Abstract
Background
Perturbation of the CDK4/6 pathway is frequently observed in advanced bladder cancer. We investigated the potential of targeting this pathway alone or in combination with chemotherapy or immunotherapy as a therapeutic approach for the treatment of bladder cancer
Methods
The genetic alterations of the CDK4/6 pathway in bladder cancer were first analyzed with The Cancer Genome Atlas database and validated in our bladder cancer patient-derived tumor xenografts (PDXs). Bladder cancer cell lines and mice carrying PDXs with the CDK4/6 pathway perturbations were treated with a CDK4/6 inhibitor palbociclib to determine its anticancer activity and the underlying mechanisms. The combination index method was performed to assess palbociclib and gemcitabine drug–drug interactions. Syngeneic mouse bladder cancer model BBN963 was used to assess whether palbociclib could potentiate anti-PD1 immunotherapy.
Results
Of the 413 bladder cancer specimens, 79.2% harbored pertubations along the CDK4/6 pathway. Palbociclib induced G0/G1 cell cycle arrest but with minimal apoptosis in vitro. In mice carrying PDXs, palbociclib treatment reduced tumor growth and prolonged survival from 14 to 32 days compared to vehicle only controls (p = 0.0001). Palbociclib treatment was associated with a decrease in Rb phosphorylation in both cell lines and PDXs. Palbociclib and gemcitabine exhibited antagonistic cytotoxicity in vitro (CI > 3) and in vivo, but palbociclib significantly enhanced the treatment efficacy of anti-PD1 immunotherapy and induced CD8+ T lymphocyte infiltration in syngeneic mouse models.
Conclusions
The CDK4/6 pathway is feasible as a potential target for the treatment of bladder cancer, especially in combination with immunotherapy. A CDK4/6 inhibitor should not be combined with gemcitabine.
Electronic supplementary material
The online version of this article (10.1007/s00262-020-02609-5) contains supplementary material, which is available to authorized users.
Keywords: CDK4/6, Targeted therapy, Chemotherapy, Immunotherapy, Bladder cancer, Patient-derived xenograft
Background
Cell cycle deregulation is a common characteristic of many cancers, resulting in uncontrolled cell proliferation [1]. Cyclin-dependent kinases (CDKs) play key roles in regulating cell cycle progression by cooperating with cyclins [2, 3]. Many members of the CDK and cyclin families have been identified as drivers of the cell cycle [1]. The cyclin D-CDK4/6 complex is a major checkpoint in the pathway, and has been shown to phosphorylate the retinoblastoma protein (Rb), leading to gene transcription that releases the cell cycle checkpoint and drives cell cycle progression from the G0/G1 to S phase and allows cell division [3–5]. In addition, the cyclin D-CDK4/6 pathway exert its pro-tumorigenic effects through the Rb-independent pathways, such as MYC, SMAD3, FOXM1, and MEP50/PRMT5 [6]. The cyclin D-CDK4/6 pathway is frequently altered in bladder cancers. Based on The Cancer Genome Atlas (TCGA) database, approximately 90% of bladder cancers have alterations in cell cycle regulators, making this pathway the most affected one among all pathways [7]. Cyclin D1 is overexpressed in about 67% of bladder cancers and correlates with disease progression [8–10]. The CCND1 gene, encoding cyclin D1, is amplified in about 10% of bladder cancer specimens, while expression of its inhibitory gene CDKN2A, encoding p16INK4a, is lost in 47% of specimens [11, 12]. Therefore, the CDK4/6-RB pathway is a reasonable target for the treatment of bladder cancer [13, 14].
Several CDK4/6 small molecule inhibitors have been developed [3, 14, 15]. So far, three CDK4/6 inhibitors, palbociclib, abemaciclib, and ribociclib, have been approved by the Food and Drug Administration (FDA) for the treatment of hormone receptor-positive, human epidermal growth factor receptor 2 (HER2)-negative advanced or metastatic breast cancer with disease progression following endocrine therapy [16–18]. Palbociclib is a highly selective CDK4/6 inhibitor and shows strong anti-proliferative activity in a number of in vitro and in vivo tumor models, including breast cancer, colon cancer, osteosarcoma, glioblastoma, and mantle cell lymphoma [4, 19–22].
Bladder cancer is the second most common genitourinary malignancy after prostate cancer and the tenth most frequent cancer globally, leading to approximately 199,922 deaths in 2018 worldwide [23]. In the USA, it is estimated 80,470 people were diagnosed with bladder cancer and 17,670 deaths occurred from bladder cancer in 2019 [24]. Currently, the standard first-line treatment for metastatic bladder cancer is cisplatin-based chemotherapy, which is associated with median overall survival (OS) of only 13–15 months [25, 26]. Carboplatin-based chemotherapy yields a median OS of only 8–9 months in cisplatin-ineligible patients [27]. The treatment and prognosis for bladder cancer has not changed significantly over the last few decades until the first anti-programmed cell death ligand 1 (PD-L1) atezolizumab immunotherapy was approved by the FDA in 2017 [28]. So far, five anti-programmed cell death 1 (PD1) or anti-PD-L1 immune checkpoint antibodies have been approved by the US FDA for post-platinum patients, but the median OS is less than a year despite responses in ~ 15 to 20% of patients. Atezolizumab and pembrolizumab are also approved for cisplatin-ineligible patients whose cancer specimens have high PD-L1 expression [29, 30]. Recurrent genetic or epigenetic alterations have been identified in bladder cancer [7, 12, 31–33]. But so far only one targeted agent, the fibroblast growth factor receptor (FGFR) inhibitor erdafitinib, has been approved by the FDA for post-platinum metastatic bladder cancers with FGFR, mainly FGFR3, activation mutations, or translocations, which occur in less than 20% of advanced bladder cancer [34, 35]. Enfortumab vedotin was recently approved by the FDA for those bladder cancer patients who have disease progression after platinum-based chemotherapy and immunotherapy but has a duration of response of around 7.6 months. Therefore, there is a clearly unmet medical need in developing novel therapy to improve bladder cancer care. Here, we determined whether targeting the CDK4/6 pathway, either alone or in combination with chemotherapy or immunotherapy, can be used to treat bladder cancer.
Methods
Analysis of the TCGA bladder cancer database
The TCGA bladder cancer database, which include 413 cancer specimens, was analyzed through the cBioPortal for Cancer Genomics (www.cbioportal.org) Web site. Mutation, putative copy number alterations, mRNA expression, and protein expression of CDK4, CDK6, cyclin D1 (CCND1), cyclin D3 (CCND3), CDKN2A, CDKN2B, and RB1 were analyzed as a whole group and of individual genes.
