Abstract
Study objective:
To assess the efficacy and safety of intranasal analgesic-dose ketamine as compared to intranasal fentanyl for pediatric acute pain.
Methods:
A systematic review and meta-analysis was performed following the PRISMA guidelines. We searched PubMed, Embase, and Scopus databases for randomized controlled trials from inception to December 2019. We conducted meta-analysis with random-effects models to evaluate pain reduction, rescue analgesia, adverse events and sedation between intranasal ketamine and intranasal fentanyl. Random-effects models were used to estimate weighted mean differences (WMD) and pooled relative risks (RR).
Results:
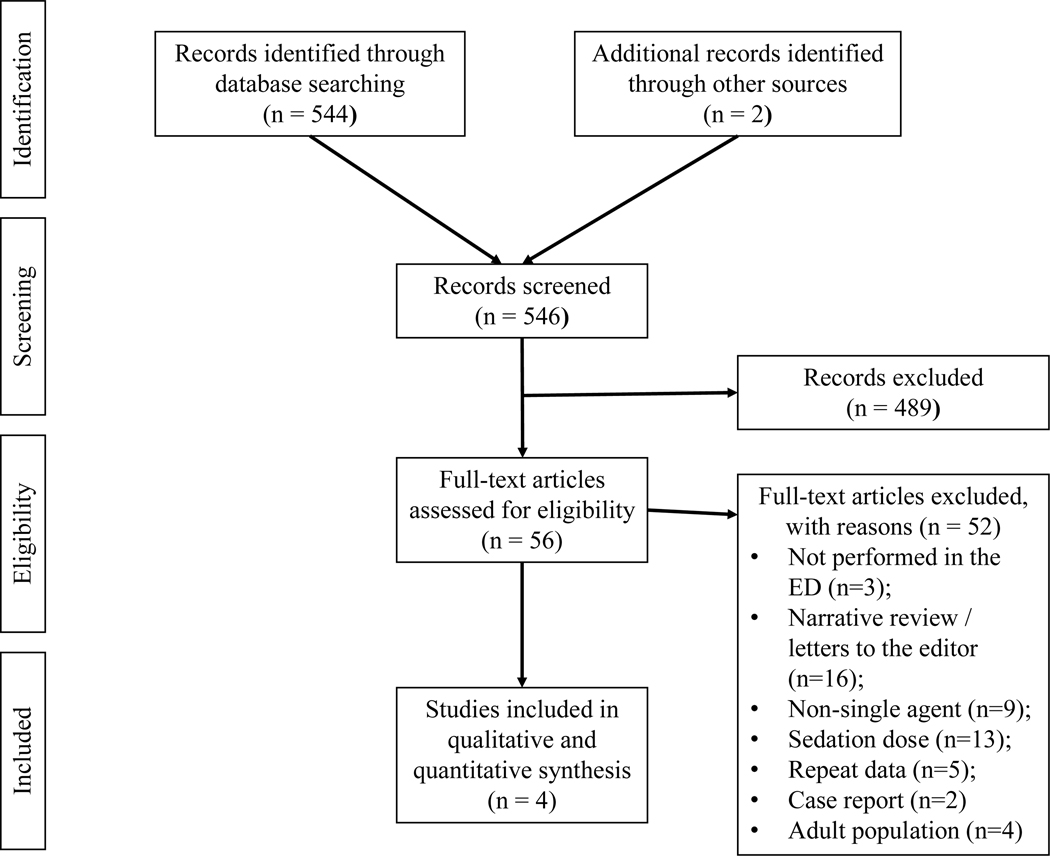
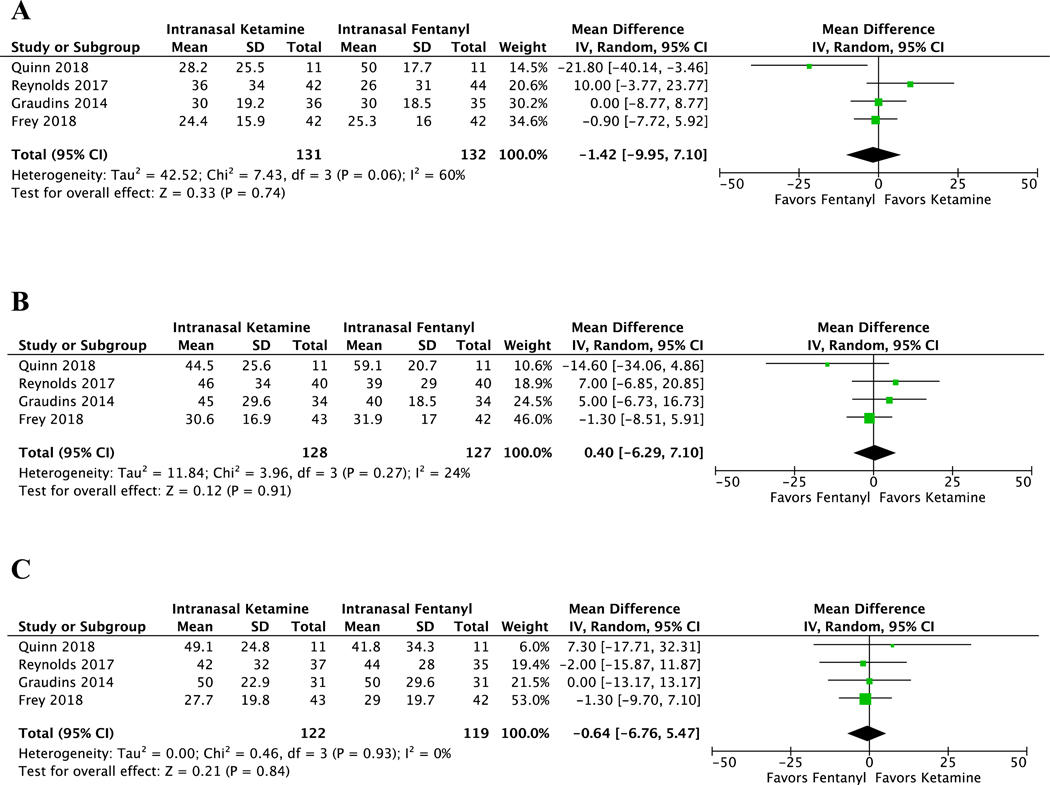
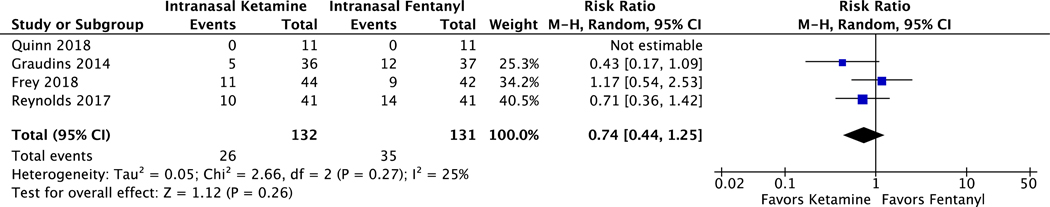
A total of 546 studies were screened and 4 trials were included. In the meta-analysis of 4 studies including 276 patients, ketamine had similar reductions in pain scores from baseline to all post-intervention times (10 to 15 minutes: WMD −1.42, 95% CI −9.95 to 7.10; 30 minutes: WMD 0.40, 95% CI −6.29 to 7.10; 60 minutes: WMD −0.64, 95% CI −6.76 to 5.47). Ketamine was associated with similar rates of rescue analgesia (RR 0.74, 95% CI 0.44 to 1.25). Ketamine had a higher risk of non-serious adverse events (RR 2.00, 95% CI 1.43 to 2.79), and no patients receiving ketamine had a serious adverse event. There was one serious adverse event (hypotension) with fentanyl that self-resolved. No patients receiving either IN fentanyl or ketamine had significant sedation.
Conclusion:
Intranasal analgesic-dose ketamine may be considered as an alternative to opioids for acute pain management in children. Its accepted use will depend on the tolerability of non-serious adverse events and the desire to avoid opioids.
INTRODUCTION
Acute pain is one of the most frequent presenting symptoms to the Emergency Department (ED).[1–3] Addressing pain promptly and appropriately is part of everyday care in the ED. In children, intranasal (IN) is a commonly utilized and easily accessible, painless route for medication administration. [4,5] Opioids are a mainstay for moderate to severe pain management in the ED, but given the concern surrounding their use, alternatives are desirable.[6,7] Ketamine administered at sub-dissociative doses (i.e. low dose) provides analgesia without significant sedation.
