||||
| Other | ||||
| Lung cancer | 3.15 | 1.54 | 6.46 | 0.002 |
| COPD | ||||
| No | ||||
| Yes | 1.67 | 0.72 | 3.90 | 0.234 |
| ICS | ||||
| No | ||||
| Yes | 4.22 | 1.93 | 9.21 | < 0.001 |
| Ever smoked | ||||
| No | ||||
| Yes | 1.54 | 0.72 | 3.29 | 0.267 |
| Line of therapy | ||||
| 1st | ||||
| 2nd | 0.48 | 0.19 | 1.23 | |
| 3rd or beyond | 0.62 | 0.26 | 1.45 | 0.244 |
| Prior chemo | ||||
| No | ||||
| Yes | 1.21 | 0.56 | 2.62 | 0.636 |
| Multivariable analysis* | ||||
| Age | ||||
| < 65 years | ||||
| ≥ 65 years | 2.20 | 1.06 | 4.58 | 0.035 |
| Cancer type | ||||
| Other | ||||
| Lung cancer | 2.29 | 1.05 | 5.00 | 0.038 |
| ICS | ||||
| No | ||||
| Yes | 3.09 | 1.32 | 7.24 | 0.009 |
Age, inhaled corticosteroid use, and lung cancer diagnosis were all significantly associated with the development of checkpoint inhibitor pneumonitis in both univariate and multivariate analysis
ICI immune checkpoint inhibitor, COPD chronic obstructive pulmonary disease, ICS inhaled corticosteroids
*Multivariable analysis includes the variables with a p value < 0.05 in the univariate analysis
The 354 (42.3%) patients with age ≥ 65 years had a statistically significant increased risk of developing CIP with hazard ratio (HR) of 2.12 (95% CI 1.02–4.40, p = 0.044) when compared to 483 (57.7%) patients who were < 65 years. In this cohort, 209 (25.0%) patients with lung cancer (198 NSCLC and 11 SCLC) had significant increased risk of developing CIP compared to other types of cancers with HR 3.15 (95% CI 1.54–6.46, p = 0.002).
The 82 (9.8%) patients who were on ICS had significant increased risk of developing CIP with HR 4.22 (95% CI 1.93–9.21) and p < 0.001. Among the 82 patients who were on ICS, 60 (73.2%) had COPD, 6 (7.3%) had asthma, 3 (3.7%) had COPD and asthma, and 13 (15.9%) had unknown or undocumented indication for ICS. COPD by itself was not a statistically significant predictor of CIP in our cohort with HR 1.67 (95% CI 0.72–3.90, p = 0.234).
A total of 489 (58.4%) patients in our cohort were previous or current smokers at time of treatment with ICI. Smoking status was not significantly associated with the development of CIP (HR 1.53, 95% CI 0.72–3.26, p = 0.274). Of the 489 former and current smokers, 336 (68.7%) had known smoking pack-year history, which was not associated with CIP (p = 0.906).
Other variables including gender, immunotherapy type, line of therapy, prior chemotherapy, and prior radiotherapy were not significantly associated with CIP in univariate analysis (Table 2).
Multivariable analysis (Table 2)
In multivariable analysis, age ≥ 65 years, lung cancer, and ICS all remained statistically significant predictors of CIP: HR 2.02 (95% CI 1.06–4.58, p = 0.035) for age ≥ 65 years, HR 2.29 (95% CI 1.05–5.00) for lung cancer with p = 0.038, and HR 3.09 (95% CI 1.32–7.24, p = 0.009) for being on ICS.
We did not detect any statistically significant difference in median OS among patients who developed CIP. Median OS was 16.36 months (95% CI 7.33-not reached) for patients who developed CIP versus 13.57 months (95% CI 11.30–15.54) in those patients who did not (p = 0.585). There was no statistically significant difference in median OS between those who received or did not receive ICS (p = 0.345).
Discussion
We identified a strong association between ICS use at the time of ICI treatment initiation and the development of CIP. To our knowledge, this is the first report of the association between ICS and ICI. Interestingly, we found no association between COPD and CIP, although patients older than 65 years and those with a diagnosis of lung cancer did have higher rates of CIP as previously reported [6, 13, 14].
ICI use has led to a significant improvement in long-term survival for patients with advanced cancer [1, 15–17]. Despite the progress made in recent years, irAEs still pose a significant challenge in cancer treatment [8]. Although mild-to-moderate irAEs have been associated with improved outcome, severe forms of irAE may lead to treatment discontinuation and even mortality [18, 19]. CIP is one of the more common serious irAE and carries one of the highest mortality [20].