Cell lines and other materials
Human bladder cancer cell lines (T24, J82, 5637, TCCSUP) were obtained from the American Type Culture Collection (Manassas, VA) and were cultured with the recommended medium at 37 °C with 5% CO2. Mouse bladder cancer cell line BBN963 was a generous gift from Dr. William Kim at University of North Carolina. Palbociclib (LC Laboratories, Woburn, MA) was dissolved in dimethyl sulfoxide (DMSO) to 10 mM stock concentrations for cell culture experiments and in phosphate-buffered solution (PBS) at 4 mg/ml for in vivo experiments. Gemcitabine (Eli Lilly, Indianapolis, IN) was diluted in PBS to 15 mg/ml stock concentrations. Anti-mouse PD1(CD279) antibody was purchased from BioXCell (West Lebanon, NH). MTS reagent was purchased from Promega (Madison, WI). Propidium iodide and Annexin V were purchased from BioLegend (San Diego, CA). The following antibodies were used for this paper: CDK4, GAPDH, total AKT, p-AKT, total ERK, p-ERK, E2F1, total RB, p-RB(Ser780), cyclin D3, cleaved caspase3, Ki-67, and CD8 (Cell Signaling Technology, Danvers, MA); Cyclin D1 (NeoMarkers, Fremont, CA); and actin (Sigma-Aldrich, St. Louis, MO). IHC kits were purchased from BioGenex (Fremont, CA).
Cell viability, cell cycle, and apoptosis assays
For cell viability, cells were seeded at 2000–5000 cells in 150 μl of medium per well in 96-well plates and incubated with different concentrations of palbociclib (0.001–10 μM), gemcitabine (0.0001–5 μM), or in combination for 72 h. For cell cycle analysis, cells were seeded at 60,000–90,000 cells/well in 6-well plates and treated with 0.1, 0.5, or 2 μM palbociclib for 48 h. Apoptosis in cell culture was measured by annexin-V-fluorescein isothiocyanate (FITC) and propidium iodide (PI) staining using the T24 cell line in which plates were seeded at 60,000 cells/well in 6-well plates and treated with 1 μM palbociclib for 48 h.
Patient-derived xenografts (PDXs) and animal studies
All animal studies were approved by the University of California Davis Institutional Animal Care and Use Committee (IACUC #17794) as previously described [36]. PDX BL0382 (passage 3) and PDX0293 (passage 3) were provided by The Jackson Laboratory (JAX, West Sacramento, CA). BL0382 was developed from an 88-year-old female patient diagnosed with cT2NxMx high-grade bladder urothelial carcinoma with lymphovascular invasion. BL0293 was developed from a 77-year-old female patient diagnosed with high-grade sarcomatoid transitional cell carcinoma. Fresh PDX specimens (3–5 mm3) were implanted subcutaneously into the flanks of 6–8-week-old NOD scid gamma (NSG) mice (JAX, West Sacramento, CA). For immunotherapy, C57BL6 albino mice (Charles River Laboratories, Hollister, CA) at 8 weeks old were implanted subcutaneously at the flanks with 5 × 106 BBN963 cells. When the tumors achieved average volumes of 150–300 mm3, mice with tumor implants were randomized to four groups (n = 10 per group) and treated with palbociclib twice weekly at 40 mg/kg, gemcitabine weekly at 150 mg/kg, or in combination, all through intraperitoneal injection. Gemcitabine was given for 4 weeks, and palbociclib was given until mice were killed. For immunotherapy, mice with BBN963 tumors were randomized to four groups (n = 10 per group) and treated with palbociclib twice weekly at 40 mg/kg, anti-PD1 twice weekly at 10 mg/kg, or in combination, all through intraperitoneal injection. Anti-PD1 was given for 4 weeks, and palbociclib was given until mice were killed. Animal weight and tumor size were measured twice a week. The tumor volume was calculated with the following formula: length (mm) × width (mm) × width (mm) × 0.5. GraphPad Prism 5.0 was used to calculate the percentage of tumor growth inhibition.
Western blotting and immunohistochemistry
Whole cells or tissue were lysed with RIPA buffer (1% NP40, 0.5% DOC, 0.1% SDS, 150 mM NaCl, and 50 mM Tris pH 8.0) containing a phosphatase inhibitor cocktail PhoStop and Complete protease inhibitor cocktail [Roche Diagnostics, Indianapolis, IN)]. Samples were spun down at 12,000 rpm at 4 °C for 20 min. The concentration of the supernatant was determined by BCA Protein Assay Kit according to the manufacturer’s instruction. Protein lysates were mixed with 4 × Laemmli loading buffer (AMRESCO, Solon, OH) and heated to 95 °C for 5 min. Proteins were separated on a 10% polyacrylamide gel and transferred to PVDF membranes (Millipore). The membranes were blocked for one hour with 5% nonfat milk and incubated with primary antibodies at a 1:1000 dilution overnight at 4 °C. Membranes were incubated with secondary antibodies at a 1:3000 dilution for 1 h at room temperature. Signal was detected by ECL reagents [SuperSignal West Pico, Thermo Scientific, Rockford, IL)] followed by exposure in a Bio-Rad Chemidoc Imaging System. Membranes incubated with phosphor-protein were washed with stripping buffer (Restore Plus Western, Thermo Scientific, Rockford, IL) before incubating for the total-protein analysis. IHC staining with anti-Ki-67 and CD8 antibodies (Cell Signaling, Danvers, MA) were performed according to the manufacturer’s protocol.
Flow cytometry analysis
Cells were plated in 6-well plates and incubated overnight. Drugs were added the next day, and cells were incubated for an additional 48 h. Cell apoptosis was measured by Annexin-V fluorescein isothiocyanate (FITC) and propidium iodide (PI) staining according to the manufacturer's protocol. Cell cycle analysis was performed as described previously (24). Flow cytometry was performed at the UC Davis Comprehensive Cancer Center Flow Cytometry Shared Resource, and data were analyzed using FlowJo software (FlowJo).
Statistics
Data are presented as mean ± standard deviation (SD). Group comparisons were carried out using one-way analysis of variance or Student's t test. Survival analysis was performed using the Kaplan–Meier method. The IC50 values and linear regression analyses were computed by GraphPad Prism 7 program (GraphPad Software Inc., San Diego, CA, USA). The combination index (CI) value of the palbociclib and gemcitabine interaction was calculated by CompuSyn program (Compusyn Inc., Paramus, NJ, USA). A p value of less than 0.05 was considered statistically significant.