The majority of ketamine’s mechanism of action is attributed to the blocking of the N-methyl-D-aspartate (NMDA) receptor site, which results in decrease activity of the excitatory neurotransmitter glutamate in the brain and spinal cord. Ketamine and its metabolites have also activity at a multitude of other sites including opioid receptors. Effects of ketamine are dose dependent, with analgesia without sedation occurring at around 10–30% of the dissociative dose. Typical analgesic ketamine dosing is 0.1 to 0.3 mg/kg intravenously (IV), 0.5 mg/kg intramuscular (IM) and 1 mg/kg IN; compared to dissociative sedation dosing of 1 to 2 mg/kg IV, 3 to 4 mg/kg IM and 6 to 9 mg/kg through the IN route.[8–10] Ketamine has most frequently been used as an adjunct to decrease opioid consumption, particularly in adult populations.[11] Analgesic-dose ketamine is increasingly considered for primary analgesia in acute pain to avoid opioid use.[2] Although ketamine administration via the IN route has been evaluated in recent trials, no previous systematic review has comprehensively evaluated its entire body of evidence.
The objective of this systematic review and meta-analysis was to evaluate the efficacy and safety of IN ketamine when compared to IN fentanyl for pediatric acute pain management in the ED. We aimed to evaluate efficacy through the outcomes of reduction in pain scores and need for rescue analgesia, and to evaluate safety through the outcomes of incidence of adverse events and sedation.
METHODS
Study design
This was a systematic review and meta-analysis conducted to assess the efficacy and safety of IN ketamine as a method for pain relief versus IN fentanyl in children undergoing pain management in the ED. A protocol was written before beginning the investigation. This report followed the recommendations made in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statements including its version focused on systematic reviews of harms (PRISMA harms).[12,13]
Search strategy and inclusion criteria
A medical librarian searched in three electronic databases, including PubMed, Embase and Scopus from inception to December, 2019. The search strategy details are presented in the Data Supplement S1. We also performed hand-searching of the reference lists of eligible articles.