The mechanism of CIP is not well defined. Current evidence suggests that CIP is an inflammatory infiltrative lung disease contributed by upregulated T-cell activities, increased level of cytokines productions, and amplified complement and auto-antibody mediated immunological processes [4, 9]. Patients with lung cancer have increased pulmonary inflammation from underlying malignancies at baseline [4, 9, 21], which may predispose to the development of inflammatory response and risk of CIP. Our finding of elevated risk of developing CIP in lung cancer compared to other cancers is consistent with the previous studies [6, 13, 14].
COPD is another condition that can result in chronic inflammation of the pulmonary system [22, 23]. Unlike lung cancer, no increased risk of developing CIP was observed in patients with COPD. Although the severity of COPD defined by pulmonary function tests and its subtypes were not available for analysis, we did not find smoking history to be a significant predictor of CIP [24].
We hypothesized that the anti-inflammatory effect exerted by ICS would result in lower risk for CIP; however, we observed a higher risk of developing CIP in patients prescribed ICS at time of ICI treatment initiation. The use of ICS is usually reserved for patients with advanced or symptomatic COPD and, therefore, may be a surrogate marker of the degree of inflammation caused by COPD. These patients may warrant closer monitoring during ICI treatment. Encouragingly, no survival difference was identified in patients who developed CIP. In this cohort, we did not find a statistically significant association between COPD and CIP, though patients on ICS had increased risk of developing pneumonitis in our study. We also identified patients with age ≥ 65 years as an independent risk factor for developing CIP.
We acknowledge that there are multiple limitations in our study. First, we were unable to establish any causal relationship through a retrospective cohort study. Second, we were not able to effectively address medication adherence through chart view. Third, the diagnosis of irAE is fraught with challenges and irAE are known to occur even after treatment discontinuation [20, 25]. Our study included patients treated over a significant time frame and our institutional approach to irAE and pneumonitis specifically has evolved with the changing guidelines for diagnosis and management of irAE. Finally, there are limitations to drawing conclusions regarding different grades of CIP given small numbers of cases. Future prospective studies are needed to delineate the relationship between ICS and CIP, as well as other risk factors to aid in patient selection and monitoring while on ICI.
Conclusion
In this study, we found no protective effect of ICS for the development of pneumonitis, and, instead, found a higher risk of pneumonitis among patients treated with ICS at the time of immune checkpoint inhibitor therapy. We also identified older adults with age ≥ 65 years and lung cancers as independent risk factors for the development of checkpoint inhibitor pneumonitis.
Acknowledgements
Research support provided by the REDCap project and The Ohio State University Center for Clinical and Translational Science grant support (National Center for Advancing Translational Sciences, Grant UL1TR002733). Dr. Owen and Dr. Presley are Paul Calabresi Scholars supported by the OSU K12 Training Grant for Clinical Faculty Investigators (K12 CA133250).
Abbreviations
- CIP
Checkpoint inhibitor pneumonitis
- ICS
Inhaled corticosteroid
- ICI
Immune checkpoint inhibitor
- irAEs
Immune-related adverse events
- OS
Overall survival
Author contributions
ML, SZ, LW, and DO contributed to the study conception and design. Material preparation, data collection, and analysis were performed by all authors. The first draft of the manuscript was written by ML, DO, SZ, and LW, and all authors commented on previous versions of the manuscript. All authors read the manuscript, revised it critically, and approved the final manuscript.
Funding
This study was supported by the National Institutes of Health (P30CA016058 and K12 CA133250).
Data availability
In accordance with local and/or U.S. Government laws and regulations, any materials and de-identified data that are reasonably requested by others will be made available in a timely fashion.
Code availability
All analyses were conducted using the SAS system, version 9.4 (SAS Institute Inc., Cary, NC). No custom codes were used.
Compliance with ethical standards
Conflicts of interest
The authors report no conflicts or competing interests. This study was not funded by any private entity.