Results
Alteration frequency of the CDK4/6 pathway in bladder cancer
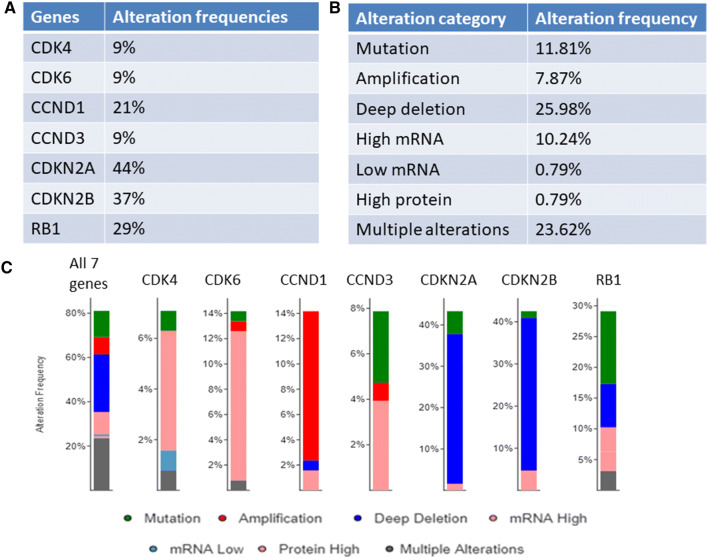
First, we analyzed the TCGA database and determined the alterations of the CDK4/6 pathways in bladder cancer. We studied the following genes, CDK4, CDK6, cyclin D1 (CCND1), cyclin D3 (CCND3), CDKN2A, CDKN2B, and RB1. Of the 413 samples, 327 (79.2%) samples had at least one of the following alterations in at least one of the genes: mutation, putative copy number alterations, mRNA expression, and protein expression (Fig. 1a, b; Supplemental Figure 1). These alterations were consistent with the corresponding functionalities as cell cycle regulators (Fig. 1c). For example, for four cell cycle promoters, CDK4, CDK6, CCND1, and CCND3, their alterations were mainly high mRNA expression (CDK4, CDK6, and CCND3), amplification (CCND1), or activation mutation (CCND3). For the two cell cycle negative regulators, CDKN2A and CDKN2B, their alterations were usually deep deletion. Alterations in RB1 were mainly inactivation mutations and deep deletion. Interestingly, alterations in RB1 and those of CDKN2A/CDKN2B were mutually exclusive (p < 0.05 both cases), while alterations of CCND1 and those of CDKN2A/CDKN2B were highly concurrent (p < 0.05 in both cases) (Supplemental Table 1).
Fig. 1.
Alteration landscape of the cyclin-CDK4/6 pathway in bladder cancer. The genomic information of 413 bladder cancer specimens was analyzed for alterations of seven genes along the cyclin-CDK4/6 pathway: CDK4, CDK6, CCND1, CCND3, CDKN2A, CDKN2B, and RB1. a Alteration frequencies of 7 genes in bladder cancer; b the alteration frequencies of each alteration subcategories; c the alteration frequencies of each gene
Cytotoxic effects of palbociclib in bladder cancer cell lines
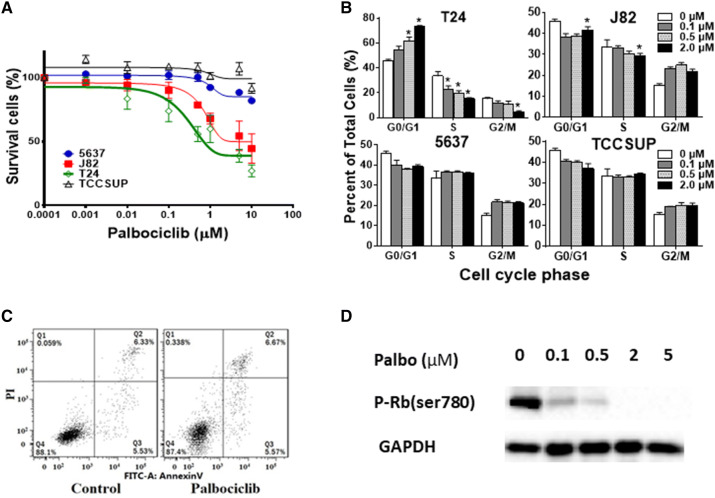
Considering the widespread alterations of cell cycle regulators in bladder cancer, we next determined whether targeting this pathway could be used for the treatment of bladder cancer. We first determined the efficacy of one of the three CDK4/6 inhibitors, palbociclib in four bladder cancer cell lines. T24 cells were highly sensitive to inhibition with an IC50 of 0.4 μM; J82 cells showed a moderate response with an IC50 of 1.0 μM; while TCCSUP and 5637 cells were resistant to palbociclib treatment with IC50 values of 97.7 and 58.1 μM, respectively (Fig. 2a). Cell cycle analysis demonstrated cell cycle arrest at the G0/G1 phase and decreased proportion at the S phase following 48 h of exposure to palbociclib in T24 cells, while no significant cell cycle disturbance was observed with resistant 5637 and TCCSUP cell lines at concentrations up to 2 µM (Fig. 2b). No significant increase in early (annexin V+ PI−) or late (annexin V+ PI+) apoptosis was detected in sensitive T24 cells after 1 μM palbociclib treatment (Fig. 2c). Consistent with this finding that palbociclib did not induce apoptosis, a large portion of sensitive T24 cells were alive and the survival curve did not go below 40% after 72 h of treatment (Fig. 2a). Palbociclib’s effects on CDK4/6-RB pathway were evaluated by Western blot. As shown in Fig. 2d, palbociclib decreased the phospho-RB. These data suggest that palbociclib could induce cell cycle arrest and inhibit cell proliferation in bladder cancer cells through the CDK4/6-RB pathway, but barely cause apoptosis.
Fig. 2.
Effect of CDK4/6 inhibitor palbociclib on bladder cancer cell lines. a Dose response curves of bladder cancer cell lines treated with palbociclib. T24, J82, TCCSUP, and 5637 cells were grown in 96-well plates and incubated for 72 h at 37 °C with increasing concentrations of palbociclib (1–10,000 nmol/L). The number of viable cells were determined by the MTS cell viability assay. b Cell cycle analysis of bladder cancer cell lines treated with palbociclib. Cells were treated with different concentrations of palbociclib (0.1, 0.5, 2 μM) or DMSO (Ctrl) for 48 h. Cell cycle progression was determined by flow cytometry. Columns mean; bars SD; n = 3. *p < 0.05. The percentage of S-phase was significantly decreased, and G1-phase was significantly increased in a dose-dependent manner in T24 and J82 cells; no significant change of cell cycle was detected in 5627 and TCCSUP cells. c Apoptosis analysis in T24 cells. Cells were treated with 2 μM palbociclib or DMSO (Ctrl) for 48 h and stained with Annexin V and propidium iodide (PI). One representative flow cytometry result was presented. Percentages of early (Annexin V+/PI−, Q3) and late apoptoses (Annexin V+/PI+, Q2) were indicated. No significant changes were observed, suggesting that palbociclib rarely induces apoptosis even in the sensitive T24 cells. d Western blot analysis of phospho-Rb protein in bladder cancer cell line T24. Cells were treated with palbociclib for 48 h with indicated palbociclib concentrations. p-Rb protein was inhibited in a dose-dependent manner
Antitumor activity of palbociclib in bladder cancer PDXs
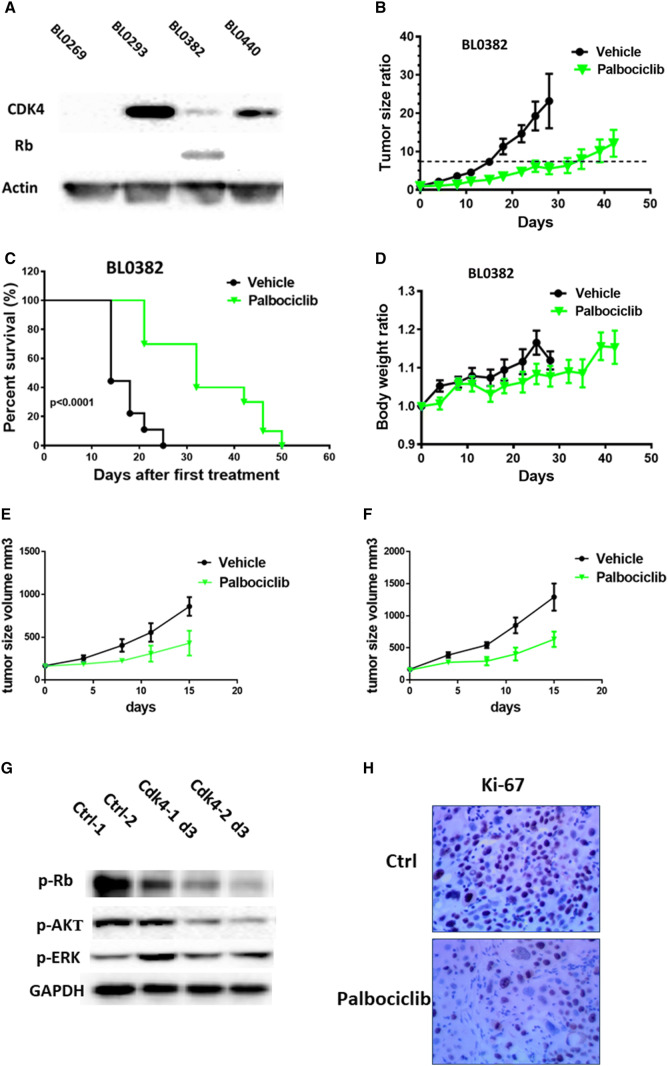