We included only randomized controlled trials (RCTs) that evaluated the analgesic efficacy and safety of IN ketamine when compared to IN fentanyl. When selecting the studies, we did not exclude articles based on language or year of publication. Specific inclusion criteria included: (1) RCT; (2) population: children (age ≤ 18) undergoing pain management in the ED; (3) intervention: IN ketamine; (4) comparison: IN fentanyl; (5) outcomes: pain reduction, rescue analgesia, adverse events, and sedation. Studies evaluating populations with chronic pain conditions such as sickle cell disease were excluded given the differences in tolerance and response to opioids.[14] Studies performed outside the ED (e.g. postoperative management) or for indications other than analgesia (e.g. sedation) were excluded. We only included trials that administered IN ketamine for analgesia (i.e. analgesic-dosing), also described as low-dose or sub-dissociative dosing. Studies using higher doses for procedural sedation were not considered, nor were studies using routes such as IV, IM or oral.
After the literature search was executed, two independent investigators screened for all titles and abstracts for eligibility. The titles and abstracts considered for inclusion by either author were retrieved in full-text and assessed for eligibility independently. Disagreements were resolved by consensus. All eligible studies were included for qualitative analysis and those with available data underwent meta-analysis.
Outcome measures
For efficacy outcomes, we included reduction in pain scores using validated pediatric pain scales from baseline to post-intervention time points, and need for rescue analgesia. The following time points were considered: 10 to 15 minutes, 30 minutes and 60 minutes.
For safety outcomes, we included the incidence of adverse events, both overall and separated into non-serious and serious designations.[13] Non-serious adverse events were defined as nausea, vomiting, dizziness, drowsiness, sleepiness, dysphoria/dissociation, unpleasant taste, pruritus, visual changes, headache, rash, light-headedness, nystagmus, salivation, vivid dreams, trouble concentrating, sore throat and hallucinations. Serious adverse events were defined as dysrhythmias, seizures, apnea, respiratory depression, anaphylaxis, hypotension and cardiac arrest. Sedation was evaluated and all studies except one[15] reported data on level of sedation using the University of Michigan Sedation Scale (UMSS). The UMSS scale has 5 possible values (0 = awake/alert; 1 = minimally sedated; 2 = moderately sedated; 3 = deeply sedated; 4 = unarousable).[16]
Data extraction and quality assessment
Data available in the full-text reports and supplementary material were extracted independently and in duplicate for all studies using a standardized predefined form. A third investigator further reviewed the accuracy of data extraction. Extracted information included study design, study size, study population, causes of pain, intervention details (doses and timing) and outcomes of interest, including pain scores pre- and post-intervention, rescue analgesia and incidences of adverse events and sedation (i.e. UMSS scores). We contacted authors by email if data were missing or unclear, and further data was obtained from one study.[17]
Risk of bias was assessed independently and in duplicate for all studies, using the version 2 of the Cochrane tool for assessing risk of bias in randomized trials (RoB 2).[18] The overall quality of evidence for each outcome was evaluated using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach.[19]
Data analysis
When outcome data such as mean differences or standard deviations were not available, we contacted the authors and if there was no response, we imputed such data using recommendations from the Cochrane handbook.[20] Data reported only in figures or graphics were extracted using plot digitizer software.[21] Meta-analysis was performed using the Review Manager (RevMan) version 5.3. For the outcome of pain reduction from baseline to post-intervention time points, we calculated weighted mean differences (WMD) of pain reduction between IN ketamine and IN fentanyl with associated 95% confidence intervals (CIs). Two studies reported reductions at 10 minutes[15,17] and two at 15 minutes[22,23]; we combined these data into a unique time stamp in the meta-analysis. For the outcomes of rescue analgesia, adverse events, and sedation (i.e. UMSS other than zero), we calculated the pooled relative risk (RR) with associated 95% CI for IN ketamine versus IN fentanyl. Only one serious adverse event was reported, hypotension from IN fentanyl that self-resolved[15]; therefore, we did not perform a meta-analysis by severity of adverse events.
Statistical heterogeneity was assessed among studies by the I2 statistic.[24] To incorporate clinical and statistical heterogeneity between studies, we used DerSimonian-Laird random-effects models.
We were unable to statistically evaluate publication bias because of the small number of studies, which makes analysis of funnel plots unreliable.[25]
RESULTS
Study characteristics
The systematic review study flow is depicted in Figure 1. From a total of 546 titles and abstracts screened, 56 potentially relevant studies were identified. After full-text review, four RCTs met the inclusion criteria.
Figure 1.
Systematic review study flow.
The baseline characteristics of each study are detailed in the Table 1. Two studies were designed as non-inferiority RCTs[17,22], one study was designed as a feasibility RCT with sample size calculation based on safety outcomes[15], and one study was designed as an equivalence RCT.[23] The included studies involved a total of 276 participants, with 138 being randomized to receive IN ketamine and 138 being randomized to receive IN fentanyl. Causes of pain included acute extremity injury in three of the studies and the fourth study included extremity injury and acute abdominal pain.[17] Only two trials[15,22] reported data on race and ethnicity. In both trials, more than 50% of included patients were White.
Table 1.