Ethical approval
This retrospective study was approved by Institutional Review Board at the Ohio State University (IRB Study ID #2016C0070, PI: Dwight H. Owen, MD, MS). All procedures performed in studies involving human participants or their data were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Consent to participate
A waiver of consent was granted by the Institutional Review Board at the Ohio State University for this retrospective study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Darvin P, Toor SM, Sasidharan Nair V, Elkord E. Immune checkpoint inhibitors: recent progress and potential biomarkers. Exp Mol Med. 2018;50(12):1–11. doi: 10.1038/s12276-018-0191-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe S, O’Brien M, Rao S, Hotta K, Leiby MA, Lubiniecki GM, Shentu Y, Rangwala R, Brahmer JR. Pembrolizumab versus chemotherapy for PD-L1–positive non–small-cell lung cancer. N Engl J Med. 2016;375(19):1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 3.Motzer RJ, Tannir NM, McDermott DF, Arén Frontera O, Melichar B, Choueiri TK, Plimack ER, Barthélémy P, Porta C, George S, Powles T, Donskov F, Neiman V, Kollmannsberger CK, Salman P, Gurney H, Hawkins R, Ravaud A, Grimm M-O, Bracarda S, Barrios CH, Tomita Y, Castellano D, Rini BI, Chen AC, Mekan S, McHenry MB, Wind-Rotolo M, Doan J, Sharma P, Hammers HJ, Escudier B. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018;378(14):1277–1290. doi: 10.1056/NEJMoa1712126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. 2018;378(2):158–168. doi: 10.1056/NEJMra1703481. [DOI] [PubMed] [Google Scholar]
- 5.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M. Safety, activity, and immune correlates of anti–PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nishino M, Giobbie-Hurder A, Hatabu H, Ramaiya NH, Hodi FS. Incidence of programmed cell death 1 inhibitor-related pneumonitis in patients with advanced cancer: a systematic review and meta-analysis. JAMA Oncol. 2016;2(12):1607–1616. doi: 10.1001/jamaoncol.2016.2453%JJAMAOncology. [DOI] [PubMed] [Google Scholar]
- 7.Sears CR, Peikert T, Possick JD, Naidoo J, Nishino M, Patel SP, Camus P, Gaga M, Garon EB, Gould MK, Limper AH, Montgrain PR, Travis WD, Rivera MP. Knowledge gaps and research priorities in immune checkpoint inhibitor–related pneumonitis. Off Am Thorac Soc Res Statement. 2019;200(6):e31–e43. doi: 10.1164/rccm.201906-1202ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang DY, Salem J-E, Cohen JV, Chandra S, Menzer C, Ye F, Zhao S, Das S, Beckermann KE, Ha L, Rathmell WK, Ancell KK, Balko JM, Bowman C, Davis EJ, Chism DD, Horn L, Long GV, Carlino MS, Lebrun-Vignes B, Eroglu Z, Hassel JC, Menzies AM, Sosman JA, Sullivan RJ, Moslehi JJ, Johnson DB. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol. 2018;4(12):1721–1728. doi: 10.1001/jamaoncol.2018.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suresh K, Naidoo J, Lin CT, Danoff S. Immune checkpoint immunotherapy for non-small cell lung cancer: benefits and pulmonary toxicities. Chest. 2018;154:1416. doi: 10.1016/j.chest.2018.08.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0 (2017). https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf
- 12.Reuss JE, Suresh K, Naidoo J. Checkpoint inhibitor pneumonitis: mechanisms, characteristics, management strategies, and beyond. Curr Oncol Rep. 2020;22(6):56. doi: 10.1007/s11912-020-00920-z. [DOI] [PubMed] [Google Scholar]
- 13.Delaunay M, Prévot G, Collot S, Guilleminault L, Didier A, Mazières J. Management of pulmonary toxicity associated with immune checkpoint inhibitors. Eur Respir Rev. 2019;28(154):190012. doi: 10.1183/16000617.0012-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cadranel J, Canellas A, Matton L, Darrason M, Parrot A, Naccache J-M, Lavolé A, Ruppert A-M, Fallet V. Pulmonary complications of immune checkpoint inhibitors in patients with nonsmall cell lung cancer. Eur Respir Rev. 2019;28(153):190058. doi: 10.1183/16000617.0058-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, Yokoi T, Chiappori A, Lee KH, de Wit M, Cho BC, Bourhaba M, Quantin X, Tokito T, Mekhail T, Planchard D, Kim Y-C, Karapetis CS, Hiret S, Ostoros G, Kubota K, Gray JE, Paz-Ares L, de Castro CJ, Wadsworth C, Melillo G, Jiang H, Huang Y, Dennis PA, Özgüroğlu M. Durvalumab after chemoradiotherapy in stage III non–small-cell lung cancer. N Engl J Med. 2017;377(20):1919–1929. doi: 10.1056/NEJMoa1709937. [DOI] [PubMed] [Google Scholar]