Next, we determined whether inhibition of the CDK4 pathway could inhibit tumor growth in PDXs. We previously showed that our bladder cancer PDXs retained the morphology fidelity and 92–97% genetic alterations of parental patient cancers [36]. It has been reported that there is a high concordance (15/17) of response between PDXs and patient tumors to treatment [37]. Therefore, findings in these PDXs can be better translated into clinical applications. We selected four PDXs and performed Western blots to determine the expression of some of the cell cycle regulators. CDK4 expression was detected in three out of four PDXs, while RB1 protein was detected in only one PDX (Fig. 3a).
Fig. 3.
Palbociclib inhibited tumor growth in bladder cancer PDX models. a Validation of CDK4 and RB1 expression in bladder cancer PDXs. b–d Studies in PDX BL0382 which expresses wild-type Rb. b Tumor growth curve of PDX BL0382 treated with palbociclib. The tumor in treatment group grew much slower than the control group. c Survival curves of control and palbociclib-treated mice. Median survival of control and palbociclib groups were 14 and 32 days, respectively, showing that treatment group had longer lifespan than the control group (p < 0.0001). d Body weight change in PDX BL0382 mice treated with palbociclib. Treatment group had slightly lower body weight than the control group, but not statistically significant. e, f Studies in PDX BL0293 which has no Rb expression. e Efficacy study in the parental PDX BL0293. Parental BL0293 was gemcitabine-sensitive and did not express RB1 as shown in a; f efficacy study in gemcitabine-resistant BL0293-subclones. Palbociclib significantly delayed tumor growth in both models. g, h Palbociclib-affected cell growth-related proteins in bladder PDX BL0382 model. g Western blot analysis of tumors obtained three days after palbociclib treatment. The expression of p-Rb and p-Akt were suppressed in treatment group. h Immunohistochemical staining of Ki-67 in tumor of BL0382 model. Palbociclib treatment reduced Ki67 expression compared to control group
We then selected PDX BL0382 for the efficacy study because it expressed CDK4, CDK6, cyclin D1, and their downstream factor Rb (Fig. 3a, Supplement Figure 2A and B) as some studies showed presence of functional RB is required for the antitumor activity of a CDK4/6 inhibitor [19, 21, 38, 39]. NSG mice bearing PDX BL0382 tumors were randomly divided into 2 groups (10 mice per treatment group). When the tumor volume reached approximately 200 mm3, mice were treated with vehicle control or palbociclib at 40 mg/kg twice per week via intraperitoneal injection until disease progression. As shown in Fig. 3b, tumor growth was significantly inhibited by palbociclib as compared to the control group. Consistent with the finding that palbociclib was cytostatic and did not induce apoptosis (Fig. 2c), the xenografts in mice treated with palbociclib grew much slower than those xenografts in control mice, but no tumor shrinkage was observed. We used the tumor volume of 7.5 × the baseline (approximately 1.5 cm3) as the study point for treatment response. Compared to 15 days in the control arm, the median time from the starting time to 7.5 × the baseline was 37 days (p < 0.0001) for the palbociclib treatment group (Fig. 3b). Compared to the overall survival (OS) of 14 days in the control arm, OS for the palbociclib group was 32 days (p < 0.0001) (Fig. 3c). Mice treated with palbociclib showed no adverse effects and maintained body weight comparable to mice in the control group (Fig. 3d). These data demonstrate that palbociclib effectively inhibits tumor growth and prolongs the overall survival in bladder cancer PDX model BL0382.
As shown in the TCGA data, approximately 29% bladder cancers have alterations of RB1, a downstream factor of cyclin/CDK, which may affect the efficacy of the CDK4/6 inhibitors. We next determined whether palbociclib was also effective in PDXs that lack Rb expression. For this experiment, we used PDX BL0293 which lacked the Rb expression (Fig. 3a). In addition, we used another PDX BL0293 sub-line BL0293-GemR that is resistant to gemcitabine. Palbociclib delayed tumor growth of both parental BL0293 and BL0293-GemR. At day 15, tumor sizes are significantly smaller in palbociclib group compared to control group (p = 0.037 in BL0293; p = 0.031in BL0293-GemR) (Fig. 3e, f). Overall, palbociclib treatment inhibited 60% of tumor growth in RB-positive PDX0382 and 47% in Rb negative PDX0293.
Palbociclib-affected proteins related to cell growth in bladder PDXs
Palbociclib treatment resulted in reduced p-RB levels (Fig. 3g), but it did not affect the expression of CDK4, CDK6, cyclin D1, and cyclin D3 (Supplement Figure 2). No significant change in cleaved caspase 3 was observed (Supplement Figure 2). As the MAPK/ERK pathway and PI3K/AKT/mTOR pathway are two major common pathways essential for tumor growth and survival, we investigated the effect of palbociclib on these pathways in the PDXs. Treatment with palbociclib resulted in significant downregulation of p-Akt compared to untreated controls, but it did not affect p-Erk levels (Fig. 3g). Immunohistochemical analysis of BL0382 xenograft tumor tissue revealed that palbociclib treatment reduced Ki-67 expression compared to control groups (Fig. 3h), suggesting decrease in cell proliferation. In summary, palbociclib treatment reduced p-Rb and p-Akt, which likely contributed to decreased cell proliferation.