Characteristics of randomized trials comparing intranasal ketamine and intranasal fentanyl for acute pain management in ED pediatric patients.
| Study | Pain Causes | Population | Intervention [Number of Patients] | Comparison [Number of Patients] |
|---|---|---|---|---|
| Graudins 2015, Australia | Acute Isolated Extremity Injury | Children aged 3-13 years and weight < 50kg with an acute isolated extremity injury and moderate to severe pain at triage. | Intranasal Ketamine (1 mg/kg) [N = 36]1 33/36 (92%) received concomitant ibuprofen |
Intranasal Fentanyl (1.5μg/kg) [N = 37]1 33/37 (89%) received concomitant ibuprofen |
| Reynolds 2017, United States | Acute Isolated Extremity Injury | Children aged 4-17 years and weight < 70kg with suspected acute isolated extremity injury and moderate to severe pain at triage. | Intranasal Ketamine (1 mg/kg) 2nd dose at least 20 minutes after the first dose at the discretion of physician. [N = 46]2 34/43 (79%) received concomitant ibuprofen, 7 (16%) received concomitant acetaminophen and 1 (2%) received both. |
Intranasal Fentanyl (1.5μg/kg) 2nd dose at least 20 minutes after the first dose at the discretion of physician. [N = 45]2 35/44 (80%) received ibuprofen, 5 (11%) received acetaminophen and 3 (7%) received both. |
| Frey 2018, United States | Acute Extremity Injury | Children aged 8-17 years with an acute extremity injury and moderate to severe pain at triage. | Intranasal Ketamine (1.5 mg/kg) [N = 45]3 4/44 (9%) received ibuprofen and 1/44 (2%) received acetaminophen prior to ED arrival. |
Intranasal Fentanyl (2 μg/kg) [N = 45]3 4/42 (10%) received ibuprofen and 2 (5%) received acetaminophen prior to ED arrival. |
| Quinn 2018, United States | Acute Pain Either Musculoskeletal or Abdominal | Children aged 3-17 years and weight < 64kg with acute moderate to severe pain at triage. | Intranasal Ketamine (1 mg/kg) [N = 11] 2/11 (18%) received ibuprofen and 2/11 (18%) received acetaminophen prior to intervention. |
Intranasal Fentanyl (1.5μg/kg) [N = 11] 2/11 (18%) received ibuprofen and 2/11 (18%) received acetaminophen prior to intervention. |
Number of patients who were randomized. Five patients in the ketamine group did not complete the 60-minute follow-up mainly because rescue analgesia was needed, and 6 patients in the fentanyl group did not complete the 60-minute follow-up mainly because rescue analgesia was needed. To note, this was predicted in their protocol as further participation in the study was terminated at rescue medication administration.
Number of patients who were randomized. Three patients randomized to ketamine withdrew prior to intervention while 1 patient randomized to fentanyl was lately recognized as a screen failure.
One patient who was randomized to ketamine did not receive the intervention and 3 patients who were initially allocated to fentanyl did not receive it. The reasons included inability to provide urine for pregnancy test, parental preference, clinical preference and unavailability of medication.
Interventions
Three studies used the dose of 1 mg/kg of IN ketamine and 1.5 μg/kg of IN fentanyl. One study[22] used higher doses for both; IN ketamine at 1.5 mg/kg and IN fentanyl at 2 μg/kg. All studies included patients who received ibuprofen or acetaminophen prior or concomitant to the interventions. The proportion of patients receiving co-interventions were similar between groups.
Efficacy outcomes
Pain reduction from baseline
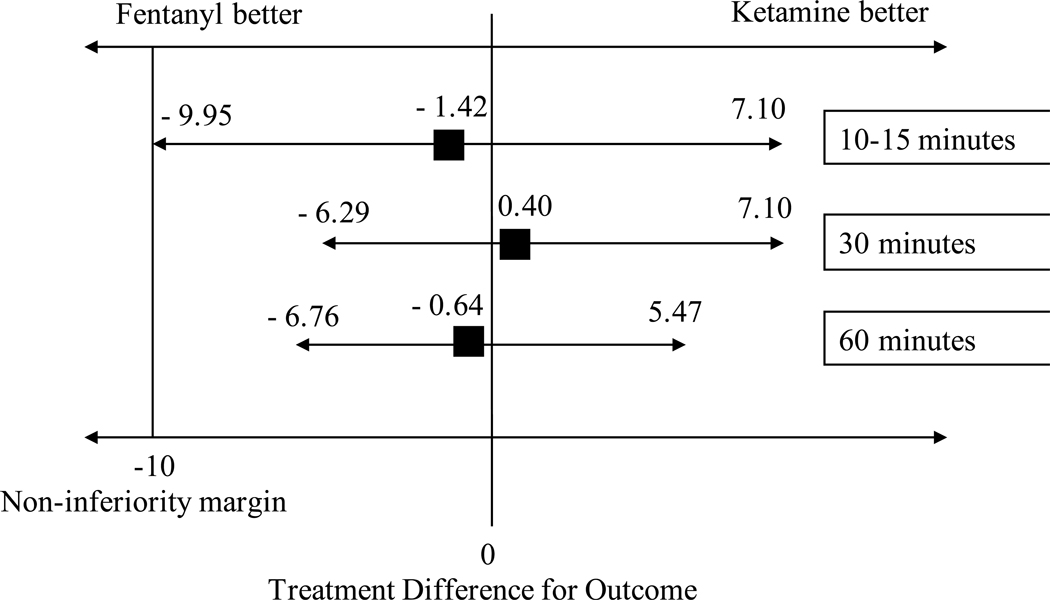
Intranasal ketamine and fentanyl resulted in similar reductions in pain scores (pain scale, 0–100) from baseline to all post-intervention time points (10 to 15 minutes, 30 minutes and 60 minutes) (Figure 2). The weighted mean difference was −1.42 (CI −9.95 to 7.10, I2 = 60%) at 10–15 minutes after intervention. For pain reduction from baseline to 30 minutes, the weighted mean difference was 0.40 (CI −6.29 to 7.10, I2 = 24%). At 60 minutes, the weighted mean difference was −0.64 (CI −6.76 to 5.47, I2 = 0%). Of note, when interpreting the weighted mean differences, positive values suggest that IN ketamine was superior to IN fentanyl in reducing pain. If we consider a non-inferiority margin of 10 points in a 0–100 scale as suggested by previous literature[26–28], IN ketamine was non-inferior to IN fentanyl across all different times, which is indicated by CIs not crossing the non-inferiority margin (Figure 3).