- 16.Marin-Acevedo JA, Dholaria B, Soyano AE, Knutson KL, Chumsri S, Lou Y. Next generation of immune checkpoint therapy in cancer: new developments and challenges. J Hematol Oncol. 2018;11(1):39. doi: 10.1186/s13045-018-0582-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qiu Z, Chen Z, Zhang C, Zhong W. Achievements and futures of immune checkpoint inhibitors in non-small cell lung cancer. Exp Hematol Oncol. 2019;8(1):19. doi: 10.1186/s40164-019-0143-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Puzanov I, Diab A, Abdallah K, Bingham CO, Brogdon C, Dadu R, Hamad L, Kim S, Lacouture ME, LeBoeuf NR, Lenihan D, Onofrei C, Shannon V, Sharma R, Silk AW, Skondra D, Suarez-Almazor ME, Wang Y, Wiley K, Kaufman HL, Ernstoff MS, Anderson J, Arrindell D, Andrews S, Ballesteros J, Boyer J, Chen D, Chonzi D, Cotarla I, Cunha R, Davies M, Dawson M, Dicker A, Eifler L, Ferguson A, Ferlini C, Frankel S, Go W, Gochett C, Goldberg J, Goncalves P, Goswami T, Gregory N, Gulley JL, Hayreh V, Helie N, Holmes W, Hsu J-Y, Ibrahim R, Larocca C, Lehman K, Ley-Acosta S, Lambotte O, Luke J, McClure J, Michelon E, Nakamura M, Patel K, Piperdi B, Rasheed Z, Reshef D, Riemer J, Robert C, Sarkeshik M, Saylors A, Schreiber J, Shafer-Weaver K, Sharfman W, Sharon E, Sherry R, Simonson C, Thomas C, Thompson JA, Trehu E, Tresnan D, Turner M, Wariabharaj D, Waxman I, Wood L, Zhang L, Zheng P, on behalf of the Society for Immunotherapy of Cancer Toxicity Management Working G Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the society for immunotherapy of cancer (SITC) toxicity management working group. J Immuno Ther Cancer. 2017;5(1):95. doi: 10.1186/s40425-017-0300-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Das S, Johnson DB. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J Immuno Ther Cancer. 2019;7(1):306. doi: 10.1186/s40425-019-0805-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang H, Guo X, Zhou J, Li Y, Duan L, Si X, Zhang L, Liu X, Wang M, Shi J, Zhang L. Clinical diagnosis and treatment of immune checkpoint inhibitor-associated pneumonitis. Thorac Cancer. 2020;11(1):191–197. doi: 10.1111/1759-7714.13240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O'Callaghan DS, O'Donnell D, O'Connell F, O'Byrne KJ. The role of inflammation in the pathogenesis of non-small cell lung cancer. J Thorac Oncol. 2010;5(12):2024–2036. doi: 10.1097/JTO.0b013e3181f387e4. [DOI] [PubMed] [Google Scholar]
- 22.Baraldo S, Turato G, Badin C, Bazzan E, Beghé B, Zuin R, Calabrese F, Casoni G, Maestrelli P, Papi A, Fabbri LM, Saetta M. Neutrophilic infiltration within the airway smooth muscle in patients with COPD. Thorax. 2004;59(4):308–312. doi: 10.1136/thx.2003.012146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sutherland ER, Martin RJ. Airway inflammation in chronic obstructive pulmonary disease: comparisons with asthma. J Allergy Clin Immunol. 2003;112(5):819–827. doi: 10.1016/S0091-6749(03)02011-6. [DOI] [PubMed] [Google Scholar]
- 24.Brusasco V, Martinez F (2014) Chronic obstructive pulmonary disease. In: Terjung R (ed) Comprehensive physiology. 10.1002/cphy.c110037 [DOI] [PubMed]
- 25.Couey MA, Bell RB, Patel AA, Romba MC, Crittenden MR, Curti BD, Urba WJ, Leidner RS. Delayed immune-related events (DIRE) after discontinuation of immunotherapy: diagnostic hazard of autoimmunity at a distance. J ImmunoTher Cancer. 2019;7(1):165. doi: 10.1186/s40425-019-0645-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
In accordance with local and/or U.S. Government laws and regulations, any materials and de-identified data that are reasonably requested by others will be made available in a timely fashion.
All analyses were conducted using the SAS system, version 9.4 (SAS Institute Inc., Cary, NC). No custom codes were used.