Antagonistic drug–drug interaction between palbociclib and gemcitabine
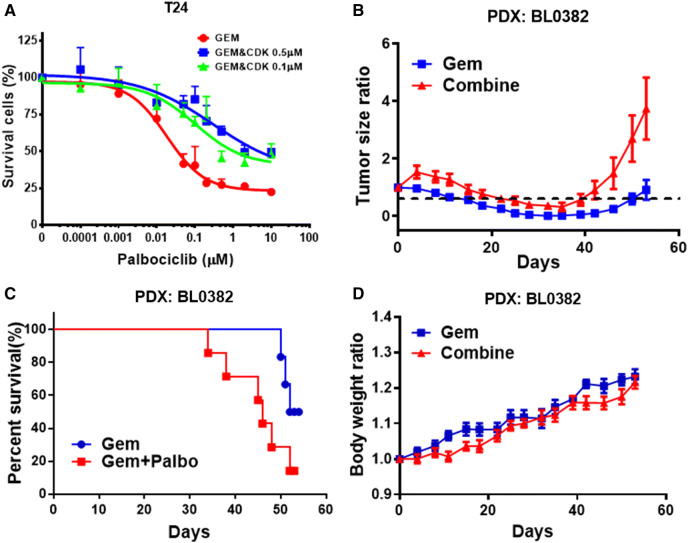
Like most other targeted therapies, response to palbociclib is usually transient. Gemcitabine is a nucleotide analog that is commonly used as a first-line therapeutic agent in the treatment of advanced bladder cancer. Here, we determined whether the antitumor activity could be improved with the combination of palbociclib and gemcitabine which targets cancer cells with different underlying mechanisms. First, we determined the combination index (CI) to assess the potential for drug–drug interactions between palbociclib and gemcitabine in cell culture with T24 cells [40]. As shown in Table 1, a strong antagonistic drug–drug interaction was observed with CI above 3 in all studies. The MTS assay showed the cytotoxicity curve of the gemcitabine and palbociclib combination shifted to the right compared to gemcitabine treatment alone. The IC50 values of gemcitabine was 0.02 µM, while the IC50s for gemcitabine + 0.5 µM palbociclib and gemcitabine + 0.1 µM palbociclib were 0.2 µM and 0.1 µM, respectively (Fig. 4a).
Table 1.
Combination index of gemcitabine and palbociclib in T24 cells
| Gem (µm) | 0.01 | 0.05 | 0.1 | 0.2 | 0.5 | 2 |
|---|---|---|---|---|---|---|
| Palbo (µm) | 0.1 | |||||
| CI value | 5.397 | 9.723 | 8.865 | 24.746 | 3.421 | 9.8 |
| Palbo (µm) | 0.5 | |||||
| CI value | 8.243 | 15.544 | 39.977 | 12.916 | 14.6374 | 15.0219 |
Fig. 4.
Effect of the palbociclib and gemcitabine combination in bladder cancer cells and PDX model. a Dose response curve of T24 bladder cancer cells treated with gemcitabine and gemcitabine + palbociclib. Cells were grown in 96-well plates and incubated with drugs for 72 h at 37 °C. Concentration of gemcitabine was 0.1–10,000 nM and palbociclib was 100 and 500 nM. b Tumor growth curve of PDX BL0382 treated with gemcitabine and gemcitabine + palbociclib. The combination treatment group grew faster after day 40 than the control group. c Survival curve of mice treated with gemcitabine or gemcitabine plus palbociclib. Mice treated with the combination lived shorter than those treated with gemcitabine alone (p = 0.016). d Body weight change in PDX BL0382 mice treated with gem and gem + palbociclib. No significant difference of body weight between the gemcitabine and gemcitabine + palbociclib groups, suggesting that the shorter survival of the combination group was not secondary to the toxicity
Next, we determined the antitumor activity of palbociclib plus gemcitabine in combination in vivo. The BL0382 PDX model was chosen for this study since our previous work showed that BL0382 was sensitive to gemcitabine and expressed CDK4 and Rb [36]. NSG mice bearing PDX BL0382 tumors were randomly divided into two groups (8–10 mice per treatment group). The gemcitabine plus palbociclib combination therapy had reduced antitumor activity when compared to gemcitabine as a single therapy. In contrast to the cytostatic effect in vitro (Fig. 2a) and no tumor shrinkage in vivo of palbociclib (Fig. 3b), gemcitabine killed cancer cells and induced tumor shrinkage (Fig. 4b). Tumor xenografts recurred much sooner when mice were treated with the gemcitabine plus palbociclib combination. Compared to 51 days in the gemcitabine treatment group, the median time from the start time to disease progression (above the baseline volume) was 44 days (p = 0.017) for the palbociclib plus gemcitabine combination group (Fig. 4b). Compared to the overall survival (OS) of 51.4 days in the gemcitabine arm, OS for the palbociclib plus gemcitabine group was 42.7 days (p = 0.016) (Fig. 4c). These data demonstrate that gemcitabine and palbociclib exhibit an antagonistic antitumor activity in vivo. Mice treated with combination therapy showed no more adverse effects and maintained body weight compared to mice with gemcitabine alone group (Fig. 4d).
Synergistic anti-tumor effect between palbociclib and anti-PD1-immunotherapy
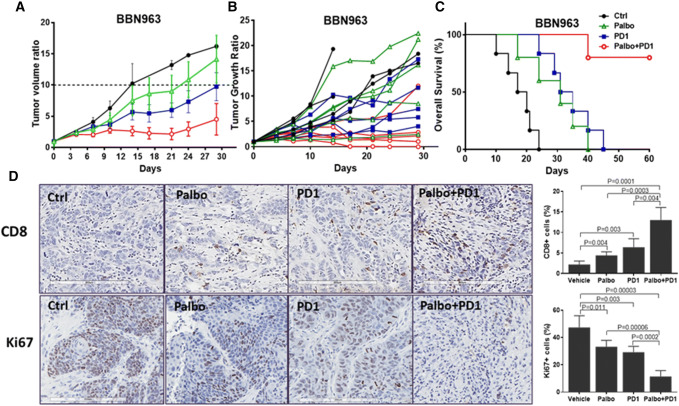
There is a great unmet need in improving the efficacy of immunotherapy in bladder cancer as the overall response rate of anti-PD1/PD-L1 immunotherapy is around 20% (Supplemental Table 2). It has been shown that CDK4/6 inhibitors could modulate the expression of antigen presentation genes [41]. Here, we determined whether palbociclib could potentiate immunotherapy in bladder cancer. A syngeneic immune-competent mouse model with a mouse bladder cancer cell line BBN963 was used for this study [42]. First, we determined and confirmed the expression of CDK4 and 6 in BBN963 (data not shown). As shown in Fig. 5a, treatment with single-agent palbociclib or anti-mouse PD1(CD279) exhibited moderate antitumor activity in mice bearing BBN963 tumors. The vehicle only control had a median time to a tenfold increase in tumor volume of 14 days, whereas the median times to this endpoint were 22.5 days (p = 0.04) and 29 days (p = 0.0001) for the palbociclib and anti-PD1 monotherapy groups. However, the palbociclib plus anti-PD1 combination group did not reach tenfold increase in tumor volume at the end of the experiment. The spider map of tumor growth is shown in Fig. 5b. Even though palbociclib potentiated the antitumor efficacy of anti-PD1, we did not observe any significant increase in toxicity based on the body weight measurement. Moreover, compared to the overall survival (OS) of 18.5 days in the control arm, OS for the palbociclib group and anti-PD1 monotherapy groups are 31 days (p = 0.04) and 33 days (p = 0.002), respectively (Fig. 5c). The median survival of mice treated with the palbociclib plus anti-PD1 combination did not reach when we terminated the experiment at day 60 (Fig. 5c). These data demonstrate that combination of palbociclib and PD1 effectively inhibits tumor growth and significantly prolongs the overall survival in the bladder cancer model.