Figure 2.
Forest plots of meta-analyses on pain reduction between intranasal ketamine and intranasal fentanyl at different timepoints (pain scale, 0–100). (A) Pain reduction from baseline to 10 or 15 minutes after intervention. (B) Pain reduction from baseline to 30 minutes after intervention. (C) Pain reduction from baseline to 60 minutes after intervention.
Figure 3.
Non-inferiority margin and interpretation of meta-analysis.
Requirements for rescue analgesia
Intranasal ketamine did not have significantly lower rates of rescue analgesia when compared to IN fentanyl (pooled RR 0.74, CI 0.44 to 1.25, I2 = 25%) (Figure 4). A detailed summary of rescue protocols is presented in the Data Supplement S2.
Figure 4.
Forest plot on the number of patients who needed rescue analgesia after either intranasal ketamine or intranasal fentanyl.
Safety outcomes
Adverse events
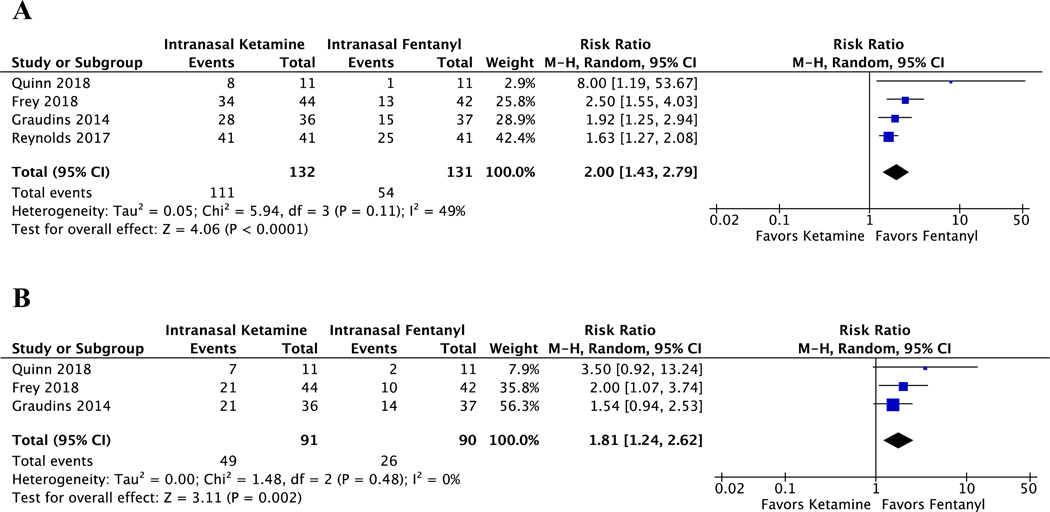
All studies reported the number of patients with an adverse event related to either the use of IN ketamine or fentanyl. There was one serious adverse event in the fentanyl group (hypotension) which resolved spontaneously.[15] No patients receiving IN ketamine had serious adverse events. The risk of having an adverse event was higher among children receiving IN ketamine than those receiving IN fentanyl (pooled RR 2.00, CI 1.43 to 2.79, I2 = 49%) (Figure 5). The raw data on adverse events, separated by serious and non-serious designations and details on how adverse events were measured are presented in Data Supplement S3 and S4.
Figure 5.
Meta-analysis of adverse events and sedation. (A) Forest plot of the incidence of any adverse event after receiving either intranasal ketamine or intranasal fentanyl. (B) Forest plot of the incidence of any sedation (UMSS score other than 0) after receiving either intranasal ketamine or intranasal fentanyl.
Sedation
In the three studies that measured level of sedation with the University of Michigan Sedation Scale (UMSS) there were a total of 181 children included in this meta-analysis. When we analyzed the incidence of “any” sedation as defined as a UMSS score greater than zero, children receiving IN ketamine had a higher risk of sedation than those receiving IN fentanyl (pooled RR 1.81, CI 1.24 to 2.62, I2 = 0%) (Figure 5). Across all studies, no children had a score of 4 (deeply sedated) or 5 (unarousable) with either of the interventions. The raw data on sedation using the UMSS scores is presented in Data Supplement S5 and S6.