Fig. 5.
Effect of the palbociclib and anti-PD1 antibody combination in a syngeneic mouse bladder cancer model. a Palbociclib potentiates immunotherapy in the BBN963 syngraft model. Tumor growth curve of BBN963 treated with palbociclib, anti-PD1 antibody, and combination of palbociclib plus anti-PD1 antibody. The tumor in the combination treatment group grew much slower than the other groups. b Spider map of tumor growth. c Survival curve of mice treated with palbociclib, anti-PD1 antibody, or combination. Mice with palbociclib, anti-PD1 antibody, or combination lived much longer than those with monotherapy. d The palbociclib plus anti-PD1 antibody combination decreased proliferation and induced T cell infiltration as determined by immunohistochemical analysis in mouse BBN963 models. Formalin-fixed paraffin-embedded BBN963 tumor sections were stained for Ki-67 and CD8. More Ki-67 positive cells were observed in the control group, but significantly decreased in the palbociclib plus anti PD1 combination group. Compared with the control group, increasing numbers of cells stained positive for CD8 were observed in the palbociclib, anti-PD1, and palbociclib plus anti PD1 combination groups. Quantitative data of Ki67 and CD8 staining in each group were generated from randomly selected 10 fields and are shown along with the images
Next, we examined if treatment of palbociclib and anti-PD1 could induce cytotoxic T cell infiltration in tumor tissue. CD8 is a T cell marker used to evaluate cytotoxic T cell expression. As shown in Fig. 5d, immunohistochemical analysis of the tumor tissue revealed a substantial increase in CD8-positive cells in the anti-PD1 antibody, palbociclib, and combination treatments compared to control group. Furthermore, Ki-67, a nuclear nonhistone protein that is preferentially expressed in dividing cells and is frequently used to assess the proliferation state of tissues, was significantly decreased in these three treatments groups compared to control group. These data demonstrate that the antitumor activity of the combination treatment with palbociclib and anti-PD1 antibody was, at least in part, due to decreased cell proliferation with increased immune response as demonstrated by cytotoxic T cell infiltration in tumor tissue and inhibited tumor proliferation in mouse bladder cancer model.
Discussion
Novel targeted therapies have revolutionized the management of some cancers, but have not had a significant impact for the majority of patients with advanced bladder cancer. Recent data from The Cancer Genome Atlas (TCGA) show that various molecules involved in cell cycle regulation are altered in 93% of muscle invasive bladder cancer [12]. The cyclin D-CDK4/6 pathway is frequently altered in bladder cancer [10, 43], making CDK4/6 a promising cancer drug target. Several CDK inhibitors have been approved by the FDA. In this study, we analyzed the TCGA database and gave a comprehensive overview of the landscape of alterations along the cyclin-CDK4/6 pathway in bladder cancer. We found that approximately 80% of bladder cancers have alterations along this pathway, making it potentially the most actionable pathway in bladder cancer. We then evaluated the antitumor effect of a CDK4/6 inhibitor palbociclib, as a single agent or in combination with chemotherapy or immunotherapy, in several bladder cancer models from cell lines, PDXs, and a syngeneic mouse model. Our results clearly showed that palbociclib, especially in combination with immunotherapy, was effective in suppressing tumor growth and prolonging overall survival of mice carrying bladder PDXs. The antitumor activity of palbociclib was achieved through downregulation of p-Rb and p-AKT, resulting in a G0/G1 cell cycle arrest without significant increase in apoptosis. Based on our study, palbociclib should not be combined with gemcitabine as this combination achieved antagonistic cytotoxic and antitumor effects. More importantly, palbociclib synergizes with anti-PD1 immunotherapy.
Considering the frequent alteration of the cyclin/CDK pathway in bladder cancer, our data strongly suggest that this pathway can be targeted for the treatment of bladder cancer. Unlike the FGFR3 alterations which occurs in less than 20% of advanced bladder cancer, alterations along the CDK4/6 pathway are very common, accounting for approximately 80% of all bladder cancers. Most alterations are overexpression and/or amplification of CDK4, CDK6, and cyclin Ds, or loss of CDKN2A and CDKN2B expression by DNA methylation. We used multiple models from cell lines to PDXs to show that a CDK4/6 inhibitor palbociclib can target this pathway and treat bladder cancer. One phase II trial evaluated palbociclib for metastatic platinum-refractory bladder cancer molecularly selected for p16 loss and intact Rb by tumor immunohistochemistry [44]. However, only 12 patients were enrolled and two patients (17%) achieved progression-free survival at 4 months with insufficient activity to advance to the next phase clinical trial. The findings from this trial suggest that CDK4/6 inhibitors, as single agents, may not induce long-term remission. Therefore, we proposed combination therapies to combine a CDK4/6 inhibitor with other agents.
Though CDK4/6 inhibitors have shown great antitumor effects in combination with endocrine therapy in breast cancer [45, 46], combining CDK4/6 inhibitors with chemotherapy has revealed controversial results. One study in bladder cancer cell lines showed a synergistic effect of palbociclib plus cisplatin when compared to cisplatin alone [47]. In contrast, an antagonistic effect was found for other cancer drugs. In an in vivo study in breast cancer, palbociclib as a single agent had antitumor activity, but the combination of palbociclib with carboplatin resulted in reduced antitumor activity compared with carboplatin alone [48]. Here, we clearly showed the antagonistic drug–drug interaction in both cell line and PDX studies when palbociclib was combined with gemcitabine. From a mechanistic standpoint, the combination of palbociclib and gemcitabine is more likely to be antagonistic. Gemcitabine is a nucleotide analog. Among several anticancer mechanisms, it kills cancer cells mainly through incorporation into DNA and termination of DNA replication and by modulating the availability of deoxynuceloside triphosphates (dNTPs) in the nucleotide pool during replication. Therefore, it is a cell cycle-specific chemotherapeutic drug that is more active in rapidly dividing cancer cells. Palbociclib inhibits CDK4/6, arrests cell cycles at G0/G1, and likely decreases the incorporation of gemcitabine into DNA and decreases the need for dNTPs. Therefore, based on these findings, combination of a CDK4/6 inhibitor with cell cycle-specific chemotherapeutic drugs, especially gemcitabine, should not be used.
Immunotherapy with immune checkpoint inhibitor anti-PD1/PD-L1 antibodies has emerged as a very promising therapeutic modality in bladder cancer and many other cancers. In most cancer types, however, only a minority of patients respond to immunotherapy. In bladder cancer, the response rates for five FDA-approved anti-PD1/PD-L1 antibodies are around 20% (Supplemental Table 2). Novel approaches are needed to improve the efficacy of immunotherapy. Here we showed that palbociclib significantly improved the efficacy of an anti-PD1 antibody in a syngeneic bladder cancer mouse model. Many mechanisms of resistance to immunotherapy have been proposed alone the anticancer immunity pathway [49]. We believe that the enhancement of antitumor activity with this combination is achieved at least through the following two mechanisms. First, palbociclib directly exerts its antitumor activity through cell cycle arrest and inhibition of tumor growth. This is evidenced by a decrease in Ki67 staining. Arrest of cancer growth provides the immune system more time and opportunity to exert its anticancer activity. Second, palbociclib potentiates immunotherapy. It has been shown that CDK4/6 inhibitors induce expression of antigen presentation genes [41]. Our findings support this observation. When tumor antigens are better presented, more immune cells are attracted to the cancer sites. As shown in our study, much more CD8+ T cells were observed when the syngeneic mice were treated with the combination of palbociclib and anti-PD1 antibody, compared to the control group or groups treated with palbociclib or anti-PD1 antibody alone.
Conclusions
The cyclin-CDK4/6 pathway is altered in approximately 80% of bladder cancers. Studies in cell lines, PDXs, and syngeneic animal models suggest that targeting the CDK4/6 pathway, alone or in combination with anti-PD1 immunotherapy, can potentially serve as a therapeutic approach in bladder cancer. The antitumor activity of palbociclib is observed in both Rb-positive and Rb-negative tumors, suggesting a wide range of bladder cancers may respond to the treatment. A CDK4/6 inhibitor should not be combined with gemcitabine. Given the strong antitumor effect of the CDK4/6 inhibitors in multiple models, these inhibitors, in combination with immunotherapy, should be considered for bladder cancer clinical trials and further investigated for potential use in combination with immunotherapy.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank Dr. Aminah Ikner and Dr. Paul Henderson for help during manuscript preparation.
Author contributions
QL and AHM designed and performed experiments, analyzed data, and contributed to writing the paper; HZ, ZC, and RX performed experiments and analyzed data; TYL, GPS, RDW, and JG provided essential experimental tools and support; CXP designed the investigation, analyzed data, supervised the study, and wrote the paper. All authors read and approved the manuscript as submitted.
Funding
Work was supported in part by U54 Grant (Grant No: 1 U54 CA233306; Contact PI: Pan), R01 Grant (Grant No: 1R01CA176803-01; PI: Pan), Merit Review (Award # 1I01 BX003840, PI: Pan) from the United States (U.S.) Department of Veterans Affairs Biomedical Laboratory Research and Development Program. The contents do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
Availability of data and materials
All data generated or analyzed during this study are included in this article and its supplementary information file.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no potential conflicts of interests.
Consent for publication
Not applicable.
Ethical approval
In vivo studies were approved by the Institutional Animal Care and Use Committee.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Qilai Long and Ai-Hong Ma contributed equally to this work.
Contributor Information
Jianming Guo, Email: guo.jianming@zs-hospital.sh.cn.
Chong-Xian Pan, Email: Chongxian_pan@hms.harvard.edu.
References
- 1.Malumbres M, et al. Mammalian cells cycle without the D-type cyclin-dependent kinases Cdk4 and Cdk6. Cell. 2004;118(4):493–504. doi: 10.1016/j.cell.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 2.Zardavas D, Baselga J, Piccart M. Emerging targeted agents in metastatic breast cancer. Nat Rev Clin Oncol. 2013;10(4):191–210. doi: 10.1038/nrclinonc.2013.29. [DOI] [PubMed] [Google Scholar]
- 3.Dickson MA. Molecular pathways: CDK4 inhibitors for cancer therapy. Clin Cancer Res. 2014;20(13):3379–3383. doi: 10.1158/1078-0432.CCR-13-1551. [DOI] [PubMed] [Google Scholar]
- 4.Fry DW, et al. Specific inhibition of cyclin-dependent kinase 4/6 by PD 0332991 and associated antitumor activity in human tumor xenografts. Mol Cancer Ther. 2004;3(11):1427–1438. [PubMed] [Google Scholar]
- 5.Choi YJ, Anders L. Signaling through cyclin D-dependent kinases. Oncogene. 2014;33(15):1890–1903. doi: 10.1038/onc.2013.137. [DOI] [PubMed] [Google Scholar]
- 6.Sheppard KE, McArthur GA. The cell-cycle regulator CDK4: an emerging therapeutic target in melanoma. Clin Cancer Res. 2013;19(19):5320–5328. doi: 10.1158/1078-0432.CCR-13-0259. [DOI] [PubMed] [Google Scholar]
- 7.Robertson AG, et al. Comprehensive molecular characterization of muscle-invasive bladder cancer. Cell. 2017;171(3):540–556.e25. doi: 10.1016/j.cell.2017.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.El-Gendi S, Abu-Sheasha G. Ki-67 and cell cycle regulators p53, p63 and cyclinD1 as prognostic markers for recurrence/progression of bladder urothelial carcinoma. Pathol Oncol Res. 2018;24(2):309–322. doi: 10.1007/s12253-017-0250-2. [DOI] [PubMed] [Google Scholar]
- 9.Kopparapu PK, et al. Expression of cyclin d1 and its association with disease characteristics in bladder cancer. Anticancer Res. 2013;33(12):5235–5242. [PMC free article] [PubMed] [Google Scholar]
- 10.Yang CC, et al. Expression of p16 and cyclin D1 in bladder cancer and correlation in cancer progression. Urol Int. 2002;69(3):190–194. doi: 10.1159/000063945. [DOI] [PubMed] [Google Scholar]
- 11.Lerner SP. Update on The Cancer Genome Atlas project on muscle-invasive bladder cancer. Eur Urol Focus. 2015;1(1):94–95. doi: 10.1016/j.euf.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cancer Genome Atlas Research Network Comprehensive molecular characterization of urothelial bladder carcinoma. Nature. 2014;507(7492):315–322. doi: 10.1038/nature12965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carneiro BA, et al. Emerging therapeutic targets in bladder cancer. Cancer Treat Rev. 2015;41(2):170–178. doi: 10.1016/j.ctrv.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 14.Pan Q, et al. CDK4/6 inhibitors in cancer therapy: a novel treatment strategy for bladder cancer. Bladder Cancer. 2017;3(2):79–88. doi: 10.3233/BLC-170105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Asghar U, et al. The history and future of targeting cyclin-dependent kinases in cancer therapy. Nat Rev Drug Discov. 2015;14(2):130–146. doi: 10.1038/nrd4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petrelli F, et al. Comparative efficacy of palbociclib, ribociclib and abemaciclib for ER+ metastatic breast cancer: an adjusted indirect analysis of randomized controlled trials. Breast Cancer Res Treat. 2019;174(3):597–604. doi: 10.1007/s10549-019-05133-y. [DOI] [PubMed] [Google Scholar]