Risk of bias and quality of evidence
All four randomized trials included were considered to have low risk of bias (Data Supplement S7). The certainty in the pooled estimates using the GRADE approach[19] was deemed to be “High” for all outcomes except for the outcome of rescue analgesia, which was downgraded because of imprecision given its wide confidence interval. (Data Supplement S8) Per-protocol rather than intention-to-treat analysis was used by 3 studies.[15,22,23] The proportion of patients across studies who did not adhere to the intended intervention, however, was minimal and balanced between groups.
LIMITATIONS
First, this meta-analysis was limited to the ED and extended inclusion criteria to other settings such as hematology-oncology and burn centers would have resulted in more studies, although at risk of selecting out populations with altered metabolisms of medications and altered physiology for pain. The main focus of the study was acute pain management in opioid naïve child. Given the potential for frequent opioid exposure in sickle cell disease, this was used as an exclusion criterion. Second, not all studies reported all the outcomes of interest, however we were able to obtained non-published de-identified data from one study.[17] Third, there was a significant statistical heterogeneity (I2 = 60%) for the outcome of pain reduction from baseline to 10 or 15 minutes, which may be explained by the fact that we merged these time stamps when performing the meta-analysis. A random effects model was conservatively used for all meta-analyses to mitigate heterogeneity even though studies were considered to be clinically and methodologically similar. Forth, these studies were limited to children with age ≥ 3 years and therefore results do not necessarily apply to patients less than 3.
DISCUSSION
In this meta-analysis of four RCTs, intranasal analgesic-dose ketamine was found to be as efficacious as IN fentanyl for management of moderate to severe acute pain for children in the ED. There were no reported serious adverse events among children that received IN ketamine, though children who received ketamine had twice the rate of non-serious adverse events compared to fentanyl. The clinically acceptable margin for non-inferiority in the children pain literature has been found to be 10 mm on a scale of 0 to 100 mm.[26–28] Combining the data of these four studies, for a total of 276 children, we were able to show that IN analgesic-dose ketamine was non-inferior to IN fentanyl as the boundary of the CI did not cross the clinically significant threshold of 10 mm of difference at any time period. (Figure 3) Factors to consider in the further exploration of its clinical utility are optimal dosing, the tolerability of non-serious adverse events, and the desire to avoid opioids.
The optimal analgesic dosing of IN analgesic-dose ketamine remains unclear, with three out of four studies using the mathematically convenient dosing of 1 mg/kg and one study[22] using 1.5 mg/kg. Similarly, the dosing of IN fentanyl was 1.5 μg/kg in three out of four studies and 2 μg/kg in one study.[22] Even though Frey et al. used higher doses of IN ketamine, the reduction in pain was lower or similar to other studies, which could be explained by the fact that the studies using lower doses had higher usage of concomitant analgesics such as ibuprofen and acetaminophen. Another difference that may have contributed to less pain reduction despite higher doses is the enrollment of older children, age 8–17 years, where the other studies enrolled patients down to 3–4 years of age. Despite pain scales being numerically reported as 0 to 100, different instruments were used for specific age groups such as the Faces Pain Scale for younger children, which might also have influenced the effect estimates.
In the children that received IN analgesic-dose ketamine no serious adverse events were observed. The rare occurrence of serious adverse events may indicate the need for much larger sample sizes in order to detect these events. However, evidence of safety may be inferred from data obtained during utilization of IN ketamine at significantly higher doses for procedural sedation. A systematic review looking at the use of IN ketamine at dissociative doses in children included 20 studies reporting adverse events with no serious adverse events identified.[29] Nausea and vomiting were the most common adverse events associated with dissociative doses of IN ketamine.
In all of the four studies, non-serious adverse events were numerous for both medications. The events that were most frequently seen with analgesic-dose ketamine were dizziness, unpleasant taste and drowsiness. Dizziness from ketamine is likely due NMDA receptor blockade in the inner ear and vestibular nuclei.[30,31] The use of a higher dose of ketamine in Frey et al. did not translate in higher incidence of dizziness, suggesting this adverse event may be more likely threshold than dose-dependent in nature. This adverse event is transient and potentially mitigated by decreased visual sensory input. Another side-effect reported in intranasal medications administration is unpleasant taste. This is mostly due to medication “run off” from the nasopharynx into the oropharynx stimulating papillary gustatory cells (aka taste buds). Ketamine was commonly noted to be “bad” tasting. Two studies[15,22] used ketamine concentrations of 50 mg/ml and the other two[17,23] used 100 mg/ml. Utilization of more concentrated formulations (i.e. 100 mg/ml) should reduce unwanted medication run-off and improve tolerability.
Rates of non-serious adverse events and depth of sedation did not vary between studies utilizing 1.0 mg/kg and 1.5 mg/kg. Although the risk of any sedation (UMSS score other than 0) was higher with IN analgesic-dose ketamine, this was driven by UMSS scores 1 or 2 where children were easily roused with verbal command or light stimulation. In theory, feeling sleepy or drowsy may actually be desirable, especially in children with pain who have surrogate decision makers involved in their care, by helping mitigate some of the anxiety that accompanies the experience.
Given the current opioid crisis in the United States, outcomes such as decreased rescue analgesia and opioid sparing are desirable. All included studies had slightly different protocols to assess for need of rescue analgesia. (Data Supplement S2) Across studies, the need of rescue analgesia for children receiving IN ketamine ranged from 0% to 25% while IN fentanyl ranged from 0% to 34.1%. The results of the meta-analysis comparing IN ketamine to IN fentanyl yielded a wide confidence interval. This confidence interval indicates that we need larger studies to evaluate the impact of IN low-dose ketamine on rescue analgesia. Future studies should also focus on its potential opioid sparing effect for children undergoing pain management in the ED.
CONCLUSIONS
In summary, this systematic review and meta-analysis showed that analgesic-dose IN ketamine is as efficacious as IN fentanyl for management of acute pain in children in the ED. Clinicians can therefore feel confident in both efficacy and safety of IN ketamine for management of acute pain in the Emergency Department setting. Tolerability of non-serious adverse events and desire for opioid sparing analgesia will influence more widespread adoption.
Supplementary Material
Data Supplement S1. Search strategy.
Data Supplement S2. Summary of Rescue Analgesia Protocols.
Data Supplement S3. Number of Patients Any Adverse Events.
Data Supplement S4. Raw Numbers of Adverse Events*.
Data Supplement S5. Level of Sedation as Measured by the University of Michigan Sedation Scale*.
Data Supplement S6. Raw Numbers for the Highest Achieved UMSS1 Score.
Data Supplement S7. Risk of Bias framework using the version 2 of the Cochrane tool for assessing risk of bias in randomized trials (RoB 2).
Data Supplement S8. Summary of findings using the GRADE approach.
ACKNOWLEDGMENTS
We thank Cynthia J. Beeler, our medical librarian, who helped us to design and conduct the literature search for this study. Also, we thank the authors of original studies who shared additional data with us upon request.
Financial support:
Mayo Clinic Small Grant Program through CTSA Grant Number UL1 TR002377 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH.
Footnotes
Presentations:
This study has not been presented in a research meeting.
Conflicts of interest:
The authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Krauss BS, Calligaris L, Green SM, Barbi E. Current concepts in management of pain in children in the emergency department. Lancet. 2016;387(10013):83–92. doi: 10.1016/S0140-6736(14)61686-X [DOI] [PubMed] [Google Scholar]
- 2.Johnston CC, Gagnon AJ, Fullerton L, Common C, Ladores M, Forlini S. One-week survey of pain intensity on admission to and discharge from the emergency department: a pilot study. J Emerg Med. 16(3):377–382. doi: 10.1016/s0736-4679(98)00012-2 [DOI] [PubMed] [Google Scholar]
- 3.Cordell WH, Keene KK, Giles BK, Jones JB, Jones JH, Brizendine EJ. The high prevalence of pain in emergency medical care. Am J Emerg Med. 2002;20(3):165–169. doi: 10.1053/ajem.2002.32643 [DOI] [PubMed] [Google Scholar]
- 4.Wolfe TR, Braude DA. Intranasal medication delivery for children: A brief review and update. Pediatrics. 2010;126(3):532–537. doi: 10.1542/peds.2010-0616 [DOI] [PubMed] [Google Scholar]
- 5.Del Pizzo J, Callahan JM. Intranasal medications in pediatric emergency medicine. Pediatr Emerg Care. 2014;30(7):496–501. doi: 10.1097/PEC.0000000000000171 [DOI] [PubMed] [Google Scholar]
- 6.Duncan RW, Smith KL, Maguire M, Stader DE. Alternatives to opioids for pain management in the emergency department decreases opioid usage and maintains patient satisfaction. Am J Emerg Med. 2019;37(1):38–44. doi: 10.1016/j.ajem.2018.04.043 [DOI] [PubMed] [Google Scholar]
- 7.Goett R, Todd KH, Nelson LS. Addressing the Challenge of Emergency Department Analgesia: Innovation in the Use of Opioid Alternatives. J Pain Palliat Care Pharmacother. 2016;30(3):225–227. doi: 10.1080/15360288.2016.1209612 [DOI] [PubMed] [Google Scholar]
- 8.Ahern TL, Herring AA, Anderson ES, Madia VA, Fahimi J, Frazee BW. The first 500: Initial experience with widespread use of low-dose ketamine for acute pain management in the ED. Am J Emerg Med. 2015;33(2):197–201. doi: 10.1016/j.ajem.2014.11.010 [DOI] [PubMed] [Google Scholar]
- 9.Hirlinger WK, Dick W. [Intramuscular ketamine analgesia in emergency patients. II. Clinical study of traumatized patients]. Anaesthesist. 1984;33(6):272–275. http://www.ncbi.nlm.nih.gov/pubmed/6476334. Accessed April 22, 2020. [PubMed] [Google Scholar]
- 10.Green SM, Roback MG, Kennedy RM, Krauss B. Clinical practice guideline for emergency department ketamine dissociative sedation: 2011 update. Ann Emerg Med. 2011;57(5):449–461. doi: 10.1016/j.annemergmed.2010.11.030 [DOI] [PubMed] [Google Scholar]
- 11.Bouida W, Bel Haj Ali K, Ben Soltane H, et al. Effect on Opioids Requirement of Early Administration of Intranasal Ketamine for Acute Traumatic Pain. Clin J Pain. February 2020. doi: 10.1097/AJP.0000000000000821 [DOI] [PubMed] [Google Scholar]
- 12.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339. doi: 10.1136/bmj.b2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zorzela L, Loke YK, Ioannidis JP, et al. PRISMA harms checklist: Improving harms reporting in systematic reviews. BMJ. 2016;352:i157. doi: 10.1136/bmj.i157 [DOI] [PubMed] [Google Scholar]
- 14.Crawford MW, Galton S, Naser B. Postoperative morphine consumption in children with sickle-cell disease. Paediatr Anaesth. 2006;16(2):152–157. doi: 10.1111/j.1460-9592.2005.01705.x [DOI] [PubMed] [Google Scholar]
- 15.Reynolds SL, Bryant KK, Studnek JR, et al. Randomized Controlled Feasibility Trial of Intranasal Ketamine Compared to Intranasal Fentanyl for Analgesia in Children with Suspected Extremity Fractures. Acad Emerg Med. 2017;24(12):1430–1440. doi: 10.1111/acem.13313 [DOI] [PubMed] [Google Scholar]
- 16.Malviya S, Voepel-Lewis T, Tait AR, Merkel S, Tremper K, Naughton N. Depth of sedation in children undergoing computed tomography: Validity and reliability of the University of Michigan Sedation Scale (UMSS). Br J Anaesth. 2002;88(2):241–245. doi: 10.1093/bja/88.2.241 [DOI] [PubMed] [Google Scholar]
- 17.Quinn K, Kriss S, Drapkin J, et al. Analgesic Efficacy of Intranasal Ketamine Versus Intranasal Fentanyl for Moderate to Severe Pain in Children: A Prospective, Randomized, Double-Blind Study. Pediatr Emerg Care. July 2018. doi: 10.1097/PEC.0000000000001556 [DOI] [PubMed] [Google Scholar]
- 18.Sterne JAC, Savović J, Page MJ, et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366. doi: 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 19.Balshem H, Helfand M, Schünemann HJ, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64(4):401–406. doi: 10.1016/j.jclinepi.2010.07.015 [DOI] [PubMed] [Google Scholar]
- 20.Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration. www.handbook.cochrane.org. Published 2011. [Google Scholar]
- 21.Rohatgi A. WebPlotDigitizer version 4.2. https://automeris.io/WebPlotDigitizer. Published 2019.
- 22.Frey TM, Florin TA, Caruso M, Zhang N, Zhang Y, Mittiga MR. Effect of Intranasal Ketamine vs Fentanyl on Pain Reduction for Extremity Injuries in Children: The PRIME Randomized Clinical Trial. JAMA Pediatr. 2019;173(2):140–146. doi: 10.1001/jamapediatrics.2018.4582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Graudins A, Meek R, Egerton-Warburton D, Oakley E, Seith R. The PICHFORK (Pain in Children Fentanyl or Ketamine) Trial: A randomized controlled trial comparing intranasal ketamine and fentanyl for the relief of moderate to severe pain in children with limb injuries. Ann Emerg Med. 2015;65(3):248–254.e1. doi: 10.1016/j.annemergmed.2014.09.024 [DOI] [PubMed] [Google Scholar]
- 24.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- 25.Lau J, Ioannidis JPA, Terrin N, Schmid CH, Olkin I. The case of the misleading funnel plot. Br Med J. 2006;333(7568):597–600. doi: 10.1136/bmj.333.7568.597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Powell C V, Kelly AM, Williams A. Determining the minimum clinically significant difference in visual analog pain score for children. Ann Emerg Med. 2001;37(1):28–31. doi: 10.1067/mem.2001.111517 [DOI] [PubMed] [Google Scholar]
- 27.Gallagher EJ, Liebman M, Bijur PE. Prospective validation of clinically important changes in pain severity measured on a visual analog scale. Ann Emerg Med. 2001;38(6):633–638. doi: 10.1067/mem.2001.118863 [DOI] [PubMed] [Google Scholar]
- 28.Bijur PE, Silver W, Gallagher EJ. Reliability of the visual analog scale for measurement of acute pain. Acad Emerg Med. 2001;8(12):1153–1157. doi: 10.1111/j.1553-2712.2001.tb01132.x [DOI] [PubMed] [Google Scholar]
- 29.Poonai N, Canton K, Ali S, et al. Intranasal ketamine for anesthetic premedication in children: a systematic review. Pain Manag. 2018;8(6):495–503. doi: 10.2217/pmt-2018-0039 [DOI] [PubMed] [Google Scholar]
- 30.Soto E, Vega R. Neuropharmacology of Vestibular System Disorders. Curr Neuropharmacol. 2010;8(1):26–40. doi: 10.2174/157015910790909511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soto E, Flores A, Eróstegui C, Vega R. Evidence for NMDA receptor in the afferent synaptic transmission of the vestibular system. Brain Res. 1994;633(1–2):289–296. doi: 10.1016/0006-8993(94)91551-2 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Supplement S1. Search strategy.
Data Supplement S2. Summary of Rescue Analgesia Protocols.
Data Supplement S3. Number of Patients Any Adverse Events.
Data Supplement S4. Raw Numbers of Adverse Events*.
Data Supplement S5. Level of Sedation as Measured by the University of Michigan Sedation Scale*.
Data Supplement S6. Raw Numbers for the Highest Achieved UMSS1 Score.
Data Supplement S7. Risk of Bias framework using the version 2 of the Cochrane tool for assessing risk of bias in randomized trials (RoB 2).
Data Supplement S8. Summary of findings using the GRADE approach.