- 17.Iwata H. Clinical development of CDK4/6 inhibitor for breast cancer. Breast Cancer. 2018;25(4):402–406. doi: 10.1007/s12282-017-0827-3. [DOI] [PubMed] [Google Scholar]
- 18.Vidula N, Rugo HS. Cyclin-dependent kinase 4/6 inhibitors for the treatment of breast cancer: a review of preclinical and clinical data. Clin Breast Cancer. 2016;16(1):8–17. doi: 10.1016/j.clbc.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 19.Finn RS, et al. PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro. Breast Cancer Res. 2009;11(5):R77. doi: 10.1186/bcr2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Higuchi T, et al. Sorafenib and palbociclib combination regresses a cisplatinum-resistant osteosarcoma in a PDOX mouse model. Anticancer Res. 2019;39(8):4079–4084. doi: 10.21873/anticanres.13565. [DOI] [PubMed] [Google Scholar]
- 21.Michaud K, et al. Pharmacologic inhibition of cyclin-dependent kinases 4 and 6 arrests the growth of glioblastoma multiforme intracranial xenografts. Cancer Res. 2010;70(8):3228–3238. doi: 10.1158/0008-5472.CAN-09-4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leonard JP, et al. Selective CDK4/6 inhibition with tumor responses by PD0332991 in patients with mantle cell lymphoma. Blood. 2012;119(20):4597–4607. doi: 10.1182/blood-2011-10-388298. [DOI] [PubMed] [Google Scholar]
- 23.Bray F, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 24.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 25.Bladder cancer: diagnosis and management of bladder cancer. BJU Int 120(6):755–765 [DOI] [PubMed]
- 26.von der Maase H, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol. 2000;18(17):3068–3077. doi: 10.1200/JCO.2000.18.17.3068. [DOI] [PubMed] [Google Scholar]
- 27.De Santis M, et al. Randomized phase II/III trial assessing gemcitabine/carboplatin and methotrexate/carboplatin/vinblastine in patients with advanced urothelial cancer who are unfit for cisplatin-based chemotherapy: EORTC study 30986. J Clin Oncol. 2012;30(2):191–199. doi: 10.1200/JCO.2011.37.3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller KD, et al. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin. 2019;69:363–385. doi: 10.3322/caac.21565. [DOI] [PubMed] [Google Scholar]
- 29.Ding X, et al. Clinicopathological and prognostic value of PD-L1 in urothelial carcinoma: a meta-analysis. Cancer Manag Res. 2019;11:4171–4184. doi: 10.2147/CMAR.S176937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ghate K, et al. PD-L1 expression and clinical outcomes in patients with advanced urothelial carcinoma treated with checkpoint inhibitors: a meta-analysis. Cancer Treat Rev. 2019;76:51–56. doi: 10.1016/j.ctrv.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 31.Rodriguez-Vida A, Lerner SP, Bellmunt J. The Cancer Genome Atlas project in bladder cancer. Cancer Treat Res. 2018;175:259–271. doi: 10.1007/978-3-319-93339-9_12. [DOI] [PubMed] [Google Scholar]
- 32.Rentsch CA, et al. Comprehensive molecular characterization of urothelial bladder carcinoma: a step closer to clinical translation? Eur Urol. 2017;72(6):960–961. doi: 10.1016/j.eururo.2017.06.022. [DOI] [PubMed] [Google Scholar]
- 33.Li HT, et al. Genetic and epigenetic alterations in bladder cancer. Int Neurourol J. 2016;20(Suppl 2):S84–94. doi: 10.5213/inj.1632752.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alifrangis C, et al. Molecular and histopathology directed therapy for advanced bladder cancer. Nat Rev Urol. 2019;16(8):465–483. doi: 10.1038/s41585-019-0208-0. [DOI] [PubMed] [Google Scholar]
- 35.Grivas P, Yu EY. Role of targeted therapies in management of metastatic urothelial cancer in the era of immunotherapy. Curr Treat Options Oncol. 2019;20(8):67. doi: 10.1007/s11864-019-0665-y. [DOI] [PubMed] [Google Scholar]
- 36.Pan CX, et al. Development and characterization of bladder cancer patient-derived xenografts for molecularly guided targeted therapy. PLoS ONE. 2015;10(8):e0134346. doi: 10.1371/journal.pone.0134346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hidalgo M, et al. Patient-derived xenograft models: an emerging platform for translational cancer research. Cancer Discov. 2014;4(9):998–1013. doi: 10.1158/2159-8290.CD-14-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dean JL, et al. Therapeutic CDK4/6 inhibition in breast cancer: key mechanisms of response and failure. Oncogene. 2010;29(28):4018–4032. doi: 10.1038/onc.2010.154. [DOI] [PubMed] [Google Scholar]
- 39.Wiedemeyer WR, et al. Pattern of retinoblastoma pathway inactivation dictates response to CDK4/6 inhibition in GBM. Proc Natl Acad Sci USA. 2010;107(25):11501–11506. doi: 10.1073/pnas.1001613107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chou TC. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010;70(2):440–446. doi: 10.1158/0008-5472.CAN-09-1947. [DOI] [PubMed] [Google Scholar]
- 41.Goel S, et al. CDK4/6 inhibition triggers anti-tumour immunity. Nature. 2017;548(7668):471–475. doi: 10.1038/nature23465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saito R, et al. Molecular subtype-specific immunocompetent models of high-grade urothelial carcinoma reveal differential neoantigen expression and response to immunotherapy. Cancer Res. 2018;78(14):3954–3968. doi: 10.1158/0008-5472.CAN-18-0173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Levidou G, et al. D-type cyclins in superficial and muscle-invasive bladder urothelial carcinoma: correlation with clinicopathological data and prognostic significance. J Cancer Res Clin Oncol. 2010;136(10):1563–1571. doi: 10.1007/s00432-010-0814-y. [DOI] [PubMed] [Google Scholar]
- 44.Rose TL, et al. Phase II trial of palbociclib in patients with metastatic urothelial cancer after failure of first-line chemotherapy. Br J Cancer. 2018;119(7):801–807. doi: 10.1038/s41416-018-0229-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Finn RS, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol. 2015;16(1):25–35. doi: 10.1016/S1470-2045(14)71159-3. [DOI] [PubMed] [Google Scholar]
- 46.Turner NC, et al. Palbociclib in hormone-receptor-positive advanced breast cancer. N Engl J Med. 2015;373(3):209–219. doi: 10.1056/NEJMoa1505270. [DOI] [PubMed] [Google Scholar]
- 47.Sathe A, et al. CDK4/6 inhibition controls proliferation of bladder cancer and transcription of RB1. J Urol. 2016;195(3):771–779. doi: 10.1016/j.juro.2015.08.082. [DOI] [PubMed] [Google Scholar]
- 48.Roberts PJ, et al. Multiple roles of cyclin-dependent kinase 4/6 inhibitors in cancer therapy. J Natl Cancer Inst. 2012;104(6):476–487. doi: 10.1093/jnci/djs002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pan C, et al. Next-generation immuno-oncology agents: current momentum shifts in cancer immunotherapy. J Hematol Oncol. 2020;13(1):29. doi: 10.1186/s13045-020-00862-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this article and its supplementary information